Key Points
Lifetime extrapolation is needed to estimate whether more relapses from omitting RT is compensated by lower risk of late effects in ESHL.
Consolidative RT was more favorable the younger the patient, when future health discounting was included, and in never smokers.
Abstract
In recent randomized trials, omitting consolidative radiotherapy (RT) in early-stage Hodgkin lymphoma (ESHL) increased relapses. However, decades of follow-up are required to observe whether lower initial disease control is compensated by reduced risk of late effects. Extrapolation beyond trial follow-up is therefore necessary to inform current treatment decisions. To this end, we developed a microsimulation model to estimate lifetime quality-adjusted life years (QALYs) after combined modality treatment (CMT) or chemotherapy-alone for stage I/IIa ESHL. For CMT, the model included risks of breast and lung cancer, coronary heart disease, and ischemic stroke. Comparative outcomes were assessed for a clinically relevant range of example patients differing by age, sex, smoking status, and representative organs at risk (OAR) radiation doses informed by the RAPID trial. Analysis was performed with and without a 3.5% discount rate on future health. Smoking status had a large effect on optimal treatment choice. CMT was superior for nearly all never smoker example patients regardless of age, sex, and OAR doses. At a maximum, CMT produced a 1.095 (95% CI: 1.054-1.137) gain in undiscounted QALYs for a 20-year-old male never smoker with unilateral neck disease. In contrast, current smokers could substantially gain from chemotherapy-alone treatment. Again at a maximum, a 20-year-old male current smoker with bilateral neck and whole mediastinum involvement gained 3.500 (95% CI: 3.400 to 3.600) undiscounted QALYs with chemotherapy-alone treatment. Overall, CMT was more favorable the younger the patient, when future health discounting was included, and in never smokers.
Introduction
Combined modality treatment (CMT) involving chemotherapy and consolidative radiotherapy (RT) is recommended for the treatment of early stage Hodgkin lymphoma (ESHL).1,2 Such treatment is highly efficacious in controlling the disease, contributing to freedom from progression of >90% 10 years after treatment.3 These excellent outcomes combined with a young median age at treatment, however, allow time for latent collateral radiation injury to organs at risk (OAR) to manifest into radiation-induced disease. Therefore, long-term survivors are at an increased risk of developing late effects, including heart disease, ischemic stroke, and second cancers, that can substantially affect quality of life and lead to early mortality.4-8
To avoid radiation-related late effects, positron emission tomography (PET) response-adaptive omission of consolidative RT has been investigated, with the aim of identifying a population that can be successfully treated with chemotherapy-alone. The recently reported RAPID, H10, and HD16 trials assessed the noninferiority of doxorubicin, bleomycin, vinblastine and dacarbazine (ABVD) chemotherapy-alone treatment after negative PET results following initial chemotherapy compared with CMT. All 3 trials demonstrated that the omission of RT led to a statistically significant loss in disease control.9-11
However, due to the relative recency of these trials and the success of treatment of relapsed HL, no randomized trial evidence exists to support an overall survival benefit from consolidative RT. In addition, due to the latency of many important radiation-related late effects, clinical data on the long-term risk from modern RT are not available. For some patients, a greater initial risk of early HL relapse may be offset by the benefit of lower incidence and mortality from late effects. In practice, persistent concerns regarding the risk of late effects seen following historical RT techniques, combined with this contemporary uncertainty, have resulted in a steady decline in the use of consolidative RT, perhaps to the detriment of some patients in whom the radiation-related risks may, in fact, be minimal with modern RT techniques.12
Ideally, the choice to omit RT should be made through shared decision-making by the patient and clinicians, with access to all relevant evidence. To better inform such choices, individualized modeling of the predicted outcomes of decisions is necessary.13 Estimation and presentation of risks alone are insufficient because a treatment decision requires explicit trade-offs between competing benefits and risks. Summarizing such trade-offs requires translation of risks into a composite and readily understandable measure, such as overall survival probability expressed as expected life years or an extension to this incorporating health-related quality of life, generally referred to as expected quality-adjusted life years (QALYs).
To this end, we have developed an individual-patient level state-transition model capable of simulating in detail the life course of a patient with stage I/IIa disease and no mediastinal bulk following the RAPID inclusion criteria, using both chemotherapy-alone treatment and CMT scenarios. For CMT, the radiation-related excess incidence and mortality risk from breast and lung cancer, coronary heart disease (CHD), and ischemic stroke are included based on a patient’s age, sex, smoking status, and radiation doses to the relevant OAR. The model provides comparative estimates of the difference in expected life years and QALYs between the two treatment choices, taking into account uncertainty in input parameters.
In this study, we outline our model and apply it to a spectrum of clinically representative stage I/IIa patient presentations to help inform whether and for whom the omission of consolidative RT may be considered. These representative examples include 4 sets of OAR radiation doses covering the range seen within the RAPID trial, 3 different ages at treatment, both sexes, and 3 smoking risk categories. Each example is assessed both with and without the addition of a discount weight to future health outcomes to demonstrate the effect of varying the degree of importance placed by individual patients on near future vs far future events, referred to as time preference.
Methods
Model overview
An individual-patient level state-transition (microsimulation) model was developed to simulate in detail a patient’s life course, including the potential for late toxicities to develop after consolidative RT. Such models simulate the health of a patient through a series of health states over time (in our case, discretized into yearly cycles) and are recommended when characterizing a disease, or disease combinations, that requires a large number of health states.14 We provide full details of the model and its derivation in supplemental Methods, alongside a less technical overview, as follows.
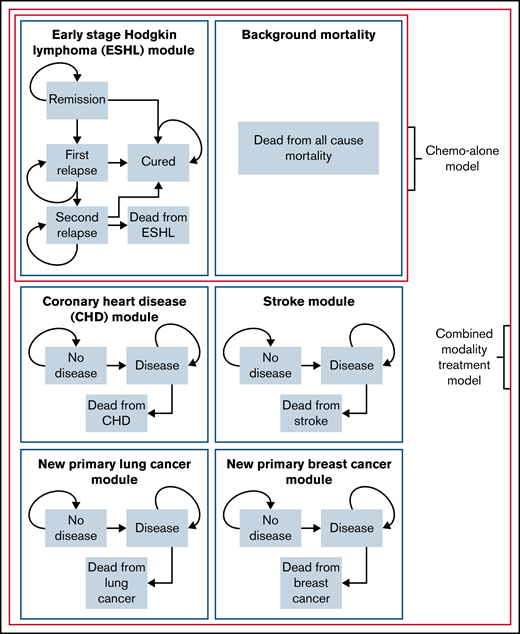
The model explicitly simulates the course of ESHL after either chemotherapy-alone treatment or CMT. For CMT, the model also simulates the potential course of second primary breast and lung cancer as well as CHD and ischemic stroke. Each disease forms an independent subcomponent of the overall model as well as background all-cause mortality, with the simulated patient beginning in the remission and no-disease states. An outline of the states and transitions in the model is given in Figure 1.
Overview of the individual-patient level state-transition model used to evaluate combined modality treatment and chemotherapy-alone. Schematic of the individual-patient level state-transition model with health states and possible transitions.
Overview of the individual-patient level state-transition model used to evaluate combined modality treatment and chemotherapy-alone. Schematic of the individual-patient level state-transition model with health states and possible transitions.
The ESHL sub-model consists of 5 health states: remission, first relapse, second relapse, cure, and death from HL. Yearly transition probabilities between states were based on data from clinical trials or, where not available, cohort studies that best reflected current treatment practice. The (per protocol) RAPID trial was used as the basis of risk of first relapse in this analysis. Transitions in the ESHL sub-model are outlined in Table 1, with full details of the methods and other studies used found in supplemental Methods.
Health state transitions in the ESHL sub-model
| Transition . | Estimate and description . |
|---|---|
| Remission to first relapse: CMT | Yearly transition probabilities for the first 5 y after initial treatment obtained from an exponential distribution: rate = 0.001061 (95% CI: 0.000603-0.001869). Using the point estimate, this corresponds to a 1.3% yearly probability of relapse over the 5 y.9 |
| Remission to first relapse: chemotherapy-alone | Yearly transition probabilities for the first 5 y after initial treatment obtained from a log-normal distribution: log mean = 7.837 (95% CI: 6.350-9.324), log SD = 7.837 (95% CI: 2.478-4.622). Using the point estimates, this corresponds to yearly relapse probabilities of 5.7%, 2.9%, 2.2%, 1.8%, and 1.6% over the 5 y.9 |
| Remission to cured | Patients were considered cured after 5 y. |
| First relapse to second relapse | Yearly transition probabilities for the first 3 y after first relapse obtained from a log-normal distribution: log mean = 3.715 (95% CI: 3.359-4.071), log SD = 1.577 (95% CI: 1.291-1.928). Using the point estimates, this corresponds to yearly relapse probabilities of 21.8%, 19.1%, and 15.8% over the 3 y. |
| First relapse to cured | Patients were considered cured after 3 y. |
| Second relapse to death from HL | Yearly transition probabilities for the first 5 y after second relapse obtained from an exponential distribution: rate = 0.00886 (95% CI: 0.00571-0.01373). Using the point estimate, this corresponds to a 10.1% yearly probability of death from HL over the 10 y. |
| Second relapse to cured | Patients were considered cured after 10 y. |
| Transition . | Estimate and description . |
|---|---|
| Remission to first relapse: CMT | Yearly transition probabilities for the first 5 y after initial treatment obtained from an exponential distribution: rate = 0.001061 (95% CI: 0.000603-0.001869). Using the point estimate, this corresponds to a 1.3% yearly probability of relapse over the 5 y.9 |
| Remission to first relapse: chemotherapy-alone | Yearly transition probabilities for the first 5 y after initial treatment obtained from a log-normal distribution: log mean = 7.837 (95% CI: 6.350-9.324), log SD = 7.837 (95% CI: 2.478-4.622). Using the point estimates, this corresponds to yearly relapse probabilities of 5.7%, 2.9%, 2.2%, 1.8%, and 1.6% over the 5 y.9 |
| Remission to cured | Patients were considered cured after 5 y. |
| First relapse to second relapse | Yearly transition probabilities for the first 3 y after first relapse obtained from a log-normal distribution: log mean = 3.715 (95% CI: 3.359-4.071), log SD = 1.577 (95% CI: 1.291-1.928). Using the point estimates, this corresponds to yearly relapse probabilities of 21.8%, 19.1%, and 15.8% over the 3 y. |
| First relapse to cured | Patients were considered cured after 3 y. |
| Second relapse to death from HL | Yearly transition probabilities for the first 5 y after second relapse obtained from an exponential distribution: rate = 0.00886 (95% CI: 0.00571-0.01373). Using the point estimate, this corresponds to a 10.1% yearly probability of death from HL over the 10 y. |
| Second relapse to cured | Patients were considered cured after 10 y. |
CI, confidence interval; SD, standard deviation.
Simple and generalizable disease sub-models were used to simulate the excess incidence and mortality of the 4 RT late effects. These consisted of 3 health states: no disease, diseased, and dead from disease. Baseline (without irradiation) age- and sex-specific yearly incidence and case fatality rates were based on the UK population and abstracted from publicly available repositories from another widely used model developed at our institution.15 Due to the strong association between smoking and increased risk of lung cancer, CHD, and stroke, we adjusted the population-based incidence rates (an aggregation of all individuals with a range of smoking behaviors) into incidence for never, former, and current smokers. The method for adjustment is outlined in supplemental Methods.
The model takes as input a patient's age at treatment, sex, smoking status, and representative doses to OARs, including some based on dosimetry data from 144 patients with negative PET results treated within the RAPID trial.9,16 The dosimetry inputs are combined with published estimates of the excess relative risk per Gray (ERR/Gy) of a late effect for a given radiation dose to an OAR. For breast and lung cancer, CHD, and ischemic stroke, mean doses to the breast tissue (MBD), lungs (MLD), heart (MHD), and common carotid arteries (MDCCA) were used as dose metrics for the respective OAR. Individualized excess incidence rates are calculated by first modifying the smoking status and age- and sex-specific incidence rates by the excess relative risk of disease. The unmodified rates are then subtracted. Additionally, the incidence and case-fatality rates are adjusted for their expected 20-year trend.15 The radiation dose-response relationships are given in Table 2 and are based on previously published epidemiological studies in HL survivors.4-7 Further details of their derivation are given in supplemental Methods. A graphic overview of the flow of inputs for CMT is given in Figure 2.
Dose-response equations
| Late toxicity . | Excess relative risk per Gy equation . | Input dosimetry variable . | Relative risk . |
|---|---|---|---|
| Breast cancer4 | No excess risk for first 5 y after treatment. Thereafter, age at treatment: <19 y, ERR/Gy = 0.257; 20-24 y, ERR/Gy = 0.097; 25-29 y, ERR/Gy = 0.057; 30-34 y, ERR/Gy = 0.043; >34 y, ERR/Gy = 0.030 | MBD | RR = 1 + (ERR/Gy × MBD × 1·608*) |
| Lung cancer5,6 | No excess risk for first 5 y after treatment. Thereafter: ERR/Gy, 0.15 (95% CI: 0.06-0.39) | MLD | RR=1+(ERR/Gy × MLD × 1·672*) |
| CHD7 | No excess risk for first 5 y after treatment. Thereafter, age at treatment: <28 y, ERR/Gy = 0.200 (95% CI: 0.052-0.070); 28-36 y, ERR/Gy = 0.088 (95% CI: 0.026-0.229); >36 y, ERR/Gy = 0.042 (95% CI: 0.006-0.111) | MHD | RR = 1 + ERR × MHD |
| Ischemic stroke8 | No excess risk for first 5 y after treatment. Thereafter, age at treatment: <21 y, ERR/Gy = 0.068 (95% CI: 0.015-0.156); 21-30 y, ERR/Gy = 0.051 (95% CI: 0.022-0.088); 31-40 y, ERR/Gy = 0.024 (95% CI: 0.005-0.051); >40 y, ERR/Gy = 0.0098 (95% CI: −0.007-0.032) | MDCCA | RR = 1 + ERR × MDCCA |
| Late toxicity . | Excess relative risk per Gy equation . | Input dosimetry variable . | Relative risk . |
|---|---|---|---|
| Breast cancer4 | No excess risk for first 5 y after treatment. Thereafter, age at treatment: <19 y, ERR/Gy = 0.257; 20-24 y, ERR/Gy = 0.097; 25-29 y, ERR/Gy = 0.057; 30-34 y, ERR/Gy = 0.043; >34 y, ERR/Gy = 0.030 | MBD | RR = 1 + (ERR/Gy × MBD × 1·608*) |
| Lung cancer5,6 | No excess risk for first 5 y after treatment. Thereafter: ERR/Gy, 0.15 (95% CI: 0.06-0.39) | MLD | RR=1+(ERR/Gy × MLD × 1·672*) |
| CHD7 | No excess risk for first 5 y after treatment. Thereafter, age at treatment: <28 y, ERR/Gy = 0.200 (95% CI: 0.052-0.070); 28-36 y, ERR/Gy = 0.088 (95% CI: 0.026-0.229); >36 y, ERR/Gy = 0.042 (95% CI: 0.006-0.111) | MHD | RR = 1 + ERR × MHD |
| Ischemic stroke8 | No excess risk for first 5 y after treatment. Thereafter, age at treatment: <21 y, ERR/Gy = 0.068 (95% CI: 0.015-0.156); 21-30 y, ERR/Gy = 0.051 (95% CI: 0.022-0.088); 31-40 y, ERR/Gy = 0.024 (95% CI: 0.005-0.051); >40 y, ERR/Gy = 0.0098 (95% CI: −0.007-0.032) | MDCCA | RR = 1 + ERR × MDCCA |
ERR/Gy, excess relative risk per Gray; MBD, mean breast dose to bilateral breast tissue; MDCCA, mean dose to the common carotid arteries; MHD, mean heart dose to the whole heart; MLD, mean lung dose to the whole lungs; RR, relative risk.
Adjustment factors to allow the use of mean organ dose for predictions (rather than point dose at site of second cancer development) with derivation provided in supplemental Material.
Inputs and outputs of the individual-patient level state-transition model. Outcomes in the model vary according to the simulated patient’s characteristics, namely age, sex, smoking status, and mean organ doses. Age and sex influence the incidence and case fatality rates of late effects as well as patient utility each cycle. Smoking status influences incidence rates. Mean organ doses, through dose-response equations, also influence the incidence rates of late effects.
Inputs and outputs of the individual-patient level state-transition model. Outcomes in the model vary according to the simulated patient’s characteristics, namely age, sex, smoking status, and mean organ doses. Age and sex influence the incidence and case fatality rates of late effects as well as patient utility each cycle. Smoking status influences incidence rates. Mean organ doses, through dose-response equations, also influence the incidence rates of late effects.
Calculating life years and QALYs
Total life years are calculated in the model as the number of cycles in which the patient remained alive. To calculate QALYs, life years were adjusted for age- and disease-related health-related quality-of-life effects using utility values sourced from the catalog of EuroQol-5D (EQ-5D) scores for the United Kingdom.17 For each cycle in which the patient is alive, a QALY is estimated based on the utility value associated with the patient’s age in that cycle, minus any decrements associated with the specific health states they are in. For the late effects, their respective decrements were applied to every cycle for which the patient is in the diseased state. For ESHL, the long-term effect of autologous stem cell transplantation (ASCT) was considered, with disutility permanently applied to every cycle lived after first relapse, even if cured. Mean utility values and standard errors are presented in supplemental Table 1. Total expected QALYs were taken as the sum of each cycle’s QALY.
Application of the model
The patient characteristics that influence model outcomes are age at treatment, sex, smoking status, and the set of representative OAR radiation doses, namely the MBD, MLD, MHD, and MDCCA. We applied the model to 90 patient presentations: a combination of 5 sets of OAR dose parameters, 3 ages, 3 smoking risk categories, and both sexes as follows.
Four of our example sets of OAR dose parameters were chosen to represent the clinical range of involved field RT (IFRT) in the modern era: (1) unilateral neck IFRT (seen in ∼35% of patients recruited to RAPID), (2) average IFRT doses for all patients with negative PET results in the RAPID cohort, (3) average IFRT doses for patients with negative PET results with mediastinal involvement in the RAPID cohort, and (4) maximal cardiovascular doses seen in patients requiring bilateral neck and whole mediastinal IFRT (<5% of patients recruited to RAPID). Finally, a fifth example corresponding to OAR doses previously seen with mantle RT was included to add historical perspective. For consistency, doses for all fields were calculated for a commonly used prescribed dose of 30 Gy. Doses for examples 1, 4, and 5 were calculated using the method published by Maraldo et al.18 Doses for examples 2 and 3 were constructed using cardiovascular doses from 144 patients in the RAPID trial, combined with the method by Maraldo et al.16,18 Full details of the derivation of dosimetry parameters are given in supplemental Methods. The MBD, MLD, MHD, and MDCCA doses from each example set are given in Table 3. Each of the 5 sets of OAR doses was run for 3 ages at treatment (20 years, 35 years, and 50 years), 3 smoking risk categories (never, former, and current smoker), and both female and male patients.
OAR doses for the five example sets
| . | Example mean OAR doses (Gy) . | ||||
|---|---|---|---|---|---|
| 1: Unilateral neck only (IFRT) . | 2: Average doses for all PET-negative RAPID patients . | 3: Average doses for PET-negative RAPID patients with mediastinal involvement . | 4: Bilateral neck and whole mediastinum (IFRT) . | 5: Mantle field RT (including bilateral axillae) . | |
| Mean breast dose (0 Gy for males) | 1.4 | 2.1 | 2.7 | 4.6 | 12.4 |
| Mean lung dose | 2.2 | 4.2 | 6.1 | 11.7 | 13.6 |
| Mean heart dose | 0.3 | 4.0 | 7.8 | 19.5 | 19.5 |
| Mean carotid dose | 13.8 | 21.5 | 28.3 | 30.0 | 30.0 |
| . | Example mean OAR doses (Gy) . | ||||
|---|---|---|---|---|---|
| 1: Unilateral neck only (IFRT) . | 2: Average doses for all PET-negative RAPID patients . | 3: Average doses for PET-negative RAPID patients with mediastinal involvement . | 4: Bilateral neck and whole mediastinum (IFRT) . | 5: Mantle field RT (including bilateral axillae) . | |
| Mean breast dose (0 Gy for males) | 1.4 | 2.1 | 2.7 | 4.6 | 12.4 |
| Mean lung dose | 2.2 | 4.2 | 6.1 | 11.7 | 13.6 |
| Mean heart dose | 0.3 | 4.0 | 7.8 | 19.5 | 19.5 |
| Mean carotid dose | 13.8 | 21.5 | 28.3 | 30.0 | 30.0 |
For each of the 90 patient combinations, we performed the analysis both with and without the addition of a discount rate applied to future health outcomes. Discounting future health outcomes is commonly performed in economic evaluations and reduces the weight given to later health outcomes when summing life years and QALYs across a lifespan; they would therefore be expected to reduce the impact of late toxicities. A 3.5% discount rate was used as recommended by the United Kingdom’s National Institute for Health and Care Excellence.
For each combination of inputs assessed, the patient’s life course was simulated 10 000 times before averaging to minimize Monte Carlo error. Known parameter uncertainty was included in our model to give not only a central estimate of mean difference but also a corresponding 95% CI reflecting joint known parameter uncertainty. For this, 1000 probabilistic iterations of the model were performed. In each iteration, the values of parameters with uncertainty estimates (the survival curves, most ERR/Gy estimates, and EQ-5D values) were sampled from their appropriately parameterized underlying parametric distributions.
Results
Smoking status had a significant effect on the choice of optimal treatment. This is highlighted in Figure 3, which shows the mean differences in expected life years and QALYs between CMT and chemotherapy-alone treatment for never smoker and current smoker example patients, stratified by their age at treatment, sex, OAR doses, and the discount rate. Full results for all 90 example patients are given in the supplemental material (supplemental Tables 5-16) as well as absolute results for chemotherapy-alone treatment (supplemental Table 4) and results had the incidence rate not been adjusted for smoking risk (supplemental Tables 17-21).
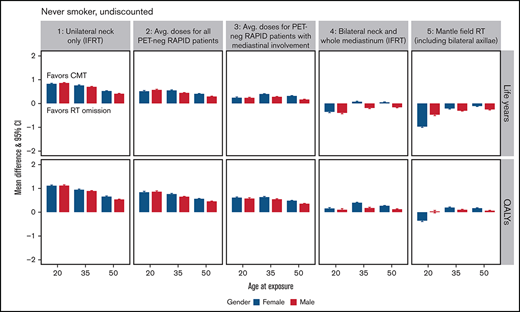
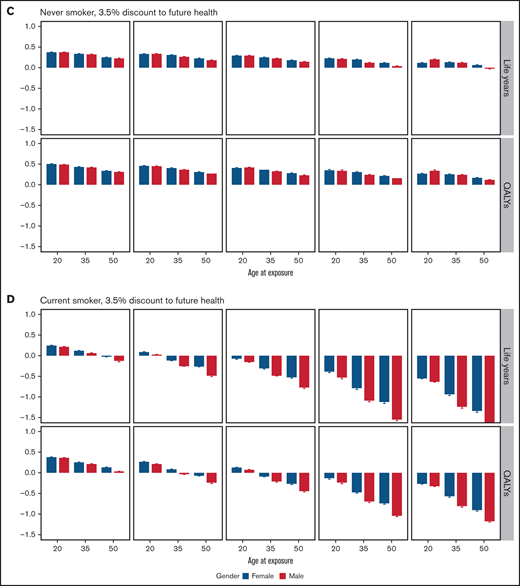
Mean difference in life years and QALYs and their 95% CI between combined modality treatment and chemotherapy-alone. Stratified results by outcome, age, sex, OAR doses, and future health discount factor for never-smokers (A and C) and current smokers (B and D). The y-axis is the estimated mean difference in the expected life years (or QALYs) if the patient were to receive CMT minus those expected if the patient received chemotherapy-alone. The OAR dose examples are as follows: (1) unilateral neck only (IFRT), (2) average doses for all PET-negative RAPID patients, (3) average doses for PET-negative RAPID patients with mediastinal involvement, (4) bilateral neck and whole mediastinum (IFRT), and (5) mantle field RT (including bilateral axillae).
Mean difference in life years and QALYs and their 95% CI between combined modality treatment and chemotherapy-alone. Stratified results by outcome, age, sex, OAR doses, and future health discount factor for never-smokers (A and C) and current smokers (B and D). The y-axis is the estimated mean difference in the expected life years (or QALYs) if the patient were to receive CMT minus those expected if the patient received chemotherapy-alone. The OAR dose examples are as follows: (1) unilateral neck only (IFRT), (2) average doses for all PET-negative RAPID patients, (3) average doses for PET-negative RAPID patients with mediastinal involvement, (4) bilateral neck and whole mediastinum (IFRT), and (5) mantle field RT (including bilateral axillae).
In never smokers, CMT produced more QALYs than chemotherapy-alone treatment for all 4 modern OAR dose strata, regardless of age at treatment, sex, and the discount rate applied to future health. A 20-year-old female patient with unilateral neck involvement only, for instance, would expect to gain 1.095 (95% CI: 1.054-1.137) undiscounted QALYs with the addition of consolidative RT. With increased MBD, MLD, and MHD doses due to mediastinal involvement, the benefit of CMT lessens but remains optimal. For instance, a 20-year-old female patient receiving IFRT to the bilateral neck and whole mediastinum would expect to gain only 0.139 (95% CI: 0.088-0.190) undiscounted QALYs.
The optimal treatment choice for former smokers depended on OAR dose, age, and whether a discount rate was applied to future health (supplemental Figure 5). With the application of the 3.5% discount rate, chemotherapy-alone treatment was only beneficial in terms of QALYs to older patients in the highest modern dose stratum. In current smokers, chemotherapy-alone treatment produced greater undiscounted life years and QALYs at average doses for RAPID patients with negative PET results, regardless of age and sex. With discounting, CMT remained optimal in the 20-year age strata. In the highest OAR dose example, a substantial number of QALYs would be lost by current smokers were they to be treated with consolidative RT. For instance, a 20-year-old man would be estimated to lose 3.500 (95% CI: 3.400-3.600) undiscounted QALYs.
Discussion
Simulation modeling makes it possible to estimate the long-term outcomes of omitting consolidative RT for the treatment of ESHL in the absence of decades of clinical follow-up.13 Such outcomes must be interpretable by both patients and clinicians, requiring the translation of individual risks from multiple diseases into a unified estimate of their overall impact, using a metric such as overall survival or quality-adjusted survival.
Ours is the first model to evaluate this treatment choice using not only individual patient characteristics such as age, sex, and smoking status but, importantly, representative RT dosimetry based on 144 patients treated in a clinical trial.16 Crucially, the model estimates the excess risks of late effects based on published dose-response relationships obtained from large cohorts of patients treated for HL. Another key strength of our model is the use of parameter inputs that are informed throughout by real data rather than assumptions or expert opinion. To our knowledge, only 1 other study has performed a model-based comparison of QALYs in this setting. This exploratory study, however, did not explicitly model specific late effects or base the probability of experiencing any late effect on radiation dosimetry and dose-response relationships.19
Our results highlight, firstly, the excellent outcomes for most patients with stage I/IIa disease regardless of treatment decision. For instance, a 20-year-old never-smoking female patient would be expected to live into her early 80s under both choices, even with considerable modern irradiation of OARs. Patients and clinicians should, therefore, be reassured by the high probability of a normal life expectancy.
Secondly, our results highlight the importance of considering a patient’s underlying health, OAR radiation doses, and time preference for health when deciding treatment choice. In our model, we accounted for a patient’s smoking status due to its well-characterized and significant effect on the incidence of lung cancer, CHD, and stroke. Comparing the results from the smoking-stratified analysis with those from the unadjusted analysis (supplemental Figure 6) demonstrates the importance of doing so because the optimal treatment choice differs greatly by smoking status. Other underlying factors that are harder to incorporate into a model, such as obesity and comorbid conditions, could also have a considerable impact on optimal treatment choice and potentially favor chemotherapy-alone treatment. Nonetheless, our results for the never-smoking patient examples suggest that CMT provides more benefit over chemotherapy-alone treatment in patients, regardless of age, sex, and OAR doses, with otherwise good underlying health.
Our graphic results also help highlight that, according to our model, CMT is comparatively more favorable the younger the patient. This finding contradicts the commonly held maxim that young patients, especially female patients, should be spared radiation. Despite younger patients having higher ERR/Gy estimates and a longer period of excess risk, due to the negligible yearly incidence rates of lung cancer, CHD, and ischemic stroke before the age of 65, there is only a small excess incidence from RT before this age. This contrasts to a high morbidity burden for young patients if they were to relapse after treatment. Additionally, we show smaller differences in outcomes between the sexes. This is due to the size and distribution of age-specific year rates for the 4 late side-effects. Although men do not share the excess risk of breast cancer, below the age of 70 years their baseline incidence and case-fatality rates for lung cancer, CHD, and ischemic stroke are higher than those of women of the same age.
The impact on results of discounting future health outcomes is also significant, greatly improving the favorability of CMT. Having a positive time preference, which has the effect of discounting future health, is a common human behavior often observed in discrete choice experiments and corresponds with surveys in HL survivors, indicating that for many patients, primary cure is of most importance.20,21 Therefore, when considering omission of RT, for instance in persons with adverse underlying health factors, this time preference with respect to health should also be considered by the clinician. Smokers, for instance, evidenced perhaps by their decision to smoke, may exhibit a strong preference toward current health.
In sum, our comprehensive set of outcomes suggests that only in patients with significant baseline risks of late effects could the radiation-related excess risks be large enough to warrant RT omission. It should be strongly emphasized that in the RAPID trial, on which our estimates of first relapse risk and OAR doses are based, <5% of patients in the trial received OAR doses close to our maximal bilateral neck and whole mediastinum example. Consequently, the proportion of patients with stage I/IIa disease who would benefit from omission of RT under our preferred quality- and discount-adjusted analysis is likely small. Furthermore, RT techniques have advanced since the RAPID trial with the introduction of the involved site concept and breath-hold techniques.18,22 The result is that, for contemporary cohorts, the radiation doses to OARs are substantially lower than those examined here.23
There are, of course, many caveats to our modeling approach and the assumptions made. The use of population-derived incidence and case-fatality rates assumes equivalence between radiation-induced disease and general population disease. New primary cancers, CHD, and ischemic stroke in ESHL survivors may be detected at an earlier stage due to survivor screening programs and increased self-monitoring. For secondary primary cancers, earlier diagnosis could lower the case-fatality rate, whereas for CHD and ischemic stroke, preventative medication might slow disease progression and reduce the incidence rate of acute events. Therefore, our model may overestimate the quality-of-life burden and mortality from late effects, making our comparative results less favorable for CMT. However, it is also important to note that the biology of radiation-related late effects may be different from sporadic forms of the same disease, and treatment may be made more difficult due to the previous treatments.
Although population-based age- and sex-specific incidence of disease may be similar among developed countries, we caution that our results are established on UK-based population statistics although the method could be applied in other countries using their own baseline disease rates. The adjustment of population-based incidence rates by 3 smoking categories is a simplification of nuanced smoking behaviors and risk. Exact risks for smokers will be closely related to the quantity and duration of their consumption, which is not possible to accurately model. Nevertheless, we thought it important to demonstrate the impact smoking has on a person’s lifetime risk of late effects using the best available data.
For clarity, our model is limited to accounting for the increased risk of breast and lung cancer, CHD, and ischemic stroke. However, irradiation increases the risk of a number of other rarer second malignancies, such as esophageal and thyroid cancer, as well as other cardiovascular events, such as valvular heart disease and heart failure.24,25 We also assume no increased risk of mortality from the use of ABVD chemotherapy, although evidence does suggest a risk of excess heart failure associated with anthracycline use.25 These may inflate our absolute estimates of life years and QALYs but would likely have modest effect on the mean difference.
The true relationships between the dose and volume of radiation received by a patient during RT and their subsequent risk of specific late effects are not yet known with certainty and can only be modeled based on the best currently available data. In this study, we chose to use published dose-response models of the ERR/Gy generated from epidemiological studies involving HL survivors, with mean organ dose acting as the summary metric of radiation dose. Alternative approaches include the use of estimates derived from the follow-up of historic low-dose radiation events such as the Life Span Study Atomic Bomb Survivor Cohort, radiobiological models, and hybrid models.26-28 We took this approach because the ERR/Gy estimates have shown robustness even when derived from survivor cohorts of other primary cancers, and it is widely accepted that with very few exceptions, dose-response relationships for cancer induction in the treatment range are linear with no threshold.29,30 Under this relationship, mean organ dose is an adequate measure for prediction because it does not vary according to the distribution of radiation dose. Nonetheless, the transferability of these dose-response models, derived from cohorts treated with historic RT techniques, to make predictions for contemporary RT cannot be tested directly due to the latency of late effects. Contemporary RT techniques such as intensity-modulated RT offer greater conformity, typically reducing the amount of normal tissue exposed to high radiation doses but can increase the volume of normal tissue receiving low doses.31 There have been theoretical concerns that the “low dose bath” from IMRT may increase risk more than 3-dimensional conformal RT. These predictions, however, strongly depend on the radiobiological models chosen, and observational data from clinical series have not thus far demonstrated any increase in risk with IMRT in adult patients.32 We lastly acknowledge that organs are not homogenous entities, with radiation dose to different tissues and substructures potentially being more critical for the pathogenesis of specific late effects. For instance, similar to breast cancer, the best data currently available for Hodgkin lymphoma suggest that the risk of CHD is linear with no threshold when related to mean heart dose.7,29 However, there are recent suggestions from other thoracic cancers, such as lung cancer, that predictions may be improved in future using additional metrics, such as coronary artery doses.33
Finally, in our ESHL sub-module, we have made the assumption that all relapsed patients would be eligible and undergo ASCT and that after such salvage therapy, the non–relapse-related subsequent all-cause mortality rate for the individual is equal to that of the general population. For the latter, we found no estimates for the standardized mortality rate after stem cell transplant that excluded deaths attributable to subsequent relapse or first line RT. However, studies suggest ASCT recipients have standardized mortality rates reaching that of the population after 10 years of event-free survival.34
We reemphasize that our results relate to stage I/IIa ESHL with no mediastinal bulk as per the RAPID inclusion criteria. The RAPID trial was chosen because we had representative dosimetry for OARs for this trial, it was based in the United Kingdom, and, significantly and in contrast to the other two trials, the authors concluded that omission of RT may be an acceptable treatment option in patients who have negative PET results after initial chemotherapy.
Similar PET response-adaptive omission of RT was assessed in the GHSG HD16 and EORTC/LYSA/FIL H10 trials, albeit with slightly different treatments, patient characteristics, and PET-negativity definitions. The GHSG HD16 trial assessed 2 cycles of ABVD chemotherapy with or without 20-Gy IFRT in PET-negative favorable (GHSG criteria) ESHL. The difference in 5-year progression-free survival (PFS) rates, which were 93.4% and 86.1%, respectively, was similar to our 5-year extrapolated PFS estimates for the RAPID trial. Additionally, with the use of 20-Gy IFRT as opposed to 30-Gy IFRT, radiation-related risks would be expected to be reduced by one-third. In H10, favorable (EORTC criteria) ESHL patients received 3 cycles of ABVD chemotherapy and 30-Gy involved-node radiotherapy in the control arm and 4 cycles of chemotherapy-alone in the PET-negative experimental arm. The difference in 5-year PFS rates, which were 99.0% and 87.1%, respectively, were greater than our 5-year extrapolated PFS estimate for the RAPID trial. Given the better survival rates and lower radiation doses administered compared with RAPID, we surmise that including HD16 and H10 trial-favorable patient data in our model would confirm and possibly increase the number of patients for whom CMT would be preferable.
The role of consolidative RT in the H10 unfavorable (EORTC criteria) group is less clear due to smaller differences in PFS. Additionally, the role of RT has been challenged by the results of the RATHL trial35 in advanced HL, which included stage IIA patients with adverse features, and recent findings of noninferiority of chemotherapy-alone with 2 + 2 intensified treatment in GHSG HD14.36
Within classifications of disease stage, variations in disease and patient factors still could influence relapse risk. For instance, in post hoc analysis of the RAPID trial, baseline maximum tumor diameter (MTD) was prognostic of subsequent relapse in patients who had achieved complete metabolic response.37 Notably, there was an approximate 19% increase in HL relapse risk per centimeter increase in MTD (P = .02). For patients with MTD of ≥5 cm, 5-year EFS in those assigned to chemotherapy-alone was 79.3% compared with 94.9% in those assigned to CMT (P = .03). This result may indicate that the benefit of consolidative RT varies with MTD. These results are based on a small number of events in the CMT group; as such, we did not incorporate MTD into our model. However, such factors could be incorporated in future, when additional supporting data become available, and may impact optimal treatment choice. For instance, were we to incorporate MTD into our model based on the above findings,37 the difference in QALYs gained from CMT for never smokers with smaller MTDs would diminish or even favor chemotherapy-alone treatment. Conversely, the benefit of CMT for never smokers with larger MTDs would increase. This highlights the need for identification of robust baseline prognostic biomarkers, which would allow for even greater personalization of predicted outcomes. Our study results demonstrate the utility of individualized modeling to inform optimal treatment decision-making in ESHL. Our model may be readily extended to compare outcomes from the other PET response-adaptive RT omission trials to incorporate prognostic biomarkers or to the context of identifying patients who might benefit most from advanced organ-sparing RT techniques, such as deep inspiration breath-hold and proton beam therapy.22,38,39
Acknowledgments
Funding support for this article was provided by the Cancer Research UK Centres Network Accelerator Award Grant to the ART-NET consortium and a Cancer Research UK program grant (A21993, C8225/A21133). A.M.G. is partly supported by the NIHR Oxford Biomedical Research Centre. R.S. is supported by a National Institutes of Health Research Doctoral Studentship (NIHR300740).
Authorship
Contribution: D.A.J., A.M.G., R.S., and D.J.C. designed the research; all authors contributed to the methodology of the research; D.A.J. and A.M.G. performed the formal analysis; and all authors contributed to the writing of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: David A. Jones, Health Economics Research Centre, Nuffield Department of Population Health, University of Oxford, Richard Doll Building, Old Road Campus, Headington, Roosevelt Dr, Oxford OX3 7LF, United Kingdom; e-mail: david.jones@dph.ox.ac.uk.
References
Author notes
A.M.G. and D.J.C. are joint senior authors.
Model input parameters derived from public domain resources have been referenced, and all other parameters are given in the manuscript and supplemental Material.
The full-text version of this article contains a data supplement.