Abstract
Although hairy cell leukemia is uniquely sensitive to interferon-α (IFN-α), the biologic basis for this phenomenon remains unclear. Here we examine the effects of IFN-α on cultured hairy cells (HCs), taking into account the possible modifying influence of cell adhesion. We make the novel observation that therapeutic concentrations of IFN-α kill nonadherent HCs by inducing apoptosis. In keeping with the persistence of HCs in tissues during therapy, such killing was inhibited by integrin-mediated adhesion to vitronectin or fibronectin. Exposure of HCs to IFN-α resulted in a marked increase in tumor necrosis factor-α (TNF-α) secretion. Furthermore, blocking antibodies to TNF-RI or TNF-RII protected HCs from IFN-α–induced apoptosis, demonstrating that such killing was mediated by TNF-α. In the absence of IFN-α, exogenous TNF-α did not induce HC apoptosis, showing that IFN-α sensitized HCs to the proapoptotic effect of autocrine TNF-α. This sensitization to TNF-α–induced killing was attributable to suppression of IAP (inhibitors of apoptosis) production known to be regulated by the cytoprotective nuclear factor–κB–dependent arm of TNF-α signaling. Moreover, engagement of the receptors for fibronectin or vitronectin prevented this IFN-α–induced down-regulation of IAPs. Understanding of the signals involved in the combined effects of IFN-α and TNF-α and abrogation of those induced by integrin engagement offers the possibility of sensitizing other malignant cells to IFN-α–induced killing and thereby extending the therapeutic use of this cytokine.
Introduction
The response of hairy cell leukemia (HCL) to interferon-α (IFN-α) is one of the most dramatic and specific effects in the whole of clinical oncology. Nevertheless, the mechanism of action of IFN-α in HCL remains unclear. The agent inhibits HC proliferation induced in vitro,1-3 but there are no reports that it shortens the survival of these cells in culture. It is difficult to reconcile these observations with the fact that the clonal expansion in HCL, as in other chronic lymphoproliferative disorders, results mainly from prolonged cell survival rather than from increased proliferation.4 The aim of this study was to explore this issue further.
Among malignant B cells, HCs are distinctive in being constitutively highly activated cells and, in a number of previous studies, we have shown that this activation is responsible for many of the characteristic features of the disease.5,6 For example, HCs have constitutively activated adhesion molecules such as integrins, with the result that these cells readily interact with extracellular matrix (ECM) components without the need for exogenous stimulation.7 Such interactions are likely to modify the propensity of these cells to undergo apoptosis.
In HCL, treatment of patients with IFN-α induces rapid disappearance of HCs from blood. In contrast, the malignant cells remain in spleen and bone marrow for much longer periods and may not become completely eliminated from these tissues.8 Previous studies of the effects of IFN-α have shown that this cytokine increases autocrine TNF-α production and inhibits HC proliferation in response to cell stimulation.3 However, these observations do not explain the therapeutic effects of IFN-α, because TNF-α is described as an autocrine rescue factor for HCs9 and because HCL is a disease of prolonged cell survival rather than of increased proliferation.4
Therefore, a plausible explanation for the therapeutic effect of IFN-α in HCL is still lacking but is important for elucidating the specific biologic properties of HCs and for understanding why IFN-α has a therapeutic effect in only a limited number of malignancies.
Here we examine how the combination of intrinsic and adhesion-generated signals influences the response of HCs to IFN-α. We show for the first time that IFN-α induces the apoptosis of HCs when they are deprived of the protective effects of cell adhesion. In contrast, IFN-α enhances the viability of chronic lymphocytic leukemia (CLL) cells cultured under identical conditions. We show that HC killing induced by IFN-α is mediated through a mechanism involving the up-regulation of autocrine TNF-α and sensitization of HCs to its proapoptotic effect via down-regulation of inhibitors of apoptosis (IAPs). Importantly, engagement of integrin receptors inhibits this latter effect, although it causes a further increase in the production of TNF-α. These observations not only shed light on the mechanism of action of IFN-α in HCL but also are of potential relevance for broadening the therapeutic applications of this cytokine.
Materials and methods
Patient samples
HCs were obtained from peripheral blood of patients with typical disease as determined by clinical presentation, malignant-cell morphology, tartrate-resistant acid phosphatase positivity, and immunophenotype. Cells from CLL patients all displayed typical morphology, CD5 and CD23 positivity, and had low-level expression of light chain–restricted surface immunoglobulin (Ig). All patients had high white cell counts (> 30 × 109/L for HCL and > 100 × 109/L for CLL). Mononuclear cells were isolated from whole blood by centrifugation over Lymphoprep (Gibco, Paisley, United Kingdom) and, when more than 95% CD19+, were used without further purification. In some cases of HCL, contaminating T cells and any residual monocytes were removed by incubating the mononuclear cell fraction with monoclonal anti-CD3 and anti-CD11b followed by separation of antibody-coated cells with magnetic beads (Miltenyi Biotech, Surrey, United Kingdom); such further purification had no effect on any of the results obtained.
Reagents and antibodies
Adhesive proteins.
Vitronectin (VN) was purified from normal plasma by heparin-Sepharose affinity chromatography according to the method of Yatohgo et al.10 The purity was determined to be more than 95% using 8% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) analysis and Coomassie blue staining. Fibronectin (FN) was purchased from Sigma (Dorset, United Kingdom).
Antibodies and reagents.
The anti-CD95 antibodies (CH11 and ZB4) were purchased from Immunotech (Marseille, France), the anti-CD120a (H398) and anti-CD120b (MR2-1) from Serotec (Oxford, United Kingdom), and the anti–PARP-1 and anti-CD95 ligand (anti-CD95L) (C-20) from Santa Cruz Biotechnology (Santa Cruz, CA). The anti–IAP-1 and anti–IAP-2 antibodies, the TNF-α enzyme-linked immunosorbent assay kit, and the TNF-α were purchased from R&D Systems Europe (Oxon, United Kingdom). The respective class-specific and nonspecific polyclonal control antibodies were purchased from Becton Dickinson (San Jose, CA), and the IFN-α was from Wellcome (Beckenham, United Kingdom).
All other chemicals used were purchased from Sigma unless otherwise stated.
Cell culture
HCL or CLL cells (106/mL) were cultured (37°C in 5% CO2) in RPMI plus 0.5% bovine serum albumin (BSA). Culture vessels (Becton Dickinson) were precoated with VN or FN overnight at 4°C or with poly(2-hydroxyethyl methacrylate) (polyHEMA) for 48 hours at 37°C.
Detection of cell death
Mitochondrial depolarization.
Cultured cells were gently resuspended and 200 μL added to an equal volume of 80 nM 3,3′-dihexyloxacarbocyanine iodide (DiOC6) in phosphate-buffered saline (PBS) containing 1% BSA. After 15 minutes of incubation at 37°C, an equal volume of propidium iodide (PI) (10 μg/mL) was added. After 30 minutes of incubation on ice, the cells were analyzed by flow cytometry. DiOC6 is a cell-permeable green fluorochrome that is selectively concentrated within the polarized mitochondria of live cells but not in the depolarized mitochondria of apoptotic cells.11 Because mitochondrial depolarization is an early apoptotic event and cell-membrane disruption a late one,12,13 double staining with DiOC6and PI allows the identification of live (DiOC6-bright/PI-dim), early apoptotic (DiOC6-dim/PI-dim), and late apoptotic/necrotic (DiOC6-dim/PI- bright) cells.14
DNA fragmentation.
DNA fragmentation was detected as previously described.15Briefly, cells were gently centrifuged and resuspended in 400 μL of a solution containing 0.1% Triton X-100, 0.1% sodium citrate, and 10 μg/mL PI. After 1 hour of incubation on ice, the cells were analyzed by flow cytometry. DNA fragmentation is one of the hallmarks of apoptosis and can be detected as a reduction in the PI staining of permeabilized cells due to loss of fragmented DNA.14
PARP-1 cleavage.
A total of 2 × 106 cells were gently washed in PBS and lysed in 100 μL buffer containing 62.5 mM Tris-HCl (pH 6.8), 2% SDS, 6 M urea, 5% β-mercaptoethanol, 10% glycerol, and 0.00125% bromophenol blue. Lysates were sonicated for 15 seconds and heated at 65°C for 15 minutes before being subjected to SDS-PAGE and Western blotting. Membranes were blocked in 5% milk and sequentially reacted with an anti–PARP-1 mouse monoclonal antibody (mAb) and a peroxidase-conjugated antimouse second-layer antibody (Transduction Laboratories, Lexington, KY). Reactive protein bands were visualized using the ECL system (Amersham, Buckinghamshire, United Kingdom). During apoptosis, full-length PARP-1 is cleaved by caspases into a characteristic 89-kd C-terminal fragment.16 17
Ethidium bromide and acridine orange staining.
To detect cell death in situ, cells were stained with a mixture of the red fluorochrome ethidium bromide (EtBr) and the green fluorochrome acridine orange (AO). A total of 50 μL PBS containing 100 μg/mL EtBr, 100 μg/mL AO, 3% ethylenediaminetetraacetic acid, and 10% bovine hemoglobin were added very slowly to the 150-μL cultures so as not to disturb the cells. After incubating for 60 minutes at 37°C, the cells were visualized using an inverted fluorescence microscope. AO is preferentially taken up by live cells, whereas EtBr (like PI) is excluded from live cells and stains dead cells.18
Fluorescence-activated cell sorter (FACS) analysis.
Washed cells were sequentially incubated with either primary mAb or class-specific controls (30 minutes at room temperature) followed by a fluorescein isothiocyanate–conjugated antimouse Ig second layer (Becton Dickinson) (30 minutes at room temperature). Following each incubation step, the cells were washed 3 times and then analyzed on a FACScan (Becton Dickinson).
TNF-α RNA isolation and reverse transcription–polymerase chain reaction
Total RNA was extracted from approximately 5 × 106 cells cultured in the presence or absence of IFN-α (100 U/mL) using an RNeasy kit (Qiagen, Hilden, Germany). Single-strand complementary DNA was synthesized from 0.7 μg total RNA using the SUPERSCRIPT RNase H− Reverse Transcriptase (Life Technologies, Paisley, United Kingdom). The TNF-α gene was amplified from the synthesized complementary DNA by polymerase chain reaction (PCR) using a human TNF-α PCR primer pair (R&D Systems), which generates a 414–base pair complementary DNA band. To normalize the product, the human L27 ribosomal RNA gene was also amplified using the sense primer, 5′-GACGCAAAGCTGTCATCGTG-3′, and the antisense primer, 5′-GCAGTTTCTGGAAGAACCAC-3′, (30 cycles, annealing at 60°C) which generates a 344–base pair band. The amplified PCR products were subjected to electrophoresis and visualized by EtBr staining. The intensity of each band was scanned and analyzed with Phoretix 1 D Advanced software (version 3.1).
IAP detection
Cultured HC samples were lysed in an equal volume of double-strength Laemmli sample buffer. The whole cell lysates were sonicated for 15 seconds and heated to 100°C for 5 minutes before being subjected to SDS-PAGE and Western blotting. Membranes were blocked, probed with an anti–IAP-1 mouse mAb, and visualized as described above.
Results
IFN-α induces the apoptosis of HCs but not CLL cells
We first sought to establish whether or not cultured HCs could be killed by IFN-α. To take into account the possibility that cell adhesion might generate signals that inhibit IFN-α–induced killing, the tissue culture plastic was coated with polyHEMA (a nontoxic hydrophilic polymer that prevents cell adhesion). When HCs were cultured in this way (Figure 1A,C), IFN-α induced progressive and concentration-dependent cell death as detected by mitochondrial depolarization (loss of staining with DiOC6 [Figure 1A]) and by increased cell membrane permeability (increased staining with PI [Figure 1A]). Importantly, IFN-α produced a small increase in the proportion of cells with depolarized mitochondria and an intact cell membrane (DiOC6-dim/PI-dim [Figure 1A]), suggesting that the cells were dying by apoptosis.19 To confirm that this was so, cells were examined for PARP-1 cleavage and DNA fragmentation because these events are specific to this mode of cell death. Exposure of HCs to IFN-α resulted in a marked increase in the number of cells with fragmented DNA (detected as a reduced DNA content following permeabilization [Figure 1B]). The cytokine also increased PARP-1 cleavage as detected by an increase in the ratio of p89 to full-length PARP-1 (Figure 1D). These findings indicate that IFN-α–induced killing was occurring, at least in part, by apoptosis.
IFN-α kills HCs by apoptosis.
HCs were cultured with or without IFN-α (0-1000 U/mL as indicated) at a density of 106/mL in RPMI plus 0.5% BSA in tissue culture plates coated with polyHEMA. Cell death was measured by FACS analysis of cells stained with DiOC6 and PI (A,C). DiOC6 is selectively concentrated in polarized mitochondria of live cells, whereas PI is excluded from such cells. Apoptosis was detected as DNA fragmentation (B) or PARP-1 cleavage (D). A representative example is shown from among the 4 cases tested. Although IFN-α produced dose-dependent killing in all 4 cases, the results were not pooled because the time taken for appreciable killing differed markedly between cases (6-14 days).
IFN-α kills HCs by apoptosis.
HCs were cultured with or without IFN-α (0-1000 U/mL as indicated) at a density of 106/mL in RPMI plus 0.5% BSA in tissue culture plates coated with polyHEMA. Cell death was measured by FACS analysis of cells stained with DiOC6 and PI (A,C). DiOC6 is selectively concentrated in polarized mitochondria of live cells, whereas PI is excluded from such cells. Apoptosis was detected as DNA fragmentation (B) or PARP-1 cleavage (D). A representative example is shown from among the 4 cases tested. Although IFN-α produced dose-dependent killing in all 4 cases, the results were not pooled because the time taken for appreciable killing differed markedly between cases (6-14 days).
We next sought to establish whether the proapoptotic effect of IFN-α in our culture system was specific to HCs. To do this, we examined the effect of IFN-α on the viability of tumor cells from patients with B-cell CLL, another mature B-lymphoproliferative disorder. In complete contrast to HCs, CLL cells cultured on polyHEMA were not killed by IFN-α; indeed, the agent enhanced cell survival to a variable extent in all cases tested (Figure 2).
IFN-α does not induce CLL apoptosis.
CLL cells from 3 patients were cultured with or without 0 U/mL (♦), 10 U/mL (▪), and 100 U/mL (▴) IFN-α at a density of 106/mL in RPMI plus 0.5% BSA in tissue culture plates coated with polyHEMA. Cell death was measured by double staining with DiOC6 and PI. The percentage of DiOC6-positive/PI-negative (live) cells is shown.
IFN-α does not induce CLL apoptosis.
CLL cells from 3 patients were cultured with or without 0 U/mL (♦), 10 U/mL (▪), and 100 U/mL (▴) IFN-α at a density of 106/mL in RPMI plus 0.5% BSA in tissue culture plates coated with polyHEMA. Cell death was measured by double staining with DiOC6 and PI. The percentage of DiOC6-positive/PI-negative (live) cells is shown.
HC adhesion to VN or FN inhibits IFN-α–induced apoptosis
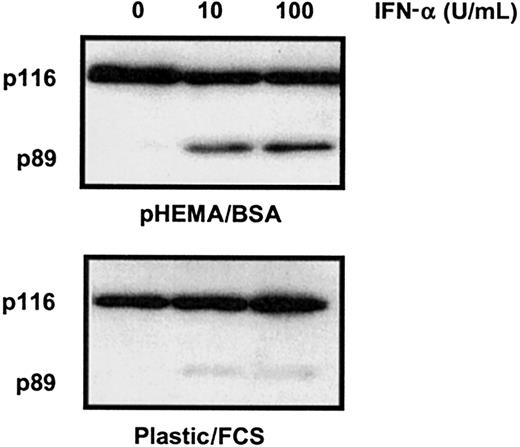
To the best of our knowledge, IFN-α has not been shown in previous studies to induce HC apoptosis. However, such studies have usually included fetal calf serum (FCS) in the culture medium and have used plastic culture vessels that become coated with adhesive proteins such as VN and FN present in the FCS. Compared with HCs cultured on polyHEMA in BSA, HCs cultured in the FCS/untreated plastic system were indeed resistant to IFN-α–induced killing (Figure3). This observation is likely to explain why the killing effect of IFN-α on HCs has not been observed in previous in vitro studies.
FCS inhibits IFN-α–induced killing of HCs.
Cells were cultured with or without IFN-α (0-100 U/mL as indicated) at a density of 106/mL in 0.5% BSA or 10% FCS on uncoated plastic. Cell lysates were separated by SDS-PAGE and Western blotted for PARP-1. Because HCs adhere to plastic, apoptosis was measured by PARP-1 cleavage because this method, unlike FACS analysis, does not exclude adherent cells from analysis. The results are representative of similar experiments performed with the cells of 4 different HCL patients.
FCS inhibits IFN-α–induced killing of HCs.
Cells were cultured with or without IFN-α (0-100 U/mL as indicated) at a density of 106/mL in 0.5% BSA or 10% FCS on uncoated plastic. Cell lysates were separated by SDS-PAGE and Western blotted for PARP-1. Because HCs adhere to plastic, apoptosis was measured by PARP-1 cleavage because this method, unlike FACS analysis, does not exclude adherent cells from analysis. The results are representative of similar experiments performed with the cells of 4 different HCL patients.
Previous work from this laboratory has shown that the ECM of spleen and bone marrow in patients with HCL contains substantial amounts of VN and FN, respectively,6,20 and that HCs interact with these proteins via specific integrin receptors.7 Because integrin engagement is capable of inhibiting apoptosis in other contexts,21 we postulated that the IFN-α–induced killing of HCs might be inhibited by integrin-mediated attachment of HCs to VN and/or FN. To examine this possibility, HCs were cultured on plates that were precoated with these ECM proteins or with BSA as a control surface. Measurement of cell death was performed by staining cells in situ with combinations of AO and EtBr and by Western blotting for PARP-1 cleavage. These methods were chosen because they enable both adherent and nonadherent cells to be analyzed.
As expected, IFN-α–treated HCs cultured on BSA underwent extensive apoptotic cell death as assessed by both methods (Figure4). In contrast, HCs cultured on FN or VN underwent very little IFN-α–induced killing. This shows that IFN-α–induced apoptosis is inhibited by HC contact with these adhesive proteins.
Contact with immobilized VN or FN inhibits the IFN-α–induced apoptosis of HCs.
Cells were cultured as in Figure 1 except that the plates were precoated with VN or FN or with BSA as a control surface. After 6 days of culture, cell death was measured by double staining with AO and EtBr (A; dead cells appear red, while live cells are stained green) and by PARP-1 cleavage (B). A representative example of the 3 cases studied is shown. Original magnification × 100.
Contact with immobilized VN or FN inhibits the IFN-α–induced apoptosis of HCs.
Cells were cultured as in Figure 1 except that the plates were precoated with VN or FN or with BSA as a control surface. After 6 days of culture, cell death was measured by double staining with AO and EtBr (A; dead cells appear red, while live cells are stained green) and by PARP-1 cleavage (B). A representative example of the 3 cases studied is shown. Original magnification × 100.
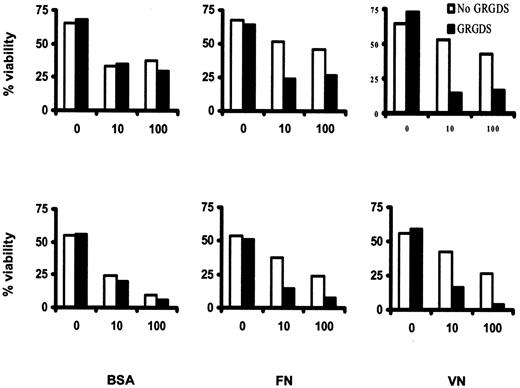
To confirm that the antiapoptotic effect of FN and VN was mediated by integrin engagement, we examined the effect of adding GRGDS peptide to the culture medium. This molecule interferes with the binding of RGD-containing ECM proteins such as FN and VN to specific binding sites on their respective integrin receptors. GRGDS peptide markedly enhanced the IFN-α–induced killing of HCs cultured on FN and VN but had no effect on cells cultured on BSA (Figure5). This indicates that the inhibition of IFN-α–induced killing by ECM proteins is mediated by integrins. For optimal signaling, these receptors are known to require extensive cross-linking,21 which in our experiments is achieved through interaction with ligand-coated plastic. Presumably, a similar cross-linking effect occurs in vivo during cell binding to adhesive proteins that have become part of the insoluble ECM of spleen and bone marrow. This may explain why, in HCL patients treated with IFN-α, HCs rapidly disappear from blood but more slowly from spleen and bone marrow.
GRGDS peptide prevents inhibition of IFN-α–induced killing by VN/FN.
HCs were cultured for 6 days as in Figure 4 but in the presence or absence of GRGDS peptide (including 60 minutes of preincubation; 200 μM). Cell survival was measured by double staining with AO and EtBr. The results are from 2 identical experiments using cells from 2 different HCL patients.
GRGDS peptide prevents inhibition of IFN-α–induced killing by VN/FN.
HCs were cultured for 6 days as in Figure 4 but in the presence or absence of GRGDS peptide (including 60 minutes of preincubation; 200 μM). Cell survival was measured by double staining with AO and EtBr. The results are from 2 identical experiments using cells from 2 different HCL patients.
CD95/CD95L is not involved in IFN-α–induced apoptosis of HCs
In other cell types, IFN-α can up-regulate CD95 (Fas) and/or its ligand (CD95L)22,23 and may sensitize cells to CD95-induced killing.24 Moreover, both CD95 and its ligand expressed by a single cell type can influence cell survival by homotypic cell interaction.25 We therefore examined the potential role of CD95/CD95L in the IFN-α–induced killing of HCs.
Here we confirm that HCs are strongly CD95 positive26 and show that CD95L expression is very low or negligible (Figure 6A). We also confirm that CD95 ligation does not induce HC apoptosis (Figure 6B), although such ligation induced killing of Jurkat cells27 (data not shown). Moreover, in contrast to many other cell types,28-31 HCs in the presence of IFN-α failed to up-regulate either CD95 (data not shown) or its ligand (Figure 6A), and IFN-α did not sensitize them to killing by CD95 ligation (data not shown). Furthermore, a blocking CD95 mAb had no effect on HC survival in the presence of IFN-α (Figure 6C). Taken together, these results exclude an involvement of CD95/CD95L in the IFN-α–induced apoptosis of HCs. IFN-α increases the production of TNF-α by HCs and sensitizes them to the induction of apoptosis by this cytokine.
CD95/CD95L does not mediate HC apoptosis.
Expression of surface CD95L was examined by FACS analysis (A) in untreated (shaded histogram) and IFN-α–treated (open histogram) HCs. (B) HCs were treated with CD95-agonist mAb (CH11; 200 ng/mL; ▴), control IgM (200 ng/mL; ▪), or left untreated (♦) and assessed for viability. (C) IFN-α–treated HCs were incubated with CD95-blocking mAb (ZB4; 500 ng/mL; ▴) or with class-specific control Ig (500 ng/mL; ▪) and compared with HCs cultured in the absence of IFN-α (♦). The percentages of viable DiOC6-positive/PI-negative cells (B,C) are shown. The results are a representative of 4 experiments involving cells from 2 different HCL patients. The results were similar in all 4 experiments but were not pooled because, as in Figure 1, the time taken for appreciable killing differed markedly between cases (6-14 days).
CD95/CD95L does not mediate HC apoptosis.
Expression of surface CD95L was examined by FACS analysis (A) in untreated (shaded histogram) and IFN-α–treated (open histogram) HCs. (B) HCs were treated with CD95-agonist mAb (CH11; 200 ng/mL; ▴), control IgM (200 ng/mL; ▪), or left untreated (♦) and assessed for viability. (C) IFN-α–treated HCs were incubated with CD95-blocking mAb (ZB4; 500 ng/mL; ▴) or with class-specific control Ig (500 ng/mL; ▪) and compared with HCs cultured in the absence of IFN-α (♦). The percentages of viable DiOC6-positive/PI-negative cells (B,C) are shown. The results are a representative of 4 experiments involving cells from 2 different HCL patients. The results were similar in all 4 experiments but were not pooled because, as in Figure 1, the time taken for appreciable killing differed markedly between cases (6-14 days).
It has been reported that IFNs can also sensitize certain cells to other death-inducing agents such as TNF-α.32 Moreover, IFN-α is known to increase TNF-α production by HCs,33and IFNs sensitize monocytes to the induction of apoptosis by TNF-α.34 Therefore, although TNF-α is normally an autocrine survival factor for HCs,9 it is possible that IFN-α may convert the effect of TNF-α from an antiapoptotic to a proapoptotic one.
We first confirmed that TNF-α production by HCs on both polyHEMA and VN is increased in the presence of IFN-α (Figure7). This increase was paralleled by an increase in TNF-α messenger RNA. Thus, culture of HCs on polyHEMA in the presence of IFN-α (100 U/mL) caused a 1.3-, 3.1-, and 4.5-fold increase in TNF-α messenger RNA relative to the controls at 6 hours, 24 hours, and 48 hours, respectively (data not shown). By incubating IFN-α–treated HCs with blocking TNF receptor antibodies or control nonspecific antibodies, we tested whether the TNF-α produced was responsible for induction of apoptosis by IFN-α. Figure8 shows that specific antireceptor antibodies inhibited IFN-α–induced cell death, with anti-TNF receptor I (CD120a) being slightly more effective than anti-TNF receptor II (CD120b) antibody. The presence of IFN-α did not significantly alter the expression of either CD120a or CD120b (data not shown), indicating that changes in receptor expression levels were not responsible for HC killing. Furthermore, in the absence of IFN-α, exogenously added TNF-α did not induce the death of HCs cultured in suspension over an 8-day period, even when used at a concentration 100-fold greater than that found in supernatants of HCs cultured with IFN-α (data not shown). This suggests that the increased TNF-α production alone was not responsible for IFN-α–induced apoptosis. Taken together, the above data show that HC treatment with IFN-α increases production of TNF-α and, on a nonadherent surface, converts the TNF-α action from a survival-promoting to a proapoptotic one.
IFN-α modulates the production of autocrine TNF-α.
Soluble TNF-α was measured by enzyme-linked immunosorbent assay in supernatants of HCs cultured on polyHEMA (A,C) or VN (B,D) in the presence (dotted line) or absence (solid line) of IFN-α (100 U/mL). The results (means ± SEM of triplicate measurements) are from experiments with HCs from 2 different patients.
IFN-α modulates the production of autocrine TNF-α.
Soluble TNF-α was measured by enzyme-linked immunosorbent assay in supernatants of HCs cultured on polyHEMA (A,C) or VN (B,D) in the presence (dotted line) or absence (solid line) of IFN-α (100 U/mL). The results (means ± SEM of triplicate measurements) are from experiments with HCs from 2 different patients.
IFN-α killing is dependent on autocrine TNF-α production.
HCs were cultured on polyHEMA in the presence (dotted line) or absence (solid line) of IFN-α (100 U/mL) together with isotype control antibodies (diamonds) or specific anti–TNF-RI- (A; ▪) or anti–TNF-RII- (B; ▪) blocking mAbs. Viability of HCs was determined by FACS analysis of HCs stained with DiOC6 and PI. The same experiment was performed with HCs from another patient, with similar results.
IFN-α killing is dependent on autocrine TNF-α production.
HCs were cultured on polyHEMA in the presence (dotted line) or absence (solid line) of IFN-α (100 U/mL) together with isotype control antibodies (diamonds) or specific anti–TNF-RI- (A; ▪) or anti–TNF-RII- (B; ▪) blocking mAbs. Viability of HCs was determined by FACS analysis of HCs stained with DiOC6 and PI. The same experiment was performed with HCs from another patient, with similar results.
To establish why comparable cell killing was not observed in adherent HCs, we then examined whether the rescue of these cells by VN from IFN-α–induced killing is mediated by inhibition of TNF-α production. However, when HCs were cultured on VN, TNF-α production was increased rather than decreased (Figure 7). Moreover, addition of IFN-α to cells on VN caused a further increase in TNF-α production (Figure 7) without marked induction of apoptosis. Thus, although TNF-α production is important in the apoptotic effect of IFN-α, modulation of the TNF-α effect by integrin signaling is able to inhibit the proapoptotic effect of IFN-α.
HC killing by IFN-α, and the rescue from this killing by adhesion, involves changes in IAP production
We next attempted to gain insight into the mechanism involved in HC killing by IFN-α and into how integrin signaling abrogates this killing. TNF-α may induce apoptosis via a pathway involving caspases and, at the same time, protect cells through activation of nuclear factor–κB (NF-κB).35 Transcriptional targets of NF-κB include cellular inhibitors of caspases known as IAPs. We have therefore measured NF-κB activation by electrophoretic mobility shift assay (EMSA) and IAP production, by Western blotting, in HCs in the presence or absence of IFN-α. Although we could not demonstrate an effect of IFN-α on NF-κB activation using EMSA (data not shown), under conditions of HC killing by INF-α (24-hour culture on polyHEMA) IAP-1 production was markedly reduced (Figure9). In contrast, when cells were cultured on FN or VN this decrease in IAP-1 production was completely abrogated (Figure 9). Similar results were observed for IAP-2 (data not shown). Although EMSA did not demonstrate an effect of IFN-α on NF-κB activation, the observed decrease in IAP production is nevertheless likely to reflect an effect of this cytokine on the NF-κB pathway. Thus, it has been demonstrated that IFN-α can attenuate gene transcription by NFκB without altering its DNA-binding activity.32 The abrogation of IAP down-regulation by integrin signaling is also likely to reflect an effect on NF-κB because integrins are also known to activate the NF-κB pathway.36-38 Because the prevention of IAP down-regulation by adhesion to ECM proteins protected cells from apoptosis, this may explain why, during IFN-α treatment, HCs persist much longer within bone marrow and spleen than in peripheral blood.
Integrin engagement abrogates IFN-α–induced IAP-1 down-regulation.
HCs were cultured for 24 hours on plates precoated with either polyHEMA, FN, or VN with or without IFN-α (100 U/mL). (A) Whole cell lysates were separated by SDS-PAGE and Western blotted for IAP-1 and then reprobed for β-actin to control for sample loading. (B) The mean and SEM determined from 2 identical experiments using cells from 2 different HCL patients are shown. The density of the IAP-1 bands was corrected for differences in loading and related to the amount of IAP-1 present in the cells before the onset of culture (t0).
Integrin engagement abrogates IFN-α–induced IAP-1 down-regulation.
HCs were cultured for 24 hours on plates precoated with either polyHEMA, FN, or VN with or without IFN-α (100 U/mL). (A) Whole cell lysates were separated by SDS-PAGE and Western blotted for IAP-1 and then reprobed for β-actin to control for sample loading. (B) The mean and SEM determined from 2 identical experiments using cells from 2 different HCL patients are shown. The density of the IAP-1 bands was corrected for differences in loading and related to the amount of IAP-1 present in the cells before the onset of culture (t0).
Discussion
One of the most striking features of HCL is its uniquely high sensitivity to IFN-α. Nevertheless, despite considerable general knowledge concerning IFN-α–induced signals,39 40 the mechanism through which these signals specifically mediate the therapeutic effect of this cytokine in HCL is unclear. We have therefore examined the effects of IFN-α on HCs in vitro. It is well established that drug effects can be modulated by external microenvironmental influences such as adhesion and cytokines. Therefore, to eliminate such influences, we have cultured HCs on a nonadhesive surface (polyHEMA) and substituted BSA for FCS. Using this culture system, we now demonstrate the novel finding that IFN-α kills HCs through the induction of apoptosis.
To preserve cell viability, HCs have in the past been cultured using standard tissue culture plates in the presence of FCS. Our results indicate that such a culture system rescues cells from IFN-α–induced apoptosis. Indeed, previous reports indicate that the presence of IFN-α under standard culture conditions only serves to limit HC proliferative potential.1,41 In our experiments, despite the absence of adhesion and FCS, good viability (> 80%) was maintained for up to 14 days of culture. When serum is present in the culture medium, HCs avidly adhere to the surface of the plastic vessel, which is known to become coated by serum proteins that include integrin ligands such as VN and FN. Cell binding to the surface-immobilized adhesive proteins results in the extensive integrin cross-linking required for optimal generation of survival signals. We therefore coated tissue culture plastic with purified VN or FN and showed that integrin engagement by these surface-immobilized proteins is sufficient to rescue HCs from the apoptotic effect of IFN-α. Although these proteins are present in the circulation in soluble form, they are abundant in tissues as part of the insoluble ECM of bone marrow and splenic red pulp where HCs accumulate and from where these cells disappear much more slowly during IFN-α therapy.8 We therefore propose that integrin receptor cross-linking by HC adhesion to ECM is responsible for the persistence of malignant cells in these organs during IFN-α therapy long after they have disappeared from the blood.
We next studied the mechanism of the observed IFN-α–induced apoptosis of HCs in the absence of environmental rescue. Several previous studies examining the killing of other cell types by IFN have implicated CD95/CD95L.22,24,28,30,42 We therefore examined the role of these proteins in the IFN-α–induced killing of HCs. Our demonstration that CD95/CD95L is not involved suggests that HCs may belong to the category of type II cells, which express CD95 but fail to form the death-inducing signaling complex.43
We next considered the involvement of autocrine TNF-α in the effect of IFN-α because this cytokine has been implicated in HC survival9 and because IFN-α is known to modulate HC TNF-α production.33 Early studies1 proposed that autocrine TNF-α has a cytoprotective effect on malignant B cells and that IFN-α has a beneficial therapeutic effect in HCL by interrupting this autocrine growth factor loop through inhibition of TNF-α production. However, in accord with the more recent studies of Billard et al44 and those of Jansen et al,45we found that IFN-α caused an increase in TNF-α production by HCs. Moreover, we demonstrate that blocking antibodies to TNF receptors inhibit IFN-α–induced killing. This involvement of TNF-α in the IFN-α–induced killing of HCs could not be attributed to increased TNF receptor expression because in our study we found that the levels of both receptors (CD120a and CD120b) remained unchanged in the presence of IFN-α.
Because addition of exogenous TNF-α had no death-inducing effects in the absence of IFN-α, the killing of HCs by IFN-α could not be attributed simply to increased TNF-α production. Instead, our results indicate that IFN-α sensitizes HCs to the proapoptotic effect of TNF-α, a phenomenon that has also been observed in other cell types.46 47 We therefore examined the mechanism by which IFN-α and TNF-α cosignaling induces HC death and how integrin signaling protects HCs from this killing.
Cell stimulation by TNF-α can induce apoptosis through caspase activation, but this effect can be suppressed through the concomitant induction of IAP synthesis through the NF-κB pathway.48Our study demonstrates that the induction of HC death by IFN-α involves suppression of IAP production and consequent sensitization of these cells to proapoptotic effects of TNF-α. Moreover, our experiments demonstrate that integrin engagement by FN and VN inhibits IFN-α–induced killing of HCs and that the restoration of IAP production is involved in this cytoprotective effect.
In conclusion, the present study clarifies the mechanism of action of IFN-α in HCL. We show for the first time that malignant HCs, when deprived of adhesion, are killed by apoptosis in response to therapeutically relevant concentrations of IFN-α. This apoptosis is mediated by increased autocrine TNF-α production and by sensitization of HCs by IFN-α to the death-inducing effect of such autocrine TNF-α. Our novel observation that integrin engagement by adhesive proteins protects cells from the killing effects of combined IFN-α and TNF-α explains why in previous work where culture vessels become coated with such proteins, no killing of HCs by IFN-α was observed. In addition, our findings explain why during IFN-α therapy HCs disappear rapidly from the blood but persist much longer in spleen and bone marrow, where they are likely to be rescued by adhesion to ECM.
The challenge now is to elucidate the signaling basis of the modulation of the action of TNF-α by IFN-α resulting in cell killing and protection by integrin engagement from this killing. This could offer the possibility of using pharmacologic manipulation of relevant signals to extend the therapeutic use of IFN-α to malignancies that are currently resistant to the cytokine.
Supported by the Leukaemia Research Fund of the United Kingdom.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Peter K. Baker, Royal Liverpool University Hospital, 3rd Floor, Duncan Building, Daulby Street, Liverpool, L69 3GA, United Kingdom; e-mail: pbaker@liv.ac.uk.