Distinct forms of tyrosine kinase domain (TKD), juxtamembrane domain, exon 8, and internal tandem duplication (ITD) mutations of c-KIT, were observed in about 46% of core binding factor leukemia (CBFL) patients. To evaluate their prognostic significance, 67 adult patients with CBFL were analyzed to ascertain the c-KIT mutation status. In acute myeloid leukemia (AML) with t(8;21), the presence of c-KIT TKD mutation at codon 816 (TKD816) was associated with a high white blood cell count at diagnosis (median, 29.60 × 109/L) and a higher incidence (33%) of extramedullary leukemia (EML) during the course of the disease. Data also showed that the TKD816 mutated patients (n = 12) had a significantly higher incidence of relapse and a lower overall survival (OS) at 24 months, compared with the 17 c-KIT unmutated (c-KIT–) patients (90% vs 35.3%, P = .002; 25% vs 76.5%, P = .006, respectively). No difference in relapse incidence (P = .126) and OS (P = .474) was observed between the c-KIT mutated other than TKD816 (n = 7) and the c-KIT– patients. These findings indicate that c-KIT TKD816 mutation has a negative impact on the outcome of AML with t(8;21).
Introduction
A more comprehensive prognostic evaluation of specific markers is important for the management of therapeutic interventions in acute myeloid leukemia (AML), as treatments can be optimized if the estimation of outcome is more accurate. Cytogenetic and molecular genetic aberrations provide important insights into the biology and pathogenesis of AML. Chromosomal abnormalities targeting the core binding factor include t(8;21)(q22;q22) and inv(16)(p13;q22). These abnormalities form a distinct subgroup, with common clinical features and outcomes, defined as core binding factor leukemia (CBFL).1,2
t(8;21)(q22;q22) occurs in about 8% of patients with de novo AML. In most cases, this disorder is morphologically associated with the French-American-British (FAB) AML-M2 subtype, showing a granulocytic maturation along the neutrophil pathway and, in a minority of cases, bone marrow eosinophilia or mastocytosis.3-5 Not infrequently, AML carrying t(8;21)(q22;q22) may develop extramedullary leukemia (EML), involving either orbital or head and neck sites in pediatric series or, more frequently, paraspinal sites in adults.6,7 The French Intergroup study has recently confirmed a high white blood cell (WBC) count at diagnosis to be the most important predictor of an inferior outcome.8
AML carrying inv(16)/t(16;16)(p13;q22) is frequently associated with monocytic and eosinophilic differentiation (AML-M4Eo) and, in some patients, with EML.9 A high WBC count at presentation and age were identified as the main risk factors for relapse, disease-free survival (DFS), and overall survival (OS).10,11
Clinically, both inv(16)/t(16;16) and t(8;21)(q22;q22) AML show a high rate of complete remission (CR) and prolonged CR duration, especially following consolidation chemotherapy with high-dose cytarabine (HD-ARAC), and are thought to have a better prognosis than patients with normal karyotype or other chromosomal aberrations.1,2,12-16
Recent advances in the understanding of molecular events leading to AML genesis include the description of activating mutations involving gene coding for class III tyrosine kinase receptors (RTKs), such as c-KIT, H-RAS, and c-FMS.17-20 Activating mutations of the c-KIT gene have been reported in between 12.8% and 46.1% of adult CBFL and in 12% of pediatric non–acute promyelocytic leukemia (APL) AML.21-23 Mutations may affect either the juxtamembrane domain proposed to regulate an otherwise normal enzymatic site of the c-KIT receptor, such as internal tandem duplication (ITD) of exon 11 or insertion/deletion of exon 8 of the c-KIT gene, or may involve the structure of the tyrosine kinase domain (TKD) mutation such as the substitution of a single amino acid at codon 816 (TKD816).
Recent results suggest that RTK mutations in patients with AML have an adverse effect on the outcome, even though prognostic implications of c-KIT mutations are still unclear.21,24,25
In this study, we evaluated the clinical features and the prognostic significance of c-KIT mutations in 67 adult patients with CBFL. We confirmed the prevalence of these mutations in this subset and the profile of associated clinical features and found that there is a greater relapse incidence (RI) and a worse OS in patients with t(8;21) carrying the c-KIT TKD816 mutation.
Patients, materials, and methods
Patients and data collection
All patients with untreated AML with either inv(16)/t(16;16) or t(8;21)(q22; q22), diagnosed in 6 Italian centers, were included in this analysis. Bone marrow samples from 67 patients were collected and cryopreserved at diagnosis. In blocks, the samples were then analyzed at the Department of Biology and Genetics for Medical Sciences, University of Milan, Italy. Each patient gave his or her informed consent for collection and cryopreservation of bone marrow (BM) samples and the performance of DNA analysis for scientific purposes, in accordance with institutional guidelines. The study design adhered to the Declaration of Helsinki, and approval for these studies was obtained from the Niguarda Hospital Review Board. A questionnaire containing a predefined set of data including hematologic presentation, cytogenetics, presence of EML, treatment, and outcome was filled out for each patient. In January 2005, the referring hematologists from each center reported any new observations (ie, date of relapse, type of relapse, presence of EML, hematologic data at relapse, cytogenetics at relapse, treatment administered, and outcome). The databases of the 6 centers were carefully checked for data accuracy by the respective referring doctors and then reviewed centrally for consistency and completeness. A unique database was subsequently created and submitted for the statistical analysis.
Screening of mutations in the entire coding region of c-KIT gene
All bone marrow samples cryopreserved and stored at diagnosis were centrally analyzed. Exon 17 mutations in the c-KIT gene were detected using sequencing and various other more sensitive assays such as: HinfI assay for Asp816Val,18,22 Tsp509I assay for Asn822Lys20 and ARMS (amplification refractory mutation system) polymerase chain reaction (PCR) for Asp816Tyr and Asp816His.22 Direct sequencing of DNA and cDNA products was performed using Thermo Sequence Dye Terminator sequencing reaction and ABI Prism 3100 sequencing analyzer (Applied Biosystems, Warrington, United Kingdom).
Treatment protocols
CBFL patients (53 of 67, aged ≤ 60 years) were enrolled in intensive chemotherapy protocols. As induction therapy, 35 patients received 12 mg/m2 idarubicin for 3 days (days 1, 3, and 5) and a 7-day continuous infusion (days 1-7) of 100 mg/m2/d cytarabine. Postremission therapy consisted of 3 consolidation courses. The first was a course of 10 mg/m2/d idarubicin for 2 days (days 1 and 3) and 3 g/m2/12 h cytarabine for 3 consecutive days. The second and the third were courses of 3 g/m2/12 h cytarabine for 3 consecutive days (patients older than 50 years received a reduced dose of cytarabine at 2 g/m2/12 h on days 1-3). Some (18) patients received induction therapy with ICE (10 mg/m2 idarubicin on days 1, 3, and 5; continuous infusion on days 1-7 of 100 mg/m2/d cytarabine and 100 mg/m2 etoposide on days 1-5). Postremission chemotherapy consisted of 1 course of NOVIA (12 mg/m2 mitoxantron on days 1-4 and 500 mg/m2/12 h cytarabine on days 1-6) and 2 courses of cytarabine (3 g/m2/12 h for 3 consecutive days).26 Patients without a human leukocyte antigen (HLA)–matched family donor received granulocyte colony-stimulating factor (G-CSF), 300 μg subcutaneously 1 day after having completed the second consolidation course and underwent leukaphereses to collect peripheral blood stem cells (PBSCs) with a target of more than 3 × 106 CD34+ cells/kg.27 Eight patients underwent autologous stem cell transplantation (ASCT) instead of the third consolidation course. No patients received allogeneic stem cell transplantation (allo-SCT) in first CR. Nine transplantations (2 ASCTs and 7 allo-SCTs) were performed in patients experiencing relapse after attempting to achieve a second or subsequent hematological response. The preparative treatment for both allo- or auto-SCT was 120 mg/kg cyclophosphamide over 2 days and total-body irradiation in 6 fractions of 200 cGy (1200 cGy) or 16 mg/kg busulfan over 4 days and 50 mg/kg cyclophosphamide over 4 days.
Criteria for response and definitions
CR was defined as less than 5% blasts in normocellular bone marrow with a peripheral neutrophil count greater than 1500/μL and platelet count greater than 100 000/μL. EML was defined as any leukemic collection outside the bone marrow. The diagnosis of EML could be established by either biopsy, clinical, or cerebrospinal fluid (CSF) criteria. Paraspinal masses that were symptomatic and documented by a computed tomography (CT) or magnetic resonance imaging (MRI) were accepted as EML. All patients with clinically diagnosed EML had to show no evidence of an active infection at the potential site of EML.6 Relapse was defined as at least 5% leukemic blasts in a bone marrow aspirate or new extramedullary leukemia in patients with a previously documented CR.12 OS was calculated from the date of diagnosis until death and all living patients, in CR or not, were censored at the time of last contact. The duration of CR was calculated from the date of the first CR until the date of the first relapse. DFS was calculated from the date of the first CR until the date of relapse or death in first CR.
Statistical analysis
Before inference, all data underwent descriptive analysis and was investigated by means of the Shapiro-Wilk and Kolmogorov-Smirnov tests to evaluate its distribution pattern. The normally distributed variables were summarized with mean and standard deviations, while the not-normal variables were summarized with median and range. Suitable parametric tests were used to compare the normally distributed variables. Nonparametric Pearson (with the Yate correction for continuity when applicable) or Fisher exact test and Mann-Whitney U test were used for contingency tables and for not-normal variables, respectively. When applicable, parametric tests were also used for these latter variables, following suitable nonlinear transformation (logarithmic, or box-cox) or adjusting for nonhomogeneous variances between groups (Welch test). To assess the survival analysis, the cumulative risk was calculated using the Nelson-Aalen estimator, then analysis was carried out using the Kaplan-Meier method, followed by the log-rank test, in order to evaluate the differences between the survivor function. Statistical analysis was carried out using Stata SE 9.0 (StataCorp LP, College Station, TX).
Results
Frequency of c-KIT mutations in adults with CBFL
There were 42 cases with t(8;21) and 25 with inv(16). Between the 2 groups of patients, there were no differences in age (Mann-Whitney U test: P = .097), sex (Pearson test: P = .855) and EML at presentation (Fisher exact test: P = .061). However, the WBC count was considerably higher in patients with inv(16) (Mann-Whitney U test: P = .009).
Mutational screening reported c-KIT mutations in 31 (46.2%) of 67 patients: 20 (29.9%) of 67 patients showed a D816 missense mutation (TKD816), 8 (11.9%) of 67 showed an exon 8 in-frame deletion plus insertion mutations, 1 patient (1.5%) had a transmembrane mutation, and 2 patients (3.0%) had an ITD mutation within exon 11. According to cytogenetics, 19 (45.2%) of 42 patients with t(8;21) and 12 (48.0%) of 25 patients with inv(16) fell into the “c-KIT–mutated group” (c-KIT+). Among the c-KIT+ patients, we detected 12 TKD816 mutations in t(8;21) and 8 in inv(16) (Pearson test: P = .767). The 36 patients who showed no mutations were classified as the “c-KIT–negative group” (c-KIT–; Table 1).
Correlation between the c-KIT mutational status and WBC count
The median WBC of the 67 patients was 15.95 × 109/L (range, 3.5-277.5 × 109/L) versus 10.90 × 109/L (range, 2.1-130.0 × 109/L; Mann-Whitney U test: P = 0.39) for c-KIT+ and c-KIT–, respectively.
The result was more significant for the 42 patients with t(8;21); analysis revealed a median WBC count of 18.85 × 109/L (range, 3.5-165.0 × 109/L) versus (range, 2.1-70.6 × 109/L; Mann-Whitney U test: P = .027) for the 19 c-KIT+ and the 23 c-KIT– patients, respectively. Furthermore, the presence of TKD816 mutation resulted highly significant to identify patients with an elevated WBC count at diagnosis (median WBC count 29.60 × 109/L vs 7.50 × 109/L; Mann-Whitney U test; P = .031). Paralleling the results of WBC, in the subgroup of patients with t(8;21), the value of WBC-Index (WBC-I), expressed as WBC × (% marrow blasts/100), was remarkably different (Mann-Whitney U test: P = .047) in the c-KIT+ group and, more specifically, in patients carrying the TKD816 mutation (Mann-Whitney U test: P = .005). (Table 2).
In the group of patients with inv(16), there was no significant difference in WBC count (Welch test: P = .285) and WBC-I (Mann-Whitney U test: P = .828) between the c-KIT+ and c-KIT– patients and between patients with or without TKD816 mutation.
EML and c-KIT mutational status
Five patients with t(8;21) and 5 with inv(16) of the 67 patients included in this study had EML at presentation and/or during the course of AML. In 8 patients, the EML manifested as granulocytic sarcoma. According to cytogenetics, 0 of 23 c-KIT– patients and 5 (23.3%) of 19 c-KIT+ patients with t(8;21) had EML (Fisher exact test: P = .014), at diagnosis (1 patient with ITD exon 8) or at relapse (4 of 12 patients with TKD816). The group of patients carrying the c-KIT mutation at TKD816 showed a high risk of developing EML (Fisher exact test: P = .009 for TKD816 vs c-KIT–; P = .021 for TKD816 vs c-KIT– in patients aged 60 years or younger) (Table 3). The association between the c-KIT mutational status and EML was not significant in patients with inv(16) (Fisher exact test: P = .645).
Overall results of treatments
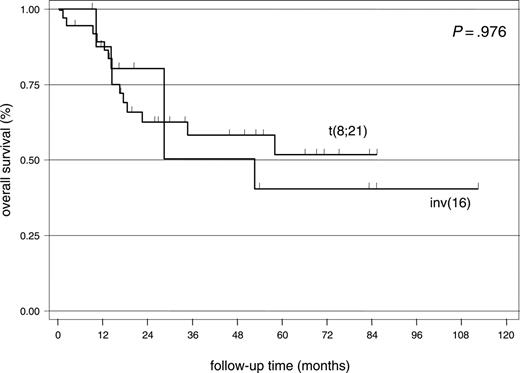
Patients (36) with t(8;21) and 17 patients with inv(16) aged 60 years old or younger (median, 40.5 years; range, 16-60 years) underwent intensive chemotherapy and were assessed for response. Most patients (51 [96.2%] of 53) achieved CR and 58.5% (31 patients) were alive after a median follow-up of 34 months (range, 1-111 months). There was no difference in RI (55.9% vs 66.7%; log-rank test: P = .435) and OS (log-rank test: P = .976) between the patients with t(8;21) and inv(16) (Figure 1).
Treatment outcome by c-KIT mutational status
Of the 53 patients who received intensive chemotherapy, 19 of 36 with t(8;21) and 8 of 17 with inv(16) resulted c-KIT+ upon mutational screening. Of these 27 c-KIT+ patients, we recorded 12 cases of TKD816 in t(8;21) and 7 in inv(16) AML. CR was reached in 92.6% (25 of 27) of c-KIT+ patients and in 100% of the remaining 26 c-KIT– patients (Fisher exact test: P = .491). Resistant disease and 1 toxic death accounted for the 2 TKD816 patients with t(8;21) who did not achieve CR.
Kaplan-Meier plot showing overall survival of patients with CBFL aged 60 years or younger.
Kaplan-Meier plot showing overall survival of patients with CBFL aged 60 years or younger.
Relapse incidence. The impact of c-KIT mutations on RI was different in AML with t(8;21) or inv(16). Among the 34 patients with t(8;21) achieving CR, with a median follow-up of 15 months (range, 3-82 months), we recorded a significantly higher incidence of relapse in c-KIT+ (13 [76.5%] of 17 patients) or TKD816 cases (9 [90.0%] of 10 patients) compared with c-KIT– (6 [35.3%] of 17 patients) (Figure 2, Tables 3, 4). Data showed no difference in RI between the c-KIT+ other than TKD816 (7 patients) and the c-KIT– cases (4 [57.1%] of 7 patients vs 35.3%; log-rank test: P = .126). In AML with inv(16), we were not able to demonstrate any difference in RI according to c-KIT mutational status (log-rank test: P = .902 for c-KIT+ vs c-KIT–; P = .974 for TKD816 vs c-KIT–).
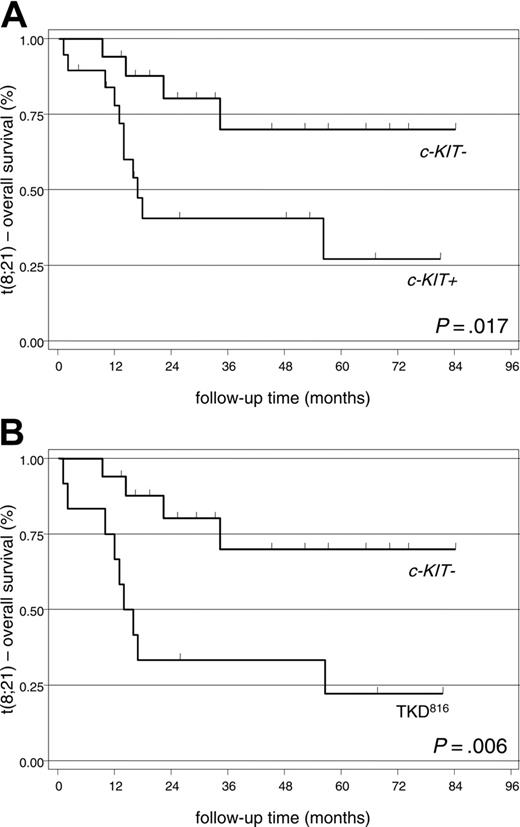
Overall survival. Most c-KIT– patients (19 [73.1%] of 26) and 12 (44.4%) of 27 of the c-KIT+ CBFL patients are still alive in January 2005 (log-rank test: P = .014). However, the impact of c-KIT mutation was only significant for the 36 patients with t(8;21). In this setting, with a median follow-up of 24 months (range, 1-84 months), we recorded a lower OS in the c-KIT+ group (8 [42.1%] of 19 patients) and more specifically, in patients carrying the TKD816 mutation (3 [25%] of 12 patients) than in the c-KIT– group (13 [76.5%] of 17 patients) (Figure 3, Tables 3,5). In contrast, the OS between c-KIT+ with mutations other than TKD816 (5 [71.4%] of 7 patients) versus the c-KIT– patients proved not significant (log-rank test: P = .474). In AML with inv(16), c-KIT mutational status appeared not to influence survival (log-rank test: P = .317 for c-KIT+ versus c-KIT–; P = .289 for TKD816 vs c-KIT–).
Kaplan-Meier plots showing relapse incidence of patients with t(8;21). (A) c-KIT+ versus c-KIT– patients. (B) TKD816 versus c-KIT– patients.
Kaplan-Meier plots showing relapse incidence of patients with t(8;21). (A) c-KIT+ versus c-KIT– patients. (B) TKD816 versus c-KIT– patients.
Discussion
No large study evaluating c-KIT mutations in CBFL patients is available, even if it has been reported that only a minority of patients have c-KIT mutations in this subset.21 In this study, we confirmed an incidence of c-KIT mutations of 46.2%, as recently reported by our group.22 These mutations were equally distributed among the t(8;21) and inv(16) patients and in most cases were represented by single amino acid substitution at TKD816 (Table 1).
Although a recent study suggested that RTK mutations in patients with t(8;21) AMLs are associated with a high cumulative incidence of relapse and poor relapse-free survival, the prognostic significance of c-KIT mutations remains unclear.25 Care et al21 reported that patients with inv(16) AML with c-KIT exon 8 mutation have a greater probability of relapse following complete remission. In a preliminary report on 18 patients with CBFL, our group demonstrated a weak statistical correlation between the presence of mutation and DFS.28 The present study, on a larger number of patients, shows that the presence of c-KIT mutation is associated with a significantly higher incidence of relapse and a worse survival in AML with t(8;21).
Kaplan-Meier plots showing overall survival of patients with t(8;21). (A) c-KIT– versus c-KIT+ patients. (B) c-KIT– versus TKD816 patients.
Kaplan-Meier plots showing overall survival of patients with t(8;21). (A) c-KIT– versus c-KIT+ patients. (B) c-KIT– versus TKD816 patients.
In a population of 53 patients with CBFL, aged between 16 to 60 years, we did not observe differences in complete remission rate, incidence of relapse, and overall survival between the 36 patients with t(8;21) and the 17 with inv(16). However, we are able to show that, within these groups, c-KIT mutations have a different impact on outcome.
The c-KIT+ patients with t(8;21) had a higher RI (P = .005) and a lower OS (P = .017) than the c-KIT– population. Furthermore, these differences resulted highly significant among the 12 patients carrying mutations at codon 816 (P = .002 for RI and P = .006 for OS). (Figures 2, 3, Tables 4, 5). In fact, of the 12 TKD816 patients, 10 achieved the first CR and 9 subsequently relapsed. The outcome after relapse was very poor in this subset: 7 patients died for resistant disease and only 2 achieved and maintained the second CR (Table 3).
Among the 7 patients with mutations other than TKD816 (5 with an exon 8 in-frame deletion plus insertion mutations and 2 with an ITD mutation within exon 11), the RI was 57% and the OS 71%, percentages not different from those observed in the c-KIT– patients.
The reasons why leukemia with kinase domain mutation has a lower DFS and is less responsive to salvage chemotherapy might be explained, on clinical grounds, by the analysis of WBC count and by the incidence of EML during the course of the disease.
It is worth noting that, in AML with t(8;21) we observed a significant difference in WBC count and WBC-I between the c-KIT– and the c-KIT+ cases at presentation, and more specifically, we recorded the highest WBC count and WBC-I in the group of patients carrying the TKD816 mutation (Table 2). The prognostic significance of high blood count (either WBC and absolute neutrophil count [ANC]) has already been reported in patients with t(8;21) in small series.29,30 Furthermore, in a recent French survey the WBC-I, calculated as WBC count × (% marrow blasts/100), was found to be the main prognostic factor for DFS, CR duration and OS.8
As an unexpected clinical feature, we noted an increased incidence of EML in the group of c-KIT+ patients with t(8;21); it is important to note that none of the c-KIT– cases developed EML and that 33.3% of patients with mutation at TKD816 had EML at relapse (Table 3). Existing literature suggests that the presence of EML at presentation and during the course of the disease is associated with a poor outcome in patients with t(8;21). However, authors substantially fail to prove significant differences in age, WBC count, percentage of marrow or peripheral blood blasts, FAB distribution, presence of del(9)(q22), and CD56 expression between the patients with and without EML.6,31,32 Although the association with EML and c-KIT mutational status in t(8;21) is suggestive, we are continuing the recruitment of patients to further confirm the link.
In AML with inv(16), our data indicate that c-KIT mutation does not affect the outcome, WBC count (P = .285), WBC-I (P = .828), or EML (P = .645).
In conclusion, this study shows that t(8;21) AMLs with TKD816 mutations are associated with a higher incidence of relapse following CR, a poor chance of salvage after relapse, and an inferior survival. Molecular characterization of t(8;21) may be a useful tool to identify a subset of CBFL patients with higher-risk disease.
Prepublished online as Blood First Edition Paper, December 29, 2005; DOI 10.1182/blood-2005-09-3640.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.