Key Points
SPAG6 is associated with AML progression and prognosis.
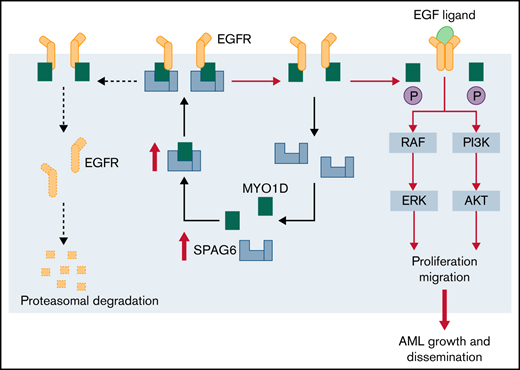
MYO1D is the regulatory node that connects cell survival with SPAG6.
Abstract
Chromosomal aberrations and gene mutations have been considered to be the major reasons for high recurrence rates and poor survival among acute myeloid leukemia (AML) patients. However, the underlying molecular mechanism of AML gene mutation remains largely unclear. Here, we show that SPAG6 (sperm-associated antigen 6), one of the most markedly increased SPAG genes in AML, significantly contributed to the proliferation and migration of leukemic cells. SPAG6 was highly expressed in AML, and its upregulation was negatively correlated with the prognosis of the disease. In vitro, SPAG6 promoted the proliferation and migration of leukemia cells and promoted cell cycle progression from the G1 phase to the S phase. In vivo, low expression of SPAG6 reduced the proliferation and infiltration of leukemia cells and prolonged the survival of xenograft tumor mice. Furthermore, immunoprecipitation and mass spectrometry analysis showed that SPAG6 interacts with MYO1D (myosin 1D). Specifically, overexpression of SPAG6 promoted the translocation of MYO1D into the cell membrane, thus upgrading the expression level of the EGFR family and thereby promoting the progression of AML. Overall, our study found that SPAG6 combined with MYO1D and translocated MYO1D from the cytosol to the cytomembrane, which induced the PI3K (phosphoinositide 3-kinase)/AKT (protein kinase B) signaling and ERK (extracellular signal-regulated kinase) signaling pathway to regulate the growth and prognosis of AML. SPAG6 may become a new target gene for the treatment of AML.
Introduction
Acute myeloid leukemia (AML) is the most common type of acute leukemia in adults, and its incidence increases with age. Although patient outcomes have improved greatly in the past 20 years, more than two-thirds of patients eventually face relapse and death.1-3 Hematopoietic stem cell transplantation can cure AML, but early identification of high-risk patients suitable for transplantation remains difficult. Currently, an increasing number of chromosomal aberrations and point mutations have been found in AML and are considered to be the initiators of AML and have important prognostic implications.4,5 In addition, changes in gene expression lead to leukemogenesis", indicating that it may become a therapeutic target for leukemia.6,7 However, there are still many unknown genes playing important roles in disease progression in AML patients. Therefore, there is an urgent need to identify more novel prognostic biomarkers and therapeutic targets to improve the risk assessment of adult AML and understand their role in leukemia to improve treatment options and benefit patients.
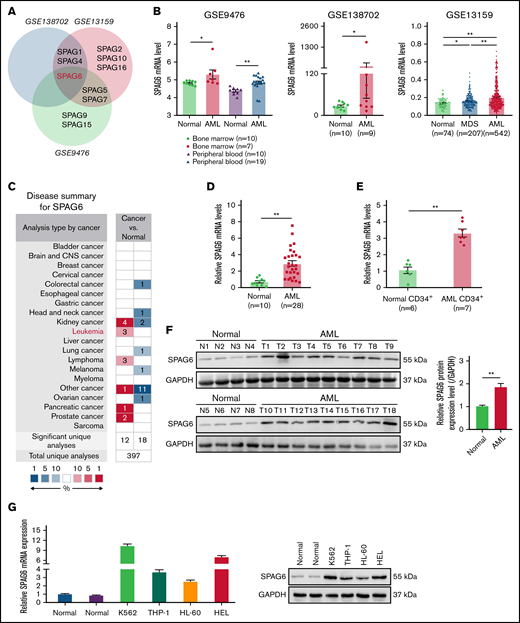
The SPAG (sperm-associated antigen) family has expanded to more than 14 members in mammals and performs specialized functions in many important biological processes.8-10 Dysregulation of SPAG genes (SPAGs) has a profound impact on human diseases, including cancer.11,12 However, the expression profile of SPAGs remains unknown in human AML. Therefore, we screened for the SPAG gene family expression signature in 3 independent datasets of AML patients and found that SPAG6 was the most notably upregulated SPAG gene in AML patients compared with normal samples (Figure 1A; supplemental Figure 1).
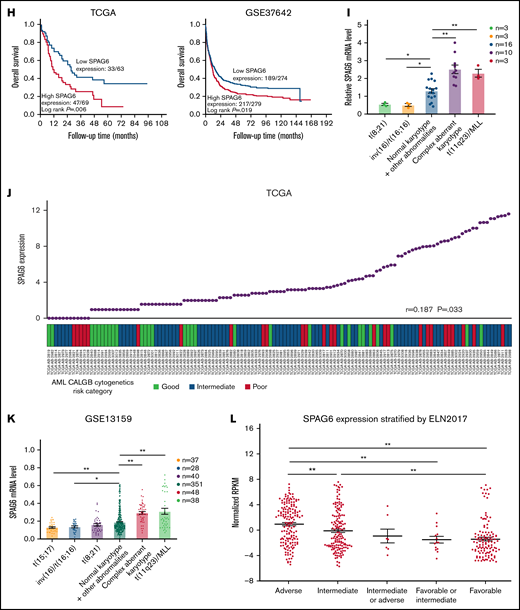
Upregulation of SPAG6 is a frequent event in AML patients and indicates a poor prognosis in human AML. (A) The public data on AML were obtained from GEO (Gene Expression Omnibus) databases, and the mRNA expression levels of SPAG6 in AML and control samples were statistically analyzed. (B) The mRNA level of SPAG6 in 3 independent datasets GSE9476, GSE138702, and GSE13159. (C) SPAG6 expression in tumor and normal tissues was analyzed on the website https://www.oncomine.org/. (D) Real-time polymerase chain reaction analysis of SPAG6 mRNA expression in AML patients (n = 28) and control samples (n = 10). (E) SPAG6 expression in CD34+ leukemia stem cells from AML patients (n = 7) and control samples (n = 6). (F) Western blot analysis of SPAG6 protein expression in AML patients (n = 18) and control samples (n = 8). (G) The mRNA and protein level of SPAG6 in normal hematopoietic cells and K562, THP-1, HL-60, and HEL cells. (H) Overall survival analyses between patients with high or low SPAG6 expression in TCGA and GSE37642 dataset. (I) SPAG6 expression from AML patients (n = 35) with various cytogenetic aberrations. (J) The correlation between SPAG6 expression and AML CALGB cytogenetics risk category in TCGA database. (K) SPAG6 expression from AML patients with various cytogenetic aberrations (GSE13159). (L) SPAG6 expression from AML patients with different risk status in Vizome-ELN2017. *P < .05; **P < .01. LCSs, leukemia stem cells.
Upregulation of SPAG6 is a frequent event in AML patients and indicates a poor prognosis in human AML. (A) The public data on AML were obtained from GEO (Gene Expression Omnibus) databases, and the mRNA expression levels of SPAG6 in AML and control samples were statistically analyzed. (B) The mRNA level of SPAG6 in 3 independent datasets GSE9476, GSE138702, and GSE13159. (C) SPAG6 expression in tumor and normal tissues was analyzed on the website https://www.oncomine.org/. (D) Real-time polymerase chain reaction analysis of SPAG6 mRNA expression in AML patients (n = 28) and control samples (n = 10). (E) SPAG6 expression in CD34+ leukemia stem cells from AML patients (n = 7) and control samples (n = 6). (F) Western blot analysis of SPAG6 protein expression in AML patients (n = 18) and control samples (n = 8). (G) The mRNA and protein level of SPAG6 in normal hematopoietic cells and K562, THP-1, HL-60, and HEL cells. (H) Overall survival analyses between patients with high or low SPAG6 expression in TCGA and GSE37642 dataset. (I) SPAG6 expression from AML patients (n = 35) with various cytogenetic aberrations. (J) The correlation between SPAG6 expression and AML CALGB cytogenetics risk category in TCGA database. (K) SPAG6 expression from AML patients with various cytogenetic aberrations (GSE13159). (L) SPAG6 expression from AML patients with different risk status in Vizome-ELN2017. *P < .05; **P < .01. LCSs, leukemia stem cells.
The SPAG6 gene is a brand new human sperm antigen that was screened by Neilson and colleagues in sterile male serum containing a high titer antisperm antibody through a testicular expression library in 1999.13 SPAG6 acts as an oncogene or tumor suppressor in human cancers.14-17 Our previous studies have demonstrated that SPAG6 is upregulated in patients with myelodysplastic syndromes (MDS) and MDS-transformed AML. SPAG6 knockdown promoted apoptosis and differentiation while suppressing proliferation and autophagy in SKM-1 (human myelodysplastic syndrome cells) cells.18-20 Zhang and colleagues reported that SPAG6 promotes cell proliferation and inhibits apoptosis through the PTEN/PI3K/AKT pathway in Burkitt lymphoma.21 On the other hand, SPAG6 and L1TD1 (LINE1 type transposase domain containing 1) are tumor-specifically methylated in nonsmall-cell lung cancer, thereby suppressing tumor-cell growth in nonsmall-cell lung cancer cells.22 The above results indicate that the role of SPAG6, serving as an oncogene or tumor suppressor gene, in different tumor types is ambiguous, and these contradictory phenomena need further investigation. In addition, the specific role and mechanism of SPAG6 in AML remain unclear.
In this study, we found that SPAG6 was significantly upregulated in AML and that its upregulation predicted poor patient survival. SPAG6 interacts with MYO1D and promotes the translocation of MYO1D to the cell membrane. SPAG6 activates the PI3K (phosphoinositide 3-kinase)/AKT (protein kinase B) signaling and ERK (extracellular signal-regulated kinase) signaling pathways by MYO1D (myosin 1D)-mediated upregulation of the EGFR (epidermal growth factor receptor) family. SPAG6 promotes AML proliferation and thus may be a promising biomarker and therapeutic target for the treatment of AML.
Methods and materials
Patient samples and CD34+ cell separation
Bone marrow mononuclear cells (BMMNCs) were collected from newly diagnosed patients with AML (n = 35) and iron deficiency anemia (n = 16) at the Hematology Department of the First Affiliated Hospital of Chongqing Medical University between 2019 and 2022. The demographic information and clinical data of each patient were collected by clinical specialists and are summarized in supplemental Table 1. The BMMNCs were extracted using Ficoll density gradient centrifugation. CD34+ cells of AML patients and control samples were purified using the EasySep Human CD34 Positive Selection Kit II (Stemcell Technologies, Canada) according to the manufacturer’s instructions. All patients signed informed consent forms, and the study was approved by the Ethics Committee of the First Affiliated Hospital of Chongqing Medical University (2021-491).
Cell lines and cell culture
The human leukemia cell lines K562, HEL (human erythroleukemia), THP-1, and HL-60 were purchased from the cell bank of the typical Culture Preservation Committee of the Chinese Academy of Sciences; K562 cells were cultured in Iscove's Modified Dulbecco's Medium (IMDM) containing 10% fetal bovine serum (FBS); HEL and THP-1 cells were cultured in RPMI 1640 containing 10% FBS; and HL-60 cells were cultured in IMDM containing 20% FBS. BM blasts from AML patients and control samples were cultured in IMDM containing 20% FBS, 100 ng/mL stem cell factor, 100 ng/mL FLT3 ligand, 100 ng/mL TPO, 10 ng/mL interleukin-3 (IL-3) and 10 ng/mL IL-6 (PeproTech). All the cells were cultured at 37°C in a humidity incubator under 5% CO2.
LC–MS/MS (liquid chromatography-tandem mass spectrometry) analysis for SPAG6-interacting proteins
To find proteins interacting with SPAG6, immunoprecipitated proteins from K562 cells were used for gel electrophoresis, and then Coomassie Brilliant Blue staining was performed. The differential gel strips between the SPAG6 and IgG groups were cut off and sent to Beijing Genomics Institute (BGI; Shenzhen, China) for LC–MS/MS analysis, while immunoprecipitated protein lysates from K562 cells were also sent to the BGI for LC–MS/MS analysis.
Animal model
All animal experiments were approved by the Committee of Animal Use and Care of Chongqing Medical University. All experiments complied with the guidelines for the care and use of laboratory animals published by the National Institutes of Health of the United States and were supervised by the Animal Care and Use Committee of Chongqing Medical University in accordance with the approved procedures. Male NOD/SCID (nonobese diabetic/severe combined immune-deficient) mice (3-weeks) were purchased from Chongqing Tengxin Biotechnology Co., Ltd., and raised under specific pathogen free grade conditions. Mice were randomly divided into 2 groups, and their tail veins were injected with cells treated as indicated (5 × 106 per mouse). The mice were sacrificed after 21 days, and the spleen weight was measured. Peripheral blood(PB) was collected in Ethylene Diamine Tetraacetic Acid anticoagulant tubes, and white blood cells were counted by a blood cell analyzer. Mouse femurs, spleens, livers, and kidneys were collected and soaked in 4% paraformaldehyde, and femurs were slowly decalcified. Tissues were embedded in paraffin, sectioned (thickness, 4 μm), dewaxed, stained with hematoxylin and eosin, and immunohistochemically stained with anti-CD45.
Statistical analysis
All data were analyzed using GraphPad Prism (Version 8.0.1) software. All data were expressed as the mean ± standard error of the mean. An unpaired t test was used for comparisons between groups. The survival analysis of NOD/SCID mice was performed by the log-rank test. P < .05 was considered as statistically significant (*), while a P < .01 was considered as extremely significant (**).
Results
SPAG6 is overexpressed in AML patients and cell lines
To determine the role of SPAG family genes in AML, we first screened the expression profile of SPAG genes (SPAG1, SPAG2, SPAG4, SPAG5, SPAG6, SPAG7, SPAG8, SPAG9, SPAG10, SPAG11, SPAG12, SPAG15, SPAG16, and SPAG17) in AML patients compared with that of normal samples from 3 public datasets and found that SPAG6 exhibited a significant upregulation in AML patients (Figure 1A; supplemental Figure 1). Compared with normal control samples, the expression of SPAG6 in BM and PB of AML patients was significantly increased. Meanwhile, the expression of SPAG6 was different between AML and MDS patients from the GSE138702, GSE13159, and GSE9476 datasets (Figure 1B). Moreover, differences were observed in messenger RNA (mRNA) expression with both SPAG6 probes individually (210032-s_at and 210033-s_at) from GSE13159. Significantly higher expression of SPAG6 was observed in AML (supplemental Figure 2A). We found that SPAG6 expression was significantly increased in renal tumors, leukemia, and lymphoma compared with their corresponding normal tissues (Figure 1C), and the expression of SPAG6 was the highest in leukemia cells among different cancer cell lines (supplemental Figure 2B). To validate this result, we assessed the expression level of SPAG6 in 28 AML patients and 10 normal samples. Real-time polymerase chain reaction (RT-qPCR) showed that SPAG6 was significantly upregulated in AML at the mRNA level compared with normal BM (Figure 1D). We further isolated CD34+ leukemia stem cells from 7 AML patients and 6 normal samples. RT-qPCR showed that SPAG6 was elevated in CD34+ AML cells compared with normal samples (Figure 1E). However, the AML RNA sequencing data (Vizome-ELN2017) showed that there was no significant difference in the expression of SPAG6 between AML and healthy-pooled CD34+ cells (supplemental Figure 2C). We also found that the mRNA expression of SPAG6 in CD34+ cells was higher than that in CD34− cells from AML patients (supplemental Figure 2D).
We randomly selected 18 out of 28 AML patients and 8 out of 10 normal control samples to extract protein for Western blot analyses. Consistently, SPAG6 expression was elevated in AML compared with normal control samples (Figure 1F). RT-qPCR and Western blot analyses showed that the expression of SPAG6 was significantly higher in 4 AML cell lines than in the 2 normal BMMNCs, and the data also revealed that SPAG6 was strongly expressed in K562 and HEL cells and weakly expressed in THP-1 and HL-60 cells (Figure 1G), which was consistent with the expression of SPAG6 in different leukemia cell lines from the TCGA (The Cancer Genome Atlas) database (supplemental Figure 2E). Meanwhile, the expression of SPAG6 in patients with different French–American–British morphology types was not significantly different (supplemental Figure 2F). Moreover, Kaplan-Meier analysis revealed that AML patients with high SPAG6 expression had significantly poorer overall survival than those with low SPAG6 expression (Figure 1H). Among the cytogenetics of AML patients, SPAG6 was most highly expressed in the complex aberrant karyotype and t(11q23)/mixed lineage leukemia groups, which was consistent with the analysis results from the GEO(Gene Expression Omnibus) database (Figure 1I,K; supplemental Figure 2G). In addition, we found that high expression of SPAG6 was associated with a high risk rating in the AML Cancer and Acute Leukemia Group B cytogenetics risk category (r = 0.187; P = .033) (Figure 1J). Likewise, SPAG6 expression was highest in the adverse group according to risk status from Vizome-ELN2017 (Figure 1L). Based on the aforementioned results, we found that SPAG6 expression was elevated in AML patients and related cell lines, and high SPAG6 expression was associated with poor prognostic factors.
SPAG6 promotes proliferation and regulates the cell cycle in AML cells
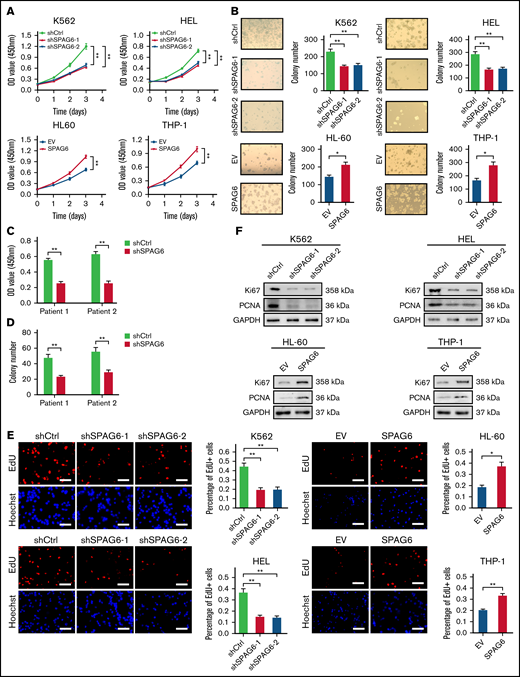
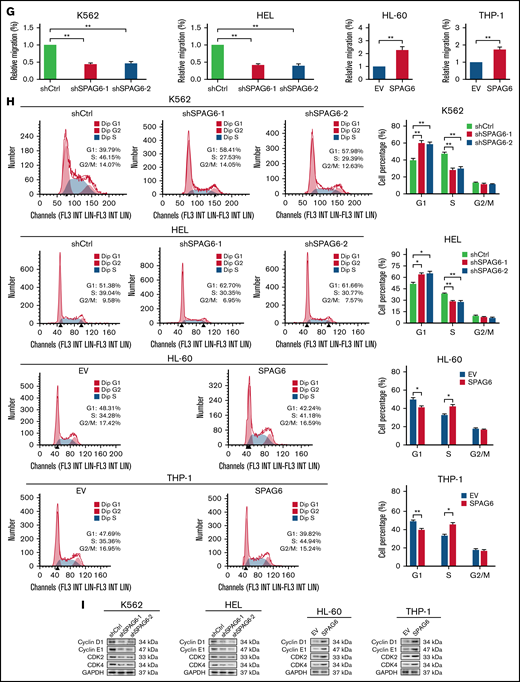
Considering that the high expression of SPAG6 was negatively correlated with the prognosis of AML patients, we investigated whether SPAG6 affected AML cell proliferation, migration, apoptosis, and differentiation. We established stable SPAG6 knockdown K562 and HEL cells and stable SPAG6 overexpression THP-1 and HL-60 cells (supplemental Figure 3A-B). Cell Counting Kit 8 (CCK-8) and methylcellulose-based colony formation assays confirmed that the cells with high expression of SPAG6 had strong growth ability (Figure 2A-B). Furthermore, we selected a patient with favorable risk (Patient 1) and a patient with poor risk (Patient 2) for SPAG6 knockdown and verified the effect of SPAG6 knockdown (supplemental Figure 3C). Similar to the observation in K562 and HEL cells, SPAG6 knockdown in CD34+ cells from AML patients significantly weakened proliferation and decreased colony formation (Figure 2C-D). The EdU (5-ethynyl-2’-deoxyuridine) assay verified that knockdown of SPAG6 resulted in a significant decrease in proliferation ability, while forced expression of SPAG6 in THP-1 and HL-60 cells exhibited the opposite effect (Figure 2E). As expected, the levels of the cell proliferative markers Ki67 and proliferating cell nuclear antigen were substantially decreased upon SPAG6 knockdown in K562 and HEL cells, but overexpression of SPAG6 markedly increased these protein levels in THP-1 and HL-60 cells (Figure 2F). The knockdown of SPAG6 impaired the migration ability of K562 and HEL cells, while the upregulation of SPAG6 increased the migratory ability of THP-1 and HL-60 cells (Figure 2G).
SPAG6 promoted proliferation and G1 to S phase transition in leukemic cells. (A-B,E). K562 and HEL cells were transduced with lentiviral particles expressing different shRNA clones directed against SPAG6 (shSPAG6-1 and shSPAG6-2). The cell lines HL-60 and THP-1 were transduced with lentiviral particles expressing SPAG6 or EV. The cell proliferative capacities were detected by CCK-8, colony formation, and EdU assays in K562, HEL, HL-60, and THP-1 cells with treatment as indicated. (C-D) Proliferation and colony formation of CD34+ cells from a patient with favorable risk (Patient 1) and a patient with poor risk (Patient 2) when SPAG6 was knocked down. (F) Western blot detection for expression levels of Ki67 and PCNA. (G) AML cell migration was determined by Transwell assay. (H) Flow cytometry analysis showing cell cycle distribution in K562, HEL, THP-1, and HL-60 cells with treatment as indicated. (I) Western blot analysis showing the expression levels of cyclin D1, cyclin E1, CDK2, and CDK4 in AML cells with treatment as indicated. Scale bar, 50 μm. *P < .05; **P < .01. PCNA, proliferating cell nuclear antigen.
SPAG6 promoted proliferation and G1 to S phase transition in leukemic cells. (A-B,E). K562 and HEL cells were transduced with lentiviral particles expressing different shRNA clones directed against SPAG6 (shSPAG6-1 and shSPAG6-2). The cell lines HL-60 and THP-1 were transduced with lentiviral particles expressing SPAG6 or EV. The cell proliferative capacities were detected by CCK-8, colony formation, and EdU assays in K562, HEL, HL-60, and THP-1 cells with treatment as indicated. (C-D) Proliferation and colony formation of CD34+ cells from a patient with favorable risk (Patient 1) and a patient with poor risk (Patient 2) when SPAG6 was knocked down. (F) Western blot detection for expression levels of Ki67 and PCNA. (G) AML cell migration was determined by Transwell assay. (H) Flow cytometry analysis showing cell cycle distribution in K562, HEL, THP-1, and HL-60 cells with treatment as indicated. (I) Western blot analysis showing the expression levels of cyclin D1, cyclin E1, CDK2, and CDK4 in AML cells with treatment as indicated. Scale bar, 50 μm. *P < .05; **P < .01. PCNA, proliferating cell nuclear antigen.
Next, we examined the apoptosis rate and the expression of CD11b and CD14 by flow cytometry. As shown in supplemental Figure 4A, in K562 and HEL cells with SPAG6 knockdown, the apoptosis rate of the cells was only marginally affected, but the difference was not statistically significant. Similarly, after overexpression of SPAG6 in HL-60 and THP-1 cells, the apoptosis rate was slightly lower than that in the control group, but the difference was still not statistically significant. In the myeloid differentiation of AML cells, the results showed that with knockdown of SPAG6 in K562 and HEL cells, there were no significant changes in the expression of CD11b and CD14 on the cell surface compared with the control group (supplemental Figure 4B).
Although K562 and HEL cells are often used in AML research, HL-60 and THP-1 cells are more representative of AML disease in terms of cellular origin. We knocked down SPAG6 in HL-60 and THP-1 cells to analyze the effect of SPAG6 on AML cell growth. CCK-8 and colony-formation assays also confirmed that HL-60 and THP-1 cells with SPAG6 knockdown had a much slower growth rate than control cells (supplemental Figure 5B-C). Furthermore, the EdU assay verified that SPAG6 knockdown resulted in a significant decrease in proliferation ability (supplemental Figure 5D). Similarly, SPAG6 knockdown decreased the proliferation and colony formation of CD34+ cells from normal human cells (supplemental Figure 5E-F).
Considering that uncontrolled cell proliferation could be caused by dysregulation of the cell-cycle machinery, we evaluated the effect of SPAG6 on cell cycle progression in AML cells. Flow cytometry showed that SPAG6 knockdown arrested the cell cycle in the G1 phase, together with a significant decrease in cells in the S phase in K562 and HEL cells, while overexpression of SPAG6 in THP-1 and HL-60 cells promoted G1 to the S cell-cycle progression (Figure 2H). In addition, we observed that HL-60 and THP-1 cells with SPAG6 knockdown exhibited a significant accumulation in the G1 phase and a remarkable decrease in the S phases (supplemental Figure 5G). Consistent with the results of flow cytometry, Western blot detection of the expression of cell cycle-related proteins indicated that SPAG6 knockdown decreased the expression levels of cyclin D1, cyclin E1, CDK2, and CDK4 in K562 and HEL cells, while SPAG6 overexpression increased these protein levels in THP-1 and HL-60 cells (Figure 2I). Collectively, the above results suggest that SPAG6 promotes G1/S transition and thus cell proliferation in AML cells.
Knockdown of SPAG6 inhibits the growth and dissemination of AML in vivo
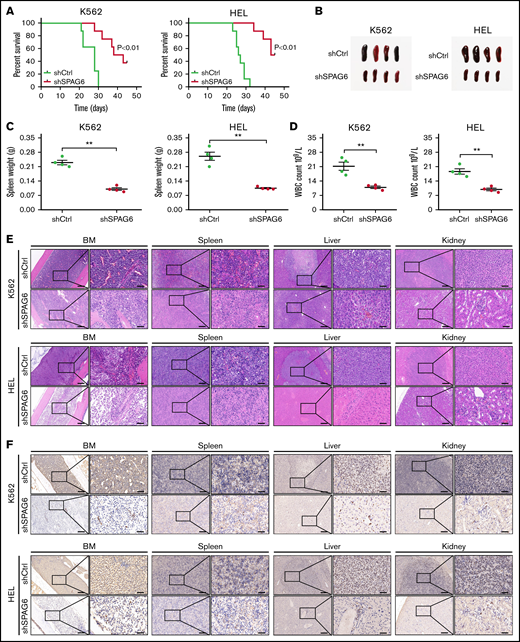
We constructed a NOD/SCID xenotransplantation assay to examine the role of SPAG6 in AML by using K562 and HEL cells with stable SPAG6 knockdown in vivo. The mice were sacrificed after 45 days of continuous observation. The Kaplan-Meier plot demonstrated that mice bearing SPAG6 knockdown survived longer than control mice (Figure 3A). The leukemia burden in mice was assessed by spleen size and AML cell infiltration. As shown in Figure 3B-C, spleen enlargement and weight were significantly lower in SPAG6 knockdown AML mice than in control mice. The PB white cell count of SPAG6 knockdown mice was also significantly lower than that in the control mice (Figure 3D). Hematoxylin and eosin staining and CD45 immunohistochemistry confirmed that the degree of AML dissemination was reduced in the BM, spleen, liver, and kidney of mice with SPAG6 knockdown compared with the control samples (Figure 3E-F). In summary, SPAG6 promotes the growth and in vivo dissemination of K562 and HEL AML cells.
Knockdown of SPAG6 inhibits the growth and dissemination of AML in vivo. (A) Kaplan-Meier curves showed the survival rate of mice injected with K562-shSPAG6 cells and HEL-shSPAG6 cells (n = 8 mice per group). (B-C) Representative examples of size and weight of spleens derived from leukemic mice with treatment as indicated. (D) White blood cell count in all the study groups. (E-F) Representative hematoxylin and eosin and immunohistochemical staining images of CD45 in BM, spleen, liver, and kidney, treated as indicated. Scale bar, 50 μm. **P < .01.
Knockdown of SPAG6 inhibits the growth and dissemination of AML in vivo. (A) Kaplan-Meier curves showed the survival rate of mice injected with K562-shSPAG6 cells and HEL-shSPAG6 cells (n = 8 mice per group). (B-C) Representative examples of size and weight of spleens derived from leukemic mice with treatment as indicated. (D) White blood cell count in all the study groups. (E-F) Representative hematoxylin and eosin and immunohistochemical staining images of CD45 in BM, spleen, liver, and kidney, treated as indicated. Scale bar, 50 μm. **P < .01.
SPAG6 interacts with MYO1D and promotes translocation of MYO1D to the cell membrane
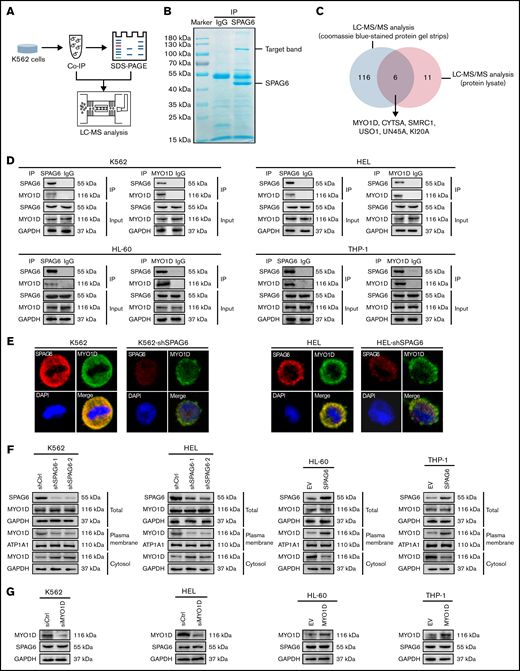
To further explore the mechanism of SPAG6 in AML, we performed a co-immunoprecipitation experiment. The experimental process is shown in Figure 4A. Protein electrophoretic bands and co-immunoprecipitation protein lysates were sent for LC–MS/MS analysis. Coomassie Brilliant Blue staining showed that the most obvious band was SPAG6 between 40 kDa and 55 kDa, and there was also an obvious band between 100 kDa and 130 kDa (Figure 4B). Furthermore, we screened the protein profile of LC–MS/MS analysis and found 6 SPAG6-interacting proteins (Figure 4C). The SPAG6-interacting protein MYO1D was selected for further analysis (Table 1). To verify the interaction between SPAG6 and MYO1D, we selected different antibodies for immunoprecipitation experiments in K562, HEL, HL-60, and THP-1 cells. As expected, an obvious interaction between SPAG6 and MYO1D was detected (Figure 4D). Meanwhile, confocal immunofluorescence showed that SPAG6 and MYO1D proteins were colocalized in the cytoplasm and cell membrane (Figure 4E).
SPAG6 interacts with MYO1D and promotes the translocation of MYO1D to the plasma membrane. (A) A schematic representation of mass spectrometry detection. (B) Immunoprecipitation (IP) assay was carried out using SPAG6 antibody or IgG (negative control antibody). Samples were electrophoresed and Coomassie Brilliant Blue stained. Arrows indicate SPAG6 and the target bands. (C) Venn diagram of the protein strip and the protein lysate detected by LC-MS/MS analysis. (D) Interaction between SPAG6 and MYO1D was demonstrated via Co-IP in K562, HEL, HL-60, and THP-1 cells. (E) Representative images showing immunofluorescence staining of SPAG6 (red), MYO1D (green), and DAPI (blue) in K562 and HEL cells with treatment as indicated. Scale bar, 25 μm. (F) Western blot verified the different distribution of MYO1D in leukemia cells. (G) Western blot demonstrated SPAG6 expression with knockdown or overexpression of MYO1D in 4 AML cells. Co-IP, co-immunoprecipitation.
SPAG6 interacts with MYO1D and promotes the translocation of MYO1D to the plasma membrane. (A) A schematic representation of mass spectrometry detection. (B) Immunoprecipitation (IP) assay was carried out using SPAG6 antibody or IgG (negative control antibody). Samples were electrophoresed and Coomassie Brilliant Blue stained. Arrows indicate SPAG6 and the target bands. (C) Venn diagram of the protein strip and the protein lysate detected by LC-MS/MS analysis. (D) Interaction between SPAG6 and MYO1D was demonstrated via Co-IP in K562, HEL, HL-60, and THP-1 cells. (E) Representative images showing immunofluorescence staining of SPAG6 (red), MYO1D (green), and DAPI (blue) in K562 and HEL cells with treatment as indicated. Scale bar, 25 μm. (F) Western blot verified the different distribution of MYO1D in leukemia cells. (G) Western blot demonstrated SPAG6 expression with knockdown or overexpression of MYO1D in 4 AML cells. Co-IP, co-immunoprecipitation.
Mass spectrum analysis of SPAG6-interacting proteins
| Protein ID . | Protein Q score . | Protein mass (kDa) . | Unique peptide number . | Abundance . |
|---|---|---|---|---|
| sp|O94832|MYO1D | 129.88 | 116.13 | 36 | 1.34 × 108 |
| sp|Q69YQ0|CYTSA | 29.28 | 124.53 | 8 | 5.90 × 106 |
| sp|Q92922|SMRC1 | 18.62 | 122.79 | 6 | 1.12 × 107 |
| sp|O60763|USO1 | 17.84 | 107.83 | 5 | 1.23 × 108 |
| sp|Q9H3U1|UN45A | 13.34 | 103.01 | 4 | 2.22 × 106 |
| sp|O95235|KI20A | 11.83 | 100.22 | 3 | 7.90 × 105 |
| Protein ID . | Protein Q score . | Protein mass (kDa) . | Unique peptide number . | Abundance . |
|---|---|---|---|---|
| sp|O94832|MYO1D | 129.88 | 116.13 | 36 | 1.34 × 108 |
| sp|Q69YQ0|CYTSA | 29.28 | 124.53 | 8 | 5.90 × 106 |
| sp|Q92922|SMRC1 | 18.62 | 122.79 | 6 | 1.12 × 107 |
| sp|O60763|USO1 | 17.84 | 107.83 | 5 | 1.23 × 108 |
| sp|Q9H3U1|UN45A | 13.34 | 103.01 | 4 | 2.22 × 106 |
| sp|O95235|KI20A | 11.83 | 100.22 | 3 | 7.90 × 105 |
CYTSA, cytospin-A; KI20A, kinesin-like protein KIF20A; MYO1D, unconventional myosin ID; SMRC1, SWI/SNF complex subunit SMARCC1; UN45A, protein unc-45 homolog A; USO1, general vesicular transport factor p115.
Following co-immunoprecipitation assays of acute myeloid leukemia cells and visualization by electrophoresis, the target band (SPAG6-interacting proteins, between 90 kDa and 130 kDa) was excised and assessed by LC–MS/MS.
A previous study reported that MYO1D binds to the nonphosphorylated domain of the EGFR family and anchors it to the cell membrane to prevent it from being degraded, while overexpressed MYO1D activates the downstream RTK (receptor tyrosine kinase) signaling pathway by upregulating EGFR on tumor cell membranes.23 Considering that MYO1D is localized in the cytoplasm and transferred around the cell membrane, we found that K562 and HEL cells with SPAG6 knockdown significantly blocked the translocation of MYO1D into the cell membrane without affecting total cellular MYO1D expression, while HL-60 and THP-1 cells with SPAG6 overexpression promoted the translocation of MYO1D into cell membrane without affecting total cellular MYO1D expression (Figure 4F). Moreover, MYO1D knockdown or overexpression in AML cells did not affect the expression of SPAG6 (Figure 4G). Taken together, our data prove that SPAG6 deficiency influences the intracellular translocation of MYO1D in AML cells.
SPAG6 activates the PI3K/AKT and ERK signaling pathways in a MYO1D-dependent manner
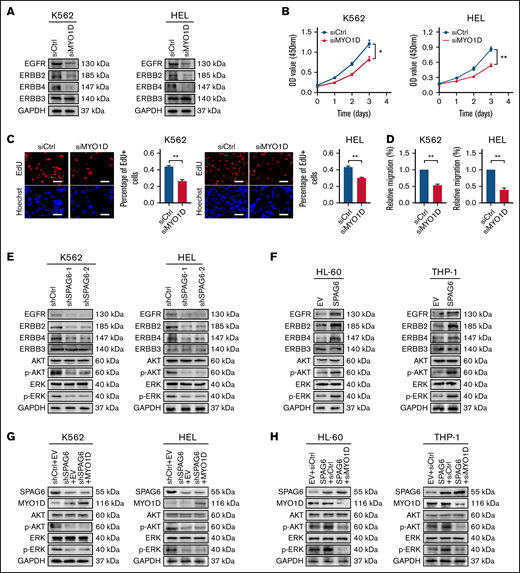
Previous studies have shown that MYO1D combined with the EGFR family (except ERBB3[Erb-B2 receptor tyrosine kinase 3]) affects EGFR downstream signals.23 Dysregulation of the EGFR family overactivates downstream prooncogenic signaling pathways, including the PI3K/AKT and ERK signaling pathways.24,25 Thus, we next investigated whether MYO1D modulates EGFR family expression in AML cells. We observed that compared with the siCtrl group, MYO1D knockdown suppressed the expression of EGFR, ERBB2, and ERBB4 (Figure 5A). To address the effect of MYO1D on leukemia progression, we analyzed CCK-8, EdU, and migration after MYO1D knockdown in K562 and HEL cells. Knockdown of MYO1D reduced the proliferation ability and impaired the migration ability of K562 and HEL cells (Figure 5B-D). Subsequently, we examined whether SPAG6 acts on EGFR downstream signals. Western blot analysis showed that the expression levels of EGFR, ERBB2, ERBB4, p-AKT, and p-ERK were decreased upon SPAG6 silencing in K562 and HEL cells (Figure 5E). Consistently, the expression levels of EGFR, ERBB2, ERBB4, p-AKT, and p-ERK were increased upon SPAG6 overexpression in THP-1 and HL-60 cells (Figure 5F). However, the expression of ERBB3 showed no significant change in the 4 cell lines (Figure 5E-F). To further confirm the interaction between SPAG6 and MYO1D, MYO1D was overexpressed in K562 and HEL cells with SPAG6 stable knockdown. Western blot analysis showed that p-AKT and p-ERK concentrations in the short hairpin RNA (sh)SPAG6+MYO1D group were not different from those in the shSPAG6+EV (empty vector) group but were significantly lower than those in the shCtrl+EV group (Figure 5G). However, when MYO1D was knocked down in THP-1 and HL-60 cells with stable SPAG6 overexpression, the expression levels of p-AKT and p-ERK in the SPAG6+siMYO1D group were significantly lower than those in the SPAG6+siCtrl and EV+siCtrl groups (Figure 5H). In summary, these results show that SPAG6 activates the PI3K/AKT and ERK signaling pathways in a MYO1D-dependent manner.
SPAG6 activates the PI3K/AKT and ERK signaling pathways in a MYO1D-dependent manner. (A) Western blot showing EGFR, ERBB2, ERBB3, and ERBB4 expression in K562 and HEL cells with MYO1D knockdown. (B-C) The proliferative capacities of K562 and HEL cells with MYO1D knockdown were detected by CCK-8 and EdU assays. (D) Migration of K562 and HEL cells when MYO1D was knocked down. (E-F) Western blot showing the concentrations of EGFR, ERBB2, ERBB3, ERBB4, AKT, p-AKT, ERK, and p-ERK in 4 AML cells treated as indicated. (G-H) Western blot showing the concentrations of AKT, p-AKT, ERK, and p-ERK in K562, HEL, HL-60, and THP-1 cells with treatment as indicated. *P < .05; **P < .01.
SPAG6 activates the PI3K/AKT and ERK signaling pathways in a MYO1D-dependent manner. (A) Western blot showing EGFR, ERBB2, ERBB3, and ERBB4 expression in K562 and HEL cells with MYO1D knockdown. (B-C) The proliferative capacities of K562 and HEL cells with MYO1D knockdown were detected by CCK-8 and EdU assays. (D) Migration of K562 and HEL cells when MYO1D was knocked down. (E-F) Western blot showing the concentrations of EGFR, ERBB2, ERBB3, ERBB4, AKT, p-AKT, ERK, and p-ERK in 4 AML cells treated as indicated. (G-H) Western blot showing the concentrations of AKT, p-AKT, ERK, and p-ERK in K562, HEL, HL-60, and THP-1 cells with treatment as indicated. *P < .05; **P < .01.
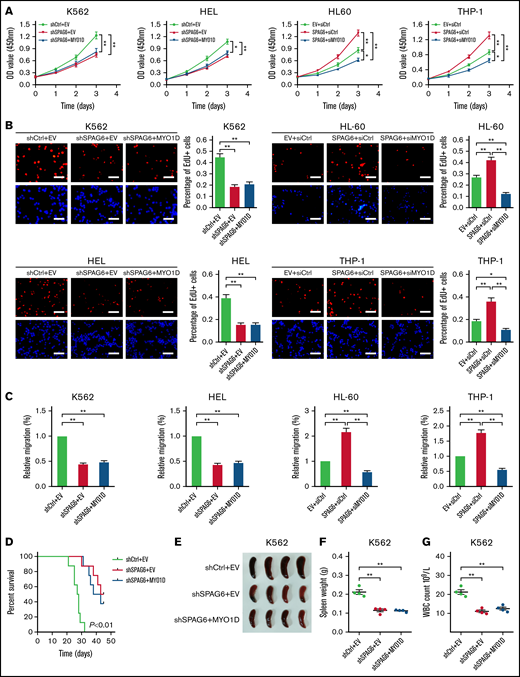
SPAG6, in association with MYO1D, promotes cell growth and migration of AML cells
As MYO1D was regulated by SPAG6, we explored its role in SPAG6-induced growth and migration of AML cells. As shown in Figure 6A-C, overexpression of MYO1D had no significant effect on the cell growth rate and migration ability decreased by knockdown of SPAG6 in K562 and HEL cells, but the silencing of MYO1D attenuated SPAG6 overexpression promoted AML growth and migration in THP-1 and HL-60 cells. In vivo, MYO1D was overexpressed in K562 cells with SPAG6 knockdown and then injected into NOD/SCID mice via the tail vein. It was observed that the survival time of mice in the shSPAG6+EV and shSPAG6+MYO1D groups was significantly longer than that in the shCtrl+EV group, but there was no difference in shSPAG6+EV vs shSPAG6+MYO1D groups (Figure 6D). Similarly, we found that the spleen size and weight in the shSPAG6+EV and shSPAG6+MYO1D groups were smaller than those in the shCtrl+EV group. As expected, there was no difference in shSPAG6+EV vs shSPAG6+MYO1D groups (Figure 6E-F). Consistent with the decreased infiltration in the shSPAG6+MYO1D and shSPAG6+EV groups, we also observed reduced numbers of circulating leukemia cells (Figure 6G).
SPAG6, in association with MYO1D, promotes cell growth and migration of AML cells. (A-B) K562 and HEL cells with SPAG6 knockdown and HL-60 and THP-1 cells with SPAG6 overexpression were transfected with MYO1D forced expression vector or small interfering RNA as indicated. The cell proliferative capacities were detected by CCK-8 and EdU assays in K562, HEL, HL-60, and THP-1 cells with treatment as indicated. (C) Transwell assay in K562, HEL, HL-60, and THP-1 cells treated as indicated. (D) K562 cells with SPAG6 knockdown were transfected with MYO1D forced expression or control vector as indicated. Kaplan-Meier curves showed the survival of mice treated as indicated. (E-F) Size and weight of the spleen between different treatment groups. (G) White blood cells (WBCs) in mice were treated as indicated. *P < .05; **P < .01.
SPAG6, in association with MYO1D, promotes cell growth and migration of AML cells. (A-B) K562 and HEL cells with SPAG6 knockdown and HL-60 and THP-1 cells with SPAG6 overexpression were transfected with MYO1D forced expression vector or small interfering RNA as indicated. The cell proliferative capacities were detected by CCK-8 and EdU assays in K562, HEL, HL-60, and THP-1 cells with treatment as indicated. (C) Transwell assay in K562, HEL, HL-60, and THP-1 cells treated as indicated. (D) K562 cells with SPAG6 knockdown were transfected with MYO1D forced expression or control vector as indicated. Kaplan-Meier curves showed the survival of mice treated as indicated. (E-F) Size and weight of the spleen between different treatment groups. (G) White blood cells (WBCs) in mice were treated as indicated. *P < .05; **P < .01.
In conclusion, we characterized the role of SPAG6 in AML and explored the underlying mechanism. We showed that SPAG6 promotes cell proliferation and infiltration in AML by translocating MYO1D to the cell membrane and activating the downstream PI3K/AKT and ERK signaling pathways.
Discussion
Despite increasing evidence implicating the SPAG family in cancer, little is known about the roles of SPAG6 in the initiation and progression of AML and its underlying mechanism. In this study, we demonstrated that SPAG6 was frequently upregulated in AML, which significantly promoted cell proliferation and migration both in vitro and in vivo and thus led to a poor prognosis for AML patients. Mechanistically, we demonstrated that the survival-promoting role of SPAG6 was mediated by binding with MYO1D and subsequent activation of the PI3K/AKT and ERK signaling pathways.
Some previous studies have confirmed that SPAG6 plays crucial roles in malignancy, but its role in tumorigenesis appears to be a double-edged sword. Frequent methylation of the promoter-associated CpG island of the SPAG6 gene occurs in bladder cancer, which may suppress tumor initiation.16 In contrast, Zhang and colleagues reported that SPAG6 promotes the growth of Burkitt lymphoma cells but inhibits cell apoptosis, and increased expression of SPAG6 is associated with poor patient survival.21 Our previous studies have demonstrated that SPAG6 promotes SKM-1 cell growth by affecting different signaling pathways and confers a worse clinical prognosis to MDS patients.26 Skou and colleagues reported that SPAG6 overexpression in PB may predict relapse in childhood AML patients.27 In line with these studies in hematological neoplasms, we found that SPAG6 was significantly upregulated in adult AML, which was negatively correlated with the overall survival rate of AML patients. In addition, functional assays of leukemia cells show a tumor-promoting function for SPAG6 in AML. The above findings suggest that the expression level and function of SPAG6 may be tumor type-specific.
Our previous studies showed that SPAG6 silencing led to cell cycle arrest at the G0/G1 phase in MDS cells.28 Li and colleagues also reported that the migration capacity of mouse embryonic fibroblasts with SPAG6 knockdown was reduced.10 In the present study, we demonstrated that SPAG6 significantly promotes AML cell proliferation in vitro by inducing the cell cycle G1/S transition. In addition, it has recently been well documented that SPAG6 is a component of cilia or flagellum axon centerpieces and plays an important role in the regulation of cell motility.29,30 Our present study shows that SPAG6 overexpression increases the migration of AML cells. Our previous study showed that SPAG6 regulated the apoptosis of MDS cells,18,19 but in this study, we found that SPAG6 had no significant effect on the apoptosis of AML cells. For cell differentiation, Jiang and colleagues found that after knockdown of SPAG6 in MDS cells, the expression of CD11b on the cell surface did not change significantly, but the expression of CD14 increased compared with the control group, suggesting that SPAG6 might play a role in the differentiation of SKM-1 cells.28 However, we found in our study that SPAG6 had no significant effect on the differentiation of AML cells.
Previous studies have confirmed that full-length SPAG6 consists of 10 exons and 16 domains, including 8 conserved ARM (Armadillo)-repeat proteins, and the tandem ARM-repeat units fold together to form a super helix, constituting a universal platform for interaction with many proteins.31 As a whole, ARM-repeat proteins in eukaryotes play a variety of important functions by mediating protein–protein interactions.32,33 We speculate that SPAG6 folds through the ARM-repeat protein in AML cells and builds a platform to combine with MYO1D to promote tumor cell growth.
MYO1D belongs to the myosin-I family, a class of ATP-dependent molecular motors in eukaryotic cells, which has been shown to play an important role in cell movement and intracellular material transport.34,35 Ko and colleagues reported that the proliferation, motility, and survival of nonsmall-cell lung cancer cells were obviously reduced when MYO1D was knocked down, resulting in reduced cytomembrane levels of EGFR or mutant ERBB2 after MYO1D deficiency.36 A previous study reported that MYO1D binds to the nonphosphorylated domain of the EGFR family and anchors it to the cytomembrane to avoid degradation, while overexpressed MYO1D activates the downstream RTK signaling pathway by upregulating EGFR on the tumor cytomembrane.23 EGFR signaling is activated in multiple tumors, such as lung cancer, colorectal cancer, and leukemia, and it plays a key role in tumorigenesis and progression by promoting various cellular processes.37-39 Our present study showed that the survival-promoting role of SPAG6 was mediated by binding with MYO1D and promoting the translocation of MYO1D to the cytomembrane for the regulation of the EGFR family.
There are some limitations to our research. First, we did not use SPAG6-KO mice in our experiment. Considering that most mice with SPAG6 gene deficiency died of hydrocephalus in the early stage and adult male mice with abnormal sperm could not reproduce normally,29 we chose the recognized xenograft tumor model of NOD/SCID mice for research. Second, the number of patients recruited was small, so we used public datasets of AML to further support our analysis. Third, the specific dynamic process by which SPAG6 promotes the translocation of MYO1D to the cytomembrane in AML is still unclear. Further studies are needed to explore the binding domain of SPAG6 and MYO1D and obtain more clinical data on SPAG6 from AML patients.
Conclusions
We identified a new mechanism by which SPAG6 promotes the translocation of MYO1D from the cytosol to the cytomembrane, which results in the activation of the PI3K/AKT and ERK signaling pathways and the subsequent promotion of cell proliferation and tumor progression in AML. Therefore, blocking the function of SPAG6 may be a new method to block the downstream signaling pathway of the EGFR family in AML cells and could provide novel guidance for the treatment of AML in the future.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (nos. 82070130 and 81570109), Air Force Military Medical University Talent Support LingYun Project-Eagle Program (2019cyjhwb and 2020cyjhyp), and Shaanxi Province Youth Science and Technology Rising Star Project (2017KJXX-13).
Authorship
Contribution: J.M. and P.Y. conceived, performed research, and cowrote the paper; J.L. collected and analyzed clinical samples; Y.C. and Y.T. performed in vitro research; L.D. and B.Z. performed in vivo research; B.W. and X.W. supervised the experiment and revised the paper; and L.L. obtained funding, gave advice on design, and critically revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Lin Liu, Department of Hematology, The First Affiliated Hospital of Chongqing Medical University, No. 1 Youyi Rd, Chongqing 400042, China; e-mail: liulin@cqmu.edu.cn; Bao Wang, Department of Neurosurgery, Tangdu Hospital, Air Force Medical University, No. 1, Xinsi Rd, Xi’an 710038, China; e-mail: bob.kane@163.com; and Xiaocheng Wang, Department of Aviation Medicine, The First Affiliated Hospital, Air Force Medical University, 169 Changle West Rd, Xi’an 710032, China; e-mail: wxcnose@126.com.
References
Author notes
J.M. and P.Y. contributed equally to this study as joint first authors.
E-mail the corresponding author for data sharing: liulin@cqmu.edu.cn.
The full-text version of this article contains a data supplement.