Abstract
Leukemic cells from 30% of patients with acute myeloid leukemia (AML) have an activating mutation in the FLT3 (fms-like tyrosine kinase) gene, which represents a target for drug therapy. We treated 20 patients, each with mutant FLT3 relapsed/refractory AML or high-grade myelodysplastic syndrome and not believed to be candidates for chemotherapy, with an FLT3 tyrosine kinase inhibitor, PKC412 (N-benzoylstaurosporine), at a dose of 75 mg 3 times daily by mouth. The drug was generally well tolerated, although 2 patients developed fatal pulmonary events of unclear etiology. The peripheral blast count decreased by 50% in 14 patients (70%). Seven patients (35%) experienced a greater than 2-log reduction in peripheral blast count for at least 4 weeks (median response duration, 13 weeks; range, 9-47 weeks); PKC412 reduced bone marrow blast counts by 50% in 6 patients (2 of these to < 5%). FLT3 autophosphorylation was inhibited in most of the Corresponding patients, indicating in vivo target inhibition at the dose schedule used in this study. PKC412 is an oral tyrosine kinase inhibitor with clinical activity in patients with AML whose blasts have an activating mutation of FLT3, suggesting potential use in combination with active agents, such as chemotherapy.
Introduction
Current therapy of acute myeloid leukemia (AML) relies on remission induction with cytosine arabinoside and an anthracycline, followed by postremission therapy with additional intensive chemotherapy or stem cell transplantation.1 The 5-year survival rate with this approach is 30% to 40% in patients under the age of 60 years, but less than 15% in older patients. In addition, the chemotherapy regimens currently in use are associated with significant morbidity and mortality, particularly in elderly patients.2 The development of less toxic and more specific therapies in AML is clearly needed, prompting an intensive search for appropriate target molecules, especially those in the pathways that cause AML. The potential value of targeting specific oncogenes in leukemia has been well demonstrated by the clinical effectiveness of all-trans-retinoic acid (RAR) for acute promyelocytic leukemia (PML; inhibiting the biologic activities of PML-RARα, the oncogene created by t(15;17)3 ) and imatinib mesylate for chronic myeloid leukemia (inhibiting BCR/ABL, the oncogene created by t(9;22)4 ).
Recent studies have shown that the class III receptor tyrosine kinase FLT3 is mutated and activated in about 30% of adult patients with AML.5 The mutations involve either an internal tandem duplication (ITD) of between 3 and more than 100 amino acids in the juxtamembrane region of the receptor (in about 25% of AML patients) or a point mutation in the activating loop (in about 7% of patients).5 Both types of activating FLT3 mutations confer growth factor independence in factor-dependent leukemia cell lines6,7 and cause a fatal myeloproliferative syndrome in mice.8 Patients with mutations in FLT3, particularly those with ITD mutations, have a worse prognosis, with lower rates of complete remission, and lower overall survival.9,10 Thus, inhibition of activated FLT3 kinase by a pharmacologic agent is an attractive therapeutic strategy in AML.
We have previously described the preclinical development of a small-molecule inhibitor of FLT3.5 PKC412 (N-benzoylstaurosporine) was developed originally as a protein kinase C (PKC) inhibitor,11 but it also has potent inhibiting activity against a variety of class III tyrosine kinase receptors including vascular endothelial growth factor receptor 2 (VEGFR-2), c-kit, platelet-derived growth factor receptor α (PDGFR-α), and PDGFR-β.12 A phase 1 trial with this agent in a variety of solid tumors demonstrated the feasibility of giving doses up to 75 mg orally 3 times a day with less than dose-limiting toxicity.13 Although the drug was highly protein bound, evidence of biologic activity was seen due to inhibition of cytokines regulated by PKC in peripheral blood obtained from patients on the phase 1 trial.14 A trial in which PKC412 was given to 13 patients with B-cell chronic lymphocytic leukemia (B-CLL) at doses of 25 to 225 mg/d documented a dose-dependent reduction in PKC activity in the malignant B cells.15 More recently, this compound was found to potently inhibit the growth of leukemic cells lines rendered factor independent by transfection of either an ITD or an activation loop mutation containing FLT3.12 Moreover, mice with lethal activated FLT3-induced myeloproliferative syndrome survived longer following administration of PKC412.8 The current phase 2 trial of PKC412 was undertaken to determine if inhibition of FLT3 would have biologic activity in patients with advanced AML and myelodysplastic syndrome (MDS) whose cells were documented to have an activating mutation of FLT3. As such, this study represents one of the first efforts in AML to provide targeted therapy based on tumor-specific genotype.
Patients, materials, and methods
Patients
Eligibility for treatment with PKC412 in this phase 2 clinical trial included a diagnosis of advanced MDS or AML. Patients with AML were required to have either relapsed or refractory disease that did not require cytoreductive treatment within 1 month of starting the study drug or were felt not to be candidates for induction chemotherapy due to age or medical considerations. FLT3 gene sequencing was performed on DNA isolated from patients with AML or myelodysplasia within 14 days of starting the drug; an ITD mutation or a D835Y activating loop mutation was required for study entry. Hydroxyurea was not allowed within 7 days of starting the drug or thereafter while on study. Patients had to be more than 2 months past bone marrow transplantation (BMT), be older than age 18, and have an Eastern Cooperative Oncology Group (ECOG) performance of 0, 1, or 2. After the first 8 patients were enrolled, the study was amended to require AML patients to have a white blood cell (WBC) count of equal to or more than 5 × 109/L (5000/μL) to allow for more robust ancillary studies and to require a baseline creatinine level of less than or equal to 1.5 × the upper limit of normal (ULN), aspartate aminotransferase (AST) and alanine aminotransferase (ALT) less than or equal to 3 × ULN, total bilirubin concentration less than or equal to 2.0 × ULN, no more than 2 prior AML treatment regimens, and no uncontrolled infection.
Objectives
The primary objectives of the trial were to evaluate the antileukemic activity of PKC412 at a dose of 75 mg orally 3 times a day in patients with AML and MDS with an ITD or D835 mutation to assess the pharmacokinetics of PKC412 and to measure targeted inhibition of the FLT3 kinase using pharmacodynamic studies of autophosphorylation of FLT3 in cells obtained from patients on treatment. A 2-stage design with a total accrual of 12 patients was initially proposed for this trial. After the first 8 patients were enrolled, biologic responses allowing for complete accrual were noted, but the ancillary end point was not met due to the rapid clearance of blasts and inability to detect FLT3 at the time points chosen for analysis. The protocol was revised to enroll 12 additional patients with multiple sampling time points during the first 48 hours of therapy. Based on 12 evaluable patients, the confidence interval (CI) for inhibition of autophosphorylation would be no more than 50%; based on 20 patients treated, the total CI for the clinical response rate would be no more than 40%.
Treatment plan
Patients signed an informed consent approved by the institutional review board before they were screened to determine eligibility. At that time, bone marrow examination and confirmation of an activating mutation of FLT3 was accomplished. Once eligibility was confirmed, patients were started on PKC412 at a dose of 75 mg orally 3 times a day and were treated until toxicity or disease progression occurred. A complete blood count (CBC), blood chemistry, and toxicity assessment were performed weekly. In addition to the baseline bone marrow examination, patients had follow-up marrow examinations every 28 days while on therapy. Dose modification for grade 3 or 4 nonhematologic toxicity mandated stopping drug administration until the toxicity resolved to grade 1 and then restarting at 50 mg orally 3 times a day. If resolution did not occur within 1 week or if severe or life-threatening toxicity recurred, the patient was removed from the study.
National Cancer Institute (NCI) criteria for complete response (CR) included the absence of blasts in the presence of more than 1.5 × 109 neutrophils/μL and more than 100 × 109 platelets/μL in the peripheral blood as well as bone marrow with more than 20% cellularity with less than 5% blasts. Partial response (PR) criteria included a 50% reduction in the blast count compared with pretreatment and a platelet count of more than 50 000/μL. Significant clinical benefit was defined as a more than 2-log reduction in the absolute number of peripheral blood blasts compared with baseline, lasting for at least 4 weeks.
FLT3 DNA mutation assessment
Heparinized or EDTA (ethylenediaminetetraacetic acid)–treated peripheral blood or bone marrow was processed by Ficoll-Hypaque centrifugation for the isolation of the mononuclear cell fraction. DNA was isolated using a kit (Gentra, Minneapolis, MN). The detection and characterization of FLT3 mutations in the nucleotides encoding the juxtamembrane region and in codons 835 and 836 of the activation loop were performed as described.16
FLT3 autophosphorylation
For the first 8 patients, peripheral blood was drawn into heparinized tubes at pretreatment day 8 and day 29. For patients 9 to 20 samples were obtained before treatment, at 4 hours, 24 hours, 48 hours, day 8, and day 29. The monitoring schedule was altered because the rapid decrease in blasts in many of the first 8 patients led to the absence of detectable FLT3 signal at the longer time points originally chosen
Patient blood or bone marrow samples were pipetted into 50-mL conical tubes. Chilled red blood cell (RBC) lysis solution supplemented with 0.4 mM Na3VO4 was added to bring the total volume to 50 mL. Samples were lysed on ice for 5 minutes then spun down at 4°C, 10 000 g [1800 rpm], for 8 minutes and supernatants were discarded. WBC pellets were resuspended in an additional 50 mL RBC lysis solution supplemented with 0.4 mM Na3VO4 and again lysed and spun down as described. WBC pellets were then lysed in 50 to 1000 μL freshly prepared lysis buffer: 20 mM Tris (tris(hydroxymethyl)aminomethane)–HCl, pH 7.4, 1% Triton X-100, 5 mM EDTA, 150 mM NaCl, 10% glycerol, 1 mM Na3VO4,10 μg/mL phenylmethylsulfonyl fluoride (PMSF), complete protease inhibitor cocktail (Roche, Indianapolis, IN), and 20 μM phenylarsine oxide (Sigma, St Louis, MO). Lysates were incubated for 5 minutes on ice and then cleared by centrifugation.
Immunoprecipitations were performed by incubating 1000 μg total protein from cell lysates with rabbit polyclonal S-18 antibody against FLT3 (Santa Cruz Biotechnology, Santa Cruz, CA) on a rotating wheel at 4°C for 1 hour. Immunoprecipitates were collected with protein A–Sepharose beads (Amersham-Pharmacia, Piscataway, NJ). Immunoprecipitates were washed 3 times in lysis buffer and boiled for 5 minutes in sodium dodecyl sulfate (SDS) sample buffer.
Immunoblotting was performed as previously described.17 Briefly, samples were separated by SDS–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred electrophoretically to a ProTran nitrocellulose membrane (Schleicher & Schuel, Keene, NH). Membranes were blocked with 2% bovine serum albumin (Sigma Fraction V; Sigma Chemicals) in wash buffer: 10 mM Tris-HCL, pH 7.4, 0.1% Triton X-100, and 0.9% NaCl. Samples were then incubated with mouse monoclonal 4G10 antibody against phosphotyrosine (Upstate Biotechnology, Lake Placid, NY) for 1 hour at room temperature. The nitrocellulose membrane was then washed and incubated with horseradish peroxidase (HRP)–conjugated antimouse IgG (Amersham-Pharmacia). Blots were again washed and then visualized by chemiluminescence. To test for equal loading of protein, blots were subsequently stripped at 55°C for 30 minutes in stripping solution of 64 mM Tris-HCl, pH 6.8, 2% SDS, and 7 mM β-mercaptoethanol, and then reprobed with rabbit polyclonal S-18 antibody against FLT3 (Santa Cruz Biotechnology).
PKC412 pharmacokinetics
PKC412 was administered with water following breakfast, lunch, and dinner at a 75-mg oral dose 3 times a day. Blood samples (3 mL each) were drawn into heparin-coated tubes immediately preceding the first dose, 4 hours after the first dose, and premorning dose samples on day 2, day 3, and day 8 of cycle 1 and on day 1 of each subsequent cycle. Blood samples were centrifuged for 10 minutes for plasma at 2500g, 3 to 5°C. Immediately after centrifugation plasma was transferred to brown polypropylene tubes and kept frozen at –18°C or colder until analysis. Plasma samples were processed and protected from light because PKC412 is sensitive to light. The concentrations of unchanged PKC412 and metabolites CGP52421 (an epimer of the monohydroxy metabolite) and CGP62221 (the desmethyl metabolite) in plasma were determined using a high-performance liquid chromatography method with fluorescence detection, with a limit of quantitation (LOQ) of 5.1 ng/mL.13 The maximum trough analyte concentration (Ctrough, max) and the time to Ctrough, max were recorded as observed, and the steady-state trough concentration (Ctrough, SS) was calculated based on concentrations measured after 28 days.
Results
Patients
Twenty eligible patients were enrolled; each received at least 2 weeks of study drug. Sixteen of the patients were treated at Dana-Farber Cancer Institute, 3 at Memorial Sloan Kettering Cancer Center, and 1 at the MD Anderson Cancer Center. The patient characteristics are shown in Tables 1 and 2. The median age was 62 years (range, 29-78 years). Ten patients had relapsed AML, 7 had never achieved remission at any point and were deemed refractory, and 1 had MDS (chronic myelomonocytic leukemia [CMML]). Patients were relatively heavily pretreated having received a median of 4 prior regimens (range, 0-9 regimens). Three patients, aged 67, 76, and 78 years, had no prior treatment except hydroxyurea. Fourteen patients had primary AML and 5 had secondary AML (2 after prior MDS and 3 after chemotherapy for another neoplasm).
Twelve patients had a normal karyotype on standard cytogenetic analysis, as occurs commonly in patients with FLT3 mutations.5 One patient had a translocation between chromosomes 6 and 9, which has been reported to be typically associated with an ITD mutation of FLT3,5 one had an inversion of chromosome 16, 3 had miscellaneous abnormalities, and 3 were unevaluable. An FLT3 mutation was confirmed in all patients. In 18 of the 20 patients this was an ITD mutation of between 18 and 189 base pair (bp) in length (median, 48 bp). In 2 patients a D835Y mutation was documented.
Toxicity
The drug was generally well tolerated. Thirteen patients had grade 1 or 2 nausea and vomiting (Table 3). In some patients, particularly those in whom a reduction in peripheral blasts was noted, a concomitant reduction in neutrophils was noted. The neutrophil count later increased although the blast count remained low. In these patients, it was difficult to determine whether myelosuppression was related to the drug or the disease.
Three patients had fatal pulmonary events, one of which was in the context of progressive leukocytosis and was almost certainly unrelated to study drug administration. In another patient, a 72-year-old man treated for his second relapse of AML, shortness of breath and diffuse pulmonary infiltrates developed after 11 days on therapy. The drug was stopped, he refused open lung biopsy, and died 7 days later from progressive pulmonary failure. An autopsy was not performed. The third patient was a 67-year-old man with CMML. He had pneumonia, which responded to antibiotics 2 weeks prior to starting therapy; he was doing well at the time of study entry. However, on day 9 he developed shortness of breath and hypoxemia. The drug was stopped. An extensive workup for infectious causes, including open lung biopsy, failed to reveal an etiology. Progressive pulmonary failure ensued and the patient died on day 26. Postmortem examination as well as the open lung biopsy showed nonspecific findings. Two other patients sustained grade 3 pulmonary toxicity, but this pertained to the transient use of oxygen in the context of an infection.
Response
Twelve of the patients received more than 29 days of PKC412 treatment. Eight patients discontinued drug on or before day 29, 3 due to adverse events (hyperbilirubinemia, elevated AST, and bronchopneumonia), 3 due to disease progression, and 2 died from pulmonary complications as noted. The drug was held in one patient due to grade 3 soft-tissue infection on day 48; however, this patient continued to show improvement in his blood counts. He underwent a marrow examination on day 165, which was compatible with remission except the cellularity was below 20%. By day 210 he had frank AML relapse. After retreatment on a compassionate basis, he responded again for 90 days until progressing at that point. Six patients progressed after 72, 72, 90, 162, 213, and 330 days on treatment. All these 6 patients had an initial peripheral blood count response. Two patients were removed from study on day 74 and day 99 to undergo BMT after having had significant clinical benefit.
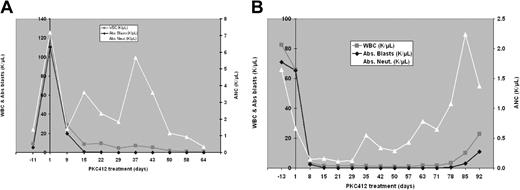
Fourteen of the 20 patients (70%; 90% CI, 49%-86%) achieved greater than a 50% reduction in the peripheral blast count compared to baseline values. In 7 of these 14, at least a 2-log reduction in peripheral blast count lasting for at least 4 weeks was seen. For example, one patient started with a blast count of 110 000/μL and went to 0 on day 15 (Figure 1A); another went from 65 000/μLon day 0 to 0 to 0.1/μL between day 15 and day 78 (Figure 1B).
Serial blood counts obtained in 2 patients receiving PKC412. (A) A 55-year-old man with refractory AML. (B) A 38-year-old man with relapsed AML.
Serial blood counts obtained in 2 patients receiving PKC412. (A) A 55-year-old man with refractory AML. (B) A 38-year-old man with relapsed AML.
Six of the 20 patients achieved a more than 50% reduction in the bone marrow blast count. Three patients exhibited fewer than 5% blasts: one on day 28 with continued low blood counts, one on day 64 who met CR criteria except for a bone marrow cellularity of only 10% (20% was required), and the third on day 58 (Tables 4 and 5). Several patients were removed from the study for reasons indicated in Table 6.
Pharmacokinetic studies
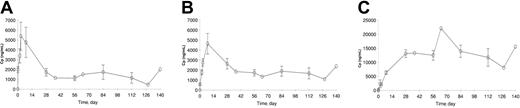
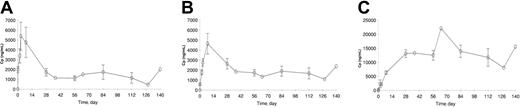
The plasma concentration data were available from 15 of 20 patients. The concentration-time profiles of PKC412 and its metabolites, CGP62221 and CGP52421, are plotted in Figure 2A-C and the kinetic parameters are listed in Table 7. PKC412 trough concentrations increased during the first 2 to 3 days and peaked between 3 to 7 days. As the oral dose continued at the same dose level, mean PKC412 concentration decreased by about 70% with time, from 5.39 μg/mL on day 3 to 1.34 μg/mL at steady state. The concentration of CGP62221, the active metabolite, showed a similar concentration and concentration-time profiles to the parent drug PKC412. Its concentration peaked on day 8 and decreased by about 63% from 4.65 μg/mL on day 8 to 1.71 μg/mL at steady state. However, the concentration of CGP52421 showed a continuous increase up to day 28 and remained relatively stable during the remaining treatment period.
Plasma concentrations. Trough plasma concentration (± SE)–time profiles of (A) PKC412 and its metabolites, (B) CGP62221, and (C) CGP52421 following oral doses of 75 mg PKC412 3 times a day in patients with mutated FLT3 AML (data from 15 of 20 patients studied, n = 1 for data points without SE bar).
Pharmacodynamic studies
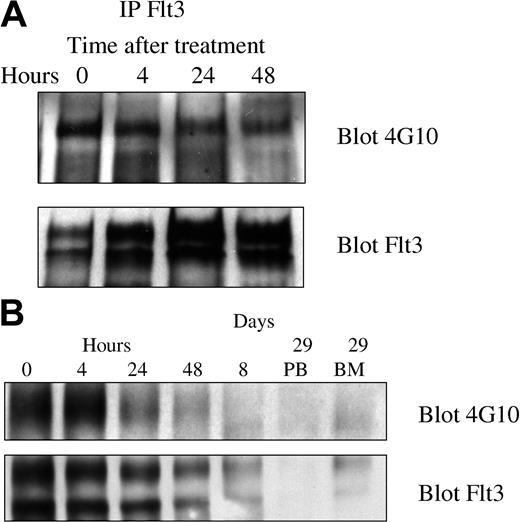
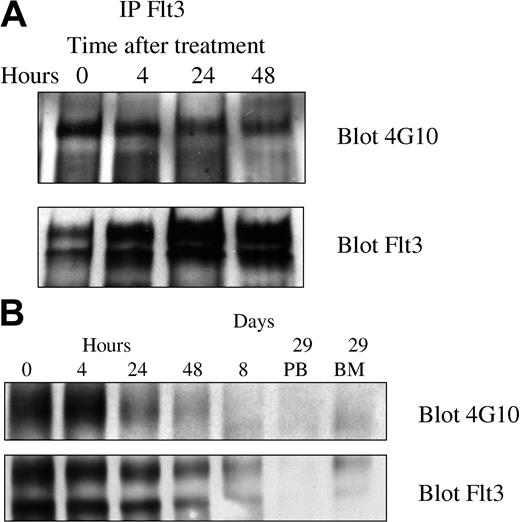
Because PKC412 inhibits several different kinases, it was particularly important to demonstrate inhibition of FLT3 tyrosine kinase activity in vivo. We therefore looked for inhibition of FLT3 autophosphorylation, a characteristic measure of FLT3 tyrosine kinase activity. In the first 8 patients the initial posttherapy time point chosen (day 8) was well after clearance of blasts and yielded undetectable levels of FLT3. The next 12 patients had blood mononuclear cells obtained at 0, 4, 24, and 48 hours and days 8 and 29. All 5 assessable specimens exhibited a decrease in the phosphotyrosine FLT3 signal relative to total FLT3 expression. All 5 patients responded to PKC412. Representative blots are depicted in Figure 3.
Pharmacodynamic response of 2 representative patients. FLT3 was immunoprecipitated and Western blotting was performed using 4G10 antiphosphotyrosine antibodies and anti-FLT3 antibodies as described in “Patients, materials, and methods.” (A) Patient 501-0008 had a significant decrease in relative phosphotyrosine content of FLT3 by 48 hours after initiation of therapy. The absolute blast count (ABC) was 15 600 on day 1 and had decreased to 200 by day 8 of therapy. (B) Patient 501-00017 started therapy with an absolute blast count of 4530 on day 1, which decreased to 100 by day 8.
Pharmacodynamic response of 2 representative patients. FLT3 was immunoprecipitated and Western blotting was performed using 4G10 antiphosphotyrosine antibodies and anti-FLT3 antibodies as described in “Patients, materials, and methods.” (A) Patient 501-0008 had a significant decrease in relative phosphotyrosine content of FLT3 by 48 hours after initiation of therapy. The absolute blast count (ABC) was 15 600 on day 1 and had decreased to 200 by day 8 of therapy. (B) Patient 501-00017 started therapy with an absolute blast count of 4530 on day 1, which decreased to 100 by day 8.
Discussion
We performed a proof-of-concept study of PKC412, a multitargeted kinase inhibitor in patients whose leukemias were characterized by the presence of an activating mutation of the FLT3 transmembrane tyrosine kinase. The activating mutation was either an ITD in the juxtamembrane region or a mutation from aspartate to tyrosine in the 835th amino acid residue, in the activation loop region. Because both of these 2 forms of FLT3 confer constitutive tyrosine kinase activity and contribute to the development of leukemia in model systems,6,7 we hypothesized that blasts from patients with this abnormality would be at least partially dependent on this mutation for growth and survival. We further reasoned that inhibiting this tyrosine kinase in patients with AML and MDS with the FLT3 mutations could result in leukemic cell death and potentially clinical responses.
PKC412, or N-benzoylstaurosporine, was initially developed as an inhibitor of PKC,11 and was previously shown to also inhibit VEGFR-2, PDGFR-α, PDGFR-β, and the c-kit receptor, but did not inhibit members of the src, fibroblast growth factor receptor, epidermal growth factor receptor families, BCR/ABL, and others.12 PKC412 was previously evaluated in a dose-escalation phase 1 clinical trial for single-agent clinical activity in 32 patients with a variety of solid tumors.13 The maximum tolerated dose (MTD) was determined to be 75 mg orally 3 times a day. Two additional phase 1/2 single-agent trials in CLL and lymphoma and melanoma suggested that 75 mg orally 3 times a day was well tolerated. More recently Weisberg et al12 identified PKC412 as an inhibitor of FLT3 in a screen that examined a wide variety of known kinase inhibitors for cross-reactivity against mutant FLT3 kinase. Preclinical studies demonstrated that PKC412 inhibited the growth of leukemic cell lines made factor independent by transfecting with an activated FLT3 kinase (inhibitory concentration of 50% [IC50] of 10 nM for the FLT3 ITD and or IC50 of 5 nM for the D835Y point mutation),12 and significantly prolonged the survival of mice with a fatal myeloproliferative syndrome engendered by a FLT3 mutation in a BMT model.8
We treated 20 patients with a fixed dose of PKC412, 75 mg orally 3 times a day. In most patients the drug was extremely well tolerated. However, serious toxicities in 2 patients who suffered fatal pulmonary events may have been related to the drug. One of these 2 patients underwent open lung biopsy and eventually a postmortem examination revealed only nonspecific findings. These 2 patients could have had an occult infection given their disease status; however, none was found despite extensive workup. Some distinctive characteristics of these patients included an elevated serum creatinine level in one patient and CMML with a fairly low burden of leukemic blasts in the other. Pulmonary toxicity was not a feature of previous trials with PKC412. Other VEGFR inhibitors have been reported to be associated with pulmonary toxicity in patients with lung cancer.15 Future trials with this agent should include careful monitoring of pulmonary findings to determine the actual incidence and severity of pulmonary side effects. For the majority of patients treated with PKC412, however, prolonged administration of this agent resulted in no obvious significant or cumulative side effects.
Pharmacokinetic analysis revealed that the plasma concentration of PKC412 increased substantially in the first week, and then declined thereafter despite continued dosing, consistent with the early findings in patients with advanced solid cancer.13 The exact mechanism for the declining concentration of PKC412 is still unclear, but it could either be due to decreased oral absorption or induced metabolism/excretion. Because PKC412 and its metabolites are all highly bound to plasma proteins including albumin and α-acid glycoprotein, a protein-binding interaction between PKC412 and its metabolites especially with CGP52421, whose concentration showed a continuous increase with time, cannot be excluded as the mechanism for the observed time-dependent kinetics of PKC412 in patients with AML. In addition, drug interactions with other medications, as well as noncompliance, might be critical factors for the observed interpatient variability and declining PKC412 plasma concentrations.
The declining concentrations of PKC412 and its active metabolite CGP62221 after day 7 may play an important role in the brief duration of blast remission and clinical efficacy in patients with FLT3-mutated AML treated with single-agent PKC412 in this preliminary study. The peak concentration of PKC412 on day 3, 5.39 μg/mL, was about 10 times higher than the IC50 value for mutated FLT3 ITD (1 μM or ∼0.57 μg/mL) corrected for plasma protein binding (99%, Novartis internal report, January 1996). Corresponding with the high plasma concentration, peripheral blast reduction was observed mostly within the first week after the dose, as described. After 1 month of treatment, the plasma concentrations of both PKC412 and CGP62221 dropped to 1 to 2 μM, only about 2 to 3 times the corrected IC50 value.
Blast reduction appeared to be generally correlated with a high plasma level of PKC412, although the small patient numbers preclude certain cause and effect. Nonetheless, maintaining a stable and biologically effective plasma concentration might be necessary for durable activity. The oral dose of 75 mg 3 times a day selected in the present study was the MTD defined in patients with solid tumors13 ; thus, no higher doses were tested in the present study. Because the plasma exposure of PKC412 decreases with time, further studies are needed to understand the mechanism underlying the drop in concentration over time and the pharmacokinetic/pharmacodynamic relationship for peripheral blast reduction and clinical efficacy. In addition, the safety and tolerability of PKC412 at doses higher than 225 mg/d (> 75 mg 3 times a day) after the fall in concentration has occurred should be investigated. Considering the large interpatient variability in PKC412 plasma concentrations, intrapatient dose titration might be required to maintain an efficacious level, thereby eliciting more frequent and durable clinical responses.
Given the advanced nature of leukemias in most of our patients, the rate of clinical activity with PKC412 was noteworthy. Significant rapid reductions in blast cell counts in the peripheral blood occurred in 7 patients, often in a few days. In another patient, an initial blast count of 10 000/μL fell to 2000/μL, correlating with a disappearance of lymphadenopathy. The patient remained stable on the drug for 11 months when the disease finally progressed. One of the patients achieved a near complete remission, with less than 5% marrow blasts and normal peripheral blood counts but had moderate marrow hypocellularity at 10% (now considered to be compatible with a complete remission based on recently published guidelines).18 One of the 7 significant responders, a 53-year-old man with refractory AML, achieved a low enough disease burden to undergo allogeneic BMT from an unrelated donor. Patient selection criteria, which did not allow patients likely requiring cytoreductive therapy within 1 month to enroll or which (after the first 8 patients) required AML patients to have a WBC count of 5000/μL or higher, may have influenced the results. Nonetheless, as can be seen in Table 2 and Figure 1, patients with indolent-behaving disease did not appear to be overrepresented and individual patients had high disease burdens at study entry. Moreover, only one patient who was eligible at screening progressed prior to beginning the drug.
Imatinib mesylate in chronic myeloid leukemia (CML) can be considered a model for tyrosine kinase inhibition therapy in hematopoietic neoplasms. In early chronic phase CML, the complete hematologic response rate is 95% and the major cytogenetic response rate is 81%. However, the outcome is markedly different when this agent is used in patients with blast crisis CML, a leukemia more similar genetically and clinically to AML. In this setting, the hematologic response rate to imatinib mesylate is 30% and the complete cytogenetic remission rate is 7%.19 Patients with CML in myeloid blast crisis are presumed to have additional genetic abnormalities that promote proliferation and impair differentiation beyond the activation of c-abl by the BCR/ABL translocation. Therefore, it was not reasonable to expect a response rate with this drug to be greater than that observed with imatinib mesylate in CML blast crisis. Similar clinical results have been observed in early reports of clinical trials with other FLT3 inhibitors in AML.20,21 A tolerable dose of MLN518 was determined and several patients treated at or near the MTD achieved both a reduction in myeloblast burden and a concomitant decrease in FLT3 autophosphorylation in blasts.21 Five of 14 patients receiving a dose of CEP701 adequate to inhibit FLT3 autophosphorylation experienced a reduction in peripheral blood blasts; in contrast to our trial, coadministration of hydroxyurea was allowed.
There may be other reasons for the lack of overt activity of PKC412 as a single agent in this patient population. Pharmacokinetic interpatient variability and declining concentration over time may contribute to lack of efficacy or the short duration of the efficacy or both. Emergence of new mutations, either in FLT3 kinase itself (resistant to PKC412) or in other genes known to be involved in leukemogenesis, may also play a role. Studies are underway to address both these possibilities, but at this time, no new PKC412-resistant FLT3 mutations have been detected in patients with an initial blast response whose blasts then recurred.
Efforts to determine if the mechanism of the activity of PKC412 is actually inhibition of activated FLT3 were undertaken. If clinical responses were seen in the absence of inhibition of the enzyme, then one might presume an alternative mechanism. Documenting inhibition of enzyme activity in cells obtained from responding patients on the drug is useful but does not exclude the possibility that there may be other targets more or equally important than FLT3. Ancillary studies on clinical samples that measure autophosphorylation on FLT3, the surrogate designated marker for inhibition of the enzyme, are technically difficult to perform. In the first 8 patients treated in this study we selected suboptimal time points to assess autophosphorylation because the malignant cells were not present in sufficient quantities on days 8 or 29 due to the rapid reduction in blasts engendered by PKC412. However, inhibition of FLT3 autophosphorylation was detected in 5 of the responding patients who had pharmacodynamic assessments at earlier time points.
The encouraging results described in this clinical trial with PKC412 indicate that additional clinical studies with this agent in patients with AML are warranted. In particular, the activity of this agent in patients with AML whose blasts are not found to have an activating mutation of FLT3 should be determined. PKC412 has activity either directly as a VEGFR-2, and c-kit kinase inhibitor, and indirectly in K-ras–mutated cells to cause inhibition of proliferation; VEGFR-2, c-kit, and ras mutations are all targets thought to contribute to myeloid leukemogenesis. Furthermore, separate from its effects as a FLT3 kinase inhibitor, PKC412 has been shown in a variety of settings to enhance apoptosis of chemotherapeutic agents, perhaps through its activity as PKC serine-threonine kinase inhibitor.22 PKC412 should be combined with other effective antileukemic drugs, such as anthracyclines and cytarabine. Because patients with an FLT3 mutation have a uniformly poor prognosis, the use of this tyrosine kinase inhibitor may make it possible to improve outcome with standard chemotherapy.
Prepublished online as Blood First Edition Paper, September 2, 2004; DOI 10.1182/blood-2004-03-0891.
Supported in part by a Leukemia and Lymphoma Society SCOR grant (R.M.S., D.J.D., D.G.D., J.D.G.) and Leukemia and Lymphoma Society grants (V.K. and S.D.N.) and by National Institutes of Health grant PO1 CA66996-06.
P.C., D.L., and Y.W. are employed by Novartis, whose potential product was studied in the present work. This study was supported by research funding from Novartis.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.