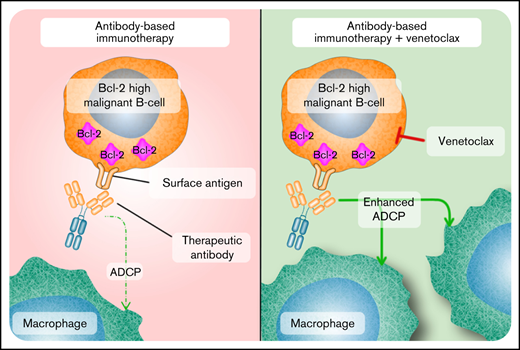
Key Points
Combination of venetoclax and therapeutic antibodies increases in vitro phagocytosis in an apoptosis-independent manner.
Survival benefit in vivo after cotreatment with venetoclax and therapeutic antibodies is dependent on macrophages.
Abstract
Immunotherapy has evolved as a powerful tool for the treatment of B-cell malignancies, and patient outcomes have improved by combining therapeutic antibodies with conventional chemotherapy. Overexpression of antiapoptotic B-cell lymphoma 2 (Bcl-2) is associated with a poor prognosis, and increased levels have been described in patients with “double-hit” diffuse large B-cell lymphoma, a subgroup of Burkitt’s lymphoma, and patients with pediatric acute lymphoblastic leukemia harboring a t(17;19) translocation. Here, we show that the addition of venetoclax (VEN), a specific Bcl-2 inhibitor, potently enhanced the efficacy of the therapeutic anti-CD20 antibody rituximab, anti-CD38 daratumumab, and anti-CD19-DE, a proprietary version of tafasitamab. This was because of an increase in antibody-dependent cellular phagocytosis by macrophages as shown in vitro and in vivo in cell lines and patient-derived xenograft models. Mechanistically, double-hit lymphoma cells subjected to VEN triggered phagocytosis in an apoptosis-independent manner. Our study identifies the combination of VEN and therapeutic antibodies as a promising novel strategy for the treatment of B-cell malignancies.
Introduction
Antibodies provide promising options in the treatment of B-cell malignancies. For example, management of relapsed/refractory B-cell precursor acute lymphoblastic leukemia (BCP-ALL) has changed since the introduction of blinatumomab, a bispecific antibody targeting the CD19 antigen.1,2 Additionally, the application of naked CD19 antibodies has moved forward in preclinical and early clinical studies.3 A benefit for children and adults with Burkitt lymphoma (BL) has been described with the CD20 antibody rituximab (RTX).4-6 Likewise, RTX added to chemotherapy increases event-free survival in “double-hit” diffuse large B-cell lymphoma (DLBCL) in adults by 10% to 15% at 3 years.7 Last, the CD38 antibody daratumumab (DARA), highly efficient in multiple myeloma, is subject of clinical trials in B-cell non-Hodgkin lymphoma (B-NHL) and ALL.8
However, patients with a high Bcl-2 expression (eg, adults with “double-hit lymphomas” [DHLs]) characterized by translocations involving MYC and BCL-2 or BCL-6,9 and children with BCP-ALL harboring a t(17;19) translocation, have a poor prognosis.10 Furthermore, some BLs also express Bcl-2, although its role in disease progression is unclear.11 Bcl-2 belongs to a family of proteins harboring a BH3 domain, balancing cell survival and death. It acts as an antiapoptotic protein favoring tumor cell survival.12 Venetoclax (VEN), a selective Bcl-2 inhibitor, has been approved for the treatment of chronic lymphocytic leukemia (CLL) showing efficacy as single agent or in combination with RTX/bendamustine.13 In a randomized phase 3 trial, VEN/RTX at least doubled 2-year rates of progression-free survival in patients with relapsed/refractory CLL.13-15 Moreover, patients with treatment-naïve acute myeloid leukemia (AML) benefit from combinations of VEN and hypomethylating agents like azacytidine, which led to its recent approval for AML.16 However, patients with DHL treated with VEN monotherapy showed only minor benefits,17 and resistance is frequently observed.18
Here, we aimed to examine the impact of VEN on the efficacy of RTX, DARA, and CD19-DE, a variant of the CD19 antibody tafasitamab (MOR208).19-21 We hypothesized that VEN enhances antibody efficacy by inducing antibody-mediated effector functions in Bcl-2–expressing B-cell malignancies. Our data reveal that VEN enhances in vitro antibody-dependent cellular phagocytosis (ADCP) by macrophages in Bcl2-expressing DHL, BL, and t(17;19)-positive ALL. Mechanistically, no changes of antigen surface expression or well-known factors associated with phagocytosis were observed after exposure to VEN. Notably, elevated phagocytosis was independent of caspase-mediated apoptosis or necroptotic cell death. Exposure to VEN most likely activates yet unknown “eat-me-signals” making cancer cells more vulnerable to macrophage-induced phagocytosis. Our in vivo data further suggest that VEN/antibody combinations are transferable into clinical trials in BCP-ALL and B-NHL and may be clinically efficient in further hematologic malignancies beyond these entities.
Methods
Cell lines and standard assays
CARNAVAL, WILL-2 cells, and Oci-Ly7 were purchased from DSMZ. Trypan blue exclusion and Western blotting were performed as previously described.22 For immunoprecipitation, cells were lysed in NaCI/EDTA/Tris buffer, and 1 mg protein was used. After preclearing using Protein G agarose beads from Cell Signaling (#37478), 2% input was removed. For immunoprecipitation, antibodies were added to the lysates overnight before Protein G agarose beads. Beads were washed 4 times with NaCI/EDTA/Tris buffer, and immunoprecipitated proteins were eluted. Detection of proteins was performed as previously described.22 Quantification of Western blot was done using ImageJ.
Cellular metabolic activity was determined using an 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide assay (Roche Diagnostics). Briefly, cells were seeded in 96-well plates at a density of 2 × 104 cells per well and treated with serial dilutions of VEN for 12 hours. After 4-hour incubation with 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide reagent, 100 µL solubilization solution was added to each well. Absorption at 550 nm (reference, 650 nm) was measured after overnight culture. Cell growth was quantified as percent relative growth compared with medium control. All experimental points were set up in triplicate. CD20, CD38, CD19, and Annexin V levels were determined by flow cytometry (BD Accuri).
CARNAVAL and WILL-2 cells depleted for Bax/Bak were generated using Clustered Regularly Interspaced Short Palindromic Repeats/Cas9. Cell lines were first infected with a lentiviral vector coding Cas9 and NeonGreen (pL40C_PGKintron_Cas9_Green, Addgene #134966). Cells with stable Cas9 expression were sorted and then transduced with the sgRNA vector (sg_shuttle_RFP657) targeting BAX and BAK and were purified by sorting.
Antibodies and drugs
Cetuximab (CTX), RTX, and DARA were provided by the institutional pharmacy. CD19-DE and the appropriate control antibody were generated and produced as previously described.19 VEN was purchased from LC Laboratories, navitoclax (NTX) from Selleckchem, N-benzyloxycarbonyl-Val-Ala-Asp(O-Me) fluoromethyl ketone (Z-VAD-FMK) from MedChemExpress, and AZD5991 from selleckchem. Antibodies for Western blot analyses were from Cell Signaling: apoptosis (#9915) antibody sampler kit, Bcl-2 (Rb, #4223: clone: D55G8), Bcl-2 (M, # 15071, clone: 124), Bim (Rb, #2933 clone: C34C5), Mcl-1 (Rb, #5453), pRB (8516P), Rb (9309). Anti-tubulin antibody was purchased from Abcam (ab15246). Anti-Bim antibody (sc-374358, clone H-5) and anti-p21 (sc-6246, F-5) were from Santa Cruz. Fluorescence-activated cell sorter antibodies were purchased from Beckman Coulter: CD19- fluorescein isothiocyanate (FITC) (A07768), CD20-FITC (A07772), CD38-FITC (A07778), and Biolegend (Annexin V, #640930). Anti-murine macrophages markers F4-80 and CD11b were obtained from Miltenyi.
Xenografts and patient cells
Animals were maintained as approved by governmental institutions (Schleswig-Holstein Ministerium für Energiewende, Landwirtschaft, Umwelt, Natur und Digitalisierung; V242-2504/2020 [39-4/19]). NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) mice (Charles River) were maintained as approved by the governmental animal care and use committees. Cell line xenografts were generated by IV injection of 1 × 105 cells per animal. Patient-derived xenograft (PDX) mice were established by intrafemoral injection of 1 × 105 ALL cells per animal. Patients were treated according to acute lymphoblastic leukemia-Berlin-Frankfurt-Münster 2009 and B-cell non-Hodgkin lymphoma-Berlin-Frankfurt-Münster 2004 protocols. Informed consent was obtained in accordance with institutional regulations. The study was approved by the ethical committee of the Christian-Albrechts University Kiel (D 437/17). Antibody therapy in mice was applied by intraperitoneal injection of 1 mg/kg body weight once per week. For depletion of macrophages, 100 μL liposomal clodronate (Clodronate Liposomes.com) was applied by intraperitoneal injection on day −1 and then once per week. VEN was applied at a dose of 100 mg/kg for 5 days per week by oral gavage. In all in vivo approaches, therapy was terminated when all mice in the control group for CARNAVAL at 4 weeks of therapy and for t(17;19)-ALL at 7 weeks of therapy were killed because of leukemic engraftment. The remaining mice were observed until they show leukemic engraftment.
51Chromium release assay
In vitro phagocytosis assay
Mononuclear cells were isolated from residual cells in leukoreduction chambers after routine platelet collection by Percoll centrifugation3,23 : Monocytes were differentiated into macrophages using X-vivo media supplemented with 0.5% 1% penicillin/streptomycin and 50 ng/mL recombinant macrophage colony-stimulating factor (R&D Systems) for 10 days. Murine macrophages were isolated from the peritoneal cavity of NSG mice.25 A total of 1 × 104 macrophages were seeded in chambered coverslips (ibidi) and allowed to adhere. First, cell lines were pretreated with 1 nm VEN or dimethyl sulfoxide (DMSO) as solvent control for 12 hours and then were washed to remove residual drug before labeling with carboxyfluorescein succinimidyl ester (BioLegend). A total of 3 × 104 labeled tumor cells were added to macrophages, and antibodies were applied to a final concentration of 10 µg/mL. Cells were incubated for 2 hours at 37°C, and phagocytosis was analyzed by microscopy (Nikon).19 For phagocytosis assays with the pan-caspase inhibitor Z-VAD-FMK, cell lines were pretreated with Z-VAD-FMK (1 h/50 µM) before treatment with VEN or DMSO (1 nM/12 h). Subsequently, phagocytosis was continued as described above. Phagocytosis was determined by counting 100 random macrophages by at least 3 independent observers. All macrophages were counted, regardless of engulfment of leukemic cells. We did not discriminate if a macrophage had digested 1 or more than 1 leukemic cell.
In vitro phagocytosis using the IncuCyte technology was performed as recently described.26
Data processing and statistical analyses
Statistical analyses were performed using GraphPad Prism (La Jolla, CA). Statistical significance was assessed using nonparametric Mann-Whitney U test. Survival was analyzed using the Kaplan-Meier method and log-rank statistics. P < .05 was considered statistically significant.
Results
VEN in low nanomolar range induces cell cycle arrest in Bcl-2–expressing DHL cells
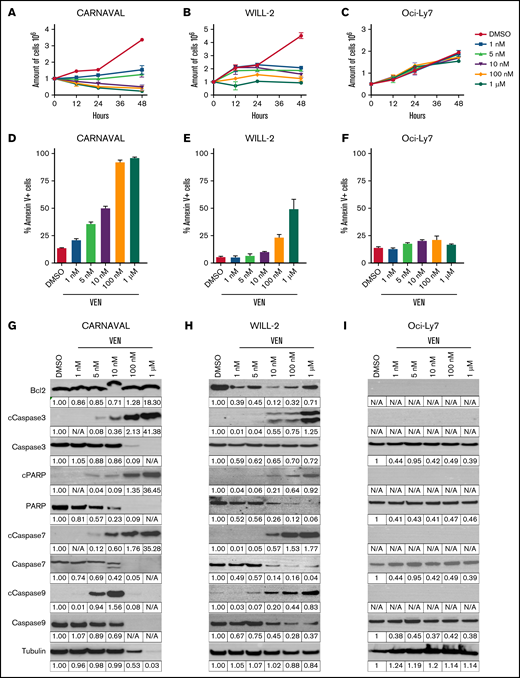
We started by investigating the effect of VEN on DHL cells using CARNAVAL (CD20+), WILL-2 (CD20−/CD38+), and Bcl-2 negative Oci-Ly7 (CD20+/CD38+) cells. First, EC50 values (supplemental Figure 1A-C) were determined, and VEN in low nanomolar range up to only 1 nM was sufficient to impact cell proliferation in CARNAVAL and WILL-2 cells as assessed by trypan blue counting, whereas no effect was observed in Oci-Ly7 (Figure 1A-C; supplemental Figure 1D-F). Moreover, cell cycle arrest was induced on treatment with 1 nM VEN in CARNAVAL cells as evidenced by increased levels of Rb and p21 and decreased levels of phospho V-Akt Murine Thymoma Viral Oncogene Homolog and phospho Retinoblastoma protein (supplemental Figure 1G). In WILL-2, an increased level of Rb, no p21, no phospho V-Akt Murine Thymoma Viral Oncogene Homolog, and reduced levels of phospho Extracellular Signal-Regulated Kinase and phospho Retinoblastoma protein were detected (supplemental Figure 1H). Furthermore, escalating concentrations of VEN resulted in an increase of annexin V–positive cells measured by flow cytometry (Figure 1D-F) and augmented cleavage of proapoptotic Caspases 3, 7, and 9 and cleaved poly-(ADP-ribose) polymerase (cPARP) levels in Bcl-2–positive CARNAVAL and Caspases 3 and 7 in WILL-2 cells, whereas no induction of apoptosis was observed in Bcl-2–negative Oci-Ly7 (Figure 1G-I). Bcl-2 levels persisted in CARNAVAL cells, which has been described,27,28 but decreased in WILL-2 cells (Figure 1G-H). In most BL and all t(17;19) BCP-ALL PDX samples, Bcl-2 levels also decreased (supplemental Figure 1I-J). Finally, the Bcl-2/Bim interaction was also disrupted with increasing concentrations of VEN in CARNAVAL and WILL-2 cells (supplemental Figure 2A-B). Hence, VEN altered Bcl-2 levels in a cell type–specific manner, and VEN had an impact on cell proliferation at low doses, whereas escalating concentrations of VEN resulted in induction of apoptosis in a Bcl-2–dependent manner.
Effects of venetoclax in DHL cells. (A-C) Trypan blue exclusion test of cell viability in CARNAVAL (A), WILL-2 (B), and Oci-Ly7 (C) cells treated with increasing concentrations of venetoclax (VEN) for up to 48 hours. Technical triplicates were counted per experiment. Graphs show results of 3 independent experiments (n = 3, SD). (D-F) Annexin V staining measured by flow cytometry in CARNAVAL (D), WILL-2 (E), and Oci-Ly7 (F) cells subjected to escalating concentrations of 1 nM to 1 µM VEN and DMSO for 12 hours. (G-I) Protein expression of Bcl-2 and apoptotic markers Caspase 3, 7, 9, cPARP, and total levels analyzed by Western blot in CARNAVAL (G), WILL-2 (H), and Oci-Ly7 (I) cells after treatment with VEN for 12 hours with concentrations as indicated. Tubulin served as a loading control. Quantification of Western blot was done with ImageJ. Intensities were calculated relative to tubulin and normalized to DMSO solvent control. N/A, not analyzable.
Effects of venetoclax in DHL cells. (A-C) Trypan blue exclusion test of cell viability in CARNAVAL (A), WILL-2 (B), and Oci-Ly7 (C) cells treated with increasing concentrations of venetoclax (VEN) for up to 48 hours. Technical triplicates were counted per experiment. Graphs show results of 3 independent experiments (n = 3, SD). (D-F) Annexin V staining measured by flow cytometry in CARNAVAL (D), WILL-2 (E), and Oci-Ly7 (F) cells subjected to escalating concentrations of 1 nM to 1 µM VEN and DMSO for 12 hours. (G-I) Protein expression of Bcl-2 and apoptotic markers Caspase 3, 7, 9, cPARP, and total levels analyzed by Western blot in CARNAVAL (G), WILL-2 (H), and Oci-Ly7 (I) cells after treatment with VEN for 12 hours with concentrations as indicated. Tubulin served as a loading control. Quantification of Western blot was done with ImageJ. Intensities were calculated relative to tubulin and normalized to DMSO solvent control. N/A, not analyzable.
VEN increases antibody-dependent phagocytosis in DHL cells
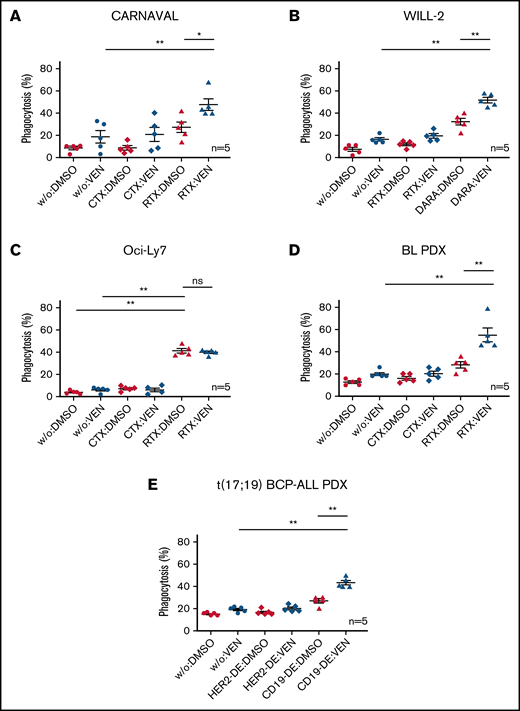
We next examined antibody-mediated immunologic effector functions. First, surface expression levels of antibody targets CD20, CD38, and CD19 were determined after treatment with VEN. Flow cytometry revealed no altered surface expression of CD20, CD38, and CD19 in cell lines and PDX samples when subjected to VEN (supplemental Figure 3A-E). Moreover, neither mononuclear cell–mediated Antibody-dependent cellular cytotoxicity (ADCC) nor CDC increased when VEN was added to antibody therapy in vitro in CARNAVAL and WILL-2 cells (supplemental Figure 4). However, ADCP significantly increased when specific antibodies were combined with VEN in phagocytosis assays with macrophages from human donors and labeled target cells, with the exception of the Bcl-2–negative Oci-Ly7 cells (Figure 2A-E). ADCP increased when CARNAVAL cells were subjected to VEN/RTX or when WILL-2 cells were treated with VEN/DARA compared with VEN or antibody alone (P = .0079/P = .0317 and P = .0079/P = .0079, respectively; Figure 2A-B). In Bcl-2–negative Oci-Ly7 cells, phagocytosis was increased on RTX treatment compared with control or VEN alone (P = .0079/P = .0079; Figure 2C), but the combination VEN/RTX did not further enhance ADCP. In BL PDX cells, VEN/RTX increased mean phagocytosis levels to an even higher extent compared with VEN (P = .0079) or RTX alone (P = .0079; Figure 2D). Finally, ADCP assays with t(17;19)-positive ALL-PDX cells and CD19-DE confirmed these results (P = .0079/P = .0079; Figure 2E). Interassay heterogeneity was minimized by multiple replications with different human donors. To confirm our findings and predict potential in vivo effects, murine macrophages were isolated from the peritoneal cavity of NSG mice, and in vitro phagocytosis was re-evaluated. Combination of VEN with appropriate antibodies also showed enhanced efficacy compared with VEN or antibody alone in CARNAVAL (P = .0317/P = .0476), WILL-2 (P = .0079/P = .0476), BL-PDX (P = .0079/P = .0079), and t(17;19)-positive ALL-PDX (P = .0079/P = .0079; supplemental Figure 5A-E), but not in Bcl-2–negative Oci-Ly7 cells (P = .0079/not significant).
ADCP in Bcl-2–expressing DHL cell lines and PDX samples on combination of VEN and therapeutic antibodies. Percentages of cells phagocytosed by human macrophages with VEN/antibody combinations compared with VEN or antibody (RTX, DARA, CD19-DE) alone. (A) CARNAVAL cells after treatment with 1 nM VEN for 12 hours, RTX, and the control antibody CTX. (B) WILL-2 cells treated with 1 nM VEN for 12 hours, DARA, and the control antibody RTX. (C) Oci-Ly7 cells after treatment with 1 nM VEN for 12 hours, RTX, and the control antibody CTX. (D) ADCP in BL PDX sample subjected to 1 nM VEN for 12 hours, RTX, and the control antibody CTX. (E) t(17;19)-positive BCP-ALL PDX sample treated with 1 nM VEN for 12 hours, CD19-DE, and the control antibody HER2-DE (a version of trastuzumab containing the same modification in the Fc part of the antibody as CD19-DE). DMSO, solvent control; w/o, no antibody. Phagocytosis was determined as the percentage of macrophages with completely ingested carboxyfluorescein succinimidyl ester green–positive cells by counting in total 100 macrophages by at least 3 independent observers. Each dot represents an independent experiment with different human donors. Data are presented as mean ± standard error of the mean (SEM) from independent experiments with 5 healthy blood donors. Statistical analysis: ns, not significant; *P < .05; **P < .005; Mann-Whitney test. All antibodies used in vitro were applied to a final concentration of 10 µg/mL.
ADCP in Bcl-2–expressing DHL cell lines and PDX samples on combination of VEN and therapeutic antibodies. Percentages of cells phagocytosed by human macrophages with VEN/antibody combinations compared with VEN or antibody (RTX, DARA, CD19-DE) alone. (A) CARNAVAL cells after treatment with 1 nM VEN for 12 hours, RTX, and the control antibody CTX. (B) WILL-2 cells treated with 1 nM VEN for 12 hours, DARA, and the control antibody RTX. (C) Oci-Ly7 cells after treatment with 1 nM VEN for 12 hours, RTX, and the control antibody CTX. (D) ADCP in BL PDX sample subjected to 1 nM VEN for 12 hours, RTX, and the control antibody CTX. (E) t(17;19)-positive BCP-ALL PDX sample treated with 1 nM VEN for 12 hours, CD19-DE, and the control antibody HER2-DE (a version of trastuzumab containing the same modification in the Fc part of the antibody as CD19-DE). DMSO, solvent control; w/o, no antibody. Phagocytosis was determined as the percentage of macrophages with completely ingested carboxyfluorescein succinimidyl ester green–positive cells by counting in total 100 macrophages by at least 3 independent observers. Each dot represents an independent experiment with different human donors. Data are presented as mean ± standard error of the mean (SEM) from independent experiments with 5 healthy blood donors. Statistical analysis: ns, not significant; *P < .05; **P < .005; Mann-Whitney test. All antibodies used in vitro were applied to a final concentration of 10 µg/mL.
We also examined the role of NTX, a structural homolog of VEN, in triggering phagocytosis in combination with RTX in CARNAVAL cells. CARNAVAL cells were less sensitive to NTX compared with VEN (EC50 NTX = 50 nM vs VEN = 1.2 nM; supplemental Figure 6A; supplemental Figure 1A). Accordingly, p21, cCaspase3, and cPARP levels started increasing only with doses as high as 100 nM NTX (supplemental Figure 6B). ADCP assays using human macrophages displayed an increase in mean phagocytosis when CARNAVAL cells were treated with RTX compared with control and NTX alone (P = .0286/P = .0286; supplemental Figure 6C). However, no further increase was observed when cells were subjected to dual treatment of RTX and NTX. In addition, ADCP was also examined using the myeloid leukemia cell differentiation protein (MCL1) inhibitor AZD5991, because resistance to VEN has been shown to be associated with overexpression of Mcl-1 and is a frequently observed phenomenon in hematologic malignancy.18,29 EC50 of CARNAVAL and WILL-2 cells were determined with increasing concentration of AZD5991, and both (supplemental Figure 7A) were less sensitive to AZD5991 (EC = 0.5 µM) than VEN (supplemental Figure 1A-B). Subjection to escalating concentrations of AZD5991 resulted in increased cleavage of Caspase 3 and PARP (supplemental Figure 7B). Cotreatment with AZD5991/RTX resulted in elevated phagocytosis in CARNAVAL cells compared with single treatment with AZD5991 (P = .0286) or RTX alone (P = .0143). Similar results were observed in WILL-2 after AZD5991/DARA combination compared with treatment with AZD5991 (P = .0079) or DARA alone (P = .0476; supplemental Figure 7C-D). Similar to treatment with VEN, co-administration of AZD5991 and therapeutic antibodies elevated ADCP in DHL cells.
Taken together, in vitro assays with human and murine macrophages demonstrate that VEN augments the activation of ADCP by macrophages in different Bcl-2–expressing cell type/antibody combinations of B-NHL and BCP-ALL.
Combination of VEN and therapeutic antibodies prolongs survival in vivo
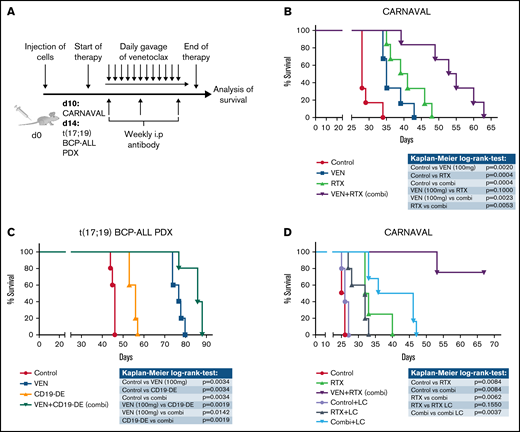
The effects of VEN on antibody therapy were next examined in vivo. CARNAVAL cells were injected into NSG mice (n = 6/group) and animals treated with VEN (100 mg/d),10 RTX, or the combination of both (Figure 3A) when 1% blasts were detected in peripheral blood to mimic overt leukemia. Mice treated with VEN displayed a slight survival advantage, which was more marked with RTX (P = .0020/P = .0004, respectively; Figure 3B). Importantly, mice treated with the combination showed significantly superior survival compared with VEN or RTX alone (P = .0023/P = .0053, respectively; Figure 3B). We also injected PDX cells from a patient with t(17;19)-positive BCP-ALL into NSG mice (n = 5/group) and treated with VEN,10 CD19-DE, or the combination. As expected,19 CD19-DE alone was modestly efficient in this overt leukemia situation (P = .0034; Figure 3C). VEN clearly prolonged survival, which supports published data10 (P = .0034; Figure 3C). Most importantly, combination treatment with VEN/CD19-DE resulted in an even more marked prolongation of animal survival (P = .0019 to CD19-DE alone and P = .0142 to VEN alone; Figure 3C). To substantiate ADCP as the underlying immunologic effector function of the observed in vivo effects, CARNAVAL cells were injected into NSG mice (n = 6/group) and animals treated with RTX or VEN/RTX, with and without a depletion of macrophages using liposomal clodronate (LC).19,30 Macrophage depletion was verified by flow cytometry analysis of murine (m)F4/80 and mCD11b (supplemental Figure 8). Most importantly, removal of macrophages had no effect on leukemic development (Figure 3D). Mice treated with VEN/RTX showed prolonged survival compared with RTX alone or control (P = .0084 for both; Figure 3D). Of note, the addition of LC to VEN/RTX resulted in a reversal of the additive synergistic effects (P = .0037 comparing LC/VEN/RTX to VEN/RTX; Figure 3D), confirming that macrophages are highly important for the combinatorial effect in vivo. Our data suggest substantial effects of VEN and antibodies in vivo in Bcl-2 expressing leukemia and lymphoma in these model systems.
Combination of VEN and antibodies in xenograft mice in vivo. (A) Experimental scheme: CARNAVAL cells or t(17;19) BCP-ALL PDX were injected IV into NSG mice. VEN (100 mg/kg per day) was given daily via oral gavage. Therapeutic antibodies (1 mg/kg) were applied weekly via the intraperitoneal route. (B) CARNAVAL cells were injected into NSG mice and left untreated (control), treated with 100 mg/kg per day VEN, 1 mg/kg per week RTX, or the combination (combi) of both. (C) One t(17;19)-positive BCP-ALL PDX sample was injected into NSG mice and either left untreated (control), treated with 100 mg/kg per day VEN, 1 mg/kg per week CD19-DE, or the combination (combi) of both. (D) NSG mice were injected with CARNAVAL cells. Animals were left untreated (control), treated with RTX (1 mg/kg per week intraperitoneal), VEN (100 mg/kg per day oral gavage), or the combination (VEN+RTX). For macrophage depletion, mice received weekly injection of LC (100 µL/wk intraperitoneally, i.p.). Survival was analyzed using the Kaplan-Meier method and log-rank statistics. P < .05 was considered statistically significant. n, animals per group.
Combination of VEN and antibodies in xenograft mice in vivo. (A) Experimental scheme: CARNAVAL cells or t(17;19) BCP-ALL PDX were injected IV into NSG mice. VEN (100 mg/kg per day) was given daily via oral gavage. Therapeutic antibodies (1 mg/kg) were applied weekly via the intraperitoneal route. (B) CARNAVAL cells were injected into NSG mice and left untreated (control), treated with 100 mg/kg per day VEN, 1 mg/kg per week RTX, or the combination (combi) of both. (C) One t(17;19)-positive BCP-ALL PDX sample was injected into NSG mice and either left untreated (control), treated with 100 mg/kg per day VEN, 1 mg/kg per week CD19-DE, or the combination (combi) of both. (D) NSG mice were injected with CARNAVAL cells. Animals were left untreated (control), treated with RTX (1 mg/kg per week intraperitoneal), VEN (100 mg/kg per day oral gavage), or the combination (VEN+RTX). For macrophage depletion, mice received weekly injection of LC (100 µL/wk intraperitoneally, i.p.). Survival was analyzed using the Kaplan-Meier method and log-rank statistics. P < .05 was considered statistically significant. n, animals per group.
VEN-induced phagocytosis in DHL cells is independent of apoptosis
We next examined the mechanism of synergism between VEN and antibody therapy. First, we hypothesized that the observed effects could be caused by distinct influences of VEN on macrophage activation (eg, myeloid immune checkpoints). One of the major proteins associated with tumor immune evasion is the “don’t eat me” protein CD47. Blocking of CD47 restores phagocytosis and CD47 is becoming an attractive target for cancer therapy.31 CARNAVAL and WILL-2 cells treated with low doses of VEN (up to 5 nM) displayed no differences in CD47 surface expression (supplemental Figure 9A). Also, expression of Calreticulin, an “eat me” protein present on cells prone to phagocytosis, was not altered in both cell lines (supplemental Figure 9B). In addition, phosphatidylserine (PS) exposure on the outer cell membrane essential for phagocytosis of apoptotic cells by macrophages remained unchanged (Figure 1D-E). Finally, gene expression profiling performed in CARNAVAL after treatment with VEN revealed no altered expression of phagocytic proteins like CD24 or major histocompatibility complex (data not shown).
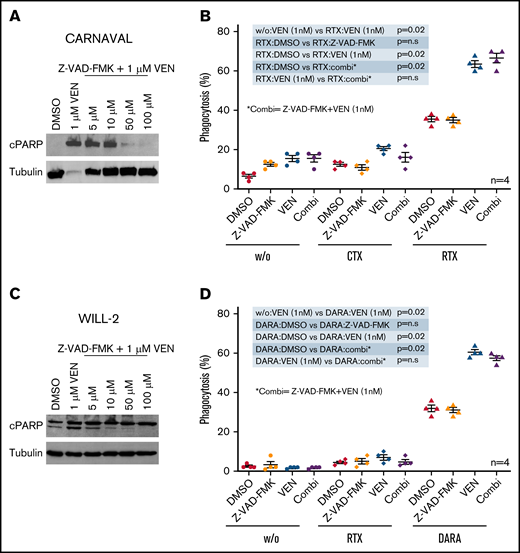
Elevation of phagocytosis was increased starting at a very low dose of 1 nM VEN without any induction of apoptosis. Interestingly, phagocytosis didn’t increase any further with escalating concentrations up to 100 nM VEN in combination with antibody therapy (supplemental Figure 10A-B). Hence, we examined whether macrophage-induced phagocytosis on VEN exposure is an apoptosis-independent event. Therefore, CARNAVAL cells were subjected to VEN and ascending concentrations of the pan-Caspase inhibitor Z-VAD-FMK.32 The induction of cPARP levels by high VEN doses could be hampered by Z-VAD-FMK, suggesting that apoptosis can be efficiently blocked in CARNAVAL cells (Figure 4A). As already seen (Figure 2A), combination of VEN/RTX significantly boost phagocytosis compared with single treatments (P = .02/P = .02) However, the addition of Z-VAD-FMK had no effect on combined VEN/RTX treatment in ADCP assays (Figure 4B). Similar results were obtained for WILL-2 cells when applying Z-VAD-FMK to the combination of VEN/DARA (Figure 4C-D). Z-VAD-FMK mainly prevents caspase-associated apoptosis. To examine whether VEN-treated cells may use alternative routes like necroptosis to enter cell death, we analyzed mixed lineage kinase domain like pseudokinase (MLKL), a major necroptotic factor. However, CARNAVAL and WILL-2 cells subjected to VEN displayed no altered phosphorylation of MLKL, although total levels were decreased in CARNAVAL cells (supplemental Figure 11A-B), suggesting that cells do not enter a necroptotic state on VEN treatment. Moreover, Z-VAD-FMK dampened expression of PS as measured by annexin V staining on CARNAVAL and WILL-2 cells treated with VEN (supplemental Figure 11C-D), suggesting that cells mainly undergo caspase-mediated apoptosis.
Apoptosis independence in VEN-induced phagocytosis. (A) CARNAVAL cells were pretreated with increasing concentrations of the pan caspase inhibitor Z-VAD-FMK for 1 hour before being subjected to 1 µM VEN for 12 hours. Protein levels of cPARP were analyzed by Western blot. (B) Percentages of cells phagocytosed by human macrophages with VEN/antibody combinations compared with VEN, the pan-caspase inhibitor Z-VAD-FMK (Z-VAD) for 1 hour, RTX, or combination of VEN and Z-VAD-FMK (combi). CARNAVAL cells were treated with 50 µM Z-VAD-FMK for 1 hour or/and 1 nM VEN for 12 hours. (C) WILL-2 cells were pretreated with increasing concentrations of the pan caspase inhibitor Z-VAD-FMK/1 hour before subjected to 1 µM VEN for 12 hour. Protein expression of cPARP was analyzed by Western blot. (D) In vitro phagocytosis assays with human macrophages in WILL-2 cells treated with 1 nM VEN for 12 hours or/and 50 µM Z-VAD-FMK for 1 hour alone, the combination (combi), DARA, and the control antibody RTX. Phagocytosis was analyzed as described above. Data are presented as mean ± SEM from independent experiments with 4 healthy blood donors. Tubulin served as a loading control.
Apoptosis independence in VEN-induced phagocytosis. (A) CARNAVAL cells were pretreated with increasing concentrations of the pan caspase inhibitor Z-VAD-FMK for 1 hour before being subjected to 1 µM VEN for 12 hours. Protein levels of cPARP were analyzed by Western blot. (B) Percentages of cells phagocytosed by human macrophages with VEN/antibody combinations compared with VEN, the pan-caspase inhibitor Z-VAD-FMK (Z-VAD) for 1 hour, RTX, or combination of VEN and Z-VAD-FMK (combi). CARNAVAL cells were treated with 50 µM Z-VAD-FMK for 1 hour or/and 1 nM VEN for 12 hours. (C) WILL-2 cells were pretreated with increasing concentrations of the pan caspase inhibitor Z-VAD-FMK/1 hour before subjected to 1 µM VEN for 12 hour. Protein expression of cPARP was analyzed by Western blot. (D) In vitro phagocytosis assays with human macrophages in WILL-2 cells treated with 1 nM VEN for 12 hours or/and 50 µM Z-VAD-FMK for 1 hour alone, the combination (combi), DARA, and the control antibody RTX. Phagocytosis was analyzed as described above. Data are presented as mean ± SEM from independent experiments with 4 healthy blood donors. Tubulin served as a loading control.
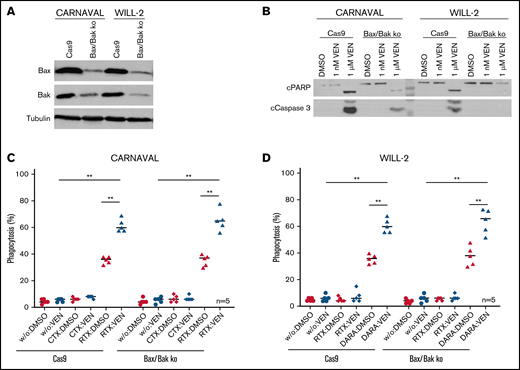
To further address apoptosis independence of ADCP on VEN exposure, CARNAVAL and WILL-2 cells lacking Bax and Bak were generated using the Clustered Regularly Interspaced Short Palindromic Repeats/Cas9 system (Figure 5A). To show that cells were indeed apoptosis deficient, cPARP and cCaspase 3 levels were determined after treatment with 1 nM or 1 µM VEN. Compared with control cells (Cas9 only), CARNAVAL cells lacking Bax/Bak showed minor activation of cPARP and cCaspase3, whereas WILL-2 cells deficient for Bax/Bak displayed no activation of both apoptotic proteins at all (Figure 5B). Finally, ADCP was re-evaluated in Bax/Bak depleted CARNAVAL and WILL-2 cells after treatment with VEN/antibody. In both control cell lines (Cas9 only), combination of VEN with appropriate antibodies significantly elevated phagocytosis levels compared with single treatments (P = .0079/P = .0079, CARNAVAL, P = .0079/P = .0079, WILL-2; Figure 5C-D). The same increase in ADCP after combination of VEN/antibodies was also observed in apoptosis-deficient Bax/Bak cells (P = .0079/P = .0079, CARNAVAL; P = .0079/P = .0079, WILL-2), underpinning that VEN-induced phagocytosis is an apoptosis-independent event (Figure 5C-D).
VEN-mediated phagocytosis in Bax/Bak-deficient DHL cell lines. (A) Protein levels of Bax and Bak in CARNAVAL and WILL-2 Bax/Bak ko cells and control cells (Cas9) analyzed by Western blot. (B) Examination of cPARP and cCaspase 3 in CARNAVAL and WILL-2 Bax/Bak ko cells control cells (Cas9) subjected to 1 nM or 1 µM VEN for 12 hours. (C) ADCP in CARNAVAL deficient for Bax/Bak or control cells (Cas9) after treatment with 1 nM VEN for 12 hours, RTX, and the control antibody CTX. (D) ADCP in WILL-2 lacking Bax/Bak or control cells (Cas9) treated with 1 nM VEN for 12 hours, DARA, and the control antibody RTX. Data are presented as mean ± SEM from independent experiments with 5 healthy blood donors. Statistical analysis: *P < .05; **P < .005; Mann-Whitney test. All antibodies used in vitro were applied to a final concentration of 10 µg/mL. Tubulin served as a loading control.
VEN-mediated phagocytosis in Bax/Bak-deficient DHL cell lines. (A) Protein levels of Bax and Bak in CARNAVAL and WILL-2 Bax/Bak ko cells and control cells (Cas9) analyzed by Western blot. (B) Examination of cPARP and cCaspase 3 in CARNAVAL and WILL-2 Bax/Bak ko cells control cells (Cas9) subjected to 1 nM or 1 µM VEN for 12 hours. (C) ADCP in CARNAVAL deficient for Bax/Bak or control cells (Cas9) after treatment with 1 nM VEN for 12 hours, RTX, and the control antibody CTX. (D) ADCP in WILL-2 lacking Bax/Bak or control cells (Cas9) treated with 1 nM VEN for 12 hours, DARA, and the control antibody RTX. Data are presented as mean ± SEM from independent experiments with 5 healthy blood donors. Statistical analysis: *P < .05; **P < .005; Mann-Whitney test. All antibodies used in vitro were applied to a final concentration of 10 µg/mL. Tubulin served as a loading control.
Taken together, these data suggest that VEN triggers phagocytosis in DHL cells in an apoptosis-independent manner.
Discussion
Application of antibodies marked a milestone in the treatment of hematologic malignancies and combination approaches with cytotoxic agents are a matter of ongoing investigations.33,34 In this study, we show that VEN in combination with 3 distinct antibodies targeting CD19, CD20, and CD38 increases ADCP by macrophages in an apoptosis-independent manner in Bcl-2 expressing DHL, BL, and t(17;19) ALL. Further in vivo experiments demonstrate an important survival benefit when combining VEN with antibodies in a macrophage-dependent manner.
Historically, conventional chemotherapy has been described to directly induce death of malignant cells by intervening in cellular processes like DNA synthesis or replication.35 However, increasing evidence suggests that the antitumor effects of several drugs can also rely on stimulation of the innate immune system by activating natural killer (NK) cells,36 human complement,37 or macrophages,38 thus triggering an eradication of tumor cells.39 Furthermore, combining cytotoxic drugs with antibodies can increase therapy effects compared with single treatments.40 First studies provide evidence that VEN restores NK cell functionality in CLL and may for that reason be a valuable add-on to therapy.41 A clear role of VEN in complement activation has not been described yet, although in vitro studies have shown that Bcl-2 prevents activation of complement in T-ALL.42 In our study, no increase in NK cell– or complement-mediated cytotoxicity was observed in DHL, suggesting a minor role of these immune components in our therapeutic setting.
Macrophage-induced ADCP in vitro has also been shown after combination of antibodies with other cytotoxic drugs. AML cells subjected to dual treatment with a CD47-targeting antibody and azacitidine displayed increased phagocytosis in vitro.43 In addition, tumor cells treated with cyclophosphamide secrete interleukin-8, vascular endothelial growth factor, and tumor necrosis factor-α, rendering cells more susceptible to phagocytosis when simultaneously subjected to the CD52 antibody alemtuzumab.44 Finally, CLL cells preincubated with VEN and subjected to RTX displayed elevated levels of phagocytosis in vitro,45 which is in line with our in vitro data in DHL, BL, and t(17;19) ALL cells. Notably, elevated in vitro phagocytosis was also observed after combination of the Mcl-1 inhibitor AZD5991 with antibodies. Because resistance to VEN is a commonly observed phenomenon,18 combination of Mcl-1 inhibitors with therapeutic antibodies might represent an alternative approach. Of interest, elevated phagocytosis was restricted to VEN/antibody or AZD5991/antibody combinations in our study, because combination of RTX and NTX, a structurally related homolog of VEN, had no phagocytosis-promoting effect. Although common features for NTX and VEN have been described, both drugs might also regulate divergent cell type–specific processes.46 For instance, NTX combined with the Chk1 inhibitor prexasertib induces apoptosis in solid cancer cells, an observation that was not observed for VEN.47 Besides Bcl-2, NTX mainly inhibits Bcl-W and Bcl-xL; hence, different signaling pathways might be additionally activated by NTX counteracting the observed effect on VEN treatment.48 Finally, the crucial role of dual treatment was underpinned by further in vivo experiments demonstrating a macrophage-dependent survival advantage on treatment with VEN/RTX, as macrophage depletion reverted the survival benefit in mice.
Mechanistically different strategies of how cytotoxic agents might activate the innate and adaptive immune system have been described.35 Demethylating agents augmented antigen presentation of CD38 in myeloma cells rendering tumor cells more susceptible to NK cell–mediated cell death when treated with DARA.49 Similar results were obtained in malignant B-cells showing elevated CD19 expression after VEN exposure.50 In our study, no altered surface expression of CD19, CD20, and CD38 were observed in DHL cell lines or PDX samples treated with VEN, suggesting an alternative mechanism of additive synergy.
Moreover, exposure of malignant cells to cytotoxic drugs might induce the upregulation of characteristics and markers that render the cells detectable by the immune system. CLL cells exposed to VEN displayed increased levels of prophagocytic PS.45 In our study, none of the classical phagocytosis associated proteins like CD47, PS, or Calreticulin38 were differentially expressed after subjecting DHL cells to VEN. Moreover, gene expression arrays in VEN-treated CARNAVAL exhibited no differently regulation of known phagocytic factors (data not shown).
Although macrophages have always been considered as the main phagocytes of apoptotic cells,51 ADCP in our study was elevated at very low concentrations of VEN without any induction of apoptosis. Notably, DHL cells deficient for Bax/Bak displayed a comparable rise in phagocytosis as control cells when cotreated with VEN/antibodies. Alternative strategies of how dying cells might facilitate engulfment by macrophages in a caspase-independent manner have been described by activation of necroptosis. However, phospho mixed lineage kinase domain like pseudokinase was not altered in our DHL cells after being subjected to VEN. Thus, VEN-driven phagocytosis is not just a clearance of dying cells by macrophages, but phagocytosis appears to be an apoptosis-independent event in our model system, suggesting additional functions of VEN apart from its known role in apoptosis induction.52 A role of VEN in cancer cell metabolism in an apoptosis-independent manner has been recently described.53 Metabolic reprogramming on VEN exposure could attract macrophages, leading to elevated phagocytosis when combined with antibodies, which is a subject of future investigations. Only very recently unknown phagocytic markers like adipocyte plasma membrane–associated protein or small cell adhesion glycoprotein54 have been discovered, which could be potentially involved. We, however, assume that there are many more unknown factors associated with phagocytosis, which could contribute to VEN-driven phagocytosis.
Overall, our data reveal that VEN markedly boosts ADCP in Bcl-2–expressing hematologic malignancies. Therefore, the application of antibody-based immunotherapy and VEN may contribute to the yet limited therapy options in relapsed and refractory Bcl-2–expressing cancer entities and other hematologic malignancies.
Acknowledgments
The authors thank Katrin Neumann and Katrin Timm-Richert for excellent technical assistance.
The manuscript contains data in partial fulfillment of the requirements for theses by J.H. and K.M. at the Medical Faculty of the Christian-Albrechts University Kiel.
This work was funded by Wilhelm Sander Stiftung (2016.110.1), Deutsche José-Carreras Leukämiestiftung (DJCLS 17 R/2017), and Deutsche Krebshilfe (70113524, 70113533 Mildred-Scheel-Professorship program) grants to D.M.S., C.K., and M.P.
Authorship
Contribution: F.V., J.H., and K.M. designed and performed experiments and analyzed data; T.R., L.L., C.L.G., and M.H. performed experiments and analyzed data; G.C., M. Schrappe, B.B. and J.-P.B. contributed ALL PDX material; A.C. and S.B. provided BL PDX material; D.A. designed experiments and analyzed data; F.-S.F. provided statistical advice; F.V., T.V., B.M.-K., M. Stanulla, M.P., C.K., and D.M.S. supervised the research direction; D.M.S. and F.V. wrote the manuscript; and all authors discussed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Denis M. Schewe, Department of Pediatrics, Otto-von-Guericke University, Leipziger Str 44, 39120 Magdeburg, Germany; e-mail: denis.schewe@med.ovgu.de.
References
Author notes
Main Figure 1 A and 1B / 2 A,B,D,E and complete Main Figure 3 have been part of the poster presented at the 60th Annual Meeting of the American Society of Hematology, 1-4 December 2018, San Diego, CA.
Requests for original data may be submitted to the corresponding author: denis.schewe@med.ovgu.de.
The full-text version of this article contains a data supplement.