Abstract
In the pathogenesis of sepsis and disseminated intravascular coagulation (DIC), dysfunctional anticoagulant pathways are important. The function of the protein C system in DIC is impaired because of low levels of protein C and down-regulation of thrombomodulin. The administration of (activated) protein C results in an improved outcome in experimental and clinical studies of DIC. It is unknown whether congenital deficiencies in the protein C system are associated with more severe DIC. The aim of the present study was to investigate the effect of a heterozygous deficiency of protein C on experimental DIC in mice. Mice with single-allele targeted disruption of the protein C gene (PC+/–) mice and wild-type littermates (PC+/+) were injected with Escherichia coli endotoxin (50 mg/kg) intraperitoneally. PC+/–mice had more severe DIC, as evidenced by a greater decrease in fibrinogen level and a larger drop in platelet count. Histologic examination showed more fibrin deposition in lungs, kidneys, and liver in mice with a heterozygous deficiency of protein C. Interestingly, PC+/– mice had significantly higher levels of proinflammatory cytokines, tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and IL-1β, indicating an interaction between the protein C system and the inflammatory response. Survival was lower at 12 and 24 hours after endotoxin in the PC+/– mice. These results confirm the important role of the protein C system in the coagulative-inflammatory response on endotoxemia and may suggest that congenital deficiencies in the protein C system are associated with more severe DIC and adverse outcome in sepsis.
Introduction
Severe infection, or sepsis, is often associated with hemostatic abnormalities; in its most severe form, it is manifested as disseminated intravascular coagulation (DIC).1 Several pathways contribute to the prothrombic state in patients with systemic inflammatory response to severe infection or sepsis.2 Besides tissue factor–induced activation of coagulation and plasminogen activator inhibitor type 1 (PAI-1)–mediated inhibited fibrinolysis, dysfunctional physiological anticoagulant pathways represent important mechanisms in the pathogenesis of the hemostatic derangement. One of the most important physiological anticoagulant pathways capable of modulating the activation of coagulation is the protein C system.3,4 There is indeed ample evidence for an impaired function of the protein C system, with relevance for hemostatic activation, morbidity, and mortality, during severe sepsis.5 First, plasma levels of protein C are markedly reduced in patients with sepsis, and low levels of protein C have been shown to be closely associated with morbidity and mortality.6,7 Second, the activation of protein C may be severely compromised by down-regulation of the endothelial surface receptor thrombomodulin by proinflammatory cytokines.8 Recently, these in vitro findings were corroborated by the observation of down-regulated thrombomodulin on endothelium in skin biopsy specimens from patients with severe Gram-negative septicemia associated with decreased activated protein C.9 Third, the administration of activated protein C has been shown to reduce mortality and morbidity in experimental models of bacteremia and in a recently published randomized controlled trial in patients with sepsis.10,11 In addition to the coagulation-modulating properties of activated protein C in sepsis, in vitro and in vivo studies point to an additional anti-inflammatory effect.12-14
It is unknown whether patients with congenital heterozygous deficiency of protein C have a different hemostatic or inflammatory response to severe infection or sepsis. Mice with a single-allele targeted disruption of the protein C gene, resulting in heterozygous deficiency of protein C, may serve as a tool to study the effect of such a deficiency on the endotoxin-induced hemostatic derangement and to more precisely analyze the inflammatory response during protein C deficiency. In this study we show that heterozygous deficiency of protein C considerably affects the hemostatic and the inflammatory response to endotoxin in mice.
Materials and methods
Mice
The experiments were approved by the Institutional Review Board of the University of Leuven, Belgium, and were conducted according to the guidelines for animal experiments of the National Institutes of Health. Mice with a heterozygous deficiency of protein C were generated as described previously.15 Briefly, a targeting vector was introduced by homologous recombination into R1 embryonic stem (ES) cells. This targeting vector contained a 6.5-kb 5′ flanking region of the protein C gene, ranging from a 5′ XbaI site to an XhoI site 456 bp upstream of exon 2 (the first translated exon). The 2.8-kb 3′ flanking region of the protein C gene ranged from a HindIII site 390 bp further than the stop codon to an EcoR1 site. Aggregation of recombinant R1 ES cells with morula-stage embryos led to the generation of chimeric mice, from which a germline-transmitting mouse and subsequently heterozygous protein-C–deficient mice were generated. The mice were backcrossed to the F4 generation in C57BL/6J mice. Protein C deficiency was confirmed by Northern blot analysis of RNA and measurement of protein C level in plasma. As described previously, mice with a heterozygous deficiency of protein C were apparently healthy, had normal lifespans, and were capable of delivering subsequent generations of mice.15 Genotypic screening from the offspring of protein C+/– mice was performed by Southern blot hybridization of DNA extracted from mouse tails, using a 0.9-kb EcoRI/XbaI 5′ external probe, as described previously.15 EcoRI digests of genomic DNA yielded a differential restriction pattern of 11 kb for the mice with a wild-type protein C gene and a 7.5-kb band for recombinant alleles. We used wild-type (protein C+/+ mice) littermates as controls in our experiments.
Experimental design
Protein C+/– mice and their wild-type littermates, weighing 25 to 30 g, were injected intraperitoneally with Escherichia coli endotoxin (serotype O111:B4) at a dose of 50 mg/kg (Sigma, St Louis, MO) or saline (control). Blood for the assay of platelet count, coagulation factors, cytokines, and clinical chemistry was collected at 4, 8, and 12 hours after injection of endotoxin from the inferior caval vein of anesthetized mice and anticoagulated with EDTA (ethylenediaminetetraacetic acid; 10 mM) or citrate (final concentration, 3.2%). Each time point consisted of observations in 6 mice from each experimental group. Histologic studies were performed at 8 and 12 hours after the injection of endotoxin. Survival was investigated in groups of 16 mice at 12 and 24 hours after the administration of endotoxin.
Assays
Protein C plasma activity levels were measured as previously described with an amidolytic assay using chromogenic substrate S2366 (Chromogenix, Milan, Italy). Platelets were measured using an automated cell counter (Cell-Dyn 1330 system; Abbott, Abbott Park, IL). Fibrinogen was assayed according to Clauss and plasma levels of coagulation factor activity by a 1-stage clotting assay on an automated clotting analyzer (STA-R; Roche Diagnostics, Almere, The Netherlands) with pooled mice plasma as reference material. PAI-1 was measured using enzyme-linked immunosorbent assay (ELISA), as previously described.16 Circulating levels of tumor necrosis factor-α (TNF-α), interleukin (IL)–6, IL-1β, and IL-10 were measured with each respective ELISA for murine plasma (TNF-α: Genzyme, Cambridge, MA; IL-1β: R&D Systems, Minneapolis, MN; IL-6 and IL-10: PharMingen, San Diego, CA), as previously described.17,18 Aspartate aminotransferase (AST), alanine aminotransferase (ALT), and urea levels were determined with commercially available kits (Sigma) using a Hitachi analyzer (Boehringer Mannheim, Mannheim, Germany).
Histology
A cannula was introduced in the right atrium, and mice were perfused with 1% phosphate-buffered paraformaldehyde at 100 cm H2O pressure for 5 minutes. Subsequently, the trachea was cannulated, and 1% phosphate-buffered paraformaldehyde was perfused at 30 cm H2O through the airways. The heart and lungs were removed en bloc, and the heart was separated from the lungs and the large vessels. Furthermore, the kidneys, liver, and brain were removed. Samples were cryoembedded or postfixed for 24 hours in 1% phosphate-buffered paraformaldehyde, dehydrated, and embedded in paraffin. Four-micrometer sections were immunostained with a monoclonal antibody directed toward fibrinogen. For granulocyte staining, slides were deparaffinized and rehydrated. Slides were incubated in 10% normal goat serum (DAKO, Glostrup, Denmark) and then were exposed to fluorescein isothiocyanate (FITC)–labeled antimouse Ly-6-G monoclonal antibody (BD Biosciences PharMingen, San Diego, CA). Secondary incubation with rabbit anti-FITC antibody (DAKO), with a biotinylated swine anti-rabbit antibody (DAKO), and ultimately with a streptavidin–avidin-biotin complex (ABC) solution (DAKO) was performed, and the slides were developed using 1% H2O2 and 3,3′-diaminobenzidine tetrahydrochloride (Sigma) in Tris-HCl.
Statistics
Results are presented as mean values ± SD. Statistical analysis was performed using analysis of variance (ANOVA) and subsequent Newman-Keuls test. P < .05 was considered statistically significant.
Results
Protein C, platelets, and coagulation factors
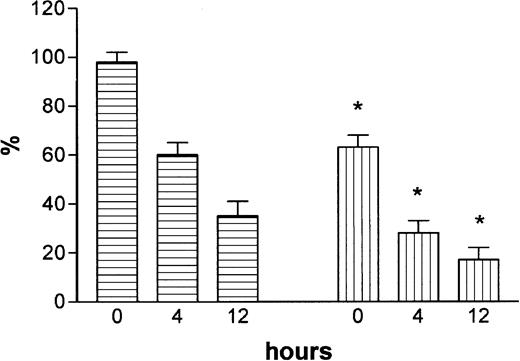
Baseline protein C activity levels were 63% ± 5% in protein C+/– mice compared with 98% ± 4% in wild-type littermates (n = 6), which is consistent with earlier observations.13 After the injection of endotoxin, protein C levels dropped in both groups, reaching levels as low as 17% ± 5% in the heterozygous protein-C–deficient group and 35% ± 6% in the wild-type mice at 12 hours after endotoxin administration (Figure 1) (P = .01 for protein C+/– compared with protein C+/+ mice). Protein-C–deficient mice injected with saline had stable protein C activity over time (data not shown).
Plasma levels of protein C activity. Activity took place before and at 4 and 12 hours after the injection of endotoxin in mice with a heterozygous deficiency of protein C (▤) and in wild-type littermates (▧). Mean values and SD are shown. Statistical significance between protein-C–deficient mice and wild-type mice at each time point is indicated (*P = .01).
Plasma levels of protein C activity. Activity took place before and at 4 and 12 hours after the injection of endotoxin in mice with a heterozygous deficiency of protein C (▤) and in wild-type littermates (▧). Mean values and SD are shown. Statistical significance between protein-C–deficient mice and wild-type mice at each time point is indicated (*P = .01).
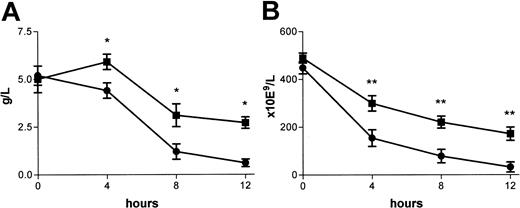
As shown in Figure 2, platelet counts dropped from 489 × 109/L ± 21 × 109/L to 171 × 109/L ± 28 × 109/L at 12 hours after the injection of endotoxin in wild-type mice. In mice with a heterozygous protein C deficiency, the drop in platelet count was significantly more profound, reaching levels as low as 32 × 109/L ± 21 × 109/L at 12 hours after endotoxin administration (n = 6; P < .001). Fibrinogen plasma levels followed a similar trend, with significantly lower levels in protein C+/– mice than in wild-type mice (Figure 2). The fibrinogen plasma level at 12 hours after endotoxin injection was 0.6 ± 0.2 g/L in the protein C+/– group compared with 2.7 ± 0.3 g/L in the protein C+/+ group (n = 6; P = .01). In addition, plasma levels of factor VII and factor V activity were lower in the protein-C–deficient mice than in wild-type mice (Table 1). PAI-1 plasma levels increased with the administration of endotoxin from 2.5 ± 1.1 ng/mL to 12.9 ± 2.7 ng/mL, but this increase was not different between the 2 groups of mice studied. Protein C+/+ and protein C+/– mice that received saline had stable levels of platelets and coagulation factors at subsequent time points during the experiment.
Plasma fibrinogen concentration and platelet count. Fibrinogen concentration (A) and platelet count (B) were determined after the injection of endotoxin (at t = 0) in mice with a heterozygous deficiency of protein C (▪) and wild-type littermates (▪). Mean values and SD are shown. Statistical significance is indicated (*P = .01; **P = .001).
Plasma fibrinogen concentration and platelet count. Fibrinogen concentration (A) and platelet count (B) were determined after the injection of endotoxin (at t = 0) in mice with a heterozygous deficiency of protein C (▪) and wild-type littermates (▪). Mean values and SD are shown. Statistical significance is indicated (*P = .01; **P = .001).
Fibrin deposition and granulocyte invasion
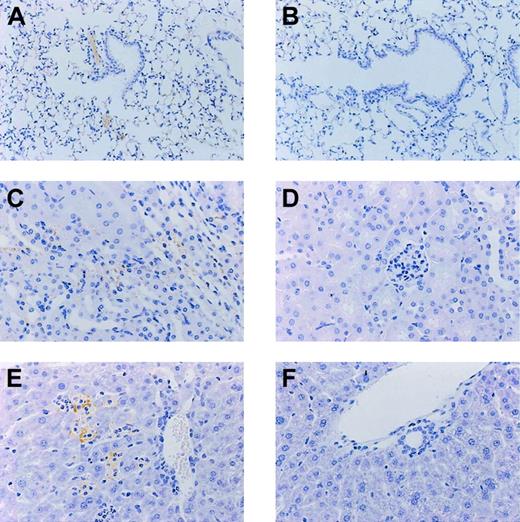
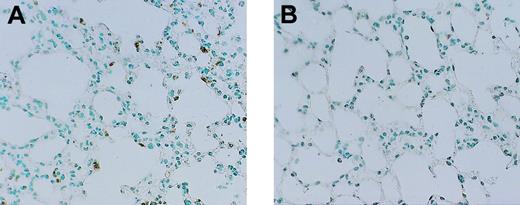
Histologic studies showed more extensive fibrin deposition in various organs at 12 hours after endotoxin administration in mice with heterozygous deficiency of protein C than wild-type mice. Fibrin deposition was most pronounced in small and mid-sized vessels in kidneys, small pulmonary arterioles, and liver vasculature. Figure 3 demonstrates the difference in fibrin deposition in lung, kidney, and liver at 12 hours after endotoxin administration between protein C+/– mice and protein C+/+ mice. In general, intravascular fibrin deposition was associated with signs of inflammation, evidenced by small infiltrates of granulocytes, in particular in lung and liver (Figure 4). Quantitative analysis revealed 2.1-fold and 1.8-fold increases in the number of granulocytes in lung and liver of protein-C–deficient mice and wild-type mice, respectively.
Fibrin staining. Lung (A-B), kidney (C-D), and liver (E-F) of mice at 12 hours after administration of endotoxin. Mice with a heterozygous deficiency of protein C (A,C,E) are compared with wild-type littermates (B,D,F). Slides are representative for 6 mice per group. Original magnification for all panels, × 50.
Fibrin staining. Lung (A-B), kidney (C-D), and liver (E-F) of mice at 12 hours after administration of endotoxin. Mice with a heterozygous deficiency of protein C (A,C,E) are compared with wild-type littermates (B,D,F). Slides are representative for 6 mice per group. Original magnification for all panels, × 50.
Anti-granulocyte staining (counterstaining with methylene green) of lungs. Staining was performed in protein C+/– mice (A) and wild-type mice (B) at 12 hours after the administration of endotoxin. Slides are representative for 6 mice per group. Original magnification for both panels, × 50.
Anti-granulocyte staining (counterstaining with methylene green) of lungs. Staining was performed in protein C+/– mice (A) and wild-type mice (B) at 12 hours after the administration of endotoxin. Slides are representative for 6 mice per group. Original magnification for both panels, × 50.
Inflammatory mediators
Interestingly, circulating levels of inflammatory cytokines on endotoxemia were significantly higher in the protein-C–deficient mice than in the wild-type mice, suggesting that protein C may indeed modulate inflammatory responses to endotoxin (Table 2). Peak plasma levels of TNF-α were approximately 4-fold higher in the protein C+/– group than in the wild-type group 4 hours after endotoxin administration (842 ± 42 pg/mL vs 231 ± 31 pg/mL; n = 6; P = .01), and they remained significantly higher in the protein-C–deficient group at 8 hours. Levels of IL-6 and IL-1β were significantly higher in the protein C+/– mice at 4 hours after endotoxin administration. At 12 hours after endotoxin administration, there was no difference in the levels of TNF-α, IL-6, and IL-1β between the 2 groups (data not shown). Levels of the anti-inflammatory cytokine IL-10 showed a trend toward higher values in the protein-C–deficient group compared with wild-type mice, but this did not reach statistical significance.
Organ failure and death
Liver damage was assessed by measuring liver enzymes (ALT and AST), whereas as an estimate of kidney failure blood urea nitrogen (BUN) was measured. Protein C+/– mice had higher ALT and AST levels at 12 hours than wild-type mice. Peak ALT and AST levels in protein C+/– mice were 362 ± 41 U/L and 291 ± 38 U/L compared with 275 ± 46 and 196 ± 23 U/L in wild-type mice (P = .01 for ALT and AST). BUN increased from 30 ± 4 mg/dL to 52 ± 7 mg/dL at 12 hours after endotoxin administration in wild-type mice and was also more elevated in protein C+/– mice (63 ± 6 mg/dL; P = .03). As shown in Figure 5, there was significantly fewer deaths in protein C+/+ mice than in protein C+/– mice at 12 and 24 hours after the administration of endotoxin (P = .02 and P = .04, respectively).
Survival. Mice (n = 16) with a heterozygous deficiency of protein C (▤) and wild-type littermates (▧) at 12 and 24 hours after the administration of endotoxin. Differences are statistically significant (P = .02 at 12 hours; P = .04 at 24 hours).
Survival. Mice (n = 16) with a heterozygous deficiency of protein C (▤) and wild-type littermates (▧) at 12 and 24 hours after the administration of endotoxin. Differences are statistically significant (P = .02 at 12 hours; P = .04 at 24 hours).
Discussion
The protein C system plays a fundamental role in regulating the balance between adequate hemostasis and blood fluidity as an endothelial-cell–associated anticoagulant system.3 For the activation of protein C, endothelial-cell–bound thrombomodulin, which is abundant in the microcirculation, is essential and renders the system of pivotal importance in the maintenance of microvascular patency. If there is systemic endothelial cell perturbation, as exemplified by the systemic inflammatory response in sepsis, a dysfunctional protein C system is likely to contribute to microvascular failure and may contribute to ensuing organ failure. Ample experimental and clinical evidence demonstrates the importance of the protein C system in sepsis.5,13 Many studies show dysfunction of the protein C system in sepsis, and the extent of the defect appears to be directly related to the severity of the disease.19,20 In addition, restoration of the protein C pathway (for example, by the administration of activated protein C) results in an improved outcome in experimental bacteremia or clinical sepsis.10,11
The present observation adds to that notion, demonstrating that mice with single-allele targeted disruption of the protein C gene, leading to a heterozygous deficiency of protein C, develop more severe signs of DIC. Presumably, the lower level of zymogen protein C, in combination with the endotoxin-induced down-regulation of thrombomodulin, results in less activated protein C and thereby less inhibition of thrombin generation.8 As a consequence, these mice have more extensive fibrin deposition in the vasculature of various organs than wild-type littermates. Protein-C–deficient mice demonstrate more extensive organ damage on the injection of endotoxin, and we observed a trend toward a lower survival rate in mice with a heterozygous deficiency of protein C. These findings underscore the pivotal importance of the protein C system in the development of endotoxin-induced activation of coagulation, fibrin deposition, and organ failure and the delicate role of the protein C plasma concentration in the severity of the response.
Interestingly, not only was there a difference in the coagulation response between protein-C–deficient mice and wild-type mice, significant differences in inflammatory responses on endotoxin administration occurred, as shown by circulating levels of proinflammatory cytokines. This observation is in agreement with previous studies, pointing to a role for the protein C system in modulating the systemic inflammatory response in sepsis and endotoxemia.13,14 Indeed, activated protein C has been found to inhibit endotoxin-induced production of TNF-α, IL-1β, IL-6, and IL-8 by cultured monocytes/macrophages.21,22 Further, activated protein C also abrogated endotoxin-induced cytokine release and leukocyte activation in rats in vivo.23 Experiments in which the protein C pathway was blocked in septic baboons exacerbated the inflammatory response.24 Conversely, administration of activated protein C ameliorated the inflammatory activation on the intravenous infusion of E coli.25 Similar experiments in rodents showed identical results and demonstrated a beneficial effect on inflammatory effects in various tissues.23 It was demonstrated in vitro that monocytes bear an activated protein C binding site that may mediate downstream inflammatory processes26 and that activated protein C can block NFκB nuclear translocation, which is a prerequisite for increases in proinflammatory cytokines and adhesion molecules.27 Binding of activated protein C to the endothelial protein C receptor may mediate another pathway by which activated protein C modulates inflammation.13 This binding was shown to affect gene expression profiles of cells expressing the protein C receptor. Recent experiments demonstrated that binding of activated protein C to the protein C receptor (that can also be detected on mononuclear cells) inhibited endotoxin-induced tissue factor expression on monocytes. Finally, patients with sepsis receiving recombinant human activated protein C had lower IL-6 levels than placebo-treated controls.11 Taken together, compelling evidence indicates that activated protein C may act as an inflammatory mediator, and this is supported by our present observations.
Human subjects with heterozygous protein C deficiency have a higher risk for venous thromboembolism. Other defects involving the protein C system, such as protein S deficiency or resistance toward activated protein C (most frequently caused by the factor V Leiden mutation), are classified as thrombophilia. It is unknown whether these patients have a higher risk for disseminated intravascular coagulation during sepsis or whether deficiency contributes to an adverse outcome. Low protein C level on admission is an independent predictor of death caused by sepsis, but it is unclear to what extent a patient's pre-existing protein C plasma level affects the protein C level on admission.7 Our experimental results may indicate that patients with protein C deficiency have more extensive levels of coagulation activation, potentially leading to increased microvascular fibrin deposition, than patients with normal protein C levels. However, additional analyses from clinical studies are required to achieve a more solid clinical basis for this hypothesis. Interestingly, a recent study in mice with antithrombin deficiency also showed more severe coagulation activation on endotoxemia.28 In line with that observation, thrombophilia in general might hypothetically be associated with severe coagulopathy and adverse outcome in sepsis.
In conclusion, mice with single-allele targeted disruption of the protein C gene, leading to heterozygous protein C deficiency, have severe derangement of coagulation on endotoxemia, leading to extensive intravascular fibrin deposition and organ failure. In addition, protein-C–deficient mice have higher levels of circulating pro-inflammatory cytokines, suggesting that the protein C system is indeed involved in the modulation of the inflammatory response in sepsis. Determining whether these findings are clinically relevant for patients with protein C deficiency or other thrombophilic defects involving the protein C system requires further study.
Prepublished online as Blood First Edition Paper, February 27, 2003; DOI 10.1182/blood-2002-10-3254.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.