We examined whether combining all-trans retinoic acid (ATRA) and arsenic trioxide (ATO) might be an alternative to ATRA plus chemotherapy in untreated acute promyelocytic leukemia (APL). Twenty-five low-risk patients (white blood cell [WBC] count less than 10 × 109/L [10 000/μL]) received ATRA (45 mg/m2 daily) and ATO (0.15 mg/kg daily, beginning day 10 of ATRA), and in complete remission (CR) received ATO plus ATRA, without chemotherapy, unless they were reverse transcriptase–polymerase chain reaction (RT-PCR)–positive 3 months from CR date or had molecular relapse. Nineteen high-risk patients were treated identically, but received chemotherapy, generally 9 mg/m2 gemtuzumab ozogamycin (GO) on day 1 of induction. The CR rate was 39 of 44 (24 of 25 in low-risk, 15 of 19 in high-risk). Disease recurred at 9, 9, and 15 months, respectively, in 3 high-risk patients. The median follow-up time from CR date in the 36 patients alive in first CR is 16 months (15 months in low-risk, 20 months in high-risk), with 9 patients followed for at least 24 months. Each of the 36 patients was PCR-negative at last follow-up. Thus, none of the low-risk patients has received chemotherapy, and only 3 high-risk patients (the 3 with relapsed disease) have received chemotherapy past induction. ATRA plus ATO may serve as an alternative to chemotherapy in low-risk untreated APL (eg, in older patients) and, when combined with GO, may improve outcome in high-risk patients.
Introduction
Although all-trans retinoic acid (ATRA) is more commonly used than arsenic trioxide (ATO) in treatment of newly diagnosed acute promyelocytic leukemia (APL), ATO may be the more effective anti-APL agent. Thus, absence of the promyelocytic leukemia–retinoic acid receptor α (PML-RARα) transcripts characteristic of APL is more frequent when ATO is used in relapsed APL than when ATRA is used, for the same length of time, in newly diagnosed APL.1,2 Several groups have successfully used single-agent ATO in untreated APL,3,4 and Shen et al randomized untreated patients to receive ATRA, ATO, or ATRA plus ATO as induction therapy.5 They observed that the combination produced the greatest reduction in PML-RARα transcript number at time of complete remission (CR).5 Although in CR patients received 9 courses of chemotherapy in addition to ATRA, ATO, or ATRA plus ATO, as originally assigned, thus potentially narrowing any differences in outcome among the 3 treatments, relapse rates were lowest with ATRA plus ATO.
Our interest in ATRA plus ATO in APL reflected the possible ability of the combination to provide an alternative to ATRA plus chemotherapy. Here we report results of a protocol that used ATRA plus ATO for both induction and postremission therapy in newly diagnosed APL. Unlike Shen et al,5 we added chemotherapy (gemtuzumab ozogamycin [GO]6,7 ) only if: (1) the presenting white blood cell (WBC) count exceeded 10 × 109/L (10 000/μL); (2) the bone marrow was polymerase chain reaction (PCR) positive for PML-RARα 3 months from CR date; (3) a negative PCR reverted to positive (molecular relapse); or (4) toxicity forced discontinuation of ATRA or ATO. The decision to give patients with WBC counts higher than 10 × 109/L (10 000/μL) GO (1 dose on day 1 of induction therapy) reflected the low CR rate (3 of 8) we had observed in such patients given liposomal ATRA without chemotherapy.8
Patients, materials, and methods
Between 2/2/02 and 7/11/05, the dates when the first and final patient went on study, we saw 49 patients with newly diagnosed APL, with the diagnosis requiring immunohistochemical evidence of the PML-RARα rearrangement.9 Eligibility for the ATRA plus ATO protocol required a Zubrod performance status of 0 to 3, serum bilirubin and creatinine each less than 2.0 mg/dL, and no history of cardiac arrhythmias; the patient could not be in the first trimester of pregnancy. Four of the 49 patients were ineligible for the protocol. The remaining 45 were treated on the protocol after providing written informed consent according to M.D. Anderson guidelines. Approval for these studies was obtained from The University of Texas M.D. Anderson Cancer Center's institutional review board. One of these 45 also had breast cancer metastatic to the bone marrow, confounding interpretation of the effect of ATRA plus ATO. Accordingly, we herein report on 44 patients. Their median age was 45 years and median presenting white blood cell (WBC) count was 3100/μL. Using the criteria of Sanz et al,10 19 patients were high risk (presenting WBC count > 10 × 109/L [10 000/μL]), 9 were low risk (WBC count < 10 × 109/L [10 000/μL] and platelet count > 40 × 109/L [40 000/μL]), and 16 were intermediate risk (WBC count < 10 × 109/L [10 000/μL] and platelet count < 40 × 109/L [40 000/μL]). Hereafter, as per common practice, we will combine the low and intermediate groups into a single “low-risk” group.
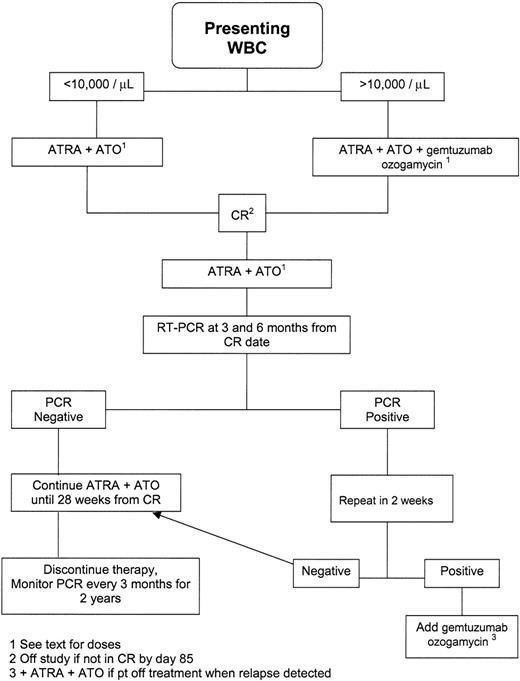
Figure 1 depicts the treatment scheme. Low-risk patients received 45 mg/m2 ATRA in 2 divided doses daily and, beginning 10 days later, 0.15 mg/kg ATO intravenously over 1 hour daily. Platelet transfusions were given to maintain the count at greater than 30 × 109/L (30 × 103/μL), while cryoprecipitate and/or fresh frozen plasma were administered to keep the serum fibrinogen above 1.5 g/L (150 mg/dL) and the internationalized normal ratio for prothrombin time less than 1.5. APL differentiation syndrome (APLDS) was treated with 45 mg methyprednisolone daily for 7 days. Marrow aspirates were obtained approximately weekly beginning 25 to 28 days after start of treatment. Once the marrow showed less than 5% blasts and no abnormal promyelocytes, ATRA and ATO were discontinued until occurrence of CR, defined by neutrophil and platelet counts greater than 1 × 109/L (1000/μL) and 100 × 109/L (100 × 103/μL), respectively, together with the noted marrow findings. Because CR occurred as late as 85 days after beginning ATO1 in the US multicenter trial in relapsed APL, patients were removed from study if not in CR by day 85.
Once in CR, patients received ATO intravenously at 0.15 mg/kg daily on Monday through Friday of weeks 1 to 4, 9 to 12, 17 to 20, and 25 to 28. They took 45 mg/m2 ATRA daily during weeks 1 to 2, 5 to 6, 9 to 10, 13 to 14, 17 to 18, 21 to 22, and 25 to 26. Therapy concluded 28 weeks after CR date. The dose of ATRA was cut in half if grades 3 to 4 toxicity (eg, headaches, rash) developed, with the drug discontinued if toxicity persisted after dose reduction. ATO was discontinued, or, depending on the severity of the toxicity, its dose reduced if peripheral neuropathy or arrhythmias were observed. If either ATRA or ATO were discontinued, patients received 9 mg/m2 GO once monthly until 28 weeks had elapsed from CR date.
Complete blood counts (CBCs) were checked every 1 to 2 months. Reverse transcriptase (RT)–PCR testing using bone marrow specimens was done at CR and every 3 months thereafter for 2 years. The test could detect PML-RARα fusion transcripts present at concentrations of 10–4 or more.6,8 If the PCR test was still positive 3 months from CR date, a repeat test was done 2 to 4 weeks later. The same procedure was followed if the PCR reverted to positive after being negative at 3 months. If the repeat PCR was also positive, a diagnosis of molecular relapse (or molecular failure if the test never became negative) was made, and patients were given 9 mg/m2 GO once monthly for 3 months while continuing ATO plus ATRA (or resuming it if relapse occurred after discontinuation of therapy). The same approach was to be used in the event of simultaneous molecular and clinical (hematologic or extramedullary) relapse. If the subsequent PCR became negative, 3 more months of GO plus ATRA plus ATO were prescribed. If not, 12 mg/m2 idarubicin daily for 3 days was to be substituted for GO, with patients referred for allogeneic transplantation if the PCR remained positive despite use of idarubicin.
High-risk patients were treated identically to low-risk patients except during induction. Most (18) of the 19 high-risk patients received chemotherapy: 15 patients received 9 mg/m2 GO on day 1, 1 patient received 12 mg/m2 idarubicin on days 1 to 4, and 2 patients received 12 mg/m2 GO on days 1 to 3 plus idarubicin. The decision to give idarubicin reflected physicians' discomfort with use of GO, as specified in the protocol.
The trial's major endpoints were CR rate and the proportion of patients entering CR who were PCR negative at 6 months from CR date. The choice of these “early” endpoints reflected the unconventional nature of our therapy in a disease where conventional therapy is quite successful. The statistical design was that described by Thall et al11 and was intended to stop the trial if, after each cohort of 5 patients was treated and evaluated, the probability was very low that either the CR rate was 80% or higher or that the proportion of patients who, after entering CR, were PCR negative at 6 months was 77% or higher, with these rates corresponding to those we had observed previously. The criterion probability 0.10 quantified the term “very low” for both endpoints. If the trial did not stop prematurely, 45 patients were to be entered. The 95% confidence limits provided by this sample size are described in Tables 1 and 3.
Results of induction therapy
Patients . | No. patients . | CR, no. (%, 95% CI) . | Median time to CR, d (range) . |
|---|---|---|---|
| All | 44 | 39 (89, 75-96) | 28 (19-48) |
| Low risk | 25 | 24 (96, 80-100) | 28 (19-48) |
| High risk | 19 | 15* (79, 54-94) | 32 (22-41) |
Patients . | No. patients . | CR, no. (%, 95% CI) . | Median time to CR, d (range) . |
|---|---|---|---|
| All | 44 | 39 (89, 75-96) | 28 (19-48) |
| Low risk | 25 | 24 (96, 80-100) | 28 (19-48) |
| High risk | 19 | 15* (79, 54-94) | 32 (22-41) |
Low risk was defined as having an initial WBC count below 10 × 109/L; high risk, as having an initial WBC count above 10 × 109/L. The low-risk patient who failed did so within 2 days of the start of therapy; the 4 high-risk patients, within 2, 2, 3, and 17 days of therapy.
Eleven of 15 patients with GO, 2 of 2 patients with GO + idarubicin, 1 of 1 patient with idarubicin, and 1 of 1 patient with only ATRA + ATO.
Molecular remission rates
Time from CR, mo . | No. patients PCR negative at or beyond date in question and positive before date in question . | Molecular remissions, no. (%, 95% CI) . |
|---|---|---|
| 3 | 29 | 29 (100, 88-100) |
| 6 | 33 | 33 (100, 89-100) |
| 12 | 23 | 20 (87,* 66-97) |
| 24 | 12 | 9 (75,† 43-95) |
| Longer than 24 | 11 | 8 (73,‡ 39-94) |
Time from CR, mo . | No. patients PCR negative at or beyond date in question and positive before date in question . | Molecular remissions, no. (%, 95% CI) . |
|---|---|---|
| 3 | 29 | 29 (100, 88-100) |
| 6 | 33 | 33 (100, 89-100) |
| 12 | 23 | 20 (87,* 66-97) |
| 24 | 12 | 9 (75,† 43-95) |
| Longer than 24 | 11 | 8 (73,‡ 39-94) |
Twenty patients were PCR negative at 12 months or later. But there were 3 patients who were PCR positive at 9 months. Counting these 3, there were 23 “evaluable” patients at 12 months, 20 of whom were PCR negative.
Nine patients were PCR negative at 24 months or later. But there were 3 patients who were PCR positive at 9 months. Counting these 3, there were 12 “evaluable” patients at 24 months, 9 of whom were PCR negative.
Eight patients were PCR negative at, respectively, 27, 28, 28, 28, 33, 33, 35, and 37 months from CR date. Counting the 3 patients who were PCR positive at 9 months, there were 11 patients who were evaluable at longer than 24 months, 8 of whom were PCR negative.
Results
Induction therapy
Table 1 depicts the outcome of induction therapy. CR rates were 89% (39 of 44) overall, 96% in low-risk patients, and 79% in high-risk patients. A few comments about the 5 patients who did not enter CR may be useful in interpreting the CR rates. In particular, as seen in Table 1, 4 of these 5 died within 3 days of beginning therapy and thus never received ATO (which was to begin on day 10); 2 of these 4 presented with intracranial or pulmonary hemorrhage. The fifth failure presented with a cerebral infarct, did not receive ATO, and died on day 17.
The APLDS was diagnosed in 6 patients (3 low-risk, 3 high-risk) and was judged probable/possible in another 3 (all low-risk) for an overall incidence of 20% (22% excluding patients dying in the first 3 days); it did not contribute to any of the deaths. All of the low-risk patients, but none of the high-risk patients, who developed APLDS had treatment-induced leukocytosis. The latter complication occurred in 20 (80%) of 25 low-risk patients and 2 (11%) of 19 high-risk patients, with the difference presumably due to use of GO in the high-risk patients. The median peak WBC count was 35 × 109/L (35 000/μL) (with the high of 193 × 109/L [193 000/μL] seen in the high-risk patient not given GO). The peak was reached at a median of 13 days (up to 26 days) after beginning treatment, with a rise apparent several days earlier. Elevations in serum aspartate aminotransferase (AST) or alanine aminotransferase (ALT) were seen in 17 patients (median peak value 125, up to 375) but were asymptomatic and unaccompanied by rises in serum bilirubin. In none of these 17 was ATRA or ATO discontinued.
Clinical outcome in CR
As seen in Table 2, relapses have occurred in 0 of 24 low-risk patients and 3 of 15 high-risk patients. Two of the relapses were simultaneously molecular and hematologic; both occurred 9 months from CR date. Both patients achieved a second CR, one with ATRA plus ATO plus GO and the second with idarubicin plus ATRA. Both remain in second CR, the first patient 21 months after an allogeneic stem cell transplantation and the second 2 months after an autologous stem cell transplantation. The third relapse was initially molecular, at 12 months from CR date, but, despite addition of GO, central nervous system (CNS) relapse occurred 3 months later, and the marrow remained PCR positive. The patient did not wish further treatment. No patients have died in CR.
Outcomes in CR
. | . | . | Median time from CR date to most recent CBC in patients remaining in first CR, mo. . | No. first CR rates, by date of most recent CBC* . | . | . | ||
|---|---|---|---|---|---|---|---|---|
| Patients . | No. CRs . | No. relapses . | . | 1-2 y from CR . | 2-3 y from CR . | 3-4 year from CR . | ||
| All | 39 | 3 | 16 | 12 | 8 | 1 | ||
| Low risk | 24 | 0 | 15 | 9 | 4 | 0 | ||
| High risk | 15 | 3 | 20 | 3 | 4 | 1 | ||
. | . | . | Median time from CR date to most recent CBC in patients remaining in first CR, mo. . | No. first CR rates, by date of most recent CBC* . | . | . | ||
|---|---|---|---|---|---|---|---|---|
| Patients . | No. CRs . | No. relapses . | . | 1-2 y from CR . | 2-3 y from CR . | 3-4 year from CR . | ||
| All | 39 | 3 | 16 | 12 | 8 | 1 | ||
| Low risk | 24 | 0 | 15 | 9 | 4 | 0 | ||
| High risk | 15 | 3 | 20 | 3 | 4 | 1 | ||
Low risk was defined as having an initial WBC count below 10 × 109/L; high risk, as having an initial WBC count above 10 × 109/L. Two high-risk patients had simultaneous molecular and clinical relapses, each at 9 months; one patient had molecular followed by clinical relapse, at 12 and 15 months, respectively.
In 12 patients, the most recent CBC was obtained 1 to 2 years from CR date; in an additional 8 patients, the most recent CBC was obtained 2 to 3 years from CR date; and in a final patient the interval between CR date and most recent CBC is 3 to 4 years.
The study remained open to new patients until very recently, thus reducing the median follow-up time in the 36 patients who remained in first CR. The median time from CR date to most recent CBC, which was normal in all cases, was 16 months in these 36. The corresponding time was 15 months in the 24 low-risk patients, and 20 months in the 12 high-risk patients (Table 2). Because of the somewhat artificial reduction in median follow-up time, it is instructive to note that 12 patients remain in first CR with their most recent CBC obtained 12+ to 24 months from CR date, 8 additional patients remain in first CR with their most recent CBC obtained 24+ to 36 months from CR date, and 1 patient's most recent CBC was 37 months from CR date (Table 2), bearing in mind that bone marrow aspirates were not always done in conjunction with blood counts.
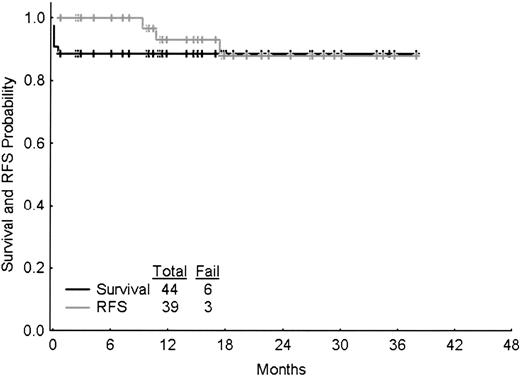
Survival and relapse-free survival
These are depicted in Figure 2. There have been 6 deaths (the 5 induction failures and 1 of the 3 relapses).
Molecular response and relapse
Most (37) of the 39 patients achieving CR had PCR testing at, or about the time of, CR. Thirty-five of the 37 were positive. In all 35, the tested marrow was morphologically normal, and in 22 of the 35 this marrow was obtained when the blood counts met the criteria for CR. The marrow was obtained a median of 1 week before formal CR date in the remaining 13. It was obtained 2 days and 18 days before this date in the 2 patients who were PCR negative at CR, with the latter observation suggesting that the virtually uniform pattern of a positive PCR at CR was not an artifact of when the test was done relative to CR date.
Most (34) of the 39 patients who entered CR had 1 or more or more follow-up PCR tests done at least 3 months from CR date. The results of these tests indicate that patients invariably became PCR negative as they continued ATRA plus ATO. For example, 29 of 29 patients tested at 3 months, and 33 of 33 patients tested at 6 months from CR, date were PCR negative (Table 3). There have been 3 molecular relapses, each in a high-risk patient, and with each having had a simultaneous or subsequent clinical relapse, as described in “Clinical outcome in CR.” Each molecular relapse occurred between 6 and 12 months from CR date, while 20 patients were PCR negative at 12 months or later. Thus, the molecular remission rate at 12 months is shown as 20 of 23 patients in Table 3. Similarly, this rate was 75% at 24 months and 73% at more than 24 months. The most recent PCR test was negative in 31 of the 36 patients remaining in first CR (the exceptions just entered CR); 11 were tested 1 to 2 years from CR date, 7 were tested 2 to 3 years from CR date, and 1 was tested 37 months from CR date. Since there have been no molecular relapses in the 24 low-risk patients who entered CR, none of these 24 has had to receive chemotherapy, with a median of 12 months between CR date and the date of the most recent negative PCR, and with 8 patients PCR negative 1 to 2 years, and 3 patients PCR negative 2 to 3 years, from CR date. Note that the difference between the 12 months median referred to immediately above and the 15 months shown for low-risk patients in Table 2 reflects the fact that marrow is not routinely sent for PCR when blood counts are checked. A median of 17 months have elapsed between CR date and the most recent negative PCR in the 12 high-risk patients who remain in molecular CR with ATO plus ATRA and only 1 dose of GO, with 3 patients PCR negative at 1 to 2 years, 4 patients PCR negative at 2 to 3 years, and 1 patient PCR negative at 3 to 4 years from CR date.
Survival and relapse-free survival (RFS). “Fail” denotes deaths for the survival graph (6 deaths) and relapse (3 relapses) or deaths in CR (none) for the relapse-free survival graph. Tick-marks denote censoring times.
Survival and relapse-free survival (RFS). “Fail” denotes deaths for the survival graph (6 deaths) and relapse (3 relapses) or deaths in CR (none) for the relapse-free survival graph. Tick-marks denote censoring times.
Early discontinuation of ATO
Five patients had toxicity (3 with arrhythmias, 1 with prolonged QT interval, and 1 with peripheral neuropathy) that led to discontinuation of ATO and its replacement by GO. ATO was discontinued at the end of induction therapy in 2 patients in whom arrhythmias were discovered during Holter monitoring with monitoring prompted by palpitations in one and syncope in the other. Since in neither patient were these symptoms reproduced during the monitoring that discovered the arrhythmias, the relation between the symptoms and the arrhythmias (atrial fibrillation, nonsustained ventricular tachycardia) remains unclear. The third patient with an arrhythmia had asymptomatic nonsustained ventricular tachycardia during a stress test; this prompted discontinuation of ATO after completion of the second postremission course of ATO. A corrected QT (QTc) interval of 513 milliseconds led to discontinuation ATO at the end of induction therapy in a 74-year-old patient, while the peripheral neuropathy was noted after 2 postremission cycles of ATO.
Excluding the 5 patients described in whom GO replaced ATRA plus ATO, this leaves 34 patients who entered CR. Three of these have relapsed as noted in “Clinical outcome in CR.” The median time from CR date to the most recent normal CBC in the remaining 31, all of whom have received only ATO plus ATRA in CR, is 14 months, with 10 patients followed for 1 to 2 years, 6 patients followed for 2 to 3 years, and 1 patient followed for 3 to 4 years. The median time from CR date to most recent negative PCR test is 12 months, with 9, 5, and 1 patient PCR negative 1 to 2, 2 to 3, and 3 to 4 years from CR date, respectively.
Older patients
Table 4 indicates that the CR rate in such patients was 10 of 12 (83%; 95% confidence interval [95% CI] 52%-98%). One of the 10 relapsed. The 9 remaining alive in first hematologic and molecular CR have been followed for a median of 17 months from CR date to both date of last normal CBC and date of last negative PCR.
Outcomes in patients aged 60 years and older
. | . | . | . | . | Time from CR, mo . | . | |
|---|---|---|---|---|---|---|---|
| Patient . | Age, y . | Risk group* . | Outcome of induction . | Outcome in CR . | Last normal CBC . | Last negative PCR . | |
| 1 | 63 | High | CR | Alive in first CR | 37 | 33 | |
| 2 | 74 | Low | CR | Alive in first CR | 33 | 33 | |
| 3 | 64 | Low | CR | Alive in first CR† | 27 | 27 | |
| 4 | 79 | High | CR | Molecular relapse at 12 mo, CNS relapse at 15 mo | — | — | |
| 5 | 74 | Low | CR | Alive in first CR | 23 | 23 | |
| 6 | 68 | Low | CR | Alive in first CR | 17 | 17 | |
| 7 | 72 | Low | CR | Alive in first CR | 14 | 11 | |
| 8 | 67 | High | Died d 3 | — | — | — | |
| 9 | 81 | Low | CR | Alive in first CR | 6 | 6 | |
| 10 | 69 | Low | CR | Alive in first CR | 2 | 2 | |
| 11 | 75 | Low | CR | Alive in first CR | 0 | 0 | |
| 12 | 66 | High | Died d 2 | — | — | — | |
. | . | . | . | . | Time from CR, mo . | . | |
|---|---|---|---|---|---|---|---|
| Patient . | Age, y . | Risk group* . | Outcome of induction . | Outcome in CR . | Last normal CBC . | Last negative PCR . | |
| 1 | 63 | High | CR | Alive in first CR | 37 | 33 | |
| 2 | 74 | Low | CR | Alive in first CR | 33 | 33 | |
| 3 | 64 | Low | CR | Alive in first CR† | 27 | 27 | |
| 4 | 79 | High | CR | Molecular relapse at 12 mo, CNS relapse at 15 mo | — | — | |
| 5 | 74 | Low | CR | Alive in first CR | 23 | 23 | |
| 6 | 68 | Low | CR | Alive in first CR | 17 | 17 | |
| 7 | 72 | Low | CR | Alive in first CR | 14 | 11 | |
| 8 | 67 | High | Died d 3 | — | — | — | |
| 9 | 81 | Low | CR | Alive in first CR | 6 | 6 | |
| 10 | 69 | Low | CR | Alive in first CR | 2 | 2 | |
| 11 | 75 | Low | CR | Alive in first CR | 0 | 0 | |
| 12 | 66 | High | Died d 2 | — | — | — | |
— indicates not applicable.
High indicates presenting WBC counts > 10 000/μL; low, presenting WBC counts < 10 000/μL).
GO replaced ATO in CR because of prolonged QTc interval.
Discussion
The results suggest that ATRA plus ATO is effective treatment for newly diagnosed APL, and that its use may provide an alternative to chemotherapy in this disease. Although the CR rate may appear relatively low (89%), it should be noted that some of our induction failures might not have received treatment on protocols reporting a higher response rate. Bearing this in mind, our survival data also appear representative of that reported with ATRA plus anthracyclines,12 as do our relapse-free survival data.
Our median follow-up of 16 months reflects the inclusion of patients who very recently entered CR, given that the study was only recently closed to new patients. For this reason we note (Table 2) that 9 patients remain in first CR for at least 25 months from CR date. Four of these 9 were low risk and never received chemotherapy. The remaining 5 were high risk and thus received chemotherapy, but only during induction. The possibility that ATRA plus ATO may be able to substitute for chemotherapy is strengthened by the high molecular remission rates illustrated in Table 3.
Burnett et al have demonstrated that similar proportions of patients who remain in CR and whose disease reappears are PCR positive at CR, and that the predictive value of a positive PCR is greatest after 3 cycles of chemotherapy have been delivered.13 Thus, there is no necessary clinical significance to the virtually 100% rate of positive PCR tests in our patients at CR, given that with continued treatment all patients became PCR negative within 3 months of CR date. Assuming that further follow-up confirms the persistence of PCR negativity, several potential uses for this combination come to mind. In particular, although treatment of newly diagnosed APL is quite successful, problems remain in treatment of older patients and high-risk patients; administration of ATRA plus ATO without chemotherapy as described here might be of use specifically in the former and the combination of ATRA plus ATO plus GO in the latter. Using idarubicin, mitoxantrone, and ATRA, Sanz et al noted that 6 of 25 patients aged 70 years and older died in remission,14 while Ades et al reported that 19% of patients aged 60 years and older died due to complications of myelosuppression during consolidation with daunorubicin plus cytarabine.15 These observations have led Fenaux et al16 and LoCoco et al17 to propose attempts to reduce the intensity of chemotherapy, at least in older patients with low-risk untreated APL. The data in Table 4 suggest that use of ATO plus ATRA may be an alternative to typical consolidation chemotherapy in such patients. We believe that the data from all our low-risk patients can be extrapolated to older patients, given that while age is predictive of death in CR, it has not been shown to be predictive of likelihood of relapse.16 ATO plus ATRA, together with GO either as administered here or given for 1 to 2 postremission courses, may also be useful in high-risk patients in whom success rates remain considerably below those seen in low-risk patients and who remain the focus of attempts to improve results.18,19 Indeed, based at least partially on our results, the US Intergroup is planning investigation of this combination in such patients (F. Appelbaum, personal communication, July 2005). It should be stressed that our approach in neither low-risk nor high-risk patients can be viewed as standard, and that the ultimate value of these approaches requires further follow-up.
Prepublished online as Blood First Edition Paper, December 22, 2005; DOI 10.1182/blood-2005-10-4006.
Supported by an R21 grant from the National Cancer Institute (R21CA101341) to E.E.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors appreciate Diana Daech's help in formatting the paper.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal