Abstract
Although TP53 mutations are rare in acute myeloid leukemia (AML), inactivation of wild-type p53 protein frequently occurs through overexpression of its negative regulator MDM2 (murine double minute 2). Recently, small-molecule antagonists of MDM2, Nutlins, have been developed that inhibit the p53-MDM2 interaction and activate p53 signaling. Here, we study the effects of p53 activation by Nutlin-3 in AML cells. Treatment with MDM2 inhibitor triggered several molecular events consistent with induction of apoptosis: loss of mitochondrial membrane potential, caspase activation, phosphatidylserine externalization, and DNA fragmentation. There was a positive correlation in primary AML samples with wild-type p53 between baseline MDM2 protein levels and apoptosis induced by MDM2 inhibition. No induction of apoptosis was observed in AML samples harboring mutant p53. Colony formation of AML progenitors was inhibited in a dose-dependent fashion, whereas normal CD34+ progenitor cells were less affected. Mechanistic studies suggested that Nutlin-induced apoptosis was mediated by both transcriptional activation of proapoptotic Bcl-2 family proteins, and transcription-independent mitochondrial permeabilization resulting from mitochondrial p53 translocation. MDM2 inhibition synergistically enhanced cytotoxicity of cytosine arabinoside and doxorubicin in AML blasts but not in normal hematopoietic progenitor cells. p53 activation by targeting the p53-MDM2 interaction might offer a novel therapeutic strategy for AML that retain wild-type p53.
Introduction
Activation of the p53 tumor suppressor protects multicellular organisms against the propagation of cells that carry damaged DNA.1 p53 is the most frequently inactivated protein in human cancer, and more than 50% of all solid tumors carry a mutation in the TP53 gene that abrogates its DNA binding and transactivation activity.2 Since the inactivation of p53 in cancer has been associated with poor survival, refractory disease, and chemoresistance,3-5 p53 gene therapy and reactivation of mutant p53 have been designed to restore p53 function.6,7 In acute myeloid leukemia (AML), TP53 mutations have been detected in only about 5% of patients, mostly those with 17p monosomy,8-11 but mutation is recognized as an adverse factor for response to chemotherapy and prognosis.11,12 Alternatively, it has been suggested that inactivation of wild-type p53 protein frequently occurs through binding to its principal cellular regulator MDM2 (murine double minute 2).13-15 MDM2 is a p53-specific E3 ubiquitin ligase, that mediates the ubiquitin-dependent degradation of p53.16 MDM2 has been found to be frequently overexpressed in AML, a process that can actively enhance tumorigenic potential and resistance to apoptosis.13-16
Depending on the degree and/or the nature of DNA damage, cells undergo either cell-cycle arrest or apoptosis in order to allow DNA repair or altruistic cellular suicide, respectively.17,18 p53 plays a critical role in determining the fate of cells by balancing these opposite cellular responses, mainly through the transcriptional induction of target genes.19 Numerous target genes of p53 have been identified that may be involved in p53-dependent apoptosis.19 Although little is known about the details of this process, central to p53-dependent apoptosis is the induction of genes encoding BH3-only members of the Bcl-2 family, which indirectly promotes Bax/Bak activation by inhibiting the functions of antiapoptotic Bcl-2 or Bcl-XL. The p53 target genes of the BH3-only members include Noxa20 and Puma.21,22 The proapoptotic multidomain Bcl-2 family member Bax, which regulates the release of apoptogenic factors from mitochondria, is also transcriptionally activated by p53.23 In addition, p53 can trigger mitochondrial outer membrane permeabilization and apoptosis in the absence of transcription, and this can occur through direct activation of Bax or Bak or through binding to Bcl-2 and Bcl-XL, which blocks their activity.24-26 Resolving the role for this mechanism versus that of transcriptional regulation will be important in understanding the apoptotic function of p53.
Recently, potent and selective small-molecule antagonists of MDM2, Nutlins, have been identified.27 Nutlins bind MDM2 in the p53-binding pocket and activate the p53 pathway in human cancer cells with wild-type p53, leading to cell-cycle arrest, apoptosis, and growth inhibition of human tumor xenografts in nude mice. In principle, induction of the p53 response in transformed cells that retain a functional p53 pathway can lead to apoptosis, as opposed to reversible growth arrest in normal cells, and targeting the p53-MDM2 interaction might offer a novel approach to treat tumors that retain wild-type p53. Here we investigate the potential therapeutic utility of p53 activation by MDM2 antagonists in AML.
Materials and methods
Reagents
The selective small-molecule antagonists of MDM2, Nutlin-3a and its 150-times-less-active enantiomer Nutlin-3b, were used.27 A stock solution of 10 mM Nutlin-3a and -3b in dimethyl sulfoxide (DMSO) was stored at –20°C. The final DMSO concentration in the medium did not exceed 0.11% (vol/vol). At this concentration, DMSO itself had no effect up to 72 hours on cell growth or viability of the AML cells used in this study. DMSO- or 10 μM Nutlin-3b–treated cells were used as controls. In some experiments, cells were cultured with 3.5 μM cycloheximide (Sigma-Aldrich, St Louis, MO) or 50 μM Z-VAD-FMK (Alexis, San Diego, CA). Cycloheximide and Z-VAD-FMK were added to the cells 1 hour before MDM2 inhibitor administration.
Cell lines, primary samples, and cell cultures
Four AML cell lines were cultured in RPMI 1640 medium containing 10% heat-inactivated fetal calf serum (FCS). OCI-AML-3 and MOLM-13 cells have wild-type p53, whereas p53 is disabled in HL-60 and NB4 cell lines by either deletion (HL-60) or missense mutation (NB4) of the p53 gene.28-30 Bone marrow and peripheral blood samples were obtained from patients with AML (> 60% blasts) and healthy donors after informed consent, according to institutional guidelines and the Declaration of Helsinki. Mononuclear cells were purified by Ficoll-Hypaque (Sigma Chemical, St Louis, MO) density-gradient centrifugation. Normal CD34+ cells were separated to more than 95% purity by positive-selection magnetic-bead sorting using a VarioMACS device (Miltenyi Biotec, Auburn, CA). Cell lines were harvested in log-phase growth, seeded at a density of 2 × 105 cell/mL, and exposed to the MDM2 inhibitor Nutlin-3a, or to a matched concentration of Nutlin-3b. Primary AML mononuclear cells seeded at 5 × 105 cells/mL in RPMI 1640 medium supplemented with 10% FCS were also exposed to the MDM2 inhibitors. In experiments involving combination of Nutlin-3a and cytosine arabinoside (AraC), the 2 agents (0, 1, 2.5, 5, or 10 μM) were added simultaneously to cells and cultured for 48 hours. In combination experiments of Nutlin-3a and doxorubicin (DOX), OCI-AML-3, primary AML, and normal CD34+ cells were treated with DOX at 0, 10, 25, 50, or 100 nM and MOLM-13 cells at 0, 4, 10, 20, or 40 nM. The concentration ratio of DOX to Nutlin was 1:100 in OCI-AML-3, primary AML, and normal CD34+ cells and 1:125 in MOLM-13 cells. In all experiments, cell viability was evaluated by triplicate counts of trypan blue dye–excluding cells. AML blast and normal hematopoietic progenitor colony assays were performed by plating 25 000 AML blast or 5000 CD34+ cells/mL into Methocult GF+ H4535 (Stem Cell Technologies, Vancouver, BC, Canada) supplemented with 3 U/mL recombinant human (rhu)–erythropoietin. Differential colony counts were assessed by morphology following incubation for 10 to 14 days at 37°C and 5% CO2 in a humidified atmosphere.31
Cell-cycle and apoptosis analysis
For cell-cycle analysis, cells were fixed in ice-cold ethanol (70% vol/vol) and stained with propidium iodide (PI) solution (25 μg/mL PI, 180 U/mL RNase, 0.1% Triton X-100, and 30 mg/mL polyethylene glycol in 4 mM citrate buffer, pH 7.8; Sigma Chemical). The DNA content was determined using a FACSCalibur flow cytometer (Becton Dickinson Immunocytometry Systems, San Jose, CA). Cell-cycle distribution was analyzed using ModFit LT software (Verity Software House, Topsham, ME). Cells with a hypodiploid DNA content were counted as apoptotic based on DNA fragmentation. Cell debris was defined as events in the lowest 10% range of fluorescence and eliminated from analysis. For annexin V binding studies, cells were washed twice with binding buffer (10 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], 140 mM NaCl, and 5 mM CaCl2 at pH 7.4; Sigma Chemical Co.) and incubated with a 1:500 solution of fluorescein isothiocyanate (FITC)–conjugated annexin V (Roche Diagnostic, Indianapolis, IN) for 15 minutes at room temperature. Stained cells were analyzed by flow cytometry, while membrane integrity was simultaneously assessed by PI exclusion. In some experiments on primary AML cells, cells were counterstained with CD34 PE (8G12; BD Biosciences, San Jose, CA) or respective isotype control antibody. To measure mitochondrial membrane potential (Δψm), cells were loaded with MitoTracker Red CMXRos (300 nM) and MitoTracker Green (100 μM; both from Molecular Probes, Eugene, OR) for 1 hour at 37°C. The Δψm was then assessed by measuring CMXRos retention (red fluorescence) while simultaneously adjusting for mitochondrial mass (green fluorescence). All experiments were conducted in triplicate.
Western blot analysis and coimmunoprecipitation
Cells were lysed in protein lysis buffer. An equal amount of protein lysate was placed on 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) for 2 hours at 100 V, followed by transfer of the protein to a Hybond-P membrane (Amersham Biosciences, Piscataway, NJ). Membranes were probed with rabbit polyclonal anti-p53 (FL-393; 1:200 vol/vol; Santa Cruz Biotechnology, Santa Cruz, CA), rabbit polyclonal anti-Bax (1:1000 vol/vol; BD Biosciences), mouse monoclonal anti-Noxa (114C307; 1:250 vol/vol; EMD Biosciences, San Diego, CA), rabbit polyclonal anti-Puma (Ab-1; 1:500 vol/vol; EMD Biosciences), rabbit polyclonal anti–caspase-3 (1:1000 vol/vol; Cell Signaling Technologies, Beverly, MA), mouse monoclonal anti–caspase-8 (1:1000 vol/vol; Cell Signaling Technologies), rabbit polyclonal anti–caspase-9 (1:1000 vol/vol; Cell Signaling Technologies), and mouse monoclonal anti–β-actin (AC-74; 1:3000 vol/vol; Sigma Chemical). The membranes were washed 3 times with phosphate-buffered saline (PBS) containing 1% Tween-20, and probed with a secondary antibody. Blots were reacted with enhanced chemiluminescence (ECL) reagent (Amersham Biosciences) and signals were detected by phosphoimager Storm 860 (Molecular Dynamics, Sunnyvale, CA). Visualized blots were analyzed by the public-domain National Institutes of Health image 1.63 program. For coimmunoprecipitation, whole-cell lysates were incubated with 30 μL agarose conjugate anti-p53 (FL-393) or anti–Bcl-XL (S-18; Santa Cruz Biotechnology) for 2 hours. Beads were washed 3 times with PBS, and equal amounts of immunoprecipitated p53 or Bcl-XL were subjected to Western blotting.
Immunofluorescence and confocal microscopy
Cells were washed twice with PBS, fixed, and permeabilized with ice-cold methanol and acetone. The cells were blocked in 5% normal goat serum for 30 minutes, followed by incubation overnight at 4°C with rabbit polyclonal anti-p53 antibodies FL-393 (1:100 vol/vol; Santa Cruz Biotechnology) and mouse monoclonal anti-cytochrome c oxidase IV (10G8; 1 μg/mL; Molecular Probes). After washing, cells were incubated with Alexa Fluor 488 chicken antirabbit secondary antibody and Alexa Fluor 594 chicken antimouse secondary antibody (Molecular Probes) diluted in 5% normal goat serum for 30 minutes at 4°C. Nuclei were counterstained with DAPI (4′,6-diamidino-2-phenylindole). Images were obtained using a 60 ×/1.40 PlanApo objective lens on an Olympus FV500 confocal microscope with Fluoview version 4.3 software (Olympus, Melville, NY). Cells not exposed to primary antibodies served as negative controls.
Reverse transcriptase–PCR and DNA sequencing of p53
Total RNAs were extracted from bone marrow and peripheral blood mononuclear cells with the RNeasy Mini Kit (Qiagen, Hilden, Germany). First-strand cDNA synthesis was performed with oligo(dT) as primer (Superscript II System; Invitrogen). Polymerase chain reaction (PCR) for p53 gene expressions followed by direct sequencing were performed as described previously.32
Statistical analysis
The statistical analysis was performed using the 2-tailed Student t test and the Pearson correlation coefficient. Statistical significance was considered when P was less than .05. Unless otherwise indicated, average values were expressed as mean plus or minus the standard error of the mean (SEM). Synergism, additive effects, and antagonism were assessed using the Chou-Talalay method33 and Calcusyn software (Biosoft, Ferguson, MO). The effect on cell viability was expressed as fraction of cells killed by the individual drugs or the combination in drug-treated versus untreated cells. The extent of apoptosis was quantified as percentage of annexin V–positive cells, and the extent of drug-specific apoptosis was assessed by the formula: % specific apoptosis = (test – control) × 100 × / (100 – control). The dose-effect curve for each drug alone was determined based on the experimental observations using the median-effect principle; the combination index (CI) for each experimental combination was then calculated according to the following equation: CI = (D)1 / (Dx)1 + (D)2 / (Dx)2 + (D)1(D)2 /(Dx)1(Dx)2, where (D)1 and (D)2 are the doses of drug 1 and drug 2 that have x effect when used in combination and (Dx)1 and (Dx)2 are the doses of drug 1 and drug 2 that have the same x effect when used alone. The CI was calculated using the more stringent statistical assumption of mutually nonexclusive modes of action. The CI provides a numerical description of combination effects. When CI equals 1, this equation represents the conservation isobologram and indicates additive effects. CI values less than 1.0 indicate a more-than-expected additive effect (synergism), whereas CI values more than 1.0 indicate antagonism between the 2 drugs.
Results
Inhibition of the p53-MDM2 interaction by Nutlin-3 induces growth arrest and apoptosis in AML cell lines with wild-type p53
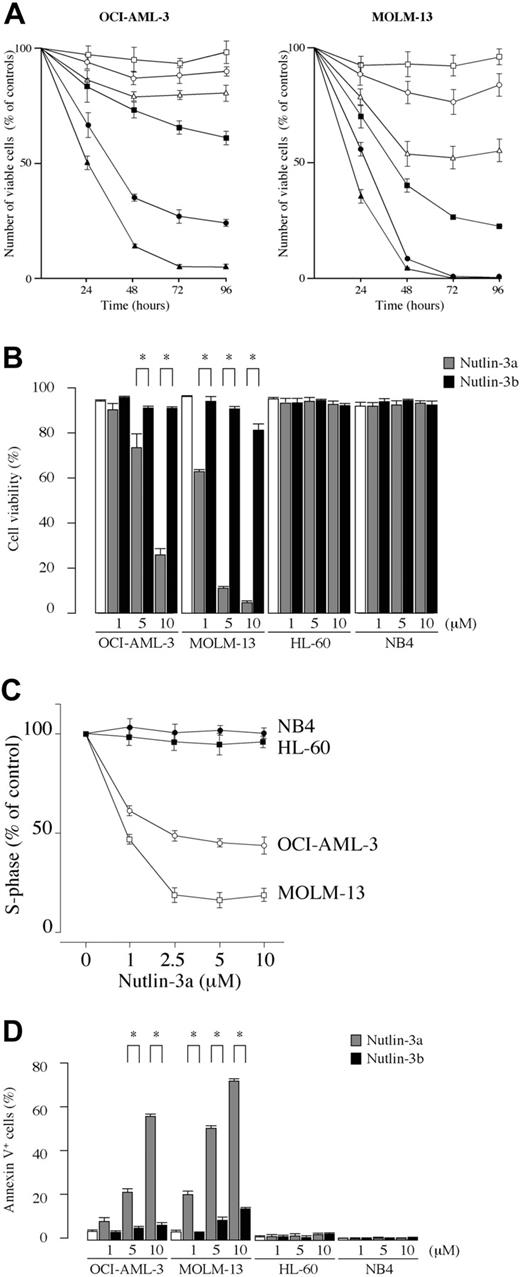
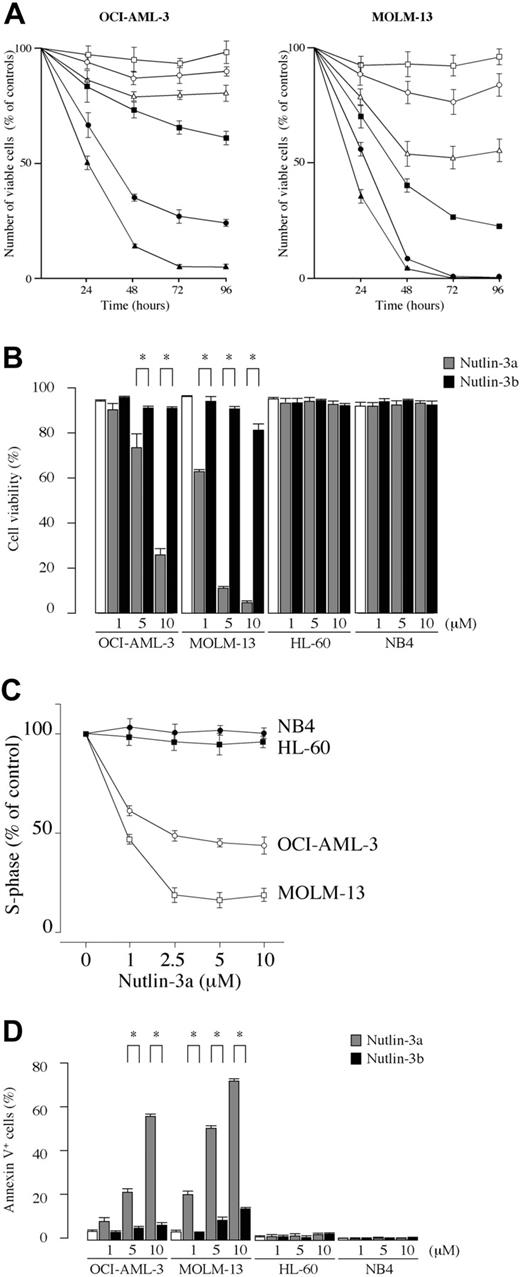
We first examined the effect of the MDM2 inhibitor on the growth and viability of cultured leukemia cell lines. The results showed that Nutlin-3a exhibited dose-dependent antiproliferative and cytotoxic activity in OCI-AML-3 and MOLM-13 cells, which have wild-type p53 (Figure 1A-B). HL-60 and NB4 cells, which have mutant p53, did not respond to the MDM2 inhibitor (Figure 1B), confirming the finding that the p53 pathway can be activated by Nutlins only in cells with wild-type p53.27 Nutlin-3a caused significant inhibition of the G1/S transition, with accumulation of cells in the G1 phase in OCI-AML-3 and MOLM-13 cell lines (Figure 1C). In contrast, the 2 cell lines with mutant p53 (HL-60 and NB4) showed a cell-cycle profile indistinguishable from untreated controls (Figure 1C). To clarify whether a significant increase of trypan blue–positive cells in wild-type p53 cells was associated with induction of apoptosis, annexin V–positive fractions and the DNA content were determined. Treatment of wild-type p53 cells with Nutlin-3a induced apoptosis in a dose- and time-dependent manner, as evidenced by high annexin V positivity (Figure 1D) and increased sub-G1 DNA content (data not shown). Thus, MDM2 inhibitor exerts antiproliferative activity through induction of both cell-cycle arrest and apoptosis, and apoptosis accounts for its cytotoxic activity. The activity of MDM2 inhibitor is limited to cells with wild-type p53.
Blockade of p53-MDM2 binding induces apoptosis and inhibits colony formation of primary AML cells
Samples from 18 patients with AML were examined for their response to MDM2 inhibition. Patient's characteristics are shown in Table 1. Six patients had newly diagnosed de novo AML, 1 had therapy-related AML, 1 had myelodysplastic syndrome (MDS)– derived leukemia, 7 had relapsed AML, and 3 had refractory disease. Among the 6 cases of de novo AML, 2 had favorable karyotypes of t(8;21) and inv(16) and 1 had an unfavorable karyotype with 11q23 translocation, according to the Southwest Oncology Group (SWOG) cytogenetic risk criteria.34 Thus, most cases had predictor(s) of poor response to chemotherapy and shorter survival.
Blockade of p53-MDM2 interaction inhibits the growth of cell lines with wild-type p53 through cell-cycle arrest and apoptosis induction. (A) Time course of effects induced in OCI-AML-3 and MOLM-13 cells by Nutlin-3a (active enantiomer) and -3b (less active enantiomer) on viable cell number. The cells were incubated with a range of concentrations of Nutlin-3a (1 μM, ▪; 5 μM, •; 10 μM, ▴) or -3b (1 μM, □; 5 μM, ○; 10 μM, ▵), and the cell viability was determined by trypan blue exclusion method. Results are expressed as the percentage of the viable cell number in an untreated group, and represent the average of triplicate cultures. (B) AML cell lines with wild-type p53 (OCI-AML-3 and MOLM-13) or mutant p53 (HL-60 and NB4) cells were incubated with the indicated concentrations of Nutlin-3a or -3b for 72 hours and the cell viability was determined by trypan blue exclusion method. MDM2 inhibitor showed significant cytotoxic activity in OCI-AML-3 and MOLM-13 cells. Results are expressed as the mean plus or minus the standard deviation (SD). *P < .05. (C) Nutlin-3a causes cell-cycle arrest in leukemia cells with wild-type p53. OCI-AML-3 (○), MOLM-13 (□), HL-60 (•), and NB4 (▪) cells were cultured for 12 hours in the presence of Nutlin-3a at the indicated concentrations, and stained for DNA content. Cell-cycle distribution was analyzed using ModFit LT software. Results are expressed as percentage of S-phase cells in DMSO-treated group. Nutlin-3a at 2.5 μM induced almost maximal cell-cycle arrest in both OCI-AML-3 and MOLM-13 cells. Results are representative of 3 independent experiments. (D) Cells were incubated with the indicated concentrations of Nutlin-3a or -3b for 48 hours (24 hours for MOLM-13 cells), and the annexin V–positive fractions were measured by flow cytometry. □ represents untreated controls. Results are expressed as mean ± SD.
Blockade of p53-MDM2 interaction inhibits the growth of cell lines with wild-type p53 through cell-cycle arrest and apoptosis induction. (A) Time course of effects induced in OCI-AML-3 and MOLM-13 cells by Nutlin-3a (active enantiomer) and -3b (less active enantiomer) on viable cell number. The cells were incubated with a range of concentrations of Nutlin-3a (1 μM, ▪; 5 μM, •; 10 μM, ▴) or -3b (1 μM, □; 5 μM, ○; 10 μM, ▵), and the cell viability was determined by trypan blue exclusion method. Results are expressed as the percentage of the viable cell number in an untreated group, and represent the average of triplicate cultures. (B) AML cell lines with wild-type p53 (OCI-AML-3 and MOLM-13) or mutant p53 (HL-60 and NB4) cells were incubated with the indicated concentrations of Nutlin-3a or -3b for 72 hours and the cell viability was determined by trypan blue exclusion method. MDM2 inhibitor showed significant cytotoxic activity in OCI-AML-3 and MOLM-13 cells. Results are expressed as the mean plus or minus the standard deviation (SD). *P < .05. (C) Nutlin-3a causes cell-cycle arrest in leukemia cells with wild-type p53. OCI-AML-3 (○), MOLM-13 (□), HL-60 (•), and NB4 (▪) cells were cultured for 12 hours in the presence of Nutlin-3a at the indicated concentrations, and stained for DNA content. Cell-cycle distribution was analyzed using ModFit LT software. Results are expressed as percentage of S-phase cells in DMSO-treated group. Nutlin-3a at 2.5 μM induced almost maximal cell-cycle arrest in both OCI-AML-3 and MOLM-13 cells. Results are representative of 3 independent experiments. (D) Cells were incubated with the indicated concentrations of Nutlin-3a or -3b for 48 hours (24 hours for MOLM-13 cells), and the annexin V–positive fractions were measured by flow cytometry. □ represents untreated controls. Results are expressed as mean ± SD.
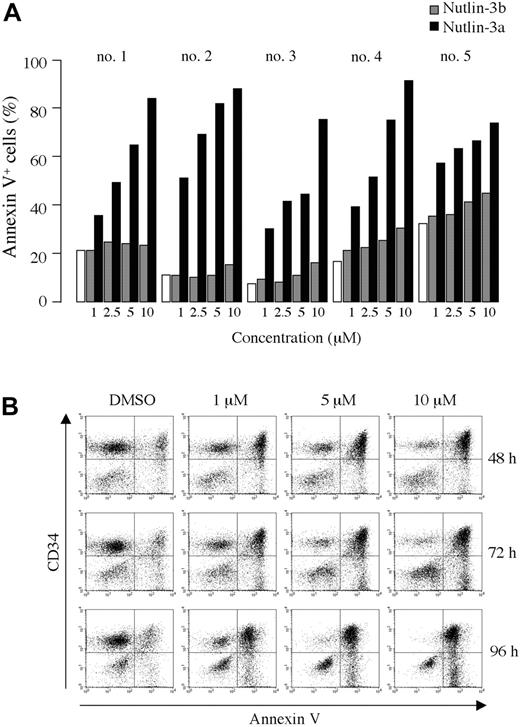
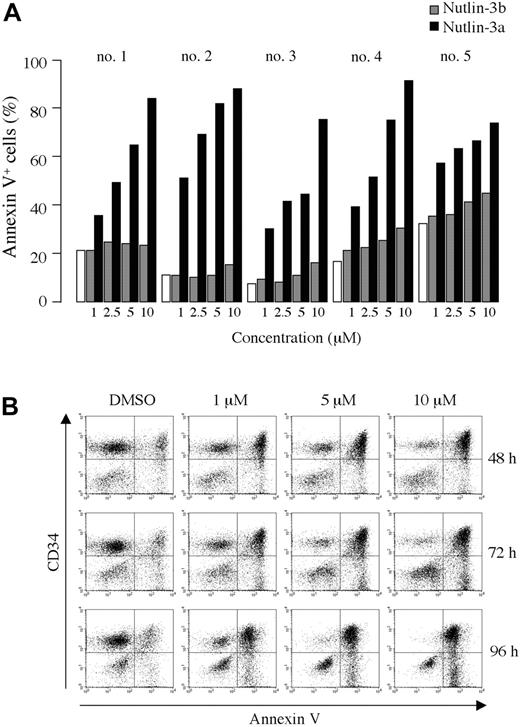
As shown in Figure 2A-B, treatment of primary AML cells with Nutlin-3a caused a dose- and time-dependent increase in the percentage of annexin V–positive cells. The enantiomer Nutlin-3b also induced apoptosis, but was significantly less potent than Nutlin-3a. A net increase in the proportion of annexin V–binding CD34+ cells of more than 40% was observed in 16 of 18 primary AML samples after exposure to 10 μM Nutlin-3a (median net apoptosis increase in sensitive samples, 66%; range, 41%-87%; Table 2). Thirteen of the 16 sensitive AML samples had a higher proportion of annexin V–binding cells in the CD34+ cell fraction than in total mononuclear cells, suggesting a higher sensitivity of immature CD34+ AML blasts compared with CD34– blasts in the majority of AML (Table 2). Consistent with the observed apoptosis induction, 10 μM Nutlin-3a caused a more than 50% decrease in the recovery of viable cells after 72 hours in vitro culture in 14 of 16 primary AML samples (median reduction in sensitive samples, 76%; range, 54%-96%; Table 2). Interestingly, disease status of AML did not affect the apoptotic response of AML blasts to Nutlin-3a. The response appeared to be tightly associated with p53 status of AML blasts. TP53 mutation was detected only in 2 Nutlin-resistant cases, in which missense mutations (CGC→GGC; R175G in case 7 and CGG→CAG; R248Q in case 17) occurred in the DNA-binding domain (Table 2).
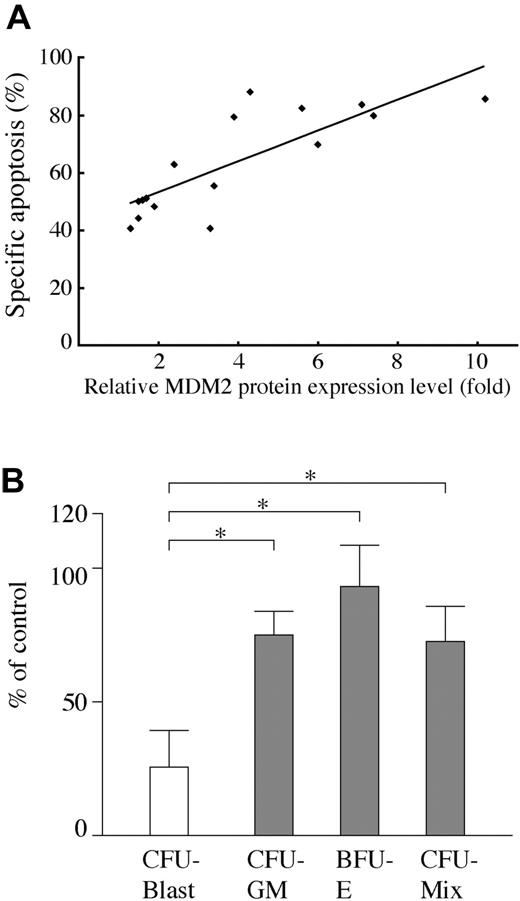
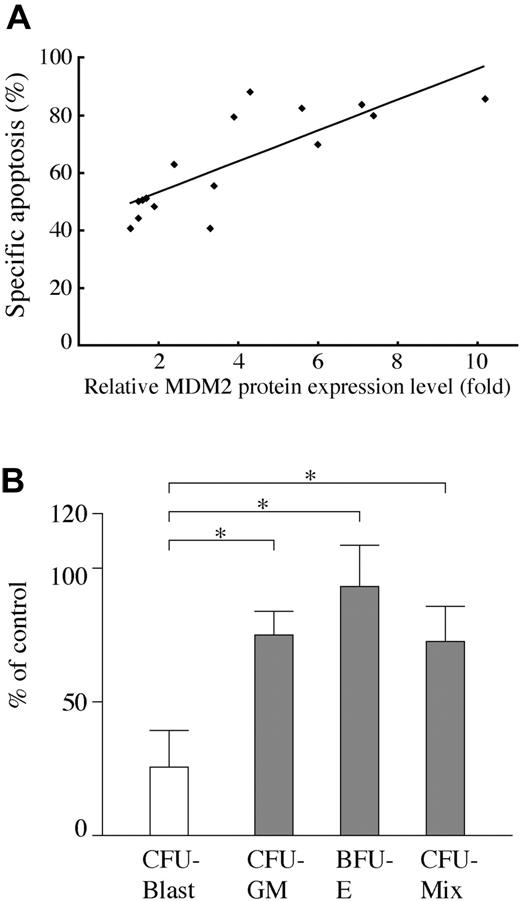
AML cells from 16 Nutlin-sensitive cases all had wild-type p53. The frequency of TP53 mutations in our series was 11% (2/18), which is similar to that reported.8-11 We then investigated the correlation between MDM2 protein level and degree of apoptosis by MDM2 inhibition in primary AML cells with wild-type p53. The MDM2 protein expression levels relative to an internal control, β-actin, were determined in each sample, and were compared with that of normal bone marrow cells from 3 donors. The levels of MDM2 were more than 2-fold higher in 10 of 16 primary AML cells than those in normal bone marrow samples. There was a significant positive correlation between relative MDM2 level and the percentage of specific annexin V induction (Figure 3A; r = 0.805, P < .01).
We next examined the effect of Nutlin-3a on clonogenic AML cells. As shown in Figure 3B, colony formation of AML progenitors was afftected by 1 μM Nutlin-3a, with 27.6% ± 14.7% surviving colonies. In contrast, 74.6% ± 10.7% granulocyte macrophage–colony-forming units (CFU-GMs), 93.8% ± 16.2% erythroid burst-forming units (BFU-Es), and 73.2% ± 13.9% mixed-lineage CFUs (CFU-Mix) from normal CD34+ cells survived treatment with 1 μM Nutlin-3a. The difference between AML and normal progenitors was statistically significant (P < .05). A higher concentration of Nutlin-3a at 5 μM almost completely (98.0% ± 1.9%) inhibited colony formation of AML progenitors, whereas 31.7% ± 12.6%, 33.1% ± 16.0%, and 27.1% ± 16.6% CFU-GMs, BFU-Es, and CFU-Mix, respectively, from normal CD34+ cells still survived treatment with 5 μM Nutlin-3a.
Blockade of p53-MDM2 binding induces apoptosis in primary AML cells. (A) Primary AML cells from patients 1 to 5 were incubated with the indicated concentrations of Nutlin-3a (active enantiomer, ▪) or -3b (less active enantiomer, ▦) for 72 hours, and the annexin V–positive fractions were measured by flow cytometry. □ represents DMSO-treated controls. (B) Primary AML cells from patient 8 were treated with the indicated concentrations of Nutlin-3a, and flow cytometry analysis using CD34-PE and annexin V–FITC antibodies was performed at the indicated times.
Blockade of p53-MDM2 binding induces apoptosis in primary AML cells. (A) Primary AML cells from patients 1 to 5 were incubated with the indicated concentrations of Nutlin-3a (active enantiomer, ▪) or -3b (less active enantiomer, ▦) for 72 hours, and the annexin V–positive fractions were measured by flow cytometry. □ represents DMSO-treated controls. (B) Primary AML cells from patient 8 were treated with the indicated concentrations of Nutlin-3a, and flow cytometry analysis using CD34-PE and annexin V–FITC antibodies was performed at the indicated times.
MDM2 inhibition results in p53-dependent expression of proapoptotic Bcl-2 family proteins and activation of caspases in AML cells
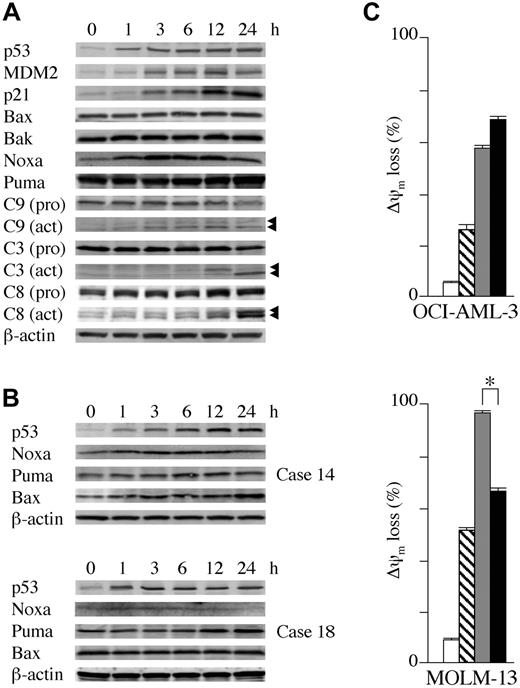
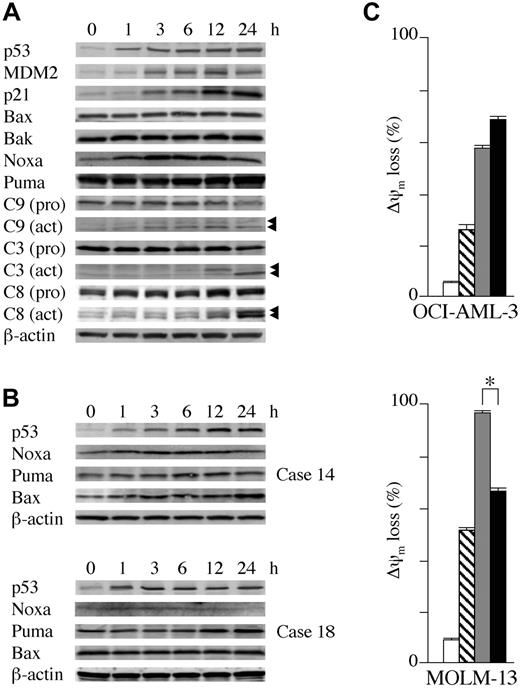
OCI-AML-3, MOLM-13, and wild-type p53 cells from 3 primary AML samples containing more than 90% blasts were cultured in the presence of 5 μM Nutlin-3a. As shown in Figure 4A, Nutlin-3a induced increased expression of p53-related proteins in OCI-AML-3 cells in a time-dependent fashion. p53 accumulated at 1 hour after exposure to Nutlin-3a, whereas increased MDM2 and p21 levels were seen at 3 hours. There was an immediate induction of Noxa at 1 hour, followed by caspase-9 cleavage. Cleavage of caspases-3 and -8 was evident at 12 hours after exposure, suggesting that an activation of the intrinsic apoptosis pathway occurs prior to caspase-8 cleavage. The protein levels of Puma, Bax, and Bak did not change. An identical protein expression pattern was seen in MOLM-13 cells (data not shown). Expression of proapoptotic Bcl-2 family proteins was determined in cells from 3 primary AML samples (nos. 6, 14, and 18 in Table 1). Nutlin-3a induced at least one proapoptotic Bcl-2 family protein in these cells (Figure 4B), followed by caspase activation (not shown). Nutlin-3a induced Noxa, Puma, and Bax in case 14, Puma in case 18, and Noxa in case 6. The role of caspase activation in the induction of apoptosis was addressed by treatment of OCI-AML-3 and MOLM-13 cells with Nutlin-3a in the absence or presence of 50 μM pan-caspase inhibitor Z-VAD-FMK. Z-VAD-FMK significantly prevented Nutlin-3a from inducing annexin V in both OCI-AML-3 (38.4% ± 0.1% reduction in % specific apoptosis compared with Nutlin-3a alone) and MOLM-13 (80.4% ± 0.2% reduction) cells from programmed cell death induced by Nutlin-3a, though the protective effect was smaller in OCI-AML-3 cells.
Both transcription-dependent and transcription-independent apoptosis pathways are operational in p53-dependent apoptosis in AML
The role of newly synthesized proteins in the initiation of apoptosis was addressed by treatment of OCI-AML-3 and MOLM-13 cells with Nutlin-3a in the absence or presence of 3.5 μM cycloheximide. Inhibition of protein synthesis with cycloheximide, which prevents apoptosis in certain circumstances,35,36 did not rescue OCI-AML-3 from ?ψm loss induced by Nutlin-3a (Figure 4C). To exclude the possibility that cycloheximide did not block the p53-dependent induction of proapoptotic proteins, we investigated Noxa protein expression and caspase-3 activation after Nutlin treatment. Cycloheximide completely inhibited the p53-dependent expression of Noxa up to 24 hours but it did not protect OCI-AML-3 cells from loss of Δψm or caspase-3 activation (not shown). These findings suggest that in OCI-AML-3 cells Nutlin-3a induces apoptosis in the absence of new protein synthesis and that induction of p53-related proapoptotic proteins may not be a sole requirement for Nutlin-induced apoptosis. In contrast, cycloheximide partially protected MOLM-13 cells from Δψm loss (Figure 4C), indicating a requirement of protein synthesis for full accomplishment of p53-induced apoptosis in these cells. To further investigate the role of protein synthesis in p53-mediated apoptosis, we examined the protective effect of cycloheximide on Δψm in 10 primary AML samples treated with 10 μM Nutlin-3a. The inhibitory effect of cycloheximide pretreatment on loss of Δψm compared with Nutlin-3a alone varied widely, ranging from 0% to 71% (median effect, 20%). More than 50% protection from Nutlin-induced apoptosis was observed in 3 primary AML samples. These findings suggest that both transcription-dependent and transcription-independent pathways mediate p53-dependent apoptosis and that their relative contribution differs significantly among AML cells.
Positive correlation between MDM2 levels and apoptosis and effect of MDM2 inhibitor on AML progenitors in primary AML samples. (A) Correlation of MDM2 protein levels relative to normal bone marrow cells with degree of apoptosis by MDM2 inhibition in 16 primary AML cells with wild-type p53. MDM2 protein expression levels relative to an internal control, β-actin, were determined in each sample, and then compared with normal bone marrow cells. There was a significant positive correlation between relative MDM2 levels and the percentage of specific annexin V induction (r = 0.805, P < .01). (B) Effect of Nutlin-3a on AML and normal clonogenic progenitors. Data represent average results from 5 different AML samples (white bar) and 5 magnetically separated normal CD34+ cells (gray bars). Results are expressed as the mean plus or minus the standard error of the mean (SEM) of the number of colonies in the presence of 1 μM Nutlin-3a compared with the number in control cells. *P < .05.
Positive correlation between MDM2 levels and apoptosis and effect of MDM2 inhibitor on AML progenitors in primary AML samples. (A) Correlation of MDM2 protein levels relative to normal bone marrow cells with degree of apoptosis by MDM2 inhibition in 16 primary AML cells with wild-type p53. MDM2 protein expression levels relative to an internal control, β-actin, were determined in each sample, and then compared with normal bone marrow cells. There was a significant positive correlation between relative MDM2 levels and the percentage of specific annexin V induction (r = 0.805, P < .01). (B) Effect of Nutlin-3a on AML and normal clonogenic progenitors. Data represent average results from 5 different AML samples (white bar) and 5 magnetically separated normal CD34+ cells (gray bars). Results are expressed as the mean plus or minus the standard error of the mean (SEM) of the number of colonies in the presence of 1 μM Nutlin-3a compared with the number in control cells. *P < .05.
MDM2 inhibition leads to nuclear and cytoplasmic accumulation of p53
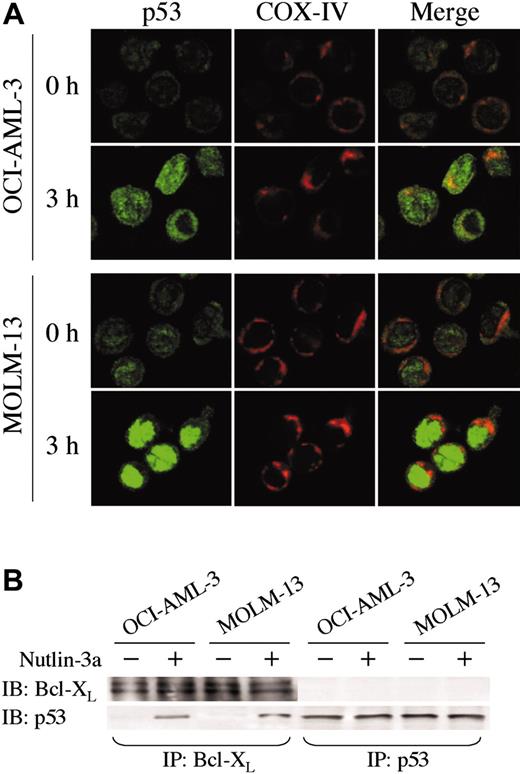
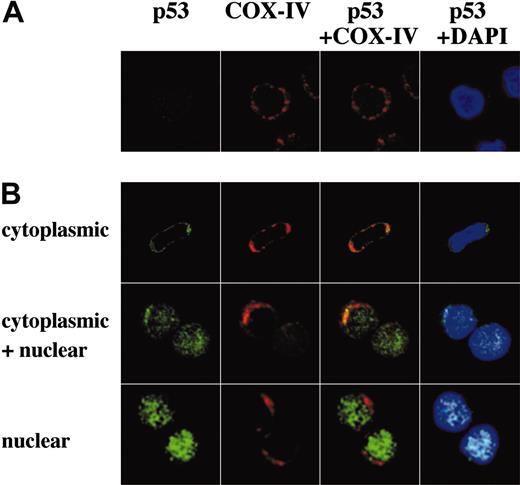
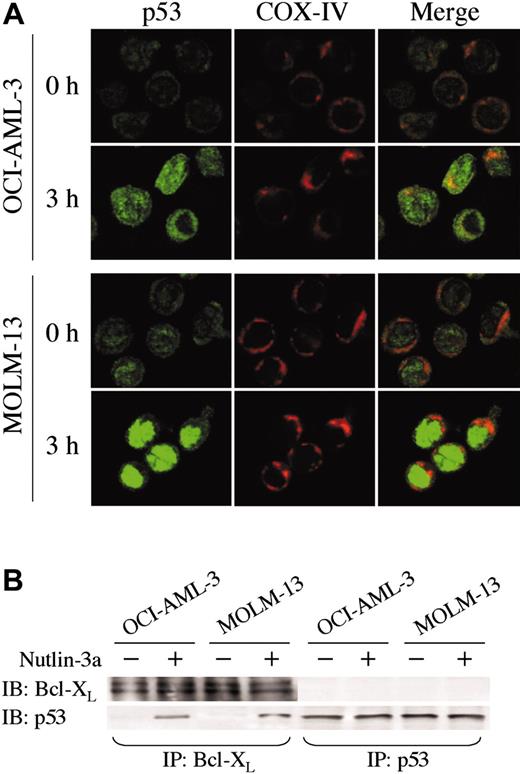
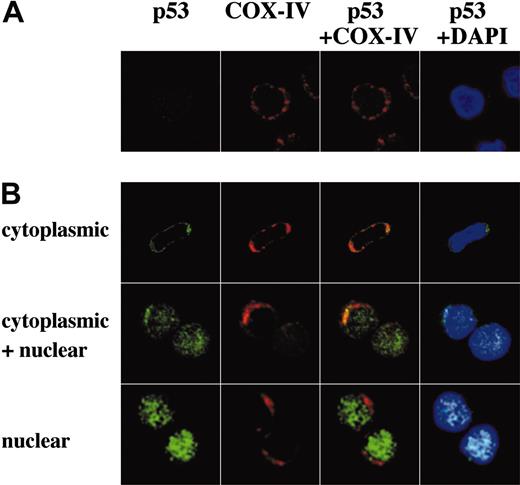
The direct transcriptional activation of numerous target genes is the major mechanism of p53-mediated apoptosis, which occurs in the nucleus. However, it has been recently reported that cytoplasmic p53 can localize to mitochondria and trigger transcription-independent apoptosis if p53 levels reach a certain threshold.24-26 To address this issue, we determined the intracellular localization of wild-type p53 protein in untreated and Nutlin-treated OCI-AML-3, MOLM-13, and cells from 3 primary AML samples using confocal microscopy. A small amount of p53 was diffusely distributed in untreated cells (Figure 5A and Figure 6A). Cells exhibited increased p53 staining after Nutlin-3a treatment, which is consistent with results obtained by Western blotting. However, the localization patterns considerably differed between the 2 cell lines. In contrast to MOLM-13, which displayed intense nuclear accumulation of p53 and smaller increase of p53 in the cytoplasm, relocation of p53 to the nucleus after Nutlin-3a was inconspicuous in OCI-AML-3 cells (Figure 5A). The accumulated cytoplasmic p53 partially colocalized with the mitochondrial marker protein cytochrome c oxidase IV (yellow-orange areas), and the mitochondrial translocation of p53 was detectable as early as 1 hour after treatment (data not shown). Specific p53/Bcl-XL complex was detectable in Nutlin-3a–treated OCI-AML-3 and MOLM-13 cell lysates by immunoprecipitation with anti–Bcl-XL antibodies and blotting with anti-p53 (Figure 5B). No complexes were seen with irrelevant antibodies against abundant nuclear or cytoplasmic proteins. Cells from 3 primary AML samples showed p53 accumulation toward either nucleus (1 case) or cytoplasm/mitochondria (2 cases) after Nutlin-3a treatment (Figure 6B). Of note, cells from a primary AML sample, which showed high percentage (90%) of cells with nuclear p53 accumulation, were most efficiently (68%) protected by cycloheximide from Nutlin-induced Δψm loss (not shown).
Transcriptional activation of proapoptotic Bcl-2 family proteins and transcription-independent apoptosis mediate Nutlin-induced apoptosis. (A) Expression of apoptosis- and cell-cycle–associated proteins in OCI-AML-3 cells, which were treated with 5 μM Nutlin-3a for the indicated times. Nutlin-3a induced increased protein expression of p53, MDM2, and p21 in OCI-AML-3 cells in a time-dependent fashion. Nutlin-3a induced Noxa, a BH3-only member of the Bcl-2 family, followed by caspase activation. β-actin was used to confirm equal loading of proteins. Arrowheads indicate cleared caspases. (B) Expression of proapoptotic Bcl-2 family proteins in primary AML cells, which were treated with 5 μM Nutlin-3a for the indicated times. Nutlin-3a induced at least 1 proapoptotic Bcl-2 family member protein in cells from 3 primary AML samples (nos. 6, 14, and 18 in Table 1) examined. Nutlin-3a induced Noxa, Puma, and Bax up-regulation in case 14 (top panel), Puma in case 18 (bottom panel), and Noxa in case 6 (not shown). β-actin was used to confirm equal loading of proteins. (C) OCI-AML-3 cells or MOLM-13 cells at a starting concentration of 2 × 105 cells/mL were cultured for 24 hours in the presence of DMSO (□), 3.5 μM cycloheximide (▧), 10 μM Nutlin-3a (▦), or a combination of cycloheximide and Nutlin-3a (▪). Δψm was assessed by flow cytometry. Results are expressed as mean ± SD of triplicate measurements. Comparable results were obtained in 2 other independent experiments. *P < .05.
Transcriptional activation of proapoptotic Bcl-2 family proteins and transcription-independent apoptosis mediate Nutlin-induced apoptosis. (A) Expression of apoptosis- and cell-cycle–associated proteins in OCI-AML-3 cells, which were treated with 5 μM Nutlin-3a for the indicated times. Nutlin-3a induced increased protein expression of p53, MDM2, and p21 in OCI-AML-3 cells in a time-dependent fashion. Nutlin-3a induced Noxa, a BH3-only member of the Bcl-2 family, followed by caspase activation. β-actin was used to confirm equal loading of proteins. Arrowheads indicate cleared caspases. (B) Expression of proapoptotic Bcl-2 family proteins in primary AML cells, which were treated with 5 μM Nutlin-3a for the indicated times. Nutlin-3a induced at least 1 proapoptotic Bcl-2 family member protein in cells from 3 primary AML samples (nos. 6, 14, and 18 in Table 1) examined. Nutlin-3a induced Noxa, Puma, and Bax up-regulation in case 14 (top panel), Puma in case 18 (bottom panel), and Noxa in case 6 (not shown). β-actin was used to confirm equal loading of proteins. (C) OCI-AML-3 cells or MOLM-13 cells at a starting concentration of 2 × 105 cells/mL were cultured for 24 hours in the presence of DMSO (□), 3.5 μM cycloheximide (▧), 10 μM Nutlin-3a (▦), or a combination of cycloheximide and Nutlin-3a (▪). Δψm was assessed by flow cytometry. Results are expressed as mean ± SD of triplicate measurements. Comparable results were obtained in 2 other independent experiments. *P < .05.
Antitumor activity of MDM2 antagonists in AML cells is enhanced by combination with AraC or doxorubicin
AraC is one of the most active chemotherapeutic agents for the therapy of AML and it remains the backbone of induction and consolidation regimens. To determine if inhibition of the p53-MDM2 interaction in AML cells might potentiate the effects of AraC, we combined Nutlin-3a and AraC (0, 1, 2.5, 5, or 10 μM), using a fixed-ratio (1:1) experimental design. A quantity of 1 μM AraC corresponds to the serum level obtained in patients treated with a conventional-dose AraC regimen (100 mg/m2 per day), whereas 10 μM AraC corresponds to the concentration achieved in serum of patients treated with high-dose AraC (1 g/m2 per day-3 g/m2 per day). An interaction study between Nutlin-3a and AraC showed highly synergistic effects on cell viability (not shown) and induction of apoptosis (Table 3) in both OCI-AML-3 and MOLM-13 cells. The experimental results fell far to the left of the ED90 line of the simulated isobolograms, and the averaged CI values calculated from the ED50, (50% effective dose) ED75, ED90, and ED95 were 0.48 for OCI-AML-3 and 0.56 for MOLM-13 for inhibition of cell viability, and 0.46 for OCI-AML-3 and 0.25 for MOLM-13 for induction of apoptosis. Potentiation effects were not seen in p53-defective HL-60 cells (not shown). We then cultured primary cells from 2 patients with AML with more than 90% blasts with Nutlin-3a and/or AraC, and evaluated apoptosis and cell number reduction after 24 and 72 hours, respectively. The AML cells had wild-type p53. Results confirmed the synergistic nature of the Nutlin-3a/AraC interaction in primary AML (Table 3).
p53 localization and its interaction with Bcl-XL. (A) Localization of p53 in OCI-AML-3 and MOLM-13 cells. Cells were treated with 10 μM Nutlin-3a for 3 hours. Cells were fixed, stained for p53 (green) and mitochondrial marker protein cytochrome c oxidase IV (red), and visualized by confocal microscopy. Localization of p53 to mitochondria is indicated by the yellow-orange color in the merged images. (B) OCI-AML-3 and MOLM-13 cells were incubated with 10 μM Nutlin-3a for 6 hours, and untreated (–) or treated (+) cells were immunoprecipitated with anti-p53 or anti–Bcl-XL antibodies and immunoblotted for Bcl-XL or p53. Immunoprecipitation with anti–Bcl-XL followed by p53 blotting showed a specific p53/Bcl-XL complex in both cells. The complex was not detectable in reverse, suggesting that a minor fraction of increased p53 protein might bind to Bcl-XL protein.
p53 localization and its interaction with Bcl-XL. (A) Localization of p53 in OCI-AML-3 and MOLM-13 cells. Cells were treated with 10 μM Nutlin-3a for 3 hours. Cells were fixed, stained for p53 (green) and mitochondrial marker protein cytochrome c oxidase IV (red), and visualized by confocal microscopy. Localization of p53 to mitochondria is indicated by the yellow-orange color in the merged images. (B) OCI-AML-3 and MOLM-13 cells were incubated with 10 μM Nutlin-3a for 6 hours, and untreated (–) or treated (+) cells were immunoprecipitated with anti-p53 or anti–Bcl-XL antibodies and immunoblotted for Bcl-XL or p53. Immunoprecipitation with anti–Bcl-XL followed by p53 blotting showed a specific p53/Bcl-XL complex in both cells. The complex was not detectable in reverse, suggesting that a minor fraction of increased p53 protein might bind to Bcl-XL protein.
Representative p53 localization patterns in primary AML cells from case 14, which were treated with 10 μM Nutlin-3a for 3 hours. Cells were stained for p53 (green) and mitochondrial marker protein cytochrome c oxidase IV (red) and visualized by confocal microscopy. Nuclei were counterstained with DAPI (blue). Localization of p53 to mitochondria is indicated by the yellow-orange color in the merged images. (A) Untreated cells showed low levels of diffusely distributed p53. (B) After treatment, individual cells showed either cytoplasmic, cytoplasmic and nuclear, or nuclear accumulation of p53. In this case, a majority (75%) of cells showed cytoplasmic accumulation of p53, and the inhibitory effect of cycloheximide on loss of Δψm compared with Nutlin-3a alone was 26%. A preferential translocation of cytoplasmic p53 to mitochondria suggests that cytoplasmic p53 mediates apoptosis mainly at the level of mitochondria.
Representative p53 localization patterns in primary AML cells from case 14, which were treated with 10 μM Nutlin-3a for 3 hours. Cells were stained for p53 (green) and mitochondrial marker protein cytochrome c oxidase IV (red) and visualized by confocal microscopy. Nuclei were counterstained with DAPI (blue). Localization of p53 to mitochondria is indicated by the yellow-orange color in the merged images. (A) Untreated cells showed low levels of diffusely distributed p53. (B) After treatment, individual cells showed either cytoplasmic, cytoplasmic and nuclear, or nuclear accumulation of p53. In this case, a majority (75%) of cells showed cytoplasmic accumulation of p53, and the inhibitory effect of cycloheximide on loss of Δψm compared with Nutlin-3a alone was 26%. A preferential translocation of cytoplasmic p53 to mitochondria suggests that cytoplasmic p53 mediates apoptosis mainly at the level of mitochondria.
Anthracyclines are another class of chemotherapeutic agents commonly used in the treatment of AML. Similar to the experiments with AraC, cells were treated with DOX and Nutlin-3a either as individual agents or in combination. The range of DOX concentration applied in this study covers the steady-state plasma concentration (15 nM-20 nM) in patients treated with 60 mg/m2 to 75 mg/m2 DOX37 and induced G2/M arrest in OCI-AML-3 and MOLM-13 cells even at the lowest DOX concentration (data not shown). Since it is possible that the timing of DOX treatment can affect the interaction between Nutlin-3a with DOX,38 DOX and Nutlin-3a were added simultaneously or sequentially with a 24-hour interval to OCI-AML-3 or MOLM-13 cells. Annexin V–positive fractions were measured 48 hours after the last drug administration in OCI-AML-3 cells and 24 hours in MOLM-13 cells, and the number of viable cells was determined 48 hours after the last drug administration. The results indicated a synergistic interaction of DOX and Nutlin-3a on cell viability (data not shown) and induction of apoptosis (Table 4), irrespective of the order of drug administration. There was no significant association of the CI values with the timing of each drug. To further confirm the synergistic nature of the interaction, we cultured wild-type primary AML cells from 2 patients with more than 90% blasts with Nutlin-3a and/or DOX, and evaluated apoptosis and cell number reduction after 72 hours. The averaged CI values calculated from the ED50, ED75, ED90, and ED95 for the 2 AML cells were 0.30 and 0.42 for cell viability, and were 0.81 and 0.82 on induction for apoptosis. Potentiation effects were not seen in p53-defective HL-60 cells (not shown). We also cultured normal CD34+ cells from 3 healthy donors with Nutlin-3a and/or DOX. The results suggest that the combination effects are slightly antagonistic (Table 4).
Discussion
TP53 mutations are rare in AML and are generally not considered to be of primary importance in the development of these malignancies.8-12 However, MDM2 has been found to be frequently overexpressed in AML, and can enhance the tumorigenic potential and resistance to apoptosis through abrogation of p53 function.13-16,39 Overproduction of MDM2 as a result of gene amplification has been shown to occur in approximately 7% of all solid tumors and, interestingly, incidence of p53 mutation and MDM2 amplification within the same tumor is rare. This suggests that MDM2 overexpression could be an effective means for inactivating p53 function.40 We here investigate the effect of p53 activation by small-molecule MDM2 antagonists on the growth and viability of AML cells. We found that AML cell lines and 16 primary AML samples with wild-type p53 responded to Nutlin treatment by induction of p53-dependent apoptosis. These findings suggest that most transformed AML cells have retained a functional p53 pathway, and p53 activation can effectively induce apoptosis. We also found a positive correlation between MDM2 protein level and degree of apoptosis by MDM2 inhibition in primary AML cells. Since primary AML cells express higher levels of MDM2 protein compared with normal hematopoietic cells, this finding further supports the rationale of targeting the p53-MDM2 interaction as a therapeutic strategy for AML.
In this study, AML cell lines and primary AML blasts harboring mutant p53 were much less sensitive to Nutlin-3a, consistent with previously published data.27,41 The differential effect of Nutlins in cells with wild-type and mutant p53 suggests that their activity is derived from selective activation of the p53 pathway. In contrast, MDM2-targeted antisense oligonucleotide therapy, which also inhibits the proliferation of tumor cells, does not differentiate between wild-type and mutant p53.42 MDM2 interacts with a number of proteins crucial for cell proliferation and survival.43 The higher selectivity of targeting the p53-MDM2 interaction by Nutlins would be advantageous in future clinical applications.
Since the MDM2 inhibition is not genotoxic, transient induction of p53 response by blockade of the p53-MDM2 interaction in normal somatic cells may lead to reversible growth arrest.44 To examine effects on normal hematopoiesis, AML and normal CD34+ hematopoietic cells were assayed for colony formation. The results showed that AML cells were more sensitive to p53 activation than normal hematopoietic cells. A preferential induction of apoptosis in AML versus normal hematopoietic cells has been described for proteasome inhibition.45 Furthermore, a recent study in which the effect of p53 activation has been investigated by manipulation of MDM2 gene dosage in transgenic mice has revealed that erythropoiesis and granulopoiesis are not significantly affected by elevated p53 levels.46 In that study, up-regulation of p53 level was tolerable in most mouse tissues except for thymus and small intestine, and the mice had a normal life span. These findings as well as those in other studies support the concept that the response of normal cells to p53 activation differs from that of neoplastic cells.27,47,48
The p53 protein controls cellular responses to stress by inducing the transcription of genes involved in cell-cycle regulation, DNA repair, and apoptosis, and transactivation is the major mechanism of p53-mediated apoptosis.17-19 Recently, some reports have shown that increased levels of cytoplasmic wild-type p53 can induce transcription-independent apoptosis if the levels of p53 reach a critical threshold.24-26 In transcription-independent p53-mediated apoptosis, p53 can localize to mitochondria and induce the release of mitochondrial cytochrome c.25,26 However, the role for this mechanism versus that of transcriptional regulation remains unknown. We found that 2 wild-type p53 AML cell lines, OCI-AML-3 and MOLM-13, may use different p53-dependent apoptosis pathways. Experiments using the protein synthesis inhibitor cycloheximide demonstrated that blockade of the p53-MDM2 interaction was capable of inducing apoptosis in OCI-AML-3 cells in the absence of new protein synthesis. In contrast, cycloheximide prevented apoptosis in MOLM-13 cells. The primary role of transcription-independent apoptosis in p53-mediated apoptosis in OCI-AML-3 was further supported by immunofluorescence and confocal microscopy, which showed cytoplasmic confinement and mitochondrial translocation of p53. The p53/Bcl-XL interaction, which was seen in both OCI-AML-3 and MOLM-13 cells after treatment, has been described as a potential mechanism of transcription-independent apoptosis mediated by p53.24-26 Since nuclear localization of p53 is essential for its function as a transcription factor, apoptosis induction in the absence of p53 relocation to the nucleus in OCI-AML-3 cells is in clear contrast with the typical nuclear p53 accumulation and apoptosis induction observed in MOLM-13 cells. The 2 different p53-dependent apoptosis pathways were also found in primary cells. Our results suggest that transcription-dependent apoptosis does not always play a major role in p53-dependent apoptosis in AML. An important role of the transcription-independent pathway in p53-mediated apoptosis has recently been suggested.49,50 Further studies are needed to clarify the specific mechanisms governing transcription-dependent and transcription-independent pathways in AML.
AraC and anthracyclines (eg, DOX) are the most effective agents in AML therapy, and are incorporated into virtually all standard induction regimens for this disease. AraC and DOX induce p53 activation and apoptosis,51,52 and induction of apoptosis has been shown to be a key determinant of drug response in AML.53 From a clinical viewpoint, p53 mutations have been associated with chemoresistance and poor prognosis in AML, suggesting that loss of functional p53 may provide a mechanism by which AML cells avoid the lethal actions of antileukemic agents.11,12 Nevertheless, the specific role of p53 activation in the modulation of cellular drug response remains unclear.54,55 A variety of mechanisms have been shown to be involved in AraC- and DOX-induced cytotoxicity, and furthermore, AraC and DOX induce cell-cycle arrest in S and G2/M phases, respectively.56,57 Since the modes of pharmacologic action of AraC and DOX depend on the drug concentration,57,58 we used clinically relevant AraC and DOX concentrations in the combination experiments with Nutlin-3a.37,57 p53 activation synergistically enhanced the cytotoxicity of both AraC and DOX in wild-type p53 AML cells. This effect is likely due to enhanced apoptosis induction, which has been shown to determine the response to therapy.59 Interestingly, the synergistic apoptotic effect between Nutlin-3a and DOX was not seen in normal CD34+ cells, probably reflecting a different response of normal cells to p53 activation from that of neoplastic cells.47,48 We have recently shown that MDM2 inhibitors can protect normal skin fibroblasts and wild-type p53 colon cancer cells HCT116 and RKO, in which p53 activation leads to prominent cell-cycle arrest and weak apoptosis, from paclitaxel-induced apoptosis.60 Taken together, these observations raise the possibility that MDM2 inhibition can antagonize chemotherapeutic agents in cells with high tolerance to p53-induced apoptosis.
In summary, our data support the concept that nongenotoxic activation of p53 by small-molecule antagonists of MDM2 may provide a novel therapeutic tool for the therapy of AML, including chemorefractory disease.
Prepublished online as Blood First Edition Paper, July 12, 2005; DOI 10.1182/blood-2005-02-0553.
Supported in part by National Institutes of Health grants PO1 CA55 164, PO1 CA49 639, and CA16 672 and the Paul and Mary Haas Chair in Genetics (M.A.); and by the Kanae Foundation for Life and Socio-Medical Science, the Mochida Memorial Foundation for Medical and Pharmaceutical Research, and the Uehara Memorial Foundation (K.K.).
L.T.V. is employed by Hoffman La-Roche Inc, whose potential product was studied in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors acknowledge Dr Yoshinobu Matsuo of Fujisaki Cell Center, Hayashibara Biochemical Laboratories, Okayama, Japan, for kindly providing MOLM-13 cells, and Rosemarie B. Lauzon of the Department of Blood and Marrow Transplantation, the University of Texas M.D. Anderson Cancer Center, for her assistance with the manuscript.