Abstract
Although the infusion of umbilical cord blood (UCB) from multiple donors can be a strategy to overcome the cell dose limitation frequently encountered in UCB transplantation, clinical trials have revealed that cells from one donor dominate engraftment. To investigate the origin of and the factors influencing this inequality, we performed mixed transplantation of 2 UCB units with varying degrees of HLA disparities into NOD/SCID mice and determined donor origins by polymerase chain reaction–sequence-specific oligonucleotide probe (PCR-SSOP) or real-time quantitative (RQ)–PCR for human short tandem repeats (STRs). When total mononuclear cells from 2 units were transplanted as a mixture, cells from one donor predominated (ratio, 81:19), despite comparable overall engraftment when infused as single units, and no augmentation in overall engraftment was observed when compared with the single-unit controls. However, lineage depletion or cotransplantation of mesenchymal stromal cells (MSCs) expanded from third-party bone marrow resulted in more balanced coengraftment. Direct comparison of double UCB transplantation in the presence or absence of MSCs showed that the reduced deviation in the donor ratio (1.8:1 vs. 2.8:1) correlated with a higher overall level of engraftment with MSC cotransplantation. These results indicate that third-party MSCs can be used to alleviate donor deviation and to facilitate engraftment of multidonor UCB.
Introduction
Umbilical cord blood (UCB) is an attractive source of hematopoietic stem cells (HSCs), and it has many advantages over bone marrow stem cells, including a higher frequency of transplantable HSCs and a higher output of progenitor cells from equivalent numbers of HSCs. In addition, public banking of UCB is creating increased accessibilty.1-3 Accumulating clinical evidence demonstrates that the number of total nucleated cells in a given UCB unit is the single most important parameter for successful outcome, with low cell numbers frequently resulting in delayed or failed engraftment.4,5 Although ex vivo expansion of cord blood cells has been explored as a method to increase the input cell number at the time of transplantation, loss of engraftment has been frequently observed,6-8 and an efficient and reliable method of expansion has yet to be determined.9-11
Interestingly, immune cells in UCB are functionally immature, and a lower incidence of severe graft-versus-host disease (GVHD) has been observed with UCB transplantation.12-14 It may be possible, therefore, to transplant UCB cells with greater HLA disparities or to transplant multiple UCB units as a mixture to increase the absolute number of HSCs in the graft. However, several clinical trials evaluating the transplantation of multidonor UCB grafts have shown that cells from one donor tend to be predominant over the cells from the other (or others) in the reconstitution of such patients, posing another challenge to this strategy.15-18
The reason for the unequal contributions from 2 UCB sources to hematopoietic reconstitution is not yet clear. It remains to be determined whether the inequality results from an unequal amount of HSCs in each UCB unit or whether it results from competition between the 2 grafts during the engrafting process. However, it has been difficult to address this issue in clinical trials because of the lack of matched, single UCB transplantation groups. Furthermore, it has not yet become clear whether the increase in cell numbers created by multiple cord transplantation indeed results in a higher overall level of engraftment compared with conventional single UCB transplantation.
To study the kinetics and the quantitative aspects of HSC engraftment, surrogate in vivo xenogeneic transplantation models have been used—including transplantation into severe combined immunodeficiency (SCID) mice,19 nonobese diabetic-SCID (NOD/SCID) mice,20 and preimmune fetal sheep21 — along with autologous transplantation models in large animals such as nonhuman primates.22 The engrafting human HSCs in NOD/SCID or SCID mice have been defined as SCID-repopulating cells (SRCs) or competitive repopulating units (CRUs),19 which are the cells that can give rise to long-term repopulation and multilineage differentiation without exhaustion23 in the recipients of transplants.
In the current study, using a series of HLA disparity-based combinations of UCB units transplanted into NOD/SCID mice, we show that the single-donor predominance phenomenon in double cord transplantation is not caused by an absence or a lack of CRU content in the nondominant graft. Rather, it occurs during the engrafting process independent of HLA disparities. Furthermore, we show that the cotransplantation of culture-expanded mesenchymal stromal cells (MSCs) from third-party bone marrow can alleviate single-donor predominance and thereby lead to additive coengraftment, resulting in higher levels of overall engraftment after double cord transplantation.
Materials and methods
Cells
Informed consent was obtained before collection of all cellular products. Low-density (1.077 g/mL) cells were isolated by Ficoll-Hypaque density centrifugation (Amersham Pharmacia, Uppsala, Sweden) from normal cord blood samples and were cryopreserved in medium containing dimethyl sulfoxide. In some experiments, low-density cord blood cells were obtained from the buffy coats that had been cryopreserved for allogeneic cord blood banking (Histostem, Seoul, Korea). In all cases, HLA typing was performed by genetic typing of the HLA-A, -B, and -DR loci.24 For lineage depletion of cord blood cells, cryopreserved light-density cells were thawed, and mature lineage-positive (Lin+) cells (CD2, CD3, CD14, CD16, CD19, CD24, CD36, CD38, CD45RA, CD56, CD66b, and glycophorin A) were depleted using an immunomagnetic column (StemCell Technologies, Vancouver, BC, Canada).
MSCs were obtained from normal donor bone marrow by culturing in Dulbecco modified Eagle medium (DMEM) containing 10% fetal bovine serum (FBS; StemCell Technologies) and 1% PenStrep (Gibco-BRL, Rockville, MD) as previously described.25 After 2 weeks of culture, adherent cells were subcultured for expansion up to 3 passages and were cryopreserved for cotransplantation with UCB. Multilineage differentiation of MSCs into adipogenic, osteogenic and neurogenic lineages was confirmed by oil red-o staining, alkaline phosphatase, Alizarine-red staining, and immunohistochemical staining against NeuN, respectively, and by their characteristic surface marker expression, as described previously.25-29
Xenotransplantation of cord blood
NOD/LtSz-scid/scid (NOD/SCID) mice,20 originally obtained from Dr L. Schultz (The Jackson Laboratory, Bar Harbor, ME), were bred and maintained in the animal facility of the Catholic Research Institutes of Medical Science (Seoul, Korea) under sterile conditions in microisolator cages located in an air-filtered, positively pressured room. The animals were provided with autoclaved food and water. Transplantation of cord blood cells into NOD/SCID mice was performed as previously described.30 Briefly, when the mice were 8 to 12 weeks of age, they received 350 cGy total body irradiation with x-rays from a linear accelerator and were given acidified drinking water supplemented with 100 mg/L ciprofloxacin (Bayer, Leverkusen, Germany) during the experimental period. Test cells were injected intravenously into the irradiated mice within 24 hours of irradiation, and they were killed 6 weeks after transplantation. Aliquots of harvested cells were incubated with 5% human serum and 2.4G2 (an antimouse Fc receptor antibody) to decrease nonspecific antibody binding.31 Cells were then stained for 30 minutes at 4°C with antihuman CD45-PE (BD PharMingen, San Diego, CA) and antihuman CD71–phycoerythrin (CD-71-PE) antibodies (BD PharMingen) and were washed twice in HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) with 2% FBS; the last wash contained 1 μg/mL propidium iodide (PI; Sigma Chemical, St Louis, MO). A detection limit of more than 1% human cells among the total PI– cells was used to identify positively engrafted mice using gates set to exclude more than 99.99% of nonspecifically stained PI– cells incubated with isotype-matched control antibodies labeled with the corresponding fluorochromes, as previously described.1 Genomic DNA was purified from aliquots of bone marrow obtained from animals engrafted with human cells using a QI Amp DNA Mini kit (Qiagen, Hilden, Germany) and was subjected to analysis for donor origin.
Mixed transplantation of cord blood
Pairs of cord blood units with varying degrees of HLA disparities were selected from the pool of cord blood that had been HLA typed at the time of freezing. Light-density cells from these cord blood units were stained with anti-CD34–fluorescein isothiocyanate (anti-CD34–FITC) (BD PharMingen) and anti-CD3–FITC (BD PharMingen) to determine CD34+ and CD3+ cell content. Cells from each single cord unit were divided into aliquots to contain equivalent numbers of CD34+ cells at limiting dose (3-5 × 104 CD34+ cells/mouse) and transplanted into irradiated NOD/SCID mice as a single unit or as part of double-unit transplantation so that the relative contribution by each donor cell could be analyzed on the basis of input HSCs transplanted. Donor origin of the engrafted cells in the NOD/SCID mice was analyzed using polymerase chain reaction–sequence-specific oligonucleotide probe (PCR-SSOP) or real-time quantitative PCR of 16 short tandem repeat (STR) markers (RQ-STRs).
Qualitative and quantitative analysis of donor origin of engrafted cells
PCR-SSOP (sequence-specific oligonucleotide probe) was used to type HLA-DRB1, -DQA1, -DQB1, and -DPB1 loci in addition to the HLA-A, -B, -DR loci to analyze the donor distribution of engrafted cells, as previously described,24 with minor modifications. Each HLA locus gene was amplified by specific primers, and the products were denatured and immobilized on a nylon membrane for probing with digoxigenin-labeled oligonucleotide probes specific for known hypervariable sequences. Stringent washing was performed in the presence of tetramethyl ammonium chloride (TMAC; Sigma Chemical). Hybridized filters were then probed with an alkaline phosphatase-conjugated antidigoxigenin antibody and were visualized with chemiluminescent substrate CSPD (Boehringer Mannheim, Germany).
For quantitative analysis of donor distribution in engrafted cells, multiplex real-time quantitative PCR (RQ-PCR) on human STR was performed as previously described32 using the AmpFlSTR Identifier PCR Amplification Kit (PE Applied Biosystems, Foster City, CA). The following 16 STR markers were amplified in this system: D8S1179, D21S11, D7S820, CSF1PO, D3S1358, TH01, D13S317, D16S539, D2S1338, D19S433, vWA, TPOX, D18S51, D5S818, FGA, and the sex marker amelogenin. All markers were amplified in a multiplex PCR reaction (PCRExpress; HYBAID, Ashford, United Kingdom) according to the manufacturer's instructions, and the resultant fragments were analyzed on the ABI Prism 310 Genetic Analyzer (PE Applied Biosystems). Then 1.5 μL PCR product was added to 24.5 μL Hi-Di formamide (PE Applied Biosystems) and 0.5 μL GeneScan-500 LIZ Size Standard (PE Applied Biosystems) and then was subjected to capillary electrophoresis using Performance Optimized Polymer (PE Applied Biosystems) with a 47 cm/50 μm capillary (PE Applied Biosystems). Fragment sizes and peak areas were analyzed using the GeneScan Analyzer and Genotyper software (PE Applied Biosystems).
The extent of double chimerism was calculated from the observed peak area of the informative markers. Only informative alleles—type 1 (nonoverlapping) and type 2 (partial overlapping)—were taken for calculation of chimerism; type 3 (overlapping) alleles were excluded.
Statistical analyses
Results are shown as mean values ± SEM from independent experiments. Differences between groups were analyzed using the Student t test.
Results
Mixed transplantation of total nucleated cells leads to single-donor predominance independent of input CRU content or degree of HLA mismatch
Light-density total mononuclear cells (MNCs) from pairs of UCB units with different degrees of HLA disparity were prepared from previously HLA-screened UCB pools and transplanted into NOD/SCID mice alone (control) or as a mixture. The percentage of CD34+ cells in these pools ranged between 0.2% and 1.0% (mean, 0.6%). Although approximately 30% of mice died within the first 2 to 3 weeks of transplantation, no signs of GVHD, such as shivering, hair loss, or gut necrosis,33,34 had been observed. Mice were killed 6 weeks after transplantation and were analyzed for human cell engraftment and donor origin of the engrafted cells.
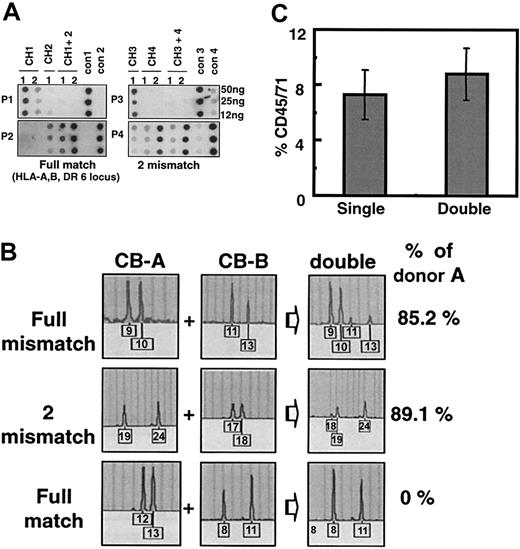
First, 3 cohorts of double cord transplantations with full matches in 6 loci (HLA-A, -B, -DR in each haploid), 2 mismatches (in HLA-B, -DR), or full mismatches were analyzed with PCR-SSOP for the relative contribution of each donor and were compared with each matched single unit control. As shown in Figure 1A, transplantation of each UCB as a single graft resulted in a readily detectable and comparable level of human cell engraftment in the mice (CH1-CH4), as evidenced by the density of hybridization of each allele-specific oligonucleotide probe (P1-P4). However, in the mixed transplantation of 2 units (CH1 + 2 or CH3 + 4), only a single probe (P2 specific for CH2 or P4 specific for CH4) hybridized to DNA obtained from the recipient's bone marrow and no specific hybridization signal was detected from the other unit (P1 for CH1 or P3 for CH3). Double transplantation of 2 fully mismatched units also demonstrated similar single-donor predominance (data not shown). These results indicate that single-donor predominance can occur in double transplantation of total MNCs even when each unit of UCB can engraft at comparable levels when infused as a single graft, suggesting that this phenomenon is not caused by a lack of input CRU in the nondominant graft.
Single-donor predominant engraftment after transplantation of 2 UCBs as a mixture of total mononuclear cells. (A) Donor origin of cord blood cells engrafted in NOD/SCID mice as determined by PCR-SSOP. Combinations of 2 UCB units were made with varying degrees of HLA disparity, and total MNCs corresponding to 5 × 104 CD34+ cells from each unit were infused into irradiated NOD/SCID mice in single (CH1-CH4) or double (CH1 + 2, CH3 + 4) transplantations. Genomic DNA harvested from recipients' bone marrow was subjected to PCR amplification by DPB1 locus-specific primers and were hybridized to allele-specific probes (P1-P4), where positive controls were DNA from donor cells (cont1-cont4). Shown are the results from 2 cohorts of double transplantations with either a full match at 6 loci (HLA-A, B, DR) (left) or 2 mismatches (HLA-B, DR) (right). (B) Profiles of RQ-PCR for the human STR to determine donor ratio of engrafted cells. Genomic DNA was harvested from mouse bone marrow and subjected to RQ-PCR analysis on 16 human STR markers. Shown are the results from a representative marker in each cohort. CB-A and CB-B were artificially nominated for the dominant and nondominant cord unit, respectively. (C) Absence of additive engraftment in transplantation of 2 UCB units as a mixture of total mononuclear cells. Overall engraftment levels of transplanted cord blood cells in NOD/SCID mice were measured by staining harvested mouse bone marrow with human cell–specific anti-CD45/CD71, as described in “Materials and methods.” Shown are the mean engraftment levels ± SEM from single or double cord transplantation (n = 22) from 9 cohorts.
Single-donor predominant engraftment after transplantation of 2 UCBs as a mixture of total mononuclear cells. (A) Donor origin of cord blood cells engrafted in NOD/SCID mice as determined by PCR-SSOP. Combinations of 2 UCB units were made with varying degrees of HLA disparity, and total MNCs corresponding to 5 × 104 CD34+ cells from each unit were infused into irradiated NOD/SCID mice in single (CH1-CH4) or double (CH1 + 2, CH3 + 4) transplantations. Genomic DNA harvested from recipients' bone marrow was subjected to PCR amplification by DPB1 locus-specific primers and were hybridized to allele-specific probes (P1-P4), where positive controls were DNA from donor cells (cont1-cont4). Shown are the results from 2 cohorts of double transplantations with either a full match at 6 loci (HLA-A, B, DR) (left) or 2 mismatches (HLA-B, DR) (right). (B) Profiles of RQ-PCR for the human STR to determine donor ratio of engrafted cells. Genomic DNA was harvested from mouse bone marrow and subjected to RQ-PCR analysis on 16 human STR markers. Shown are the results from a representative marker in each cohort. CB-A and CB-B were artificially nominated for the dominant and nondominant cord unit, respectively. (C) Absence of additive engraftment in transplantation of 2 UCB units as a mixture of total mononuclear cells. Overall engraftment levels of transplanted cord blood cells in NOD/SCID mice were measured by staining harvested mouse bone marrow with human cell–specific anti-CD45/CD71, as described in “Materials and methods.” Shown are the mean engraftment levels ± SEM from single or double cord transplantation (n = 22) from 9 cohorts.
To quantitatively measure the extent and variability of single-donor predominance with respect to HLA disparity, we next performed RQ-PCR of donor-specific STR and analyzed the distribution of donor engraftment from 9 cohorts of double cord transplantation recipients receiving grafts with varying degrees of HLA disparity (summarized in Table 1). Figure 1B illustrates a representative analysis in which STR peaks derived from the dominant party constitute 85% to 100% of donor-derived peaks amplified. Table 1 summarizes the quantitation from each HLA-matching category. As shown, minor variations in the extent of single-donor predominance were observed within each HLA-matching group, but no significant difference in the extent or frequency of single-donor predominance was observed among the various HLA-matching groups. Overall dominant cells comprised 80.74% ± 2.2% of engraftment in recipient animals (average donor cell ratio, 4.2:1). Additionally, none of the parameters, including CD34+ cell percentage, CD3+ cell percentage, or presence of a particular HLA type, correlated with the dominance observed.
We next compared the overall engraftment levels achieved after double cord transplantation with that achieved after single-unit transplantation. As shown in Figure 1C, transplantation of 2 UCB units as a mixture of total MNCs led to overall human cell engraftment ranging from 2.2% to 31.0% (mean, 8.8% ± 1.9%), whereas transplantation of matched single UCB units led to engraftment of 1.1% to 33.1% (mean, 7.3% ± 1.8%), thus showing no significant difference in the level of engraftment between single and double cord transplantation, despite the fact that twice as many cells were transplanted for the latter. This result suggests that, under circumstances of single-donor predominance, mixing 2 cord units does not lead to a significant increase in the overall engraftment level.
Lineage depletion of the graft alleviates single-donor predominance
To determine whether single-donor predominance can be attributed to an in vivo graft-versus-graft reaction between the 2 allogeneic cord blood grafts, 4 cohorts of double cord transplantations were prepared after the depletion of Lin+ cells, and the relative distribution of donor-derived cells was compared.
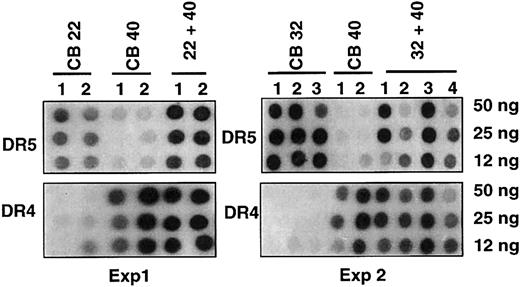
In the first 2 experiments analyzed by PCR-SSOP, 2 pairs of UCB with either 5 (CB32, CB40) or 6 (CB22, CB40) mismatches were double transplanted, with corresponding single unit grafts transplanted in parallel. As shown (Figure 2), despite a high degree of mismatch, remarkable coengraftment levels were observed in the double-transplantation recipients (22 + 40, 32 + 40), as evidenced by the comparable intensity of hybridization by each donor-specific probe (DR4-DR6).
Effect of lineage depletion on single-donor predominance. Two sets of double cord transplantations were performed with lineage-depleted cord blood pairs having 5 or 6 mismatches. Shown are results as analyzed by PCR-SSOP for the DRB1 locus using allele-specific probes (DR5 for CB22 and CB32, DR4 for CB40), where CB22, CB40, or CB32 represents single cord transplantation and CB22/40 or CB32/40 represents double cord transplantation; numbers below represent numbers of mice (n = 6).
Effect of lineage depletion on single-donor predominance. Two sets of double cord transplantations were performed with lineage-depleted cord blood pairs having 5 or 6 mismatches. Shown are results as analyzed by PCR-SSOP for the DRB1 locus using allele-specific probes (DR5 for CB22 and CB32, DR4 for CB40), where CB22, CB40, or CB32 represents single cord transplantation and CB22/40 or CB32/40 represents double cord transplantation; numbers below represent numbers of mice (n = 6).
Quantitative analysis of the donor cell engraftment ratios from 2 additional cohorts also showed similar alleviation of the single-donor predominance, with dominant donor cells comprising only 67.5% ± 4.8% (donor cell ratio, 2.1:1) (Table 2). Moreover, the engraftment levels observed after lineage-depleted double UCB transplantation were 2-fold higher than those after single UCB transplantation with lymphoid and myeloid reconstitution.
Taken together, these results show that the depletion of Lin+ cells from double UCB grafts can lead to improved donor coengraftment and that a graft-versus-graft reaction with immunologic competition may play a role in the process of single-donor predominance.
Cotransplantation of third-party MSCs can alleviate single-donor predominance and result in additive coengraftment
Although lineage depletion alleviates single-donor predominance, alternative strategies not requiring lineage depletion would be more desirable because the latter is frequently associated with cell loss,35 which might itself hamper reaching the target cell dose. To circumvent this hurdle, we postulated that cotransplantating MSCs, bone marrow–derived cells that have been implicated in the suppression of allogeneic immune response,36-40 could also suppress the graft-versus-graft reaction in double cord transplantation.
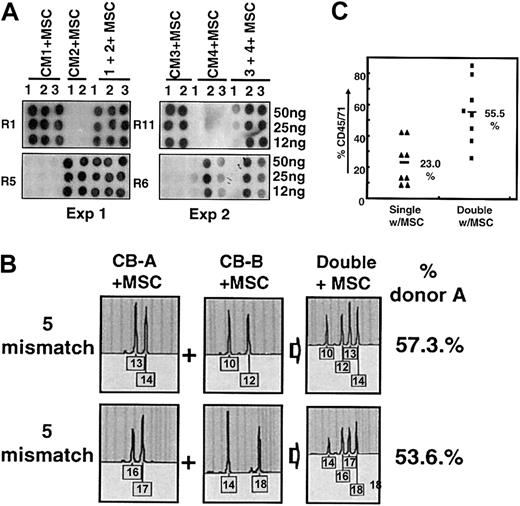
Therefore, MSC cultures were established from third-party bone marrow and were expanded in culture up to 3 passages, a passage number at which these cells retain their phenotypic characteristics and multilineage differentiation potential, as previously defined29 (data not shown). Aliquots of expanded MSCs were then cotransplanted with total MNCs from each UCB unit as part of double- or single-unit transplantation to examine the effects on donor cell distribution. In the first 2 experiments using 2 pairs of UCB units with 5 mismatches each, remarkable degrees of coengraftment by each donor unit were achieved in the mixed transplantations (1 + 2+ MSC and 3 + 4+ MSC), as evidenced by comparable intensities of hybridization by each donor-specific probe, which were also proportional to the intensities seen in the recipients of single-unit controls (CM1+MSC to CM4+MSC) (Figure 3A).
Suppression of single-donor predominance by cotransplantation of MSC from third-party bone marrow. (A) Effect of MSC cotransplantation on donor distribution as analyzed by PCR-SSOP. Total MNCs equivalent to 3 × 104 CD34+ cells from each UCB unit were infused into NOD/SCID mice in single (CM1-CM4) or mixed (CM1 + 2, CM3 + 4) transplantations, as described, except that 4 × 104 MSCs were coinfused into each recipient. The donor origin of the engrafted cells was identified by PCR on the HLA-DR locus, followed by hybridization to allele-specific probes (R1 for CM1, R5 for CM2, R11 for CM3, and R6 for CM4, respectively). Shown are the results of 2 experiments using pairs of 5 mismatch UCBs. (B) MSC-mediated coengraftment as assessed by RQ-STR. Shown are the profiles for donor distribution analyzed by RQ-PCR on representative STR markers with percentage reconstitution of dominant donor cells artificially named as donor A. (C) Increase in overall engraftment in MSC cotransplanted double cord transplantation over single-unit transplantation. Total engraftment of human cord blood cells was measured by antihuman CD45/71, as described. Shown are the engraftment levels of single or double cord transplantations (each n = 8) in cohorts including one 3-mismatch pair, two 5-mismatch pairs, and one full-mismatch pair.
Suppression of single-donor predominance by cotransplantation of MSC from third-party bone marrow. (A) Effect of MSC cotransplantation on donor distribution as analyzed by PCR-SSOP. Total MNCs equivalent to 3 × 104 CD34+ cells from each UCB unit were infused into NOD/SCID mice in single (CM1-CM4) or mixed (CM1 + 2, CM3 + 4) transplantations, as described, except that 4 × 104 MSCs were coinfused into each recipient. The donor origin of the engrafted cells was identified by PCR on the HLA-DR locus, followed by hybridization to allele-specific probes (R1 for CM1, R5 for CM2, R11 for CM3, and R6 for CM4, respectively). Shown are the results of 2 experiments using pairs of 5 mismatch UCBs. (B) MSC-mediated coengraftment as assessed by RQ-STR. Shown are the profiles for donor distribution analyzed by RQ-PCR on representative STR markers with percentage reconstitution of dominant donor cells artificially named as donor A. (C) Increase in overall engraftment in MSC cotransplanted double cord transplantation over single-unit transplantation. Total engraftment of human cord blood cells was measured by antihuman CD45/71, as described. Shown are the engraftment levels of single or double cord transplantations (each n = 8) in cohorts including one 3-mismatch pair, two 5-mismatch pairs, and one full-mismatch pair.
RQ-STR analysis performed on these cohorts with MSC cotransplantation also showed that cells from both donors made a comparable contribution to bone marrow reconstitution, as evidenced by the coexistence of each donor-specific STR peak at similar amplitudes (Figure 3B). Cumulative measurement of the donor distribution showed that the dominant cells comprised 66.5% ± 4.4% engraftment, with a donor cell ratio of 2.0:1 (Table 3), which was significantly lower than that observed after double cord transplantation without MSC cotransplantation (4.2:1) (Table 1).
Of note, in the MSC cotransplanted cohorts, though single cord blood transplantation showed an average of 23.0% ± 4.6% (n = 8) overall engraftment, double cord blood transplantation showed 55.5% ± 6.7% (n = 8), a nearly 2-fold increase (Figure 3C). This increased level of engraftment after double cord transplantation sharply contrasts that achieved without MSC cotransplantation (Figure 1), where no significant difference in the overall engraftment level was observed between the single and double cord blood transplantations.
Higher-level engraftment and balanced coengraftment can be achieved in double cord transplantation with MSC cotransplantation
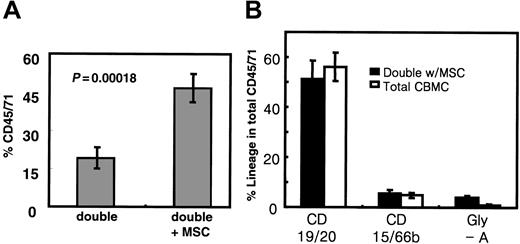
To determine whether cotransplantation of MSCs could indeed bring about a beneficial outcome in double cord transplantation, we directly compared multiple independent cohorts of double cord transplantations in the presence or absence of MSCs (Figure 4). As shown in Figure 4A, though transplanting double cord units (equivalent to 3 × 104 CD34+ cells each) without MSCs showed 19.1% ± 4.4% total human cell engraftment (n = 26), those with MSC cotransplantation showed 46.6% ± 5.8% (n = 19), demon-strating a significantly higher level of engraftment with MSC cotransplantation (P = .00018).
Cotransplantation of MSCs may result in higher engraftment in double cord blood transplantation because of alleviation of donor-deviated engraftment. (A) Total engraftment of cord blood cells achieved by double cord transplantations in the presence or absence of MSC cotransplantation. Multiple independent cohorts of double cord transplantations were performed by transplanting total MNCs equivalent to 3 × 104 CD34+ cells for each UCB unit in the presence (n = 19) and absence (n = 26) of 4 × 104 MSCs. Shown are the mean engraftment levels ± SEM of human cord blood cells in NOD/SCID mice. (B) Lineage distribution of human cells engrafted in the NOD/SCID mice in the presence or absence of MSC cotransplantation. Shown are the mean percentages of the total human cell engraftment (CD45/71) of each lineage (with SEM, n = 8 each).
Cotransplantation of MSCs may result in higher engraftment in double cord blood transplantation because of alleviation of donor-deviated engraftment. (A) Total engraftment of cord blood cells achieved by double cord transplantations in the presence or absence of MSC cotransplantation. Multiple independent cohorts of double cord transplantations were performed by transplanting total MNCs equivalent to 3 × 104 CD34+ cells for each UCB unit in the presence (n = 19) and absence (n = 26) of 4 × 104 MSCs. Shown are the mean engraftment levels ± SEM of human cord blood cells in NOD/SCID mice. (B) Lineage distribution of human cells engrafted in the NOD/SCID mice in the presence or absence of MSC cotransplantation. Shown are the mean percentages of the total human cell engraftment (CD45/71) of each lineage (with SEM, n = 8 each).
Notably, the higher-level engraftment observed with MSC cotransplantation correlated with the alleviation of single-donor predominance (Table 4). In other words, though the dominant unit represented 73.5% (donor cell ratio, 2.8:1) of the engrafted cells in conventional double cord transplantation without MSCs, it was reduced to 64.5% (donor ratio, 1.8:1) with MSC cotransplantation, showing more balanced coengraftment. Additionally, no significant difference in the lineage distribution of engrafted cells was seen in the presence or absence of MSC cotransplantation (Figure 4B), precluding the possibility that the increase in engraftment level with MSC cotransplantation was produced by distinct HSC populations with short-term, lineage-restricted potential.41
Taken together, these results show that cotransplantation of culture-expanded third-party MSCs results in higher-level engraftment after double cord transplantation and that such increased engraftment can be partly, if not completely, attributed to a reduced extent of donor deviation between the 2 grafts. In addition, these results demonstrate the importance of alleviating single-donor predominance as a means of improving outcome after double cord transplantation.
Discussion
In UCB transplantation, total cell number has been a major limiting factor, with a lower input cell number correlating with higher rates of delayed or failed engraftment.4,5,42 Although increasing total input cell numbers by admixing multidonor-derived UCB has been used as an attractive strategy to overcome this limit, clinical studies have shown variable degrees of single-donor predominance in recipients after double UCB transplantation,15,16 which increases over time after transplantation.17 Therefore, the usefulness of double UCB transplantation has remained an open question, and the origin of the unequal engraftment remains unresolved.
In this study, we have shown in the NOD/SCID model that the mixed transplantation of 2 allogeneic UCB grafts in the form of total MNCs leads to single-donor predominance independent of the degree of HLA matching between the 2 grafts.
Although the mechanism for this unequal engraftment has not been fully elucidated, it is unlikely that it originated from differences in the input CRU content of 2 grafts because this phenomenon is observed even when each unit of UCB showed comparable levels of engraftment as a single unit control. Moreover, in our clinical study of double cord transplantation in patients with chronic myelogenous leukemia, reversion between the dominant and nondominant part donor was not seen in any point analysis up to 66 days after transplantation (data not shown), suggesting that the clonal heterogeneity in HSCs that exhibit kinetic differences in their clonal contribution to repopulation during analysis may not be the reason for single-donor predominance.43,44
In contrast, removing Lin+ cells before grafting resulted in significant alleviation of the dominance with more balanced coengraftment, implicating that immunologic competition between the grafts may occur during the engrafting process. The possibility of this immune reaction in NOD/SCID mice, despite their multiple defects in immune function,45 is supported by recent reports demonstrating that functional human T cells can home and engraft in NOD/SCID mice46,47 and that functional B cells48 or dendritic cells49 can develop after transplantation of human cord blood CD34+ cells. Consistent with these findings, infusing human cytotoxic T cells into tumor-bearing NOD/SCID mice resulted in tumor cell killing, suggesting that human immune function can be reproduced to a certain extent in the NOD/SCID model.50
In addition, though immune cells in UCB are relatively immature,12,13,51 GVHD remains a common, albeit less severe, occurrence after UCB transplantation,4,5 supporting the possibility of a graft-versus-graft immune reaction between the cord blood cells.
Interestingly, MSCs have been implicated in the inhibition of lymphocyte proliferation in response to mitogenic or antigenic stimuli and in the inhibition of stimulated T cells, regardless of the origin of the lymphocytes, suggesting that MSCs could exert a potent suppressive effect on the allogeneic immune response.36-40 Taking advantage of their immunosuppressive effects and the ease of their ex vivo expansion, we have shown that the graft-versus-graft reaction that occurs in the context of double UCB transplantation can be suppressed by MSC cotransplantation, with significant alleviation of single-donor predominance.
Notably, suppressing single-donor predominance appears to be important in achieving high-level overall engraftment after double cord transplantation. When cells from one donor predominated in the recipients, as was the case for total MNC double cord transplantation, no significant improvement in the overall human cell engraftment level was achieved by such doubling of the input cell dose compared with their single-unit controls. Moreover, our multiple cohorts of double cord transplantation performed with MSC cotransplantation showed significantly higher engraftment levels, and these higher levels were well correlated with more balanced coengraftment and displayed multipotent lymphomyeloid reconstitution. Taken together, these results suggest that the higher engraftment levels achieved with MSC cotransplantation are attributed to the more balanced coengraftment of the 2 allogeneic cord blood cells, allowing a contribution by HSCs from both donor grafts.
Recently, Noort et al52 reported that cotransplanting cultured MSCs promoted hematopoietic engraftment despite the lack of homing by MSCs to the bone marrow. We also did not find evidence for hematopoietic engraftment of MSCs by flow cytometric or genomic STR analysis. However, in our model, no significant increase in the level of engraftment was seen in the single unit controls cotransplanted with MSCs at the doses tested (data not shown). Therefore, the increase in the overall engraftment levels achieved in our double cord transplantation with MSCs would be less likely because of a direct engraftment-promoting effect of MSCs. The reason for this discrepancy is not clear, yet contributing factors could include differences in cell types and ratios of MSCs to UCB CD34+ cells cotransplanted. In the study by Noort et al,52 1 × 106 fetal lung-derived MSCs were cotransplanted with 0.03 to 1.0 × 106 UCB CD34+ cells, and an MSC-mediated increase of UCB engraftment was seen only at a 10- to 33-fold excess of MSC over UCB CD34+ cells. In our model, only 4 × 104 MSCs were infused (adjusted to be between 1 to 2 × 106 cells/kg53 ), and higher numbers of UCB CD34+ cells were cotransplanted, which could explain our lack of MSC-mediated increase in engraftment in this context.
Of note, MSCs express low levels of class 2 antigens and do not express costimulatory molecules such as B7-1, B7-2, or CD4026,29,40 ; hence, major histocompatibility complex (MHC)–mismatched MSCs have been well tolerated in animal models.53,54 Furthermore, in our study, the suppressive effects of MSCs were present even for the pairs of UCBs with 5 HLA mismatches, raising the possibility that a greater extent of HLA disparity in UCB transplantation could be tolerated in the presence of MSCs, potentially extending the size of the donor pool among units matching in ABO blood type. Taken together, the finding that culture-expanded third-party MSCs can suppress single-donor predominance may have clinical advantages. For instance, after expanding the MSCs from third-party healthy volunteers to a large quantity in culture, aliquots of these cells could be cotransplanted with many sets of double cord transplants, regardless of the donor origin. In support of this possibility, LeBlanc et al39 recently reported that MSCs could modulate mixed lymphocyte reactions independent of MHC matching status.
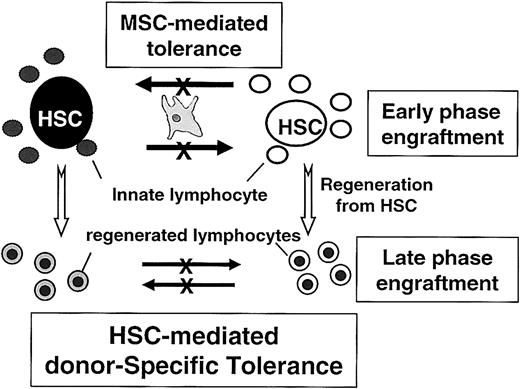
At present, it is unclear how MSCs, despite the fact that they do not home to bone marrow,52,55,56 promote the coengraftment of 2 UCB grafts. However, it has been known that HSC transplantation can induce donor-specific tolerance during bone marrow regeneration,57-59 with mechanisms involving positive and negative T-cell selection by HSCs themselves,60 or veto cell activities of CD34+ cells.61,62 Therefore, a hypothesis could be put forward wherein the innate lymphocytes contained in the cord graft are suppressed by the cotransplanted MSCs and tolerance thereafter is maintained by the HSCs from both donors (Figure 5). However, additional studies regarding the effect of MSCs on other immune cells, such as natural killer cells or dendritic cells,63,64 are also warranted.
Schematic model for MSC-mediated coengraftment of 2 allogeneic cord blood cells. UCB units contained primitive HSCs and differentiated cells, including lymphocytes. During the early phase of engraftment, the allogeneic immune responses by innate lymphocytes (lymphocytes contained in the graft) may be suppressed by cotransplanted MSCs because of the MSC's inhibitory effects on lymphocytes. Primitive HSCs are, therefore, protected from the alloimmune responses; thus, surviving HSCs induce tolerance to cells matched to their own genotypes. Therefore, though coinfused MSCs do not home to bone marrow and do not exist throughout the period of marrow reconstitution, mixed chimerism established during the early phase of engraftment with MSCs may be maintained for longer periods of engraftments.
Schematic model for MSC-mediated coengraftment of 2 allogeneic cord blood cells. UCB units contained primitive HSCs and differentiated cells, including lymphocytes. During the early phase of engraftment, the allogeneic immune responses by innate lymphocytes (lymphocytes contained in the graft) may be suppressed by cotransplanted MSCs because of the MSC's inhibitory effects on lymphocytes. Primitive HSCs are, therefore, protected from the alloimmune responses; thus, surviving HSCs induce tolerance to cells matched to their own genotypes. Therefore, though coinfused MSCs do not home to bone marrow and do not exist throughout the period of marrow reconstitution, mixed chimerism established during the early phase of engraftment with MSCs may be maintained for longer periods of engraftments.
Although xenotransplantation into NOD/SCID mice is a popular surrogate animal model for human HSCs, certain differences between this animal model and clinical situations should be considered. First, though adoptive transfer is feasible in this model,50 the cellular nature or activities of immune cells that can be reconstituted in these mice may be different from that in a clinical setting. For example, though GVHD can develop in mice that undergo transplantation with human cells, a host-versus-graft immune reaction does not operate in NOD/SCID mice. Under normal immune conditions, however, one might expect that the MHC-independent suppressive effect of MSCs36-40 may, in turn, inhibit the host immune reaction against the graft, including the possible biased immune responses toward 1 of the 2 grafts in double UCB transplantation. In support of this possibility, MSCs infused into baboons could inhibit the allogeneic immune response in vivo, increasing graft survival.36 Second, the spectrum of cellular engraftment would be different from the clinical model. Recent studies on NOD/SCID-β2M–/–41 or NOD/SCID-γc null mice65 revealed that NOD/SCID mice exhibit relative difficulty in engrafting short-term repopulating cells or in reconstituting a complete spectrum of immune cells, respectively, preferentially reflecting behaviors of the long-term repopulating cells. Furthermore, a recent gene marking study suggests that distinct HSC clones may be responsible for hematopoietic reconstitution in NOD/SCID mice versus nonhuman primates.66 These results suggest that animal models more closely reflecting human hematopoiesis would better provide insight into the clinical application of MSCs in double UCB transplantation.
In summary, though additional studies using sophisticated animal models are warranted, our present study suggests that the single-donor predominance observed after double cord transplantation may be attributed to immunologic competition during the in vivo engrafting process and that suppression by cotransplantation of the culture-expanded third-party MSCS leads to a concomitant increase in overall engraftment levels. Further studies on the long-term kinetics of MSC-mediated coengraftment and on the mechanisms for donor deviation should open the horizons for efficient multidonor UCB transplantation in severe clinical situations.
Prepublished online as Blood First Edition Paper, October 30, 2003; DOI 10.1182/blood-2003-05-1601.
Supported by grant SC13031 from the Stem Cell Research Center of the 21st Century Frontier Research Program, funded by the Ministry of Science and Technology, Republic of Korea, and supported in part by NITR/Korea FDA grant 03142BIO-031-2 for Biologics Evaluation Research.
Y.-J.C. and D.-W.K. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Histostem Corporation for its generous support to search for HLA-matched umbilical cord units and Dr Jong-Chul Shin for his support in collecting umbilical cord blood. In addition, we thank Dr Yu-Jin Kim for his contribution in clinical data acquisition and Dr Hee-Jin Kim for her generous help in manuscript preparation.