Key Points
OS (3-year) of patients with TCL getting haplo-HCT or MSD, MUD TCD+, or MUD TCD− allo-HCT was 60%, 63%, 59%, and 64%, respectively.
PFS (3-year) of patients with TCL getting haplo-HCT or MSD, MUD TCD+, or MUD TCD− allo-HCT is 50%, 50%, 48%, and 52%, respectively.
Abstract
Mature T-cell lymphomas constitute the most common indication for allogeneic hematopoietic cell transplantation (allo-HCT) of all lymphomas. Large studies evaluating contemporary outcomes of allo-HCT in mature T-cell lymphomas relative to commonly used donor sources are not available. Included in this registry study were adult patients who had undergone allo-HCT for anaplastic large cell lymphoma, angioimmunoblastic T-cell lymphoma (AITL), or peripheral T-cell lymphoma not otherwise specified (PTCL-NOS) between 2008 and 2018. Hematopoietic cell transplantation (HCT) platforms compared were posttransplant cyclophosphamide-based haploidentical (haplo-)HCT, matched sibling donor (MSD) HCT, matched unrelated donor HCT with in vivo T-cell depletion (MUD TCD+), and matched unrelated donor HCT without in vivo T-cell depletion (MUD TCD−). Coprimary end points were overall survival (OS) and progression-free survival (PFS); secondary end points included nonrelapse mortality (NRM), and relapse/progression incidence (RI). A total of 1942 patients were eligible (237 haplo-HCT; 911 MSD; 468 MUD TCD+; 326 MUD TCD−). Cohorts were comparable for baseline characteristics with the exception of higher proportions of patients with decreased performance status (PS) and marrow graft recipients in the haplo-HCT group. Using univariate and multivariate comparisons, OS, PFS, RI, and NRM were not significantly different among the haplo-HCT, MSD, MUD TCD+, and MUD TCD− cohorts, with 3-year OS and PFS of 60%, 63%, 59%, and 64%, respectively, and 50%, 50%, 48%, and 52%, respectively. Significant predictors of inferior OS and PFS on multivariate analysis were active disease status at HCT and decreased PS. AITL was associated with significantly reduced relapse risk and better PFS compared with PTCL-NOS. Allo-HCT can provide durable PFS in patients with mature T-cell lymphoma (TCL). Outcomes of haplo-HCT were comparable to those of matched donor allo-HCT.
Introduction
Mature T-cell lymphomas are a heterogenous group of non-Hodgkin lymphomas (NHLs) with varied morphological and clinical features1,2 and an overall prognosis that is generally worse than their B-cell counterparts.2 The most common subtypes of mature T-cell lymphoma are peripheral T-cell lymphoma not otherwise specified (PTCL-NOS), angioimmunoblastic T-cell lymphoma (AITL), and anaplastic large cell lymphoma (ALCL).3 Although the addition of brentuximab vedotin to anthracycline-based frontline therapies has improved the outcomes of CD30+ T-cell NHL (T-NHL; especially ALCL),4 the results after first-line treatment of PTCL-NOS and AITL remain suboptimal.5-7 In the relapsed/refractory setting, available pharmacological options typically do not provide long-term disease control,8-10 and adoptive immunotherapy in the form of allogeneic hematopoietic cell transplantation (allo-HCT) remains the only curative option for all 3 subtypes.11-16 In fact, with the recent decline in allo-HCT utilization for diffuse large B-cell lymphoma, T-NHL now constitutes the most common indication for allo-HCT for lymphomas in the United States and Europe.17 Historically, application of this option (even in relatively younger patients) was limited by the lack of donor availability and high procedure-related mortality rates (∼30% to 35% at 1 year).11-13 Large contemporary analyses evaluating outcomes after allo-HCT in mature T-NHL, especially relative to alternative donor sources, are not available.
With the introduction of posttransplant cyclophosphamide (ptCY)-based immunosuppression, allo-HCT using haploidentical related donors has emerged as a valuable alternative for patients without an available matched sibling donor (MSD) or matched unrelated donor (MUD).18 Similar to more common indications for allo-HCT,19-21 ptCY haploidentical (haplo)-HCT seems to provide outcomes comparable to MSD/MUD transplants (using conventional calcineurin inhibitor [CNI]-based prophylaxis) in patients with lymphoma.22-26 However, these results derive from retrospective analyses of patient samples with the global diagnoses of Hodgkin lymphoma and/or NHL. Because of the increasing utilization of allo-HCT in T-NHL, we evaluated the contemporary outcomes of this modality, relative to established donor sources, in the three most common nodal variants of mature T-cell lymphomas (PTCL-NOS, AITL, and ALCL).
Materials and methods
Data sources
The study was performed through collaboration between the European Society for Blood and Marrow Transplantation (EBMT) and the Center for International Blood and Marrow Transplant Research (CIBMTR). EBMT is a voluntary organization comprising >600 transplant centers, primarily from Europe. Accreditation as a member center requires submission of minimum essential data (MED-A form) from all consecutive patients to a central registry. Accredited EBMT centers are subject to on-site audits, and all centers must obtain written informed consent prior to data registration following the Declaration of Helsinki.
CIBMTR is a working group of >380 transplantation centers worldwide that contribute detailed data on hematopoietic cell transplantation (HCT) to a statistical center at the Medical College of Wisconsin. Participating centers are required to report all transplantations consecutively, patients are followed longitudinally, and compliance is monitored by on-site audits. Computerized checks for discrepancies, physicians’ review of submitted data, and on-site audits of participating centers ensure data quality. The CIBMTR collects data at 2 levels: transplant essential data in all patients and more comprehensive data (comprehensive report form) in a subset of patients selected by a weighted randomization scheme. Observational studies conducted by the CIBMTR are performed in compliance with all applicable federal regulations pertaining to the protection of human research participants. Patients provided written informed consent for research. The institutional review boards of the Medical College of Wisconsin and the National Marrow Donor Program approved this study.
Study design
This was a collaborative retrospective registry-based analysis. Adults (≥18 years) with PTCL-NOS, AITL, or ALCL, who had undergone their first allo-HCT between 2008 and 2018, were eligible for the study. Eligible donors included MSDs, 8 of 8 MUDs (allele-level match at HLA-A, HLA-B, HLA-C, and HLA-DRB1), or haploidentical related donors. Recipients of haplo-HCT were limited to those receiving graft-versus-host disease (GVHD) prophylaxis with ptCY (with or without CNI and mycophenolate mofetil). GVHD prophylaxis in MSD recipients was limited to CNI-based approaches without anti-thymocyte globulin/alemtuzumab in vivo T-cell depletion (TCD), whereas MUD recipients received CNI-based prophylaxis with or without in vivo TCD. Patients receiving ex vivo graft manipulation (eg, CD34 selection; n = 77) were excluded to reduce study heterogeneity. The CIBMTR cohort was limited to patients from the United States and Canada to avoid duplicate inclusion of European patients reported to both registries.
Definitions
The intensity of allo-HCT conditioning regimens was categorized as myeloablative conditioning or nonmyeloablative reduced-intensity conditioning (RIC) using consensus criteria.27 Disease response at the time of HCT was determined using the current International Working Group criteria during the time of this analysis.28-30
Study end points
The coprimary end points were overall survival (OS) and progression-free survival (PFS). Death from any cause was considered an event for OS, and surviving patients were censored at last follow-up. For PFS, a patient was considered to have failed treatment at the time of progression/relapse or death from any cause. Patients alive without evidence of disease relapse or progression were censored at last follow-up. Secondary outcomes included nonrelapse mortality (NRM) and progression/relapse. NRM was defined as death without evidence of prior lymphoma progression/relapse; relapse was considered a competing risk. Progression/relapse was defined as progressive lymphoma after HCT or lymphoma recurrence after a complete remission (CR); NRM was considered a competing risk. Acute GVHD and chronic GVHD were graded using established clinical criteria.31,32 GVHD-free relapse-free survival (GRFS) was calculated using the modification proposed by Ruggeri et al for registry-based studies).33 Probabilities of GRFS, PFS, and OS were calculated using Kaplan-Meier estimates. Neutrophil recovery was defined as the first of 3 successive days with absolute neutrophil count ≥ 500 per microliter after the posttransplantation nadir. Platelet recovery was considered to have occurred on the first of 3 consecutive days with platelet count ≥ 20 000 per microliter, in the absence of platelet transfusion for 7 consecutive days. For neutrophil and platelet recovery, death without the event was considered a competing risk.
Statistical analysis
The haplo-HCT cohort was compared against the MSD, MUD with TCD (MUD TCD+), and MUD without TCD (MUD TCD−) cohorts. Patient-, disease- and transplant-related variables were compared among the 4 cohorts using the χ2 test for categorical variables and the Mann-Whitney U test for continuous variables. Cumulative incidences of hematopoietic recovery, acute and chronic GVHD, relapse, and NRM were calculated to accommodate for competing risks.
Associations among patient-, disease, and transplantation-related variables and outcomes of interest were evaluated using Cox proportional-hazards regression for acute GVHD, chronic GVHD, relapse, NRM, PFS, and OS. All potential prognostic factors for outcomes and characteristics that differed among the groups were included in multivariate models (stratified on data source [CIBMTR vs EBMT]): donor source, disease status at HCT, patient age, patient sex, cell source, conditioning regimen intensity, prior autologous HCT, Karnofsky Performance Status (KPS), and histology. The proportional hazards assumption for Cox regression was tested by adding a time-dependent covariate for each risk factor and each outcome. Covariates violating the proportional hazards assumption were added as time-dependent covariates in the Cox regression model. Interactions between the main effect and significant covariates were examined. All tests were 2 sided. The type I error rate was fixed at 0.05. Statistical analyses were performed with SPSS 26 (SPSS Inc., Chicago, IL), and R 3.6.1 (R Development Core Team, Vienna, Austria) software packages.
Results
Patient and transplant characteristics
Altogether, 1942 eligible patients (haplo-HCT, n = 237; MSD, n = 911; MUD TCD+, n = 468; MUD TCD−, n = 326) were included. Of these, 23 patients were part of a previous EBMT study addressing the outcome of allo-HCT in ALCL,34 and 144 patients overlapped with a prior CIBMTR analysis of allo-HCT in AITL.35 Of note, patients receiving haplo-HCT were excluded from both of these studies. There were no significant differences among the 4 cohorts in terms of patient sex, disease status at allo-HCT, HCT-comorbidity index, median number of prior therapies, distribution of T-cell histologies, and history of autologous HCT. Compared with the MSD and MUD cohorts, patients in the haplo-HCT cohort more frequently had a KPS ≤ 90 (42% vs 32-35%; P = .03) and bone marrow as graft source (31% vs 6-7%; P < .001). Use of RIC was more frequent in the haplo-HCT (73%) and MUD TCD− (73%) cohorts compared with the MSD (62%) and MUD TCD+ (67%) cohorts (P < .001). Fewer patients in the MSD cohort were ≥60 years of age (19%) compared with other cohorts (26-29%). Details are given in Table 1. The completeness of patient follow-up at 1 year, 2 years, and 3 years was 94.5%, 86.5%, and 79.2%, respectively.
Patient characteristics
| . | Haplo-HCT . | MSD HCT . | MUD TCD+ . | MUD TCD− . | P . |
|---|---|---|---|---|---|
| Patients, n | 237 | 911 | 468 | 326 | |
| Data source | <.001 | ||||
| CIBMTR | 104 (44) | 324 (36) | 156 (33) | 209 (64) | |
| EBMT | 133 (56) | 587 (64) | 312 (67) | 117 (36) | |
| Age, y | |||||
| Median, range | 54 (18-76) | 51 (18-77) | 53 (18-75) | 54 (18-74) | .004 |
| 60-69 | 51 (21) | 164 (18) | 126 (27) | 89 (27) | |
| ≥70 | 11 (5) | 8 (1) | 10 (2) | 6 (2) | |
| Males | 84 (35) | 322 (35) | 165 (35) | 119 (37) | .98 |
| KPS | |||||
| ≥90 | 131 (58) | 584 (68) | 301 (68) | 209 (65) | .03 |
| Not reported | 10 | 47 | 24 | 5 | |
| Cell source | <.001 | ||||
| Bone marrow | 74 (31) | 61 (7) | 26 (6) | 23 (7) | |
| Peripheral blood | 163 (69) | 850 (93) | 442 (94) | 303 (93) | |
| Conditioning intensity | <.001 | ||||
| Myeloablative | 65 (27) | 345 (38) | 154 (33) | 86 (27) | |
| RIC | 172 (73) | 552 (62) | 306 (67) | 239 (73) | |
| Missing data | 0 | 14 | 8 | 1 | |
| Disease status | .84 | ||||
| CR | 120 (51) | 451 (51) | 238 (52) | 161 (51) | |
| PR | 74 (32) | 252 (29) | 124 (27) | 96 (30) | |
| Resistant/Untreated | 39 (17) | 177 (20) | 93 (20) | 60 (19) | |
| Missing data | 4 | 31 | 13 | 9 | |
| HCT-CI | |||||
| ≥3 | 52 (28) | 191 (30) | 105 (35) | 95 (37) | .08 |
| Missing data* | 52 | 280 | 164 | 72 | |
| Chemotherapy lines | |||||
| Median (IQR), n | 2 (1.8-3) | 2 (1-3) | 2 (1-3) | 2 (2-3) | .34 |
| 1 line† | 31 (23) | 89 (26) | 57 (29) | 19 (21) | |
| 2 lines† | 47 (34) | 109 (32) | 62 (32) | 32 (34) | |
| ≥3 lines† | 58 (43) | 143 (42) | 76 (39) | 42 (45) | |
| Missing data‡ | 101 | 570 | 273 | 233 | |
| Female donor/male recipient | 61 (26) | 254 (28) | 52 (11) | 45 (14) | <.001 |
| Histology | .14 | ||||
| AITL | 68 (29) | 270 (30) | 155 (33) | 101 (31) | |
| ALCL§ | 58 (24) | 170 (19) | 82 (18) | 75 (23) | |
| PTCL | 111 (47) | 471 (51) | 231 (49) | 150 (46) | |
| Prior autologous HCT | 78 (33) | 336 (37) | 195 (42) | 132 (41) | .09 |
| Follow-up, median (range) [IQR], months | 24 (1-113) [13-48] | 43 (1-128) [17-72] | 35 (1-132) [13-63] | 49 (1-134) [24-72] |
| . | Haplo-HCT . | MSD HCT . | MUD TCD+ . | MUD TCD− . | P . |
|---|---|---|---|---|---|
| Patients, n | 237 | 911 | 468 | 326 | |
| Data source | <.001 | ||||
| CIBMTR | 104 (44) | 324 (36) | 156 (33) | 209 (64) | |
| EBMT | 133 (56) | 587 (64) | 312 (67) | 117 (36) | |
| Age, y | |||||
| Median, range | 54 (18-76) | 51 (18-77) | 53 (18-75) | 54 (18-74) | .004 |
| 60-69 | 51 (21) | 164 (18) | 126 (27) | 89 (27) | |
| ≥70 | 11 (5) | 8 (1) | 10 (2) | 6 (2) | |
| Males | 84 (35) | 322 (35) | 165 (35) | 119 (37) | .98 |
| KPS | |||||
| ≥90 | 131 (58) | 584 (68) | 301 (68) | 209 (65) | .03 |
| Not reported | 10 | 47 | 24 | 5 | |
| Cell source | <.001 | ||||
| Bone marrow | 74 (31) | 61 (7) | 26 (6) | 23 (7) | |
| Peripheral blood | 163 (69) | 850 (93) | 442 (94) | 303 (93) | |
| Conditioning intensity | <.001 | ||||
| Myeloablative | 65 (27) | 345 (38) | 154 (33) | 86 (27) | |
| RIC | 172 (73) | 552 (62) | 306 (67) | 239 (73) | |
| Missing data | 0 | 14 | 8 | 1 | |
| Disease status | .84 | ||||
| CR | 120 (51) | 451 (51) | 238 (52) | 161 (51) | |
| PR | 74 (32) | 252 (29) | 124 (27) | 96 (30) | |
| Resistant/Untreated | 39 (17) | 177 (20) | 93 (20) | 60 (19) | |
| Missing data | 4 | 31 | 13 | 9 | |
| HCT-CI | |||||
| ≥3 | 52 (28) | 191 (30) | 105 (35) | 95 (37) | .08 |
| Missing data* | 52 | 280 | 164 | 72 | |
| Chemotherapy lines | |||||
| Median (IQR), n | 2 (1.8-3) | 2 (1-3) | 2 (1-3) | 2 (2-3) | .34 |
| 1 line† | 31 (23) | 89 (26) | 57 (29) | 19 (21) | |
| 2 lines† | 47 (34) | 109 (32) | 62 (32) | 32 (34) | |
| ≥3 lines† | 58 (43) | 143 (42) | 76 (39) | 42 (45) | |
| Missing data‡ | 101 | 570 | 273 | 233 | |
| Female donor/male recipient | 61 (26) | 254 (28) | 52 (11) | 45 (14) | <.001 |
| Histology | .14 | ||||
| AITL | 68 (29) | 270 (30) | 155 (33) | 101 (31) | |
| ALCL§ | 58 (24) | 170 (19) | 82 (18) | 75 (23) | |
| PTCL | 111 (47) | 471 (51) | 231 (49) | 150 (46) | |
| Prior autologous HCT | 78 (33) | 336 (37) | 195 (42) | 132 (41) | .09 |
| Follow-up, median (range) [IQR], months | 24 (1-113) [13-48] | 43 (1-128) [17-72] | 35 (1-132) [13-63] | 49 (1-134) [24-72] |
Unless otherwise noted, data are n (%).
HCT-CI, HCT-comorbidity index; IQR, interquartile range; PR, partial response; PTCL, peripheral T-cell lymphoma.
EBMT does not collect this variable for Minimum Essential Data – A patients.
Missing number was excluded when calculating percentages.
CIBMTR does not collect this variable for the transplant-essential level patients.
ALCLs included 116 ALK+ cases, 143 ALK− cases, and 126 cases for which ALK status was not known.
Hematopoietic recovery
The day-28 cumulative incidence of neutrophil recovery was significantly lower in the haplo-HCT cohort (86%; 95% confidence interval [CI], 81-90) compared with the MSD (95%; 95% CI, 94-97), MUD TCD+ (94%; 95% CI, 91-96), and MUD TCD− (97%; 95% CI, 94-98) cohorts (P < .001; Table 2; supplemental Figure 1). Similarly, the day-100 cumulative incidence of platelet recovery was significantly lower in the haplo-HCT cohort (80%; 95% CI, 74-85) compared with the MSD (96%; 95% CI, 94-97), MUD TCD+ (93%; 95% CI, 89-95) and MUD TCD− (96%; 95% CI, 93-98) cohorts (P < .001; Table 2; supplemental Figure 1).
Univariate outcomes
| Outcomes . | Haplo-HCT (N = 235) . | MSD HCT (N = 909) . | MUD TCD+ (N = 465) . | MUD TCD− (N = 325) . | P . | ||||
|---|---|---|---|---|---|---|---|---|---|
| Neutrophil recovery, 28 d | n = 230 | 86 (81-90) | n = 883 | 95 (94-97) | n = 453 | 94 (91-96) | n = 322 | 97 (94-98) | <.001 |
| Platelet recovery, 100 d | n = 219 | 80 (74-85) | n = 737 | 96 (94-97) | n = 378 | 93 (89-95) | n = 284 | 96 (93-98) | <.001 |
| Stage II-IV acute GVHD, 100 d | n = 225 | 33 (27-39) | n = 837 | 31 (28-34) | n = 429 | 30 (26-35) | n = 305 | 37 (32-42) | .03 |
| Stage III-IV acute GVHD, 100 d | n = 225 | 9 (6-13) | n = 837 | 11 (9-13) | n = 429 | 10 (8-13) | n = 305 | 18 (14-23) | .002 |
| Chronic GVHD | n = 226 | n = 770 | n = 395 | n = 296 | <.001 | ||||
| 1-y | 28 (22-34) | 41 (37-44) | 35 (30-40) | 54 (48-59) | |||||
| 2-y | 32 (25-38) | 50 (47-54) | 41 (36-46) | 64 (58-70) | |||||
| NRM | n = 237 | n = 911 | n = 468 | n = 326 | .49 | ||||
| 100 d | 11 (7-16) | 8 (7-10) | 10 (8-13) | 8 (6-12) | |||||
| 1 y | 20 (15-25) | 15 (13-18) | 18 (15-22) | 16 (13-21) | |||||
| 3 y | 22 (17-28) | 21 (18-23) | 24 (20-28) | 23 (18-28) | |||||
| Relapse | n = 237 | n = 911 | n = 468 | n = 326 | .73 | ||||
| 1 y | 22 (17-28) | 25 (22-27) | 23 (19-27) | 23 (19-28) | |||||
| 2 y | 27 (21-33) | 29 (25-32) | 26 (22-31) | 25 (20-30) | |||||
| 3 y | 28 (22-35) | 29 (26-32) | 28 (24-33) | 25 (21-30) | |||||
| PFS | n = 235 | n = 909 | n = 465 | n = 325 | .80 | ||||
| 1 y | 59 (52-65) | 61 (57-64) | 59 (54-63) | 60 (55-65) | |||||
| 3 y | 50 (43-57) | 50 (47-54) | 48 (43-53) | 52 (46-58) | |||||
| OS | n = 237 | n = 911 | n = 468 | n = 326 | .30 | ||||
| 1 y | 70 (63-75) | 74 (71-77) | 70 (65-74) | 74 (69-78) | |||||
| 3 y | 60 (52-66) | 63 (59-66) | 59 (54-64) | 64 (58-69) | |||||
| GRFS | n = 179 | n = 665 | n = 378 | n = 210 | .02 | ||||
| 1 y | 46 (38-53) | 46 (42-50) | 50 (44-55) | 38 (32-45) | |||||
| 2 y | 37 (29-45) | 36 (32-40) | 41 (36-46) | 31 (25-37) | |||||
| Outcomes . | Haplo-HCT (N = 235) . | MSD HCT (N = 909) . | MUD TCD+ (N = 465) . | MUD TCD− (N = 325) . | P . | ||||
|---|---|---|---|---|---|---|---|---|---|
| Neutrophil recovery, 28 d | n = 230 | 86 (81-90) | n = 883 | 95 (94-97) | n = 453 | 94 (91-96) | n = 322 | 97 (94-98) | <.001 |
| Platelet recovery, 100 d | n = 219 | 80 (74-85) | n = 737 | 96 (94-97) | n = 378 | 93 (89-95) | n = 284 | 96 (93-98) | <.001 |
| Stage II-IV acute GVHD, 100 d | n = 225 | 33 (27-39) | n = 837 | 31 (28-34) | n = 429 | 30 (26-35) | n = 305 | 37 (32-42) | .03 |
| Stage III-IV acute GVHD, 100 d | n = 225 | 9 (6-13) | n = 837 | 11 (9-13) | n = 429 | 10 (8-13) | n = 305 | 18 (14-23) | .002 |
| Chronic GVHD | n = 226 | n = 770 | n = 395 | n = 296 | <.001 | ||||
| 1-y | 28 (22-34) | 41 (37-44) | 35 (30-40) | 54 (48-59) | |||||
| 2-y | 32 (25-38) | 50 (47-54) | 41 (36-46) | 64 (58-70) | |||||
| NRM | n = 237 | n = 911 | n = 468 | n = 326 | .49 | ||||
| 100 d | 11 (7-16) | 8 (7-10) | 10 (8-13) | 8 (6-12) | |||||
| 1 y | 20 (15-25) | 15 (13-18) | 18 (15-22) | 16 (13-21) | |||||
| 3 y | 22 (17-28) | 21 (18-23) | 24 (20-28) | 23 (18-28) | |||||
| Relapse | n = 237 | n = 911 | n = 468 | n = 326 | .73 | ||||
| 1 y | 22 (17-28) | 25 (22-27) | 23 (19-27) | 23 (19-28) | |||||
| 2 y | 27 (21-33) | 29 (25-32) | 26 (22-31) | 25 (20-30) | |||||
| 3 y | 28 (22-35) | 29 (26-32) | 28 (24-33) | 25 (21-30) | |||||
| PFS | n = 235 | n = 909 | n = 465 | n = 325 | .80 | ||||
| 1 y | 59 (52-65) | 61 (57-64) | 59 (54-63) | 60 (55-65) | |||||
| 3 y | 50 (43-57) | 50 (47-54) | 48 (43-53) | 52 (46-58) | |||||
| OS | n = 237 | n = 911 | n = 468 | n = 326 | .30 | ||||
| 1 y | 70 (63-75) | 74 (71-77) | 70 (65-74) | 74 (69-78) | |||||
| 3 y | 60 (52-66) | 63 (59-66) | 59 (54-64) | 64 (58-69) | |||||
| GRFS | n = 179 | n = 665 | n = 378 | n = 210 | .02 | ||||
| 1 y | 46 (38-53) | 46 (42-50) | 50 (44-55) | 38 (32-45) | |||||
| 2 y | 37 (29-45) | 36 (32-40) | 41 (36-46) | 31 (25-37) | |||||
Unless otherwise noted, all data are probability, % (95% confidence interval).
GVHD
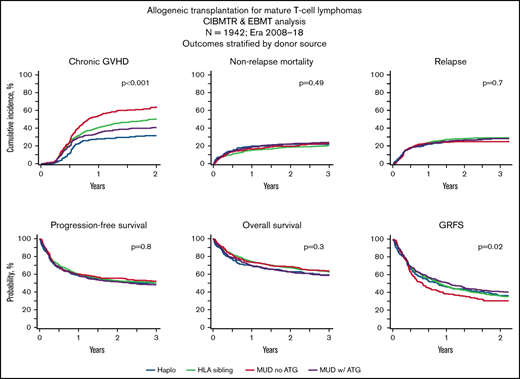
The day-100 cumulative incidence of acute GVHD grade 2-4 (grade 3-4) in the haplo-HCT, MSD, MUD TCD+, and MUD TCD− cohorts was 33% (9%), 31% (11%), 30% (10%), and 37% (18%), respectively (Table 2). Using multivariate analysis, compared with haplo-HCT, the MUD TCD− cohort was associated with a significantly higher risk for grade 3-4 acute GVHD (hazard ratio [HR], 2.0; 95% CI, 1.2-3.3; P = .01) (Table 3). The 1-year cumulative incidence of chronic GVHD in the haplo-HCT, MSD, MUD TCD+, and MUD TCD− cohorts was 28%, 41%, 35%, and 54%, respectively (Table 2). Using multivariate analysis, compared with haplo-HCT, the MSD (HR, 1.7; 95% CI, 1.3-2.2; P < .001), MUD TCD+ (HR, 1.4; 95% CI, 1.0-1.9; P = .047), and MUD TCD− (HR, 2.4; 95% CI, 1.8-3.2; P < .001) cohorts were associated with a significantly higher risk for chronic GVHD (Figure 1A; Table 3).
Multivariate analysis
| Covariates . | Reference group * . | OS . | PFS . | Relapse . | NRM . | Acute GVHD stage III-IV . | Chronic GVHD . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) . | P . | HR (95% CI) . | P . | HR (95% CI) . | P . | HR (95% CI) . | P . | HR (95% CI) . | P . | HR (95% CI) . | P . | ||
| Type of donor | |||||||||||||
| MSD | Haplo-HCT | 0.9 (0.7-1.2) | .42 | 1.0 (0.8-1.2) | .75 | 1.0 (0.8-1.4) | .89 | 0.9 (0.7-1.3) | .60 | 1.2 (0.8-2) | .39 | 1.7 (1.3-2.2) | <.001 |
| MUD TCD+ | 0.9 (0.7-1.3) | .70 | 1.0 (0.8-1.3) | .76 | 1.1 (0.8-1.5) | .54 | 1.0 (0.7-1.4) | .94 | 1.0 (0.5-1.7) | .88 | 1.4 (1-1.9) | .047 | |
| MUD TCD− | 0.9 (0.7-1.2) | .40 | 0.9 (0.7-1.2) | .71 | 0.9 (0.6-1.2) | .41 | 1.0 (0.7-1.5) | .83 | 2.0 (1.2-3.3) | .01 | 2.4 (1.8-3.2) | <.001 | |
| Disease status | |||||||||||||
| PR | CR | 1.4 (1.1-1.6) | .001 | 1.4 (1.2-1.7) | <.001 | 1.7 (1.4-2.1) | <.001 | 1.2 (0.9-1.5) | .12 | 1.2 (0.9-1.7) | .280 | 1.1 (0.9-1.3) | .25 |
| Resistant/Unt | 1.8 (1.4-2.1) | <.001 | 1.9 (1.6-2.2) | <.001 | 2.4 (1.9-3) | <.001 | 1.5 (1.2-2) | .001 | 1.8 (1.3-2.6) | .001 | 1.2 (0.9-1.4) | .19 | |
| Patient age, y | |||||||||||||
| 40-59 | 18-39 | 1.3 (1.1-1.7) | .01 | 1.2 (1-1.5) | .04 | 1.0 (0.8-1.3) | .75 | 1.6 (1.2-2.3) | .005 | 1.2 (0.8-1.8) | .37 | 1.3 (1.1-1.6) | .01 |
| ≥60 | 1.7 (1.3-2.2) | <.001 | 1.4 (1.1-1.7) | .007 | 1.0 (0.7-1.3) | .95 | 2.1 (1.5-3.1) | <.001 | 1.4 (0.9-2.3) | .11 | 1.3 (1.1-1.7) | .02 | |
| Sex | Female vs male | 0.9 (0.7-1) | .09 | 0.9 (0.8-1) | .15 | 0.9 (0.7-1) | .12 | 0.9 (0.7-1.2) | .60 | 0.7 (0.5-0.9) | .01 | 1 (0.86-1.2) | .88 |
| Female donor/male recipient | Yes vs no | 1.1 (0.9-1.3) | .50 | 1.2 (1-1.4) | .045 | 1.3 (1-1.6) | .03 | 1.1 (0.9-1.5) | .38 | 0.9 (0.6-1.3) | .64 | 1.4 (1.1-1.7) | .001 |
| Cell source | PB vs BM | 0.9 (0.7-1.2) | .63 | 0.9 (0.7-1.1) | .25 | 0.8 (0.6-1.2) | .29 | 0.8 (0.6-1.2) | .27 | 1.1 (0.6-1.8) | .79 | 0.9 (0.7-1.2) | .62 |
| Conditioning | RIC vs MAC | 1 (0.9-1.2) | .63 | 1.1 (0.9-1.2) | .47 | 1.2 (0.9-1.4) | .15 | 1.0 (0.8-1.2) | .80 | 1 (0.8-1.4) | .87 | 1 (0.9-1.2) | .99 |
| Prior auto-HCT | Yes vs no | 1 (0.8-1.2) | .90 | 1.1 (0.9-1.2) | .40 | 1 (0.8-1.2) | .850 | 1.1 (0.9-1.4) | .29 | 0.8 (0.6-1) | .08 | 1 (0.9-1.2) | .60 |
| KPS | <90 vs ≥90 | 1.5 (1.3-1.8) | <.001 | 1.3 (1.2-1.5) | <.001 | 1.3 (1-1.5) | .02 | 1.4 (1.2-1.8) | .001 | 1.2 (0.9-1.6) | .25 | 0.9 (0.8-1.1) | .28 |
| Histology | |||||||||||||
| ALCL | PTCL | 0.9 (0.8-1.2) | .59 | 1.1 (0.9-1.3) | .31 | 1.3 (1.1-1.6) | .01 | 0.8 (0.6-1.1) | .19 | 1.5 (1.1-2.2) | .02 | 1.1 (0.9-1.4) | .23 |
| AITL | 0.9 (0.7-1) | .09 | 0.7 (0.6-0.9) | <.001 | 0.5 (0.4-0.7) | <.001 | 0.9 (0.8-1.2) | .52 | 1.1 (0.8-1.5) | .63 | 1.0 (0.8-1.2) | .86 | |
| Covariates . | Reference group * . | OS . | PFS . | Relapse . | NRM . | Acute GVHD stage III-IV . | Chronic GVHD . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) . | P . | HR (95% CI) . | P . | HR (95% CI) . | P . | HR (95% CI) . | P . | HR (95% CI) . | P . | HR (95% CI) . | P . | ||
| Type of donor | |||||||||||||
| MSD | Haplo-HCT | 0.9 (0.7-1.2) | .42 | 1.0 (0.8-1.2) | .75 | 1.0 (0.8-1.4) | .89 | 0.9 (0.7-1.3) | .60 | 1.2 (0.8-2) | .39 | 1.7 (1.3-2.2) | <.001 |
| MUD TCD+ | 0.9 (0.7-1.3) | .70 | 1.0 (0.8-1.3) | .76 | 1.1 (0.8-1.5) | .54 | 1.0 (0.7-1.4) | .94 | 1.0 (0.5-1.7) | .88 | 1.4 (1-1.9) | .047 | |
| MUD TCD− | 0.9 (0.7-1.2) | .40 | 0.9 (0.7-1.2) | .71 | 0.9 (0.6-1.2) | .41 | 1.0 (0.7-1.5) | .83 | 2.0 (1.2-3.3) | .01 | 2.4 (1.8-3.2) | <.001 | |
| Disease status | |||||||||||||
| PR | CR | 1.4 (1.1-1.6) | .001 | 1.4 (1.2-1.7) | <.001 | 1.7 (1.4-2.1) | <.001 | 1.2 (0.9-1.5) | .12 | 1.2 (0.9-1.7) | .280 | 1.1 (0.9-1.3) | .25 |
| Resistant/Unt | 1.8 (1.4-2.1) | <.001 | 1.9 (1.6-2.2) | <.001 | 2.4 (1.9-3) | <.001 | 1.5 (1.2-2) | .001 | 1.8 (1.3-2.6) | .001 | 1.2 (0.9-1.4) | .19 | |
| Patient age, y | |||||||||||||
| 40-59 | 18-39 | 1.3 (1.1-1.7) | .01 | 1.2 (1-1.5) | .04 | 1.0 (0.8-1.3) | .75 | 1.6 (1.2-2.3) | .005 | 1.2 (0.8-1.8) | .37 | 1.3 (1.1-1.6) | .01 |
| ≥60 | 1.7 (1.3-2.2) | <.001 | 1.4 (1.1-1.7) | .007 | 1.0 (0.7-1.3) | .95 | 2.1 (1.5-3.1) | <.001 | 1.4 (0.9-2.3) | .11 | 1.3 (1.1-1.7) | .02 | |
| Sex | Female vs male | 0.9 (0.7-1) | .09 | 0.9 (0.8-1) | .15 | 0.9 (0.7-1) | .12 | 0.9 (0.7-1.2) | .60 | 0.7 (0.5-0.9) | .01 | 1 (0.86-1.2) | .88 |
| Female donor/male recipient | Yes vs no | 1.1 (0.9-1.3) | .50 | 1.2 (1-1.4) | .045 | 1.3 (1-1.6) | .03 | 1.1 (0.9-1.5) | .38 | 0.9 (0.6-1.3) | .64 | 1.4 (1.1-1.7) | .001 |
| Cell source | PB vs BM | 0.9 (0.7-1.2) | .63 | 0.9 (0.7-1.1) | .25 | 0.8 (0.6-1.2) | .29 | 0.8 (0.6-1.2) | .27 | 1.1 (0.6-1.8) | .79 | 0.9 (0.7-1.2) | .62 |
| Conditioning | RIC vs MAC | 1 (0.9-1.2) | .63 | 1.1 (0.9-1.2) | .47 | 1.2 (0.9-1.4) | .15 | 1.0 (0.8-1.2) | .80 | 1 (0.8-1.4) | .87 | 1 (0.9-1.2) | .99 |
| Prior auto-HCT | Yes vs no | 1 (0.8-1.2) | .90 | 1.1 (0.9-1.2) | .40 | 1 (0.8-1.2) | .850 | 1.1 (0.9-1.4) | .29 | 0.8 (0.6-1) | .08 | 1 (0.9-1.2) | .60 |
| KPS | <90 vs ≥90 | 1.5 (1.3-1.8) | <.001 | 1.3 (1.2-1.5) | <.001 | 1.3 (1-1.5) | .02 | 1.4 (1.2-1.8) | .001 | 1.2 (0.9-1.6) | .25 | 0.9 (0.8-1.1) | .28 |
| Histology | |||||||||||||
| ALCL | PTCL | 0.9 (0.8-1.2) | .59 | 1.1 (0.9-1.3) | .31 | 1.3 (1.1-1.6) | .01 | 0.8 (0.6-1.1) | .19 | 1.5 (1.1-2.2) | .02 | 1.1 (0.9-1.4) | .23 |
| AITL | 0.9 (0.7-1) | .09 | 0.7 (0.6-0.9) | <.001 | 0.5 (0.4-0.7) | <.001 | 0.9 (0.8-1.2) | .52 | 1.1 (0.8-1.5) | .63 | 1.0 (0.8-1.2) | .86 | |
Auto, autologous; BM, bone marrow; MAC, myeloablative conditioning; PB, peripheral blood; PTCL, peripheral T-cell lymphoma; PR, partial remission; Unt, untreated.
Statistically significant P values are shown in bold type.
Reference group is in bold type.
Transplantation outcomes. Cumulative incidence of chronic GVHD (A), NRM (B) and relapse (C). Probability of PFS (D), OS (E), and GRFS (F). ATG, anti-thymocyte globulin; Haplo, haplo-HCT; MU, matched unrelated donor.
Transplantation outcomes. Cumulative incidence of chronic GVHD (A), NRM (B) and relapse (C). Probability of PFS (D), OS (E), and GRFS (F). ATG, anti-thymocyte globulin; Haplo, haplo-HCT; MU, matched unrelated donor.
NRM
The 3-year cumulative incidence of NRM in the haplo-HCT, MSD, MUD TCD+, and MUD TCD− cohorts was 22%, 21%, 24%, and 23%, respectively (Figure 1B; Table 2). The risk of NRM was not significantly different among the 4 cohorts on multivariate analysis (Table 3). Resistant disease at HCT, age ≥ 40 years, and KPS < 90 were independently associated with a higher risk for NRM (Table 3).
Disease control
The 3-year cumulative incidence of relapse/progression was 28% (95% CI, 22-35) in the haplo-HCT cohort compared with 29%, 28%, and 25% in the MSD, MUD TCD+, and MUD TCD− cohorts, respectively (Figure 1C; Table 2). The risk of relapse/progression was not significantly different among the 4 cohorts on multivariate analysis (Table 3). Other factors independently associated with the risk of relapse included disease status at HCT, female donor for male recipient, KPS, and lymphoma subtype (Table 3). Across all 4 donor groups, the majority of relapse/progression events occurred within the first 6 months after allo-HCT, whereas events became rare beyond 2 years post-HCT.
Survival
The median follow-up of survivors was 38 months (range, 1-134). The 3-year PFS in the haplo-HCT group was 50% (95% CI, 52-66) compared with 50%, 48%, and 52% in the MSD, MUD TCD+, and MUD TCD− cohorts, respectively (Figure 1D; Table 2). The 3-year OS was 60%, 63%, 59%, and 64%, respectively (Figure 1F; Table 2). Multivariate analyses did not reveal a significant difference in OS or PFS among the 4 cohorts (Table 3). Disease status less than a CR, age ≥ 40 years, and decreased KPS significantly reduced OS and PFS on multivariate analysis. In addition, PFS was significantly associated with AITL histology and donor-recipient sex mismatch (Table 3). Table 4 summarizes allo-HCT univariate outcomes based on pretransplant remission status. The 3-year PFS for patients in CR or partial remission, as well as for those with refractory/untreated relapse, was 57%, 47%, and 36% respectively (P < .0001). The respective 3-year OS estimates were 68%, 59%, and 49% (P < .0001).
Univariate outcomes according to preallogeneic transplant remission status
| . | . | OS . | PFS . | Relapse/progression . | NRM . |
|---|---|---|---|---|---|
| At 1 y | CR | 79 (76-82) | 68 (65-71) | 17 (15-20) | 15 (13-17) |
| Partial remission | 70 (66-74) | 55 (51-59) | 29 (25-33) | 16 (13-19) | |
| Resistant/untreated | 61 (55-65) | 45 (39-50) | 34 (29-39) | 21 (17-26) | |
| P | <.0001 | <.0001 | <.0001 | .13 | |
| At 3 y | CR | 68 (65-71) | 57 (53-60) | 22 (19-25) | 21 (19-24) |
| Partial remission | 59 (54-63) | 47 (42-51) | 33 (29-37) | 20 (17-24) | |
| Resistant/untreated | 49 (4455) | 36 (31-41) | 39 (33-44) | 25 (21-30) | |
| P | <.0001 | <0001 | <.0001 | .13 |
| . | . | OS . | PFS . | Relapse/progression . | NRM . |
|---|---|---|---|---|---|
| At 1 y | CR | 79 (76-82) | 68 (65-71) | 17 (15-20) | 15 (13-17) |
| Partial remission | 70 (66-74) | 55 (51-59) | 29 (25-33) | 16 (13-19) | |
| Resistant/untreated | 61 (55-65) | 45 (39-50) | 34 (29-39) | 21 (17-26) | |
| P | <.0001 | <.0001 | <.0001 | .13 | |
| At 3 y | CR | 68 (65-71) | 57 (53-60) | 22 (19-25) | 21 (19-24) |
| Partial remission | 59 (54-63) | 47 (42-51) | 33 (29-37) | 20 (17-24) | |
| Resistant/untreated | 49 (4455) | 36 (31-41) | 39 (33-44) | 25 (21-30) | |
| P | <.0001 | <0001 | <.0001 | .13 |
Data are % (95% CI).
Two-year composite end point GRFS following haplo-HCT was 37% (95% CI, 29-45) compared with 36% (95% CI, 32-40) following MSD, 41% (95% CI, 36-46) following MUD TCD+, and 31% (95% CI, 25-37) following MUD TCD− (P = .02; Figure 1F).
Subgroup analysis
Information about the number of therapy lines prior to allo-HCT was not available for 1177 patients (61%; explained in Table 1, footnote †). Baseline characteristics and univariate analysis outcomes for patients with data available for prior therapy lines are shown in supplemental Tables 1 and 2). To adjust for confounding variables, the impact of the number of treatment lines prior to allo-HCT was examined in a separate exploratory multivariate model restricted to patients with available data (supplemental Table 3). More than 1 treatment line prior to allo-HCT was associated with a significantly higher risk for NRM (HR, 1.8; P = .02) but not relapse/progression or PFS. Patients receiving ≥3 treatment lines prior to allo-HCT also had a higher risk for overall mortality (HR, 1.6; P = .02).
Causes of death
The most common cause of death in all 4 cohorts was recurrent T-NHL (haplo-HCT, 38%; MSD, 36%; MUD TCD+, 30%; MUD TCD−, 31%). Overall, infections accounted for the second most common cause of death (19%), with the numerically highest proportion in the MUD TCD+ group (22%), followed by the haplo-HCT (20%), MUD TCD− (19%), and MSD (17%) groups (supplemental Table 4).
Discussion
Allo-HCT is an effective form of adoptive immunotherapy for patients with high-risk and/or relapsed/refractory T-NHL. Although mature T-cell neoplasms now constitute the most common lymphoma indication for allografting in the United States and Europe,17 published studies of allo-HCT in T-cell lymphoma have been limited by small sample size and the heterogeneity of underlying diagnoses and largely refer to transplant series performed in the early 2000s.36 In contrast, this joint analysis performed by the EBMT and the CIBMTR evaluated a 15-fold larger sample than the prior registry study,12 focuses on the 3 most important T-NHL entities, and covers a contemporary era with specific emphasis on haplo-HCT. Several important observations were made: (1) regardless of the donor source, allo-HCT provides unprecedented rates of survival outcomes (3-year OS, 60-64%; PFS, 48-52%), (2) disease recurrence appears to be a rare event for patients surviving 2 years relapse free, (3) careful patient preparation (eg, adequate disease control, patient fitness) remains a crucial factor that impacts allo-HCT outcomes, and (4) the efficacy of allo-HCT appears to differ among the 3 main T-NHL subsets, with the lowest risk of relapse in AITL.
The current collaborative analysis highlights the remarkable improvements in the outcomes of allo-HCT for T-NHL since the last CIBMTR analysis looking at a similar population.12 That study reporting allo-HCT outcomes in PTCL-NOS, AITL, and ALCL (between 1996 and 2006) was notably restricted to younger patients (age ≤ 60 years) receiving HLA-matched allografts.12 In that analysis, the 3-year rates for PFS and OS were 37% and 46%, respectively, and NRM rates ranged from 27% to 34%, depending on conditioning intensity. By contrast, in our study reflecting the period between 2008 and 2018, the 3-year NRM, PFS, and OS are substantially better (∼20%, 50%, and 60%, respectively), despite inclusion of alternative donor sources and that a sizable proportion of patients were ≥60 years of age (n = 465; 24%). These improved outcomes are potentially due to advances in unrelated donor selection, transplant supportive care, and management of posttransplant complications.
The outcomes of the haplo-HCT cohort in our study (n = 237) also validate the results of prior retrospective studies limited by small sample sizes (n = 20-35 patients), the broad variety of T-NHL subtypes investigated, and restriction to single-center analyses and/or certain types of conditioning and graft sources (n ≤ 35).37-40 Thus, this study is the first one to show comparable outcomes after haplo-HCT and matched donor transplants across the 3 main T-cell lymphoma subsets using a reasonable patient number in a multicenter setting. Based on the current analysis, we cannot speculate about scenarios in which haplo-HCT might be preferable over matched donor HCT and vice versa. However, in a recent cross-sectional EBMT study of predictors of haplo-HCT outcomes in lymphoma, chemorefractoriness at transplantation and primary diagnosis emerged as the only significant independent risk factors for survival.41
The timing of allo-HCT in T-NHL is controversial (in first remission vs for relapsed/refractory disease). A French/German randomized study compared allo-HCT with autologous HCT in younger patients with nodal T-cell lymphoma as part of first-line therapy.15 Roughly half of the patients randomized to allo-HCT (23/49) did not undergo allotransplantation, largely because of disease progression and donor unavailability. No difference in survival outcomes was seen among the 67 patients undergoing HCT. At this time, the American Society for Transplantation and Cellular Therapy guidelines do not recommend allo-HCT in first CR for nodal mature T-NHL.42 Notably, although our study suggests that survival after allo-HCT decreases with an increasing number of pretreatment lines, this effect reached statistical significance only in cases with ≥3 lines of therapy, but not if 1 vs 2 prior lines of treatment were compared. However, the design of our study does not permit valid conclusions about the impact of early vs delayed allo-HCT on the natural course of T-NHL.
This study represents the first time that it was possible to compare allo-HCT outcomes of the main T-NHL subsets using an informative sample size. Relative to PTCL-NOS, patients with AITL had a significantly lower risk for relapse and better PFS (Table 3). This is in keeping with preliminary data suggesting that AITL appears to be exquisitely sensitive to graft-versus-leukemia effects43 and with a previous CIBMTR study showing excellent survival of patients with AITL after RIC allo-HCT (4-year survival, 70%) and no relapses beyond 2 years.35 On the other hand, patients with ALCL had a significantly higher risk of relapse relative to PTCL-NOS (Table 3), which is in accordance with a previous small EBMT series also containing mismatched unrelated donors in which a 3-year relapse incidence of 40% was observed.34 The benefits of intensifying conditioning intensity in patients with lymphoma are debatable. Inline with previous studies on allo-HCT in other NHL subtypes,22,35,44-48 this study does not show an advantage for high-intensity conditioning regimens for the first time also for mature nodal T-NHL in a large data set.
Disease status at HCT was an independent outcome predictor in our analysis. Although being in CR at transplant was most favorable, patients in partial remission experienced 3-year PFS and OS of ∼47% and 58%, respectively, which seems to suggest that allo-HCT can provide durable disease control in a sizeable subset of responding patients not in CR. Outcomes are not as encouraging in resistant disease, but 3-year PFS (36%) and OS (49%) still appear to be very reasonable for patients with refractory T-NHL.
Unlike their B-cell counterparts, chimeric antigen receptor (CAR) T-cell therapies for T-cell lymphoma are in the early phases of development. Inherent challenges of CARs for T-cell malignancies include fratricide by self-targeting of antigens on the CARs themselves and a high risk of life-threatening infections due to potential T-cell aplasia. Several antigens under active investigation include CD5, CD7, CD30, CD3, CD4, and T-cell receptor β-chain constant region, among others.17 Future studies will define the safety and efficacy profile of CAR platforms in T-cell malignancies. Until then, allo-HCT remains a standard-of-care option for the management of relapsed/refractory mature T-NHL.
When interpreting the results of our study, some important shortcomings inherent to its design need to be considered. There are significant differences to consider between the patients who underwent haplo-HCT and the other groups. The haplo-HCT cohort had more patients with KPS < 90, and bone marrow grafts and RIC were used more frequently. Most importantly, GVHD prophylaxis was inherently different among the groups. Application of the ptCY platform to MUD transplants may question the equivalence of haplo-HCT and matched donor transplantation.49 This analysis is underpowered to detect small differences among the cohorts. The typical limitations of a registry analysis have to be taken into account, such as selection bias (ie, applicable only to fitter patients in remission who are able to undergo HCT), lack of central review of histology, unavailability of ALK status for one third of patients with ALCL (Table 2, footnote #3), and missing information on other confounders that could not be compensated for (eg, center practices and patient selection). In addition, during the era of this analysis the registries did not collect information on imaging modalities used for response assessment (computed tomography scan or positron emission tomography scan) or captured data regarding postrelapse therapies.
In summary, this study shows that, in the modern era, adoptive immunotherapy using allo-HCT can provide high rates of durable disease control in high-risk nodal mature T-cell lymphomas, with NRM rates that compare favorably against historical data. Outcomes are comparable across various commonly used donor sources in the contemporary era. Early evaluation by transplant physicians should be considered in all patients with refractory or relapsed nodal mature T-cell lymphomas.
Acknowledgments
This work was supported by the CIBMTR and the EBMT.
The CIBMTR is supported primarily by Public Health Service grant U24CA076518 from the National Cancer Institute, the National Heart, Lung, and Blood Institute, and the National Institute of Allergy and Infectious Diseases, grant HHSH250201700006C from the Health Resources and Services Administration, and grants N00014-20-1-2705 and N00014-20-1-2832 from the Office of Naval Research. Support is also provided by Be the Match Foundation, the Medical College of Wisconsin, the National Marrow Donor Program, and from the following commercial entities: Actinium Pharmaceuticals, ADIENNE, AlloVir, Amgen, Angiocrine Bioscience, Astellas Pharma US, bluebird bio, Bristol Myers Squibb, Celgene, CSL Behring, CytoSen Therapeutics, Daiichi Sankyo Company, ExCellThera, Fate Therapeutics, Gamida Cell, Genentech, Incyte Corporation, Janssen/Johnson & Johnson, Jazz Pharmaceuticals, Kiadis Pharma, Kite, a Gilead Company, Kyowa Kirin, Legend Biotech, Magenta Therapeutics, Merck Sharp & Dohme, Millennium, Takeda Oncology, Miltenyi Biotec, Novartis Pharmaceuticals, Omeros, OncoImmune, Orca Biosystems, Pfizer, Pharmacyclics, Sanofi Genzyme, StemCyte, Takeda Pharma, Vor Biopharma, and Xenikos BV.
Authorship
Contribution: P.D. and M.H. conceived and design the study and wrote the manuscript; C.A.L., M.N., H.F., A.B., P.D., and M.H. collected and assembled data; M.N. analyzed data; and all authors interpreted data, revised the manuscript, and approved the final version of the manuscript.
Conflict-of interest-disclosure: M.H. has received research support/funding from Takeda Pharmaceutical Company; has acted as a consultant for Incyte Corporation, ADC Therapeutics, Celgene, Pharmacyclics, Omeros, Verastem, and TeneoBio; and is a member of the speaker’s bureau for Sanofi Genzyme, AstraZeneca, and BeiGene. A.S. has received research support/funding from Takeda Pharmaceutical Company; has acted as a consultant for Incyte Corporation, Takeda Pharmaceutical Company, BMS Celgene, MSD, Gilead Kite, Bluebird, Novartis, Sanofi, Janssen, Roche, and Mundipharma; and is a member of the speaker’s bureau for Takeda. Q.B. has received research support/funding from Takeda Pharmaceutical Company, Stemline Pharmaceutical, Acrotech Biopharma, and Celgene and has acted as a consultant or as a member of the advisory board for Spectrum Pharmaceutical, Kite Pharmaceutical, and Purdue Pharmaceutical. J.Z. has acted as a consultant for Seattle Genetics, Acrotech Biopharma, Secura Bio, Kiwo Kirin, Legend Biotech, and Ono Pharmaceutical; has received research support from Seattle Genetics and Secura Bio; and is a member of the speaker’s bureau for Seattle Genetics, Acrotech Biopharma, Kiwo Kirin, and Mundi Pharma. D.B. has acted as a consultant for Jazz Pharmaceuticals and Molmed. S.D. has received research support from Janssen, AbbVie, and Astra Zeneca and honoraria from Riemser, Takeda, and Janssen. N.S. has received research support from Janssen, AbbVie, and Astra Zeneca and honoraria from Riemser, Takeda, and Janssen and owns stock in Bristol Myers Squibb. B.G. has acted as a consultant for BMS, Gilead, Novartis, and Roche; is a member of the speaker’s bureau for Gilead, Novartis, Riemser, and Roche; and has received research support from Riemser. M.S. has acted as a consultant and/or has served on advisory boards, steering committees, or data safety monitoring committees for AbbVie, Genentech, AstraZeneca, Sound Biologics, Pharmacyclics, BeiGene, Bristol Myers Squibb, MorphoSys, TG Therapeutics, Innate Pharma, Kite Pharma, Adaptive Biotechnologies, Epizyme, and Atara Biotherapeutics and has received research funding from Mustang Bio, Celgene, Bristol Myers Squibb, Pharmacyclics, Gilead, Genentech, AbbVie, TG Therapeutics, BeiGene, AstraZeneca, Sunesis, and Atara Biotherapeutics. M.A.K.-D. has acted as a consultant for Pharmacyclics and Daiichi Sankyo. C.S.S. has acted as a consultant and/or served on advisory boards for Juno Therapeutics, Sanofi-Genzyme, Spectrum Pharmaceuticals, Novartis, Genmab, Precision Biosciences, Kite/a Gilead Company, Celgene/BMS, Gamida Cell, Karyopharm Therapeutics, and GSK and has received research funding from Juno Therapeutics, BMS Celgene, Bristol Myers Squibb, Precision Biosciences, and Sanofi-Genzyme. S.M. has received travel grants from Gilead, is a member of the data monitoring committee for Bayer, and has received speaker’s fees from Janssen. A.F.H. has acted as a consultant and/or advisor for Bristol Myers Squibb, Merck, Seattle Genetics, Karyopharm, Genentech/Roche, ADC Therapeutics, Tubulis, Takeda, and AstraZeneca and has received institutional research funding from Bristol Myers Squibb, Genentech/Roche, Merck, Seattle Genetics, ADC Therapeutics, and Gilead/Kite Pharma. P.D. has acted as a consultant for AbbVie, AstraZeneca, bluebird bio, Gilead, Janssen, Novartis, Riemser, and Roche; is a member of the speaker’s bureau for AbbVie, AstraZeneca, Gilead, Novartis, Riemser, and Roche; and has received research support from Riemser. The remaining authors declare no competing financial interests.
Correspondence: Mehdi Hamadani, BMT and Cellular Therapy Program, Medical College of Wisconsin, 9200 W. Wisconsin Ave, Suite C4200, Milwaukee, WI 53226; e-mail: mhamadani@mcw.edu.
References
Author notes
Requests for data sharing may be submitted to Mehdi Hamadani (mhamadani@mcw.edu).
The full-text version of this article contains a data supplement.