Key Points
The combination of rhIL-15 and mogamulizumab is associated with immunologic toxicity.
NK cell activity increases within days of treatment and is associated with a rapid reduction in circulating tumor cells.
Visual Abstract
Recombinant human interleukin-15 (rhIL-15) is an immunotherapeutic agent that enhances natural killer (NK) cells to augment the antibody-dependent cellular cytotoxicity (ADCC) of monoclonal antibodies. Mogamulizumab is a CC chemokine receptor 4–directed monoclonal antibody that exerts cytotoxicity through ADCC and depletes regulatory T cells within the tumor microenvironment. We conducted a phase 1 clinical trial of rhIL-15 in combination with mogamulizumab. Patients with relapsed or refractory adult T-cell leukemia/lymphoma (ATLL), mycosis fungoides (MF), and Sezary syndrome (SS) received a fixed dose mogamulizumab, combined with escalating doses of rhIL-15 to identify the maximum tolerated dose (MTD). Six patients were enrolled, 4 with ATLL and 2 with MF/SS. The most common adverse events were rash, infection, and fever (67% of all). Two patients (33%) had grade 4 acute kidney injury, and in 25% of cycles, grade 3 or higher anemia was present. The MTD was dose level 1. One patient with ATLL had a partial response despite receiving only 4 cycles because of grade 4 myositis. Circulating NK cells were increased in all patients during the first cycle and a rapid reduction in tumor cells within the peripheral circulation was noted. Ex vivo assessment demonstrated increased NK cell activation and increased cell lysis in the presence of monoclonal antibodies after only 5 days. Our small study suggests that rhIL-15, in combination with mogamulizumab, leads to effector NK cell activation and regulatory T-cell depletion but has an unfavorable safety profile. Future development of combinations of immunotherapy that target the microenvironment in relapsed or refractory T-cell lymphomas remains rational. This trial was registered at www.ClinicalTrials.gov as #NCT04185220.
Introduction
Cytokines are key regulators of T-cell function.1,2 Both interleukin-2 (IL-2) and structurally similar IL-15 lead to T and natural killer (NK) cell activation and persistence. IL-2 preferentially supports peripheral regulatory T-cell maintenance, whereas IL-15 leads to persistence of CD8+ memory T cells.2-4 Therapeutically modulating T cells to treat cancer has been a productive area of investigation from the development of allogeneic stem cell transplantation to chimeric antigen receptor (CAR) T cells. Cytokines, such as interferon alpha, IL-2, IL-10, IL-12, and IL-15, also have been explored as a form of immunotherapy because of their immunostimulatory effects.5 High-dose IL-2 is associated with response rates of around 20% in patients with metastatic solid tumors, including renal cell carcinoma and melanoma, and may be curative in some patients.6-8
IL-15 has also been investigated as an immunotherapeutic agent. Studies conducted in nonhuman primates with recombinant human IL-15 (rhIL-15) showed a 1 to 2 log increase in circulating effector memory CD8+ T cells and NK cells after rhIL-15 administration.9 Based on the observed increase in T and NK cells in preclinical models, rhIL-15 was further evaluated in clinical studies. Single-agent rhIL-15 was tested in a series of phase 1 clinical trials using different administration methods and duration of therapy in patients with metastatic solid tumors. Although no responses were observed, circulating CD8+ T cells and NK cells were increased after rhIL-15, consistent with preclinical models.10-13 Despite the limited clinical activity of rhIL-15 as a single agent, it was hypothesized that rhIL-15 could be effective in combination with monoclonal antibodies by enhancing antibody-dependent cellular cytotoxicity (ADCC) through increased T and NK cell activity. Indeed, preclinical models confirmed increased ADCC and prolonged animal survival when IL-15 was combined with multiple monoclonal antibodies including rituximab, alemtuzumab, and avelumab.14,15 These results formed the scientific rationale to conduct phase 1 trials testing rhIL-15 in combination with monoclonal antibodies in patients with relapsed or refractory T-cell lymphomas.16,17
Mogamulizumab is a humanized monoclonal antibody that binds to CC chemokine receptor 4 (CCR4), which is normally expressed by regulatory and T-helper type 2 cells and effects T-cell homing to the skin and extranodal sites.18,19 CCR4 is also highly expressed on tumor cells in adult T-cell leukemia/lymphoma (ATLL), mycosis fungoides (MF), and Sezary syndrome (SS), making it a rational therapeutic target.20,21 Mogamulizumab binds to CCR4 and causes target cell death by ADCC, along with the immunomodulatory effects of depleting immunosuppressive regulatory T cells within the tumor microenvironment.22,23
The clinical activity of mogamulizumab in cutaneous T-cell lymphomas was demonstrated in the phase 3 MAVORIC study, which compared mogamulizumab with vorinostat in relapsed or refractory MF/SS and found a significantly longer median progression free survival (PFS) of 7.7 months with mogamulizumab as opposed to 3.1 months with vorinostat (P < .0001).24 In a phase 2 study of mogamulizumab in CCR4-positive peripheral and cutaneous T-cell lymphomas, the objective response rate (ORR) was 35% (95% confidence interval [CI], 20-53) in all patients and 34% in the 29 patients with peripheral T-cell lymphoma (PTCL) with a median PFS of 2 months.22 In a subsequent phase 2 study in CCR4-positive PTCL, the ORR was 11% (95% CI, 3-27) and the median PFS was 3 months (95% CI, 2-5 months).25
A phase 2 trial in relapsed or refractory ATLL compared mogamulizumab with the investigators’ choice of chemotherapy and demonstrated an ORR of 11% (95% CI, 4-23) with mogamulizumab as opposed to 0% (95% CI, 0-14) in the control arm.26 The median PFS was <1 month in both arms. When combined with chemotherapy in patients with relapsed or refractory ATLL, 2 studies demonstrated response rates of ∼65% and a median PFS of 7 to 8 months.27,28 Based on these data, mogamulizumab is approved in Japan for the treatment of relapsed or refractory ATLL, MF/SS, or PTCL and in the United States for relapsed or refractory MF/SS.
Clinical response to mogamulizumab seems to be disease-compartment specific. In the abovementioned MAVORIC trial, the ORR was 42% in skin, 68% in peripheral blood, and only 17% in lymph nodes.24 Taken together, these data suggest that the clinical activity of mogamulizumab could be enhanced with rational combinations, particularly with agents that improve activity within nodal compartments.
As is common with immunotherapeutics, autoimmune toxicities are potentially dose-limiting for mogamulizumab with a rash being reported in nearly 30% of patients that may be severe to life threatening. Notably, the rash associated with mogamulizumab may also be associated with clinical response because 1 study showed longer survival in patients with MF who developed a rash.29
We hypothesized that rhIL-15 would increase the clinical activity of mogamulizumab by enhancing ADCC and tested the combination in a phase 1 trial in patients with relapsed or refractory ATLL, MF, or SS (NCT04185220). The primary objectives of the trial were to determine the overall safety profile of the combination and to determine the maximum tolerated dose (MTD) of a continuous intravenous infusion of rhIL-15 in combination with mogamulizumab.
Methods
Inclusion criteria and trial design
Eligible patients were 18 years or older, had an Eastern Cooperative Oncology Group (ECOG) performance status of ≤2, and had histologically or cytologically confirmed relapsed or refractory ATLL (acute, lymphoma, or chronic subtypes), MF, or SS for which they had received 1 or more previous lines of systemic therapy. Patients with CD30-positive MF/SS were also required to previously have received brentuximab vedotin. Adequate organ function, defined as hemoglobin >9 g/dL, absolute neutrophil count >1 x103 cells per μL, platelet count >100 x 109/L, creatinine ≤1.5 g/dL, and liver function tests <3 times the upper limit of normal, had to be established. Exclusion criteria included current steroid use of >10 mg/d of prednisone, autoimmune disease, active graft-versus-host disease, or HIV infection. The trial was approved by the institutional review board of the National Cancer Institute and was conducted in accordance with the principles of the declaration of Helsinki. All patients provided written informed consent before study enrollment.
A 3 + 3 design was used to determine the MTD of rhIL-15 in combination with mogamulizumab. Dose-limiting toxicity was defined as any grade 3 to grade 5 toxicity within the first 28 days of treatment deemed to at least be possibly related to the study drug with the exception of grade 3 to grade 4 cytopenias (<7 days), transient laboratory abnormalities (<7 days), grade 3 rash, or grade 3 infusion reaction. During each 28-day cycle, rhIL-15 was administered through continuous IV infusion for days 1 to 5 after mogamulizumab infusion. Mogamulizumab was administered IV weekly during the first cycle starting on day 1 and then every 2 weeks thereafter.24 Dose level 1 was 2 μg/kg of rhIL-15 and 1 mg/kg mogamulizumab and dose level 2 was 4 μg/kg rhIL-15 and 1 mg/kg mogamulizumab. The maximum number of cycles was 6 with no planned maintenance.
Toxicity was measured using the revised National Cancer Institute Common Terminology Criteria for Adverse Events version 5.0. Response was investigator-assessed using the modified severity weighted assessment tool in patients with MF/SS and the International Consensus criteria for ATLL.30
Fluorescence-activated cell sorter analysis
NK cell numbers, ATLL cell percentages, and cell phenotyping were determined directly from whole blood on days 1, 5, and 8. Antibodies CD3 (SP34-2), CD4 (RPA-T4), CD7 (CD7-6B7), CD16 (3G8), CD25 (M-A251), CD56 (HCD56), CD69 (FN50), CCR4 (CD194; L291H4), CD20 (rituximab), NKp30 (p30-15), NKp46 (9E2), DNAM1 (11A8), NKG2D recognizing surface proteins were added to whole blood and incubated for 45 minutes at 4°C after blocking with human TruStain FcX BioLegend for 15 minutes at room temperature. Blood samples were then treated with Cal-Lyse (ThermoFisher) to lyse red blood cells and analyze them using a FACSVerse.
Lysis assays
Peripheral blood mononuclear cells (PBMCs), collected on days 1, 5, and 8, were isolated from blood using Ficoll centrifugation and viably frozen. These time points were selected because the expected time of maximum lymphocytosis induced by rhIL-15 infusions was around day 8.12 After thawing, PBMCs were rested overnight at 37°C in RPMI/8% human AB serum (MediaTech). To test whether rhIL-15 infusions affect ADCC mediated by NK cells, Raji cells, which are not killed by primary NK cells, were coated with the anti-CD20 antibody rituximab for 30 minutes at room temperature and used as a target; uncoated Raji cells were used as a control. Target and control cells were labeled at different carboxyfluorescein succinimidyl ester concentrations (1 vs 0.1 μmol/L; Invitrogen), mixed at equal numbers, and added to NK cells at a 5:1 fixed ratio of effector to target cells. Incubations were done in the presence of human IL-15 (2 ng/mL, 20 hours at 37°C). The ratios of surviving propidium iodide-negative target and control cells with or without NK cell exposures were determined using fluorescence-activated cell sorter analysis and used to calculate the percentages of target cell lysis.
Results
Patient characteristics, safety, and efficacy
A total of 6 patients were treated during 2019 to 2021 (Table 1). The median age was 58 years (range, 30-65), and 5 (83%) were male. Four (67%) had ATLL (3 acute and 1 lymphomatous) and 1 (6%) patient each had MF and SS. The median number of previous lines of therapy was 2 (range, 1-8). Three patients were treated at dose level 1 and 3 at dose level 2. The median number of cycles received was 2 (range, 1-4). Eight total cycles were administered at dose level 1 and 4 cycles at dose level 2. The most common adverse events were rash (67%), infection (67%), fever (67%), and fatigue (50%) primarily at grade 1 to grade 2. Two (33%) patients had grade 4 acute kidney injury (not attributable to tumor lysis), and 25% of cycles were associated with grade 3 or higher anemia, although no cases of autoimmune hemolytic anemia were observed (Table 2). Two patients had dose limiting toxicities (DLTs), which both occurred at dose level 2. The first case of DLT presented as grade 4 acidosis, capillary leak syndrome, and acute kidney injury that occurred in a patient with ATLL. This event occurred shortly after starting therapy and required hemodialysis and resolved after hospital admission for supportive care. The second case of DLT involved a fall and traumatic grade 3 hip fracture, deemed possibly related to therapy, and occurred in a patient with MF. The MTD was determined to be 2 μg/kg per day of rhIL-15 with 1 mg/kg of mogamulizumab (dose level 1).
Patient characteristics
| Age . | Disease . | Stage at diagnosis . | Previous lines of therapy . | Response to previous line of therapy . | Dose level . |
|---|---|---|---|---|---|
| 53 | ATLL, acute | 4 | Ruxolitinib, rhIL-15 with avelumab | PR | 1 |
| 62 | SS | 4B (T4N1M0B2) | Bexarotene, extracorporeal photopheresis, interferon alpha, vorinostat, alemtuzumab, mogamulizumab, pembrolizumab + TTI-621, doxil | CR | 1 |
| 65 | ATLL, acute | 4 | EPOCH | PD | 1 |
| 46 | ATLL, lymphomatous | 4 | CHOP, rhIL-15 with alemtuzumab | PD | 2 |
| 30 | ATLL, acute | 4 | CHOP, GDP | PD | 2 |
| 63 | MF | 2A (T2N0M0B0) | CHOP, brentuximab vedotin, bendamustine, rhIL-15 with avelumab | PD | 2 |
| Age . | Disease . | Stage at diagnosis . | Previous lines of therapy . | Response to previous line of therapy . | Dose level . |
|---|---|---|---|---|---|
| 53 | ATLL, acute | 4 | Ruxolitinib, rhIL-15 with avelumab | PR | 1 |
| 62 | SS | 4B (T4N1M0B2) | Bexarotene, extracorporeal photopheresis, interferon alpha, vorinostat, alemtuzumab, mogamulizumab, pembrolizumab + TTI-621, doxil | CR | 1 |
| 65 | ATLL, acute | 4 | EPOCH | PD | 1 |
| 46 | ATLL, lymphomatous | 4 | CHOP, rhIL-15 with alemtuzumab | PD | 2 |
| 30 | ATLL, acute | 4 | CHOP, GDP | PD | 2 |
| 63 | MF | 2A (T2N0M0B0) | CHOP, brentuximab vedotin, bendamustine, rhIL-15 with avelumab | PD | 2 |
CHOP, cyclophosphamide, doxorubicin, vincristine, prednisone; CR, complete response; EPOCH, etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin; GDP, gemcitabine, cisplatin, dexamethasone; PD, progressive disease; PR, partial response.
Adverse events by grade
| . | Grade 1-2 . | Grade 3-4 . |
|---|---|---|
| Hematologic | ||
| Anemia | 0 | 3 (25%) |
| Neutropenia | 4 (33%) | 0 |
| Thrombocytopenia | 2 (17%) | 0 |
| Nonhematologic | ||
| Acidosis | 0 | 1 (17%) |
| Acute kidney injury | 1 (17%) | 2 (33%) |
| ALT elevation | 1 (17%) | 1 (17%) |
| AST elevation | 3 (50%) | 0 |
| Capillary leak syndrome | 1 (17%) | 1 (17%) |
| Chills | 2 (33%) | 0 |
| Diarrhea | 2 (33%) | 0 |
| Dysgeusia | 2 (33%) | 0 |
| Dyspnea | 2 (33%) | 0 |
| Fall/fracture | 0 | 1 (17%) |
| Fatigue | 3 (50%) | 0 |
| Fever | 4 (66%) | 0 |
| Hemorrhage | 0 | 1 (17%) |
| Hypoalbuminemia | 0 | 1 (17%) |
| Hyponatremia | 0 | 1 (17%) |
| Infection | 3 (50%) | 1 (17%) |
| Maculopapular rash | 3 (50%) | 1 (17%) |
| Multiorgan failure | 0 | 1 (17%) |
| Myositis | 0 | 1 (17%) |
| Pain | 0 | 1 (17%) |
| . | Grade 1-2 . | Grade 3-4 . |
|---|---|---|
| Hematologic | ||
| Anemia | 0 | 3 (25%) |
| Neutropenia | 4 (33%) | 0 |
| Thrombocytopenia | 2 (17%) | 0 |
| Nonhematologic | ||
| Acidosis | 0 | 1 (17%) |
| Acute kidney injury | 1 (17%) | 2 (33%) |
| ALT elevation | 1 (17%) | 1 (17%) |
| AST elevation | 3 (50%) | 0 |
| Capillary leak syndrome | 1 (17%) | 1 (17%) |
| Chills | 2 (33%) | 0 |
| Diarrhea | 2 (33%) | 0 |
| Dysgeusia | 2 (33%) | 0 |
| Dyspnea | 2 (33%) | 0 |
| Fall/fracture | 0 | 1 (17%) |
| Fatigue | 3 (50%) | 0 |
| Fever | 4 (66%) | 0 |
| Hemorrhage | 0 | 1 (17%) |
| Hypoalbuminemia | 0 | 1 (17%) |
| Hyponatremia | 0 | 1 (17%) |
| Infection | 3 (50%) | 1 (17%) |
| Maculopapular rash | 3 (50%) | 1 (17%) |
| Multiorgan failure | 0 | 1 (17%) |
| Myositis | 0 | 1 (17%) |
| Pain | 0 | 1 (17%) |
All grade 3 to grade 4 adverse events and/or any adverse events that were observed in >1 patient are shown. Hematologic events are shown as events observed per patient per cycle and nonhematologic events are shown as the maximum grade observed per patient.
ALT, alanine aminotransferase; AST, aspartate aminotransferase.
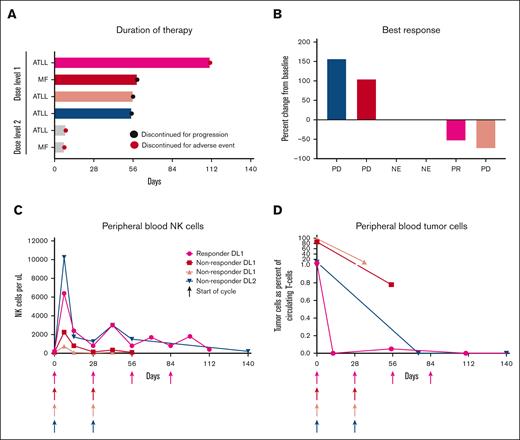
One patient with acute ATLL, treated at dose level 1, had a partial response, which lasted for 6 months. Interestingly, this patient had a complete response within the blood and skin after therapy and a partial response in lymph nodes. Treatment was stopped after 4 cycles because of grade 4 myositis, which was effectively treated with high-dose steroids. Two patients with ATLL had progressive disease by the end of cycle 2, and 1 discontinued during cycle 1 because of toxicity and was unevaluable for response. Neither of the patient with MF/SS responded (Figure 1A-B).
Clinicial response to IL15 and mogamuliziumab. Duration of response (A), best response (B), circulating NK cells (C), and circulating tumor cells (D) after treatment with rhIL-15 and mogamulizumab. Each patient is represented by a consistent color in panels A-D; gray color in panel A indicates patients who were not evaluable in other panels. NE, not evaluable; PR, partial response; PD, progressive disease.
Clinicial response to IL15 and mogamuliziumab. Duration of response (A), best response (B), circulating NK cells (C), and circulating tumor cells (D) after treatment with rhIL-15 and mogamulizumab. Each patient is represented by a consistent color in panels A-D; gray color in panel A indicates patients who were not evaluable in other panels. NE, not evaluable; PR, partial response; PD, progressive disease.
Dynamic changes in peripheral blood NK and circulating lymphoma cells
Circulating NK cells increased after administration of rhIL-15 and mogamulizumab in all 4 of the evaluable patients (Figure 1C). Among patients who were treated at dose level 1, the responding patient had a greater than threefold increase in NK cells when compared with the 2 patients who did not respond. In the responding patient, circulating tumor cells became undetectable after 2 weeks of treatment and remained undetectable at the 4 month follow-up (Figure 1D). Circulating tumor cells remained detectable in the 2 patients with the lowest peak increase in circulating NK cells after rhIL-15 and mogamulizumab infusions treated at dose level 1. The only patient who was treated at dose level 2 (ATLL) and in whom peripheral NK cells were measured had the highest peak level of NK cells observed. Although the patient did not have a clinical response, the tumor cells within the peripheral blood cleared after 2 cycles. In all patients, subsequent cycles of rhIL-15 increased NK cell numbers to a lesser extent than the first cycle.
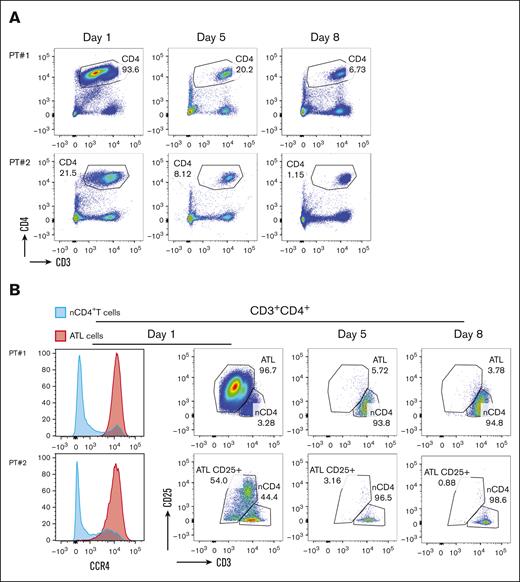
To further characterize the dynamic effects of treatment with rhIL-15 and mogamulizumab, flow cytometry was used to detect malignant cells in blood samples during the first week of therapy from 2 patients with ATLL who were both treated at dose level 1. ATLL cells resemble mature CD4+ T cells and are characterized by the expression of CD25 and CCR4, diminished expression of CD3, and absent CD7 expression.31,32 Direct staining of blood samples showed decreases in the percentage of CD4+ T cell in both patients within 8 days (Figure 2A). To determine whether the decrease in CD4+ T cells was specifically ATLL cells, we used CD3 and CD25 to differentiate between tumor cells and normal CD4+ T cells. Before therapy, we detected 2 populations among CD4+ T cells in both patient samples, namely CD25–CD3+ T cells that were comparable with normal CD4+ T cells and ATLL cells that were identified by their distinctive high levels of CD25 and lower levels of CD3 expressions. We also confirmed that ATLL cells expressed high levels of CCR4 and that the majorly lacked CD7 expression when compared with normal CD4+ T cells. Within 5 days, the number of ATLL cells were dramatically reduced in the blood of both patients, and this effect persisted at day 8 (Figure 2B). The CD4+ T cells detected at day 5 and 8 had a phenotype that was comparable with CD4+ T cells found in normal donors, demonstrating that mogamulizumab induced the clearance of circulating CCR4+ leukemic cells in patients with ATLL.
Changes in circulating T and lymphoma cells. CD4+ T cells in peripheral blood are decreased after initiation of cycle 1 rhIL-15 and mogamulizumab (A) with preferential depletion of CD4+ T cells with a phenotype consistent with ATLL (B).
Changes in circulating T and lymphoma cells. CD4+ T cells in peripheral blood are decreased after initiation of cycle 1 rhIL-15 and mogamulizumab (A) with preferential depletion of CD4+ T cells with a phenotype consistent with ATLL (B).
NK cells display increased markers of activation and tumor cell lysis
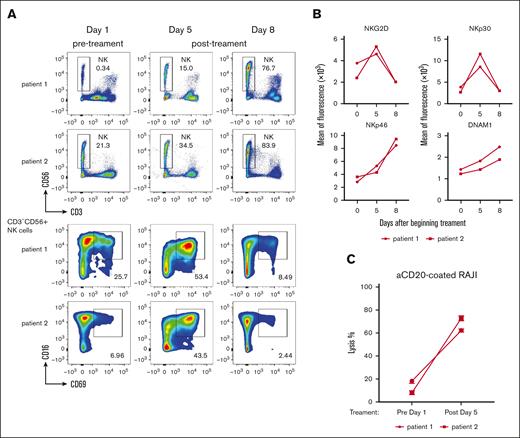
We previously demonstrated that subcutaneous rhIL-15 injections lead to increased circulating NK cells in patients with ATLL and to augmentation of their ADCC-mediated cytotoxicity.16,17 Consistent with these results, we found that a 5-day infusions of rhIL-15 led to progressive increases in NK cell percentages in both evaluated patients (Figure 3A). ADCC relies on the engagement of CD16 expressed on the cell surface of CD56dimNK cells by the Fc portion of the antibody-coated target cells.33 To assess whether NK cells responded in vivo to the presence of antibody-coated ATLL cells after infusion of rhIL-15 and mogamulizumab, we evaluated the expression of activation markers on NK cells. A transient upregulation in the NK cell activation marker CD69 in the CD16+ NK cell population was observed by 5 days in both patients (Figure 3A), which coincided with the disappearance of leukemic cells described above. We also evaluated the expression of the NK cell activating receptors NKG2D, NKp30, NKp46, and DNAM1. NKG2D and NKp30 were increased at day 5 and returned to baseline by day 8, whereas NKp46 and DNAM1 increased at both time points (Figure 3B). Finally, to investigate whether the ADCC activity of NK cells was affected by rhIL-15 infusions, we compared the lytic function of NK cells ex vivo in PBMCs isolated from blood samples taken on days 1 and 5 of therapy. In a pooled analysis of patients 1 and 3, we observed a greater than threefold increase in NK cell killing activity against anti-CD20-coated Raji cells after 5 days of therapy when compared with target cell lysis at day 1 (Figure 3C). Together, these data show that IL-15 infusions support NK cell lytic activities and suggest that NK cells participate in mogamulizumab-induced cytotoxicity.
Changes in NK cell number and function. (A) Infusion of rhIL-15 and mogamulizumab led to increased circulating NK cell numbers within 1 week and increased CD69 expression, a marker of activation, which peaked around day 5. (B) Activating NK cell receptors NKG2D, NKp30, NKp46, and DNAM1 were assessed at baseline and after treatment. (C) NK cells obtained after 5 days of rhIL-15 and mogamulizumab demonstrated increased cell lysis ex vivo when cultured with CD20 antibody-coated Raji cells.
Changes in NK cell number and function. (A) Infusion of rhIL-15 and mogamulizumab led to increased circulating NK cell numbers within 1 week and increased CD69 expression, a marker of activation, which peaked around day 5. (B) Activating NK cell receptors NKG2D, NKp30, NKp46, and DNAM1 were assessed at baseline and after treatment. (C) NK cells obtained after 5 days of rhIL-15 and mogamulizumab demonstrated increased cell lysis ex vivo when cultured with CD20 antibody-coated Raji cells.
Discussion
We investigated the combination of rhIL-15 and mogamulizumab in patients with T-cell malignancies and demonstrated expansion of NK cells shortly after therapy, along with rapid clearance of peripheral tumor cells. We also noted modest increases in NK cells over the course of several months in patients who remained on therapy. Furthermore, NK cells showed increased markers of activation and were more effective in inducing cell lysis in the presence of monoclonal antibodies ex vivo. These findings suggest that this combination may represent a novel immunotherapeutic approach to improve the ADCC of monoclonal antibodies. Despite these interesting biologic changes in immune cells, the clinical activity of this combination was limited because of an unfavorable safety profile, which prevented safe delivery of the regimen. Three (50%) patients stopped therapy prematurely because of adverse events, and only 1 (15%) patient responded. Despite the lack of clinical activity, 2 patients had durable clearance of tumor cells from the peripheral blood, suggesting that this combination has preferential activity in this anatomic compartment.
Most patients with relapsed or refractory T-cell lymphomas will ultimately die from their disease. Immunotherapy in the form of allogeneic stem cell transplantation is the most reliably curative treatment for patients with relapsed or refractory T-cell lymphomas ,but it is only used in a minority of patients.34-36 Targeted immunotherapeutic agents for patients with relapsed or refractory T-cell lymphomas are needed to fill this treatment gap. Monoclonal antibodies, CAR T cells, and bispecific T-cell engaging antibodies have revolutionized the treatment of patients with B-cell lymphomas,37-39 but these treatments have been challenging to apply to patients with T-cell malignancies because of a lack of tumor specific antigens, excess toxicity, T-cell fratricide, and poor persistence, among other challenges.40-43
The longest studied method of targeted immunotherapy for T-cell lymphoma has been with monoclonal antibodies directed against T-cell antigens. These agents have been associated with significant toxicity from T-cell depletion and are not widely used to treat patients with T-cell lymphomas. In addition, randomized trials have failed to demonstrate a survival benefit.44-47 CAR T cells and bispecific antibodies that target various T-cell antigens, such as CD5, CD7, CD30, and TRBC1, have been explored in clinical trials and may be an effective way of overcoming an immunosuppressive tumor microenvironment.48-51 For example, in 9 patients treated with a TRBC1-directed autologous CAR T-cell product, an objective response was observed in 6 patients (66%) and 5 patients (56%) had a complete response. In this study, no CAR T-cell expansion was noted after infusion, which raises concern about the durability of response.50 Similarly, early results from a phase 1 trial of a CD70-directed allogeneic CAR T-cell therapy reported an overall response rate of 71%, but longer follow-up is needed to determine the durability of these responses.52
Another immunotherapeutic approach for the treatment of T-cell lymphomas has been to use antibody drug conjugates. Brentuximab vedotin, a CD30-directed antibody drug conjugate, has arguably been the most effective targeted therapy for patients with T-cell lymphomas, particularly for those with anaplastic large cell lymphoma.53-55 Bispecific antibodies that link CD30 and CD16 are also in clinical development.41,56 The development of antibody drug conjugates offers another promising way of delivering targeted therapy while overcoming issues of fratricide and persistence encountered with CAR T cells and bispecific antibodies.57
The development of targeted immunotherapeutic approaches has been elusive but holds great potential for patients with relapsed T-cell malignancies. Augmenting the ADCC activity of therapeutic antibodies with concurrent infusions of rhIL-15 may increase the activity of these agents but, in the case of mogamulizumab, seems to exacerbate the toxicity of the antibody, potentially because of augmentation of the cytotoxic T-cell activity in the absence of regulatory T cells. The timing of antibody and rhIL-15 infusions may also have impacted the toxicity of the combination with various dosing schedules having been used in studies of rhIL-15 with alemtuzumab, avelumab, and mogamulizumab with different safety profiles (Table 3).16,17 The expression of CCR4 on a subset of NK cells has been reported previously and is a potential cause of antagonism with the combination.58 IL-15 could also theoretically cause rapid progression of T-cell lymphomas, but consistent with previous studies, we did not note rapid progression.16 Clinical use of IL-15 may still be a viable means of improving the efficacy of cellular therapies and several recent studies have developed NK CAR products that secrete IL-15 to improve the persistence and activity of the CAR.59,60 Our group is currently evaluating other approaches to target T-cell malignancies and the microenvironment, such as an approved protocol to investigate a CCR4-directed autologous CAR T-cell therapy in a phase 1 study.
Comparison of dosing schedule and toxicity of IL-15 targeted therapies in oncology clinical trials
| Regimen . | Dosing schedule . | Study population . | Adverse events . | Clinical outcome . | Reference . |
|---|---|---|---|---|---|
| rhIL15 | Bolus infusion of 0.3-3 μg/kg for 12 days | Metastatic melanoma and kidney cancer | Hypotension, thrombocytopenia, liver function test abnormalities | Maximum tolerated dose was the lowest dose level, 0.3 μg/kg | 10 |
| rhIL15 | Subcutaneous injection of 0.25-3 μg/kg Monday to Friday for 2 weeks | Metastatic solid tumor | Hypotension, cardiac chest pain, pancreatitis | No clinical responses, and study was stopped early because of chest pain episode | 11 |
| rhIL15 | Continuous infusion of 3-5 μg/kg per day for 5 d | Metastatic solid tumor | Pulmonary capillary leak | No DLT; 4 μg/kg was selected as recommended phase 2 dose | 12 |
| rhIL15 | Continuous infusion of 0.125-4 μg/kg per day for 10 days | Metastatic solid tumor | Bleeding, uveitis, pneumonitis, intestinal ischemia | Recommended phase 2 dose was 4 μg/kg per day | 13 |
| rhIL15 + alemtuzumab | Subcutaneous injection of 0.5-2 μg/kg Monday to Friday for 2 weeks of rhIL15 with fixed dose alemtuzumab | Mature T-cell malignancies | Cytopenias, infusion reaction | No DLT | 16 |
| rhIL15 + avelumab | Continuous infusion of 1-4 μg/kg per day for 5 days of rhIL15 with fixed dose avelumab | Mature T-cell malignancies | Autoimmune hemolytic anemia, infusion reaction | Study terminated early because of slow accrual | 17 |
| rhIL15 + mogamulizumab (current study) | Continuous infusion of 2-4 μg/kg per day for 5 days of rhIL15 with fixed-dose mogamulizumab | Mature T-cell malignancies | Capillary leak, rash, infection | Maximum tolerated dose was 2 μg/kg | NA |
| N-803 (IL-15 receptor agonist) + rituximab | 1-20 μg/kg N-803 weekly for 4 doses with 4 consolidation doses with fixed dose rituximab | Indolent B-cell lymphomas | Infusion reaction, hypertension, lymphopenia | Recommended phase 2 dose 15-20 μg/kg | 61 |
| N-803 + BCG | 100-400 μg N-803 with fixed dose weekly bladder instillations of BCG | Non-muscle invasive bladder cancer | Hypertension, hematuria, fatigue | No DLT | 62 |
| Regimen . | Dosing schedule . | Study population . | Adverse events . | Clinical outcome . | Reference . |
|---|---|---|---|---|---|
| rhIL15 | Bolus infusion of 0.3-3 μg/kg for 12 days | Metastatic melanoma and kidney cancer | Hypotension, thrombocytopenia, liver function test abnormalities | Maximum tolerated dose was the lowest dose level, 0.3 μg/kg | 10 |
| rhIL15 | Subcutaneous injection of 0.25-3 μg/kg Monday to Friday for 2 weeks | Metastatic solid tumor | Hypotension, cardiac chest pain, pancreatitis | No clinical responses, and study was stopped early because of chest pain episode | 11 |
| rhIL15 | Continuous infusion of 3-5 μg/kg per day for 5 d | Metastatic solid tumor | Pulmonary capillary leak | No DLT; 4 μg/kg was selected as recommended phase 2 dose | 12 |
| rhIL15 | Continuous infusion of 0.125-4 μg/kg per day for 10 days | Metastatic solid tumor | Bleeding, uveitis, pneumonitis, intestinal ischemia | Recommended phase 2 dose was 4 μg/kg per day | 13 |
| rhIL15 + alemtuzumab | Subcutaneous injection of 0.5-2 μg/kg Monday to Friday for 2 weeks of rhIL15 with fixed dose alemtuzumab | Mature T-cell malignancies | Cytopenias, infusion reaction | No DLT | 16 |
| rhIL15 + avelumab | Continuous infusion of 1-4 μg/kg per day for 5 days of rhIL15 with fixed dose avelumab | Mature T-cell malignancies | Autoimmune hemolytic anemia, infusion reaction | Study terminated early because of slow accrual | 17 |
| rhIL15 + mogamulizumab (current study) | Continuous infusion of 2-4 μg/kg per day for 5 days of rhIL15 with fixed-dose mogamulizumab | Mature T-cell malignancies | Capillary leak, rash, infection | Maximum tolerated dose was 2 μg/kg | NA |
| N-803 (IL-15 receptor agonist) + rituximab | 1-20 μg/kg N-803 weekly for 4 doses with 4 consolidation doses with fixed dose rituximab | Indolent B-cell lymphomas | Infusion reaction, hypertension, lymphopenia | Recommended phase 2 dose 15-20 μg/kg | 61 |
| N-803 + BCG | 100-400 μg N-803 with fixed dose weekly bladder instillations of BCG | Non-muscle invasive bladder cancer | Hypertension, hematuria, fatigue | No DLT | 62 |
BCG, bacillus calmette-guerin; NA, not applicable.
In our small study, we found that the combination of rhIL-15 and mogamulizumab was associated with immunologic toxicities that limit further development of this combination. Patients with relapsed or refractory T-cell lymphomas have a poor prognosis, particularly those with ATLL and advanced stage cutaneous T-cell lymphomas, which make them a particularly difficult population to study in clinical trials, and the results from this study should be interpreted within that context.
Acknowledgments
The authors thank the patients, their families, and caregivers for their participation in the study; Elaine Jaffe for pathologic review; and Jen Hsu Albert and Kim Johnson for their administrative support.
This study was supported by the Intramural Research Program of the National Institutes of Health.
Authorship
Contribution: M.J.G. and S.D. wrote the first draft; S.N., M.R., M.D.M., K.C., R.L., C.M., S.P., L.M.S., and W.H.W. revised the manuscript; S.D., B.B., M.D.M., and K.C. conducted the experiments and analyzed the data; M.J.G., S.D., S.N., M.R., M.D.M., and K.C. interpreted the data; and M.R., M.D.M., K.C., T.W., L.M.S., and W.H.W. designed the research study and supervised the project.
Conflict-of-interest disclosure: M.D.M. reports employment with and ownership interest in Cartesian Therapeutics. The remaining authors declare no competing financial interests.
Thomas Waldmann died on 25 September 2021.
Correspondence: Max J. Gordon, National Cancer Institute, Building 10, Room 3B38, Bethesda, MD 20892; email: max.gordon@nih.gov.
References
Author notes
M.J.G. and S.D. contributed equally to this study.
Data are available on request from the corresponding author, Max J. Gordon (max.gordon@nih.gov).