Key Points
Dominance of epitopes presented by prevalent HLA alleles permits use of banked CMVpp65CTLs in >90% of transplant recipients.
Immunodominant CMVpp65CTLs restricted by HLA B35 alleles fail to lyse infected cells in vitro or control infection in vivo.
Abstract
We established and characterized a bank of 138 CMVpp65 peptide-specific T-cell (CMVpp65CTLs) lines from healthy marrow transplant donors who consented to their use for treatment of individuals other than their transplant recipient. CMVpp65CTL lines included 131 containing predominantly CD8+ T cells and 7 CD4+ T cells. CD8+ CMVpp65CTLs were specific for 1 to 3 epitopes each presented by one of only 34 of the 148 class I alleles in the bank. Similarly, the 7 predominantly CD4+ CMVpp65CTL lines were each specific for epitopes presented by 14 of 40 HLA DR alleles in the bank. Although the number of HLA alleles presenting CMV epitopes is low, their prevalence is high, permitting selection of CMVpp65CTLs restricted by an HLA allele shared by transplant recipient and hematopoietic cell transplant donor for >90% of an ethnogeographically diverse population of hematopoietic cell transplant recipients. Within individuals, responses to CMVpp65 peptides presented by different HLA alleles are hierarchical. Furthermore, within groups, epitopes presented by HLA B*07:02 and HLA A*02:01 consistently elicit immunodominant CMVpp65CTLs, irrespective of other HLA alleles inherited. All dominant CMVpp65CTLs exhibited HLA-restricted cytotoxicity against epitope loaded targets and usually cleared CMV infections. However, immunodominant CMVpp65CTLs responding to epitopes presented by certain HLA B*35 alleles were ineffective in lysing CMV-infected cells in vitro or controlling CMV infections post adoptive therapy. Analysis of the hierarchy of T-cell responses to CMVpp65, the HLA alleles presenting immunodominant CMVpp65 epitopes, and the responses they induce may lead to detailed algorithms for optimal choice of third-party CMVpp65CTLs for effective adoptive therapy.
Introduction
Cytomegalovirus (CMV) infections remain a major cause of morbidity and mortality after allogeneic hematopoietic cell transplants (alloHCTs). Of the seropositive recipients reactivating CMV, up to 42% remain viremic after 4 weeks of antiviral drug therapy.1 Prolonged treatment promotes outgrowth of resistant strains.2-4 Furthermore, drug toxicities often necessitate their discontinuation,5-7 permitting development of CMV disease.8 Mortality from CMV disease is 3% to 5% after HLA-matched grafts but can be ≥15% after HLA-disparate transplants.9-12
CMV-specific T-cell reconstitution is essential for controlling CMV infections after alloHCT.13,14 Adoptive transfer of alloHCT donor-derived CMV-specific T cells (CMV-CTLs) can clear CMV infections.15-20 However, application is limited by the time required to generate CMV-CTLs and, in some cases, by transplant donor seronegativity or unavailability. Virus-specific T cells (VSTs) from third-party donors can also clear life-threatening Epstein-Barr virus (EBV), CMV, and adenovirus infections.21-27 Initially, third-party VSTs were selected based on HLA allele matching between patient and CTL donor.21 More recently, our group22-24 has shown that selection based on restriction of VSTs by an HLA allele shared by infected cells in the host is critical when CTL donor and patient are not fully HLA matched. However, continued development of evidence-based algorithms is still needed for selection of CTLs predictive of clinical response.
We have established a bank of 138 Good Manufacturing Practice–grade, CMVpp65 peptide-specific T-cell (CMVpp65CTL) lines from specifically consented third-party donors and have used these banked T cells to treat CMV disease or persistent viremia unresponsive to antiviral drugs. Characteristics of CMVpp65CTLs contributing to broad application and clinical activity are described. We found that CMVpp65CTLs, generated from a genetically diverse donor population, respond to only a limited number of CMVpp65 peptides presented by prevalent HLA alleles, thus permitting application to >90% of patients referred for alloHCT. Notably, although CMVpp65CTLs specific for immunodominant epitopes presented by most HLA alleles studied regularly control CMV infections, others, restricted by certain HLA alleles, do not. Thus, immunogenic epitopes and their presenting HLA alleles need to be considered in T-cell selection to enhance the probability of a therapeutic response.
Methods
Donor and patient characteristics
The CMVpp65CTL lines were generated from leukapheresis products obtained from 138 healthy seropositive donors, including 134 donors of alloHCTs and 4 parents of alloHCT recipients, who specifically consented to their use for generation of virus-specific T cells for the treatment of both the recipient of their donated HCT and recipients of other alloHCTs. Third-party CMVpp65CTLs were defined as CMVpp65CTLs from an allogeneic donor other than the donor or recipient of the patient’s alloHCT, or relative thereof.
Donors were typed at high resolution for HLA A, B, C, DR, and DQ alleles. Demographic characteristics of the donors are summarized in supplemental Table 1.
HCT recipients analyzed are described in the Results. All received alloHCTs after myeloablative conditioning per investigational review board–approved protocols at Memorial Sloan Kettering Cancer Center (MSKCC IRB). Those who received CMVpp65CTLs from their HCT donor or from third-party donors were treated on protocols approved by the MSKCC IRB, US Food and Drug Administration, and the National Marrow Donor Program (NMDP). Results of these trials are reported separately19 (S.P., A.H., E.D., Parastoo B. Dahi, Irene Rodriguez-Sanchez, Michael Curry, Audrey Maugen, Genovefa Papanicolaou, Yiqi Su, JinJuan Yao, Farid Boulad, Hugo Castro-Malaspina, Christina Cho, Kevin Curran, Sergio Giralt, Nancy Kernan, Guenther Koehne, Ann Jakubowski, Esperanza Papadopoulos, Miguel-Angel Perales, Ioannis Politikos, Craig S. Sauter, Roni Tamari, James W. Young, and R.J.O., manuscript submitted).
Generation of CMVpp65CTL lines
Peripheral blood mononuclear cells (PBMCs) were isolated from leukapheresis products by density gradient centrifugation.23 Autologous donor-derived cytokine-activated monocytes (CAMs) and EBV-transformed B-cell lines were generated from PBMCs as previously described.23 CD3+ T cell–enriched fractions, isolated from PBMCs by depletion of adherent monocytes and immunoadsorption of CD56+ natural killer cells to paramagnetic beads (Miltenyi Biotec, Bergisch Gladbach, Germany), were stimulated at an effector-to-stimulator ratio of 20:1 with irradiated (6000 cGy) autologous CAMs loaded with a pool of overlapping pentadecapeptides spanning the sequence of CMVpp65 (Invitrogen, Boston, MA). They were propagated in vitro with weekly restimulation at an effector-to-stimulator ratio of 4:1 and supplementation with interleukin-2 (IL-2) beginning at day 10 as previously described.19,28,29 After 28 days, T cells were harvested and tested for antigen-specific cytotoxicity, lack of alloreactivity,19,23 microbiological sterility, and endotoxin levels. Aliquots of CMVpp65CTLs meeting release criteria were cryopreserved in doses for subsequent administration.
Characterization of CMVpp65CTL lines.
Yields of CD3+ CD4+ and CD3+ CD8+ T cells and content of CD3– CD56+ natural killer cells and CD20+ B cells were quantitated by fluorescence-activated cell sorting analysis. Interferon-γ (IFN-γ)+ CD8+ and CD4+ CMVpp65CTLs were quantitated by using a technique modified from Waldrop et al,30 as previously described.19 For comparative analyses of HLA A*0201 and HLA B*3501-restricted, epitope-specific CMVpp65CTLs, these CMVpp65CTLs were isolated by immunoadsorption to CMVpp65 epitope/HLA tetramers (Beckman Coulter, Fullerton, CA) as previously described.19
Functional characterization and mapping of epitope specificity and HLA restriction.
CMVpp65CTLs were assessed for CMVpp65-specific cytolytic activity using a standard chromium-51 release assay. Targets included CMVpp65 peptide-loaded and unloaded autologous and fully allogeneic phytohemagglutinin-stimulated blasts. We identified CMVpp65CTL epitope specificities using a mapping grid of CMVpp65 peptide subpools as previously described,29 quantitating IFN-γ+ T-cell responses to specific CMVpp65 peptides or peptide subpools after secondary stimulation with autologous peptide-loaded CAMs.29
HLA restrictions of epitope-specific CMVpp65CTLs were identified by measuring their cytotoxic activity (effector-to-target ratio of 25:1) against a panel of allogenic phytohemagglutinin-stimulated blasts loaded with the epitope, each sharing a single HLA allele with the CMVpp65CTL, as previously described. Cytolysis >10% is considered reactive.21,29
HLA ligand-binding predictions were performed with netMHC pan 4.1 in BA mode,31 and results are provided as percentile ranks for each peptide epitope compared with peptides most likely to be presented by the HLA allele. For HLA class I peptides, those with 2% rank or less are considered binders by the algorithm. We did not include binding predictions for ligands presented by class II alleles because of their unreliability.
Functional avidity and cytotoxicity of epitope-specific CMV-CTLs.
To assess avidity, HLA A*02:01– and HLA B*35:01–restricted CMVpp65CTLs were incubated for 16 hours with serial 10-fold dilutions of their epitopes (ie, the NLVPMVATV peptide presented by HLA A*02:01 or the IPSINVHHY peptide presented by HLA B*35:01 [MBL International Corporation, Woburn, MA]), and their generation of intracellular IFN-γ T cells quantitated by flow cytometry. To assess T-cell cytotoxic activity against CMV-infected targets, autologous donor-derived dendritic cells (DCs) were generated and infected with a green fluorescent protein–expressing variant of the TB40/E endotheliotropic strain of CMV for 2 hours; they were then washed and incubated in RPMI 1640 supplemented with tumor necrosis factor-α (20 mg/mL), IL-6 (1000 IU/mL), IL-1β 5 ng/mL, prostaglandin E2 (25 IU/mL), granulocyte-macrophage colony-stimulating factor (1000 IU/mL), and IL-4 (500 IU/mL) for 72 hours. Cytotoxic activity of NLVPMVATV-specific HLA A*02:01–restricted and IPSINVHHY-specific HLA B*35:01–restricted T cells against infected DCs was assessed by using a standard chromium-51 release assay.
Biostatistics
In analyses of immunodominance, responses of groups of T-cell lines to specific epitopes or restricted by specific HLA alleles were compared by using the Wilcoxson rank sum test. Exact 95% binomial confidence intervals were used to infer the probability of dominance relative to other alleles. P values were generated from an exact binomial test that the probability of dominance was equal to .5. Fisher’s exact tests were used to determine if there was a relationship between HLA restriction alleles and clinical response to adoptive therapy or development of CMV viremia or disease after transplantation. Due to the exploratory nature of the latter analyses, no adjustment for multiple testing was undertaken.
Results
Characteristics of the banked CMVpp65CTLs
Of 138 CMVpp65CTL lines, 131 contained predominantly CD8+ T cells and 7 CD4+ T cells. Each line exhibited CMVpp65-specific cytotoxicity and IFN-γ production in response to CMVpp65 peptide-loaded autologous CAMs with low or absent reactivity against unloaded autologous or allogeneic CAMs.
Despite diversity of donors, CMVpp65CTL lines selectively respond to only 48 CMVpp65 epitopes presented by a limited number of prevalent HLA alleles
Demographic characteristics of donors to our bank are summarized in supplemental Table 1. These donors inherited 188 different HLA A (n = 37), B (n = 71), C (n = 40), or DR (n = 40) alleles, reflecting the ethnically diverse alloHCT candidates at our center and their related or unrelated donors.
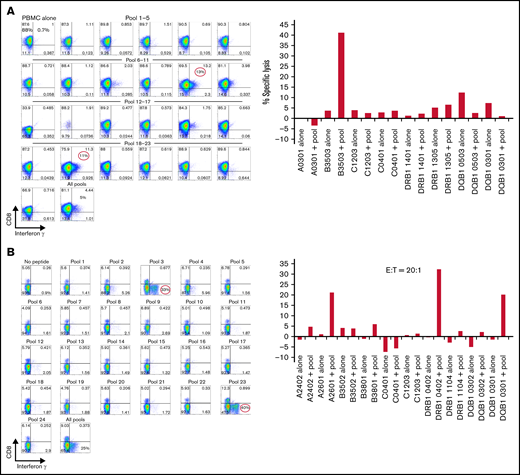
Of 138 CMVpp65CTL lines, 96 (70%) were restricted by a single class I (n = 90) or class II (n = 6) HLA allele; 31 (22%) were restricted by 2 alleles and 11 (8%) by 3 alleles. As shown for one representative CTL line in Figure 1A, epitope mapping exhibited dominant IFN-γ + CD8+ T-cell responses to peptide subpools 10 and 19 that share peptide 82 bearing the epitope QAIRETVEL, which is presented by HLA-B*35:03 as demonstrated by in vitro lysis of peptide-loaded B-cell lines selectively sharing HLA-B*35:03. In another CTL line (Figure 1B), CD4+ IFN-γ+ T cells responding to pools 3 and 23 uniquely sharing peptide 123 were dominant. These CD4+ IFN-γ+ T cells specifically lysed HLA-DR*B1 04:02 cells loaded with this 15-mer LARNLVPMVATV peptide. Both lines also contained nondominant CMVpp65CTLs specific for peptides presented by other alleles, as noted in the legend for Figure 1.
Mapping of CMVpp65 peptide epitope specificity and HLA restriction of a representative CMVpp65CTL line. (A) IFN-γ responses of T cells against a matrix of CMVpp65 peptide subpools. CD8+ T cells of the CMVpp65CTL line respond selectively to subpools #10 and #19, which uniquely share the QAIRETVEL peptide. Furthermore, these CMVpp65CTLs selectively lyse phytohemagglutinin-stimulated blasts loaded with this peptide that share with the CMVpp65CTLs the HLA-B*3503 and not peptide-loaded phytohemagglutinin-stimulated blasts sharing other HLA alleles in the CMVpp65CTL genotype. This line also contains a nondominant population of IFN-γ+ CD8+ T cells specific for peptide 44 uniquely shared by subpools 8 and 16 that contains the QWKEPDVYYT epitope that can be presented by the donor’s HLA C0401 allele. However, these T cells were not cytotoxic against peptide-loaded targets sharing any single HLA allele, with donor preventing ascertainment of HLA restriction. Note that a low-level (11%) alloresponse to DQB10503 alone and not against the same cells loaded with CMVpp65 peptides was also seen. (B) This CMVpp65CTL line contains dominant CD4+ T cells that selectively generate IFN-γ in response to subpools 3 and 23, which uniquely share the LARNLVPMVATV peptide, and lyse targets loaded with the peptide that selectively share only the HLA-DRB*1-0402 allele with the CMVpp65CTLs. This line also contains 2 other CMVpp65CTL populations: a small population of CD8+ T cells restricted by HLA A2601 specific for SHIMLDVAF, a CMVpp65 peptide in 15-mer #72 shared by pools 12 and 18, and an IFN-γ+ cytolytic CD4+ T-cell population restricted by HLA DQB, 0301, specific for subpools 8 and 20 sharing the 15-mer #92: EHPTFTSQYRIQGKL. E:T, effector-to-target ratio.
Mapping of CMVpp65 peptide epitope specificity and HLA restriction of a representative CMVpp65CTL line. (A) IFN-γ responses of T cells against a matrix of CMVpp65 peptide subpools. CD8+ T cells of the CMVpp65CTL line respond selectively to subpools #10 and #19, which uniquely share the QAIRETVEL peptide. Furthermore, these CMVpp65CTLs selectively lyse phytohemagglutinin-stimulated blasts loaded with this peptide that share with the CMVpp65CTLs the HLA-B*3503 and not peptide-loaded phytohemagglutinin-stimulated blasts sharing other HLA alleles in the CMVpp65CTL genotype. This line also contains a nondominant population of IFN-γ+ CD8+ T cells specific for peptide 44 uniquely shared by subpools 8 and 16 that contains the QWKEPDVYYT epitope that can be presented by the donor’s HLA C0401 allele. However, these T cells were not cytotoxic against peptide-loaded targets sharing any single HLA allele, with donor preventing ascertainment of HLA restriction. Note that a low-level (11%) alloresponse to DQB10503 alone and not against the same cells loaded with CMVpp65 peptides was also seen. (B) This CMVpp65CTL line contains dominant CD4+ T cells that selectively generate IFN-γ in response to subpools 3 and 23, which uniquely share the LARNLVPMVATV peptide, and lyse targets loaded with the peptide that selectively share only the HLA-DRB*1-0402 allele with the CMVpp65CTLs. This line also contains 2 other CMVpp65CTL populations: a small population of CD8+ T cells restricted by HLA A2601 specific for SHIMLDVAF, a CMVpp65 peptide in 15-mer #72 shared by pools 12 and 18, and an IFN-γ+ cytolytic CD4+ T-cell population restricted by HLA DQB, 0301, specific for subpools 8 and 20 sharing the 15-mer #92: EHPTFTSQYRIQGKL. E:T, effector-to-target ratio.
Using this strategy, we identified 48 CMVpp65 epitopes that elicited responses, including 34 presented by 57 class I alleles (17 HLA-A, 35 HLA-B, and 5 HLA-C alleles) and 14 by class II alleles (13 HLA DR and 1 HLA DQ alleles). A subset of 14 epitopes presented by 22 class I and 4 class II HLA alleles elicited immunodominant HLA-restricted T-cell responses in 121 (88%) of 138 T-cell lines in the bank (Table 1). Of the 10 most prevalent HLA class I alleles in the bank, 8 were the presenting alleles for 76% of the lines in the bank (HLA-A*02:01 [n = 51], B*07:02 [n = 35], B*08:01 [n = 28], A*01:01 [n = 27], B*35 [including B*35:01, 02, 03, 08, and 11; n = 25], A*24:02 [n = 25], B*44:01,44:02,44:03 [n = 30], and A*11:01 [n = 16]). Notably, although CMVpp65 epitopes have been predicted for HLA A*03:01 and HLA B*15 by binding algorithms, T cells restricted by these prevalent alleles were not detected among donors inheriting these alleles. However, this may, in part, reflect the immunodominance of CMVpp65CTLs restricted by coinherited alleles, as 18 of 31 HLA A*0301+ CMVpp65CTL lines coinherited the consistently immunodominant allele, HLA B0702 (with which HLA A0301 is in linkage disequilibrium), and 3 inherited HLA A0201.
List of the 14 CMVpp65 epitopes that elicited specific responses from >1 individual and their presenting HLA allele
| Recurring HLA class I and HLA class II epitopes38 . | |||||
|---|---|---|---|---|---|
| . | Epitope . | Presenting HLA allele . | CTL lines responding (n) . | Percentile rank (BA) . | Length . |
| 1 | NLVPMVATV | HLA-A*02:01 | 40 | 0.259 | 9 |
| 2 | TPRVTGGGAM | HLA-B*07:02 | 23 | 0.0094 | 10 |
| 3 | RPHERNGFTV | HLA-B*07:02 | 10 | 0.0788 | 10 |
| 4 | HERNGFTVL | HLA-A*26:01 | 2 | 10.362 | 9 |
| HERNGFTVL | HLA-B*40:01 | 2 | 0.0901 | 9 | |
| HERNGFTVL | HLA-B*40:02 | 1 | 0.0392 | 9 | |
| HERNGFTVL | HLA-B*40:06 | 1 | 0.3451 | 9 | |
| HERNGFTVL | HLA-B*44:02 | 1 | 2.2282 | 9 | |
| HERNGFTVL | HLA-B*44:03 | 3 | 2.4214 | 9 | |
| 5 | QAIRETVEL | HLA-B*35:01 | 1 | 1.288 | 9 |
| QAIRETVEL | HLA-B*35:02 | 1 | 0.241 | 9 | |
| QAIRETVEL | HLA-B*35:03 | 2 | 0.178 | 9 | |
| QAIRETVEL | HLA-B*35:08 | 2 | 1.47 | 9 | |
| QAIRETVEL | HLA-B*35:11 | 1 | 1.312 | 9 | |
| 6 | QMWQARLTV | HLA-B*52:01 | 3 | 0.1784 | 9 |
| 7 | QYDPVAALF | HLA-A*24:02 | 3 | 0.1439 | 9 |
| QYDPVAALF | HLA-A*24:07 | 1 | 0.16 | 9 | |
| 8 | YSEHPTFTSQY | HLA-A*01:01 | 4 | 0.021 | 11 |
| 9 | RPHERNGFTVL | HLA-B*42:01 | 2 | 0.024 | 11 |
| RPHERNGFTVL | HLA-B*42:02 | 1 | 0.039 | 11 | |
| 10 | NVHHYPSAAER | HLA-A*68:01 | 2 | 0.442 | 11 |
| 11 | IPSINVHHY | HLA-B*35:01 | 1 | 0.0747 | 9 |
| IPSINVHHY | HLA-B*53:01 | 1 | 0.1222 | 9 | |
| 12 | FTSQYRIQGKLEYRH | HLA-DRB1*11:01 | 2 | 15 | |
| FTSQYRIQGKLEYRH | HLA-DRB1*11:04 | 1 | 15 | ||
| FTSQYRIQGKLEYRH | HLA-DRB1*15:01 | 1 | 15 | ||
| 13 | KYQEFFWDANDIYRI | HLA-DRB1*11:01 | 4 | 15 | |
| KYQEFFWDANDIYRI | HLA-DRB1*11:04 | 1 | 15 | ||
| KYQEFFWDANDIYRI | HLA-DRB1*15:01 | 1 | 15 | ||
| KYQEFFWDANDIYRI | HLA-DRB1*07:01 | 1 | 15 | ||
| 14 | EDVPSGKLFMHVTLG | HLA-DRB1*11:01 | 1 | 15 | |
| EDVPSGKLFMHVTLG | HLA-DRB1*11:04 | 1 | 15 | ||
| EDVPSGKLFMHVTLG | HLA-DRB1*15:01 | 1 | 15 | ||
| EDVPSGKLFMHVTLG | HLA-DRB1*07:01 | 1 | 15 | ||
| Recurring HLA class I and HLA class II epitopes38 . | |||||
|---|---|---|---|---|---|
| . | Epitope . | Presenting HLA allele . | CTL lines responding (n) . | Percentile rank (BA) . | Length . |
| 1 | NLVPMVATV | HLA-A*02:01 | 40 | 0.259 | 9 |
| 2 | TPRVTGGGAM | HLA-B*07:02 | 23 | 0.0094 | 10 |
| 3 | RPHERNGFTV | HLA-B*07:02 | 10 | 0.0788 | 10 |
| 4 | HERNGFTVL | HLA-A*26:01 | 2 | 10.362 | 9 |
| HERNGFTVL | HLA-B*40:01 | 2 | 0.0901 | 9 | |
| HERNGFTVL | HLA-B*40:02 | 1 | 0.0392 | 9 | |
| HERNGFTVL | HLA-B*40:06 | 1 | 0.3451 | 9 | |
| HERNGFTVL | HLA-B*44:02 | 1 | 2.2282 | 9 | |
| HERNGFTVL | HLA-B*44:03 | 3 | 2.4214 | 9 | |
| 5 | QAIRETVEL | HLA-B*35:01 | 1 | 1.288 | 9 |
| QAIRETVEL | HLA-B*35:02 | 1 | 0.241 | 9 | |
| QAIRETVEL | HLA-B*35:03 | 2 | 0.178 | 9 | |
| QAIRETVEL | HLA-B*35:08 | 2 | 1.47 | 9 | |
| QAIRETVEL | HLA-B*35:11 | 1 | 1.312 | 9 | |
| 6 | QMWQARLTV | HLA-B*52:01 | 3 | 0.1784 | 9 |
| 7 | QYDPVAALF | HLA-A*24:02 | 3 | 0.1439 | 9 |
| QYDPVAALF | HLA-A*24:07 | 1 | 0.16 | 9 | |
| 8 | YSEHPTFTSQY | HLA-A*01:01 | 4 | 0.021 | 11 |
| 9 | RPHERNGFTVL | HLA-B*42:01 | 2 | 0.024 | 11 |
| RPHERNGFTVL | HLA-B*42:02 | 1 | 0.039 | 11 | |
| 10 | NVHHYPSAAER | HLA-A*68:01 | 2 | 0.442 | 11 |
| 11 | IPSINVHHY | HLA-B*35:01 | 1 | 0.0747 | 9 |
| IPSINVHHY | HLA-B*53:01 | 1 | 0.1222 | 9 | |
| 12 | FTSQYRIQGKLEYRH | HLA-DRB1*11:01 | 2 | 15 | |
| FTSQYRIQGKLEYRH | HLA-DRB1*11:04 | 1 | 15 | ||
| FTSQYRIQGKLEYRH | HLA-DRB1*15:01 | 1 | 15 | ||
| 13 | KYQEFFWDANDIYRI | HLA-DRB1*11:01 | 4 | 15 | |
| KYQEFFWDANDIYRI | HLA-DRB1*11:04 | 1 | 15 | ||
| KYQEFFWDANDIYRI | HLA-DRB1*15:01 | 1 | 15 | ||
| KYQEFFWDANDIYRI | HLA-DRB1*07:01 | 1 | 15 | ||
| 14 | EDVPSGKLFMHVTLG | HLA-DRB1*11:01 | 1 | 15 | |
| EDVPSGKLFMHVTLG | HLA-DRB1*11:04 | 1 | 15 | ||
| EDVPSGKLFMHVTLG | HLA-DRB1*15:01 | 1 | 15 | ||
| EDVPSGKLFMHVTLG | HLA-DRB1*07:01 | 1 | 15 | ||
Similarly, 11 of 18 HLA B15+ CMVpp65CTL lines were restricted by HLA A0201 or HLA B0702. In all, 25 of 31 HLA A*0301+ and 17 of 18 HLA B15+ CMVpp65CTLs are specific for epitopes presented by immunodominant HLA alleles in Table 1.
As shown in Table 1, certain epitopes induced T-cell responses when presented by only one HLA allele (eg, TPRVTGGGAM presented by HLA B*0:702). Other epitopes could stimulate T-cell responses when presented by several different HLA alleles (eg, the HERNGFTVL peptide induced epitope-specific CMVpp65CTLs when presented by HLA A*26:01, 3 allelic variants of HLA B*40, and 2 of HLA B*44). These peptides were potent binders to each of their presenting HLA alleles, by percentile rank, with the exception of the HERNGFTVL peptide when presented by HLA A*26:01.
Four CMVpp65 15-mer peptides contained overlapping epitopes that could be presented by both HLA class I and class II alleles (Table 2). Individuals coinheriting both the class I and class II allele presenting any of these epitopes (eg, HLA A*02:01 and DRB1*04:01, or HLA B*07:02 and DRB1*15:01) generated significantly greater CD3+ IFN-γ+ T-cell responses against these CMVpp65 peptides than donors who did not coinherit the presenting class II allele but had a CD4+ CMV-CTL response specific for an alternate, non-overlapping class II epitope (P < .001) (Figure 2), or those who generated only a class I restricted response and no class II restricted response against the CMVpp65 peptide pool (P < .001).
Peptides containing shared HLA class I and class II epitopes eliciting CTL responses
| No. . | Epitope . | Presenting HLA alleles . | CTL line responding (n) . | Immunodominant response (class I/class II) . | % CD8+ IFN-γ+ CTLs . | % CD4+ IFN-γ+ CTLs . | ||
|---|---|---|---|---|---|---|---|---|
| Maximum . | Range . | Maximum . | Range . | |||||
| 1 | AGILARNLVPMVATV (489-503) | A0201 DRB1 0401 DRB1 0402 DRB1 0404 | 5 | Class I (A0201) | 61.4 | 12-61.4 | 10.7 | 1.3-10.7 |
| 2 | QPFMRPHERNGFTVL (261-275) | B0702 B0801 DRB1 0301 DRB1 1501 | 6 B7/DR15 = 3 B8/DR03 = 3 | Class I (B0702, B0801) | 44.7 | 15.7-44.7 | 8.7 | 3-8.7 |
| 3 | PQYSEHPTFTSQYRI (361-375) | A0101 B801 DRB1 0301 | 2 | Class I (A0101, B0801) | 29.2 | 11.2-29.2 | 1.2 | 0.5-1.2 |
| 4 | DVEEDLTMTRNPQPF (249-263) | B0801 DRB1 0301 | 2 | Codominant class I and class II | 14 | 9-14 | 6.5 | 4-6.5 |
| No. . | Epitope . | Presenting HLA alleles . | CTL line responding (n) . | Immunodominant response (class I/class II) . | % CD8+ IFN-γ+ CTLs . | % CD4+ IFN-γ+ CTLs . | ||
|---|---|---|---|---|---|---|---|---|
| Maximum . | Range . | Maximum . | Range . | |||||
| 1 | AGILARNLVPMVATV (489-503) | A0201 DRB1 0401 DRB1 0402 DRB1 0404 | 5 | Class I (A0201) | 61.4 | 12-61.4 | 10.7 | 1.3-10.7 |
| 2 | QPFMRPHERNGFTVL (261-275) | B0702 B0801 DRB1 0301 DRB1 1501 | 6 B7/DR15 = 3 B8/DR03 = 3 | Class I (B0702, B0801) | 44.7 | 15.7-44.7 | 8.7 | 3-8.7 |
| 3 | PQYSEHPTFTSQYRI (361-375) | A0101 B801 DRB1 0301 | 2 | Class I (A0101, B0801) | 29.2 | 11.2-29.2 | 1.2 | 0.5-1.2 |
| 4 | DVEEDLTMTRNPQPF (249-263) | B0801 DRB1 0301 | 2 | Codominant class I and class II | 14 | 9-14 | 6.5 | 4-6.5 |
Augmented functional activity of CMVpp65CTLs responding to pentadecapeptides containing overlapping epitopes presented by HLA class I and class II alleles. Comparison of IFN-γ CD3+ T-cell responses to the CMVpp65 15-mer peptide pool in CMVpp65CTLs derived from donors who coinherited both the class I and class II HLA alleles presented epitopes in an overlapping 15-mer peptide vs responses in donors generating both CD4+ and CD8+ T cells to CMVpp65 peptides that do not overlap or to individuals generating only a CD8+ CMVpp65–specific T-cell response.
Augmented functional activity of CMVpp65CTLs responding to pentadecapeptides containing overlapping epitopes presented by HLA class I and class II alleles. Comparison of IFN-γ CD3+ T-cell responses to the CMVpp65 15-mer peptide pool in CMVpp65CTLs derived from donors who coinherited both the class I and class II HLA alleles presented epitopes in an overlapping 15-mer peptide vs responses in donors generating both CD4+ and CD8+ T cells to CMVpp65 peptides that do not overlap or to individuals generating only a CD8+ CMVpp65–specific T-cell response.
HLA alleles presenting immunogenic CMVpp65 epitopes permit treatment of a diverse patient population
Among 446 consecutive HCT recipients at our center, we identified a CMVpp65CTL line restricted by an HLA allele shared by patient and donor and matched at high resolution for at least 2 HLA alleles for 222 (93%) of 239 recipients of HLA-matched related or unrelated HCTs, 125 (91%) of 137 HLA nonidentical HCT recipients and 60 (86%) of 70 cord blood transplant recipients. These 70 were patients for whom a 7/8 HLA allele matched unrelated donor could not be identified among the 33 million unrelated donors accessible through the NMDP.32,38 We have also received 540 inquiries regarding availability of CMVpp65 T cells in the bank and identified an appropriately HLA-restricted line for 94.8%.
Epitopes eliciting immunodominant CMVpp65-specific CD8+ T-cell responses are presented by a hierarchy of HLA class I alleles
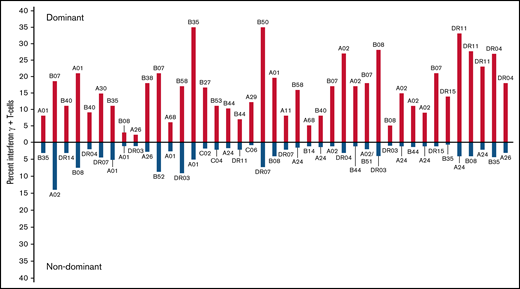
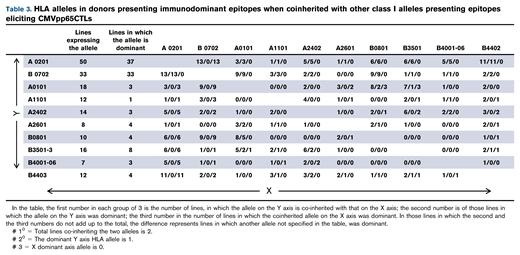
We identified the immunodominant T-cell response as the response to a peptide epitope presented by a specific HLA allele that elicited the highest proportion of IFN-γ+ T cells in the line. Figure 3 illustrates quantitative differences between the immunodominant and second largest population of IFN-γ+ CMVpp65-specific T cells for 41 of 42 CMVpp65CTL lines specific for >1 epitope. In 124 (90%) of 138 CMVpp65CTL lines, immunodominant T cells were CD8+ and specific for an epitope presented by an HLA-A (n = 17), HLA-B (n = 35), or HLA-C (n = 5) allele; 14 (10%) contained CD4+ T cells recognizing a dominant epitope presented by an HLA DR (n = 13) or DQ (n = 1) allele. Previously, CMVpp65CTLs restricted by HLA B*07:02 had been reported to be dominant among individuals coinheriting HLA A*02:01 and B*07:02.25,26 To confirm this and identify other hierarchical relationships among presenting HLA alleles, we analyzed CMVpp65CTL lines in the bank that coinherited at least 2 of 8 HLA alleles that repeatedly presented CMVpp65 epitopes eliciting IFN-γ+ CMVpp65CTL responses, using 95% binomial confidence intervals to infer the probability of dominance relative to other alleles, with P values generated from an exact binomial test that the probability of dominance is equal to 0.5. As shown in Tables 3 and 4, CMVpp65CTLs restricted by HLA B*07:02 were dominant in 33 of 33 lines inheriting this allele, irrespective of other HLA alleles inherited, including 13 of 13 coinheriting HLA A0201 (P < .001). However, epitope specificity of HLA B*07:02–restricted CMVpp65CTLs was not uniform, with 23 specific for TPRVTGGGAM and 10 for RPHERNGFTV. The second in this hierarchy is HLA A*02:01: of 50 CMVpp65CTL lines inheriting HLA A*02:01, NLVPMVATV-specific, HLA A*02:01–restricted T cells were dominant in 36 of 37 lines that did not coinherit HLA B*07:02 (P < .001). We did not detect statistically significant intragroup dominance for other coinherited HLA alleles (Table 4). However, epitopes presented by 2 of the most prevalent alleles in the bank, HLA A*01:01 and HLA A*11:01, were significantly less likely to be dominant than those presented by other alleles analyzed (P = .008 and .006, respectively). Again, this finding potentially reflects the immunodominance of coinherited HLA alleles as the other alleles presenting the epitopes in Table 1 are dominant in 26 of 29 HLA A*0101 and 12 of 13 HLA A*1101 CMVpp65CTL lines.
Comparison of dominant vs nondominant responses in 41 individuals who generated IFN-γ+ CMVpp65CTLs against >1 peptide epitope. Dominant responses are quantitated above the x-axis; nondominant responses from the same individual are quantitated below the axis. The HLA restriction of each T cell is above (dominant) or below (nondominant) each response compared.
Comparison of dominant vs nondominant responses in 41 individuals who generated IFN-γ+ CMVpp65CTLs against >1 peptide epitope. Dominant responses are quantitated above the x-axis; nondominant responses from the same individual are quantitated below the axis. The HLA restriction of each T cell is above (dominant) or below (nondominant) each response compared.
Analysis of probability that specific coinherited HLA allele will be immunodominant
| Allele . | Lines expressing an allele coinherited by other alleles in the table . | Lines in which allele is dominant . | Estimated probability of dominance relative to other alleles . | 95% Confidence interval . | P value from binomial test P = 0.5 . |
|---|---|---|---|---|---|
| A 0201 | 50 | 37 | 0.74 | 0.60, 0.85 | <.001 |
| B0702 | 33 | 33 | 1.00 | 0.89, 1.00 | <.001 |
| A0101 | 18 | 3 | 0.17 | 0.04, 0.41 | .008 |
| A1101 | 12 | 1 | 0.08 | 0.00, 0.38 | .006 |
| A2402 | 14 | 3 | 0.21 | 0.05, 0.51 | .06 |
| A2601 | 8 | 4 | 0.50 | 0.16, 0.84 | 1.0 |
| B0801 | 10 | 4 | 0.40 | 0.12, 0.74 | .75 |
| B3501-3 | 16 | 8 | 0.50 | 0.25, 0.75 | 1.0 |
| B4001-06 | 7 | 3 | 0.43 | 0.10, 0.82 | 1.0 |
| B4403 | 12 | 4 | 0.33 | 0.10, 0.65 | .39 |
| Allele . | Lines expressing an allele coinherited by other alleles in the table . | Lines in which allele is dominant . | Estimated probability of dominance relative to other alleles . | 95% Confidence interval . | P value from binomial test P = 0.5 . |
|---|---|---|---|---|---|
| A 0201 | 50 | 37 | 0.74 | 0.60, 0.85 | <.001 |
| B0702 | 33 | 33 | 1.00 | 0.89, 1.00 | <.001 |
| A0101 | 18 | 3 | 0.17 | 0.04, 0.41 | .008 |
| A1101 | 12 | 1 | 0.08 | 0.00, 0.38 | .006 |
| A2402 | 14 | 3 | 0.21 | 0.05, 0.51 | .06 |
| A2601 | 8 | 4 | 0.50 | 0.16, 0.84 | 1.0 |
| B0801 | 10 | 4 | 0.40 | 0.12, 0.74 | .75 |
| B3501-3 | 16 | 8 | 0.50 | 0.25, 0.75 | 1.0 |
| B4001-06 | 7 | 3 | 0.43 | 0.10, 0.82 | 1.0 |
| B4403 | 12 | 4 | 0.33 | 0.10, 0.65 | .39 |
Exact 95% binomial confidence intervals were used to infer the probability of dominance relative to other alleles. The P‐values were generated from an exact binomial test that the probability of dominance was equal to 0.5.
T-cell responses to epitopes presented by specific HLA alleles also influence response to adoptive therapy
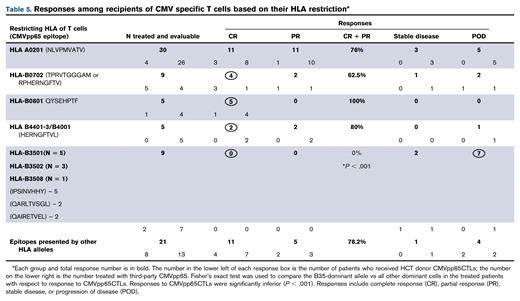
To assess influence of epitope specificity and HLA restriction on CMVpp65CTL activity in vivo, we examined responses of 79 alloHCT recipients who received either HCT donor-derived (n = 20) or HLA partially matched (≥2 HLA alleles) third-party CMVpp65CTLs (n = 59) restricted by an HLA allele shared by the patient and the patient’s HCT donor as treatment for CMV disease or persistent viremia unresponsive to >2 weeks of antiviral drugs.19,25 As shown in Table 5, response rates (complete response + partial response) among 48 patients who received CMVpp65CTLs specific for epitopes presented by HLA A*02:01, B*07:02, B*08:01 or the HERNGFTVL peptide presented by variants of HLA B44 and B40 ranged from 62% to 100%. Of the 21 patients treated with CMVpp65CTLs, restricted by other HLA alleles excluding HLA-B*35, 76.2% responded. In contrast, all 9 patients infused with CMVpp65CTLs restricted by HLA B*3501, B*3502, or B*3508 CMVpp65CTLs, including all 7 who received third-party CMVpp65CTLs, failed treatment (P = .001).
In the trial report (S.P., A.H., E.D., Parastoo B. Dahi, Irene Rodriguez-Sanchez, Michael Curry, Audrey Maugen, Genovefa Papanicolaou, Yiqi Su, JinJuan Yao, Farid Boulad, Hugo Castro-Malaspina, Christina Cho, Kevin Curran, Sergio Giralt, Nancy Kernan, Guenther Koehne, Ann Jakubowski, Esperanza Papadopoulos, Miguel-Angel Perales, Ioannis Politikos, Craig S. Sauter, Roni Tamari, James W. Young, and R.J.O., manuscript submitted), we have detailed analyses of these patients. However, at initiation of infusions, we found no differences between those treated with CMVpp65CTLs restricted by an HLA B35 allele and those treated with CMVpp65CTLs restricted by other HLA class I alleles in terms of disease parameters, level of viremia, the number of antiviral drugs previously received, their duration, or the proportion of patients with mutant CMV strain resistant to drugs. Types of transplant, degree of HLA matching, and exposures to immunosuppressive drugs also did not differ. Degree of HLA matching of CMVpp65CTLs to patient and HCT donor, their content of CD8+ T cells (including TCM, TEM, and TERA), and the doses and number of infusions were also comparable and could not explain these differences in outcome.
Because CMVpp65CTL donors in the bank were also donors of alloHCT, we evaluated whether the immunodominance of T-cell lines generated from these donors correlated with outcome of CMV infections in recipients of their transplants. Accordingly, we evaluated 113 CMV seropositive recipients of either T cell–depleted (n = 86) or unmodified (n = 27) transplants from donors of CMVpp65CTLs in the bank (Table 6). Recipients of HCT from donors whose immunodominant CMVpp65CTLs were specific for epitopes presented by HLA B*07:02 or A*02:01 had a low incidence of CMV disease and cleared CMV with antiviral agents in 92% and 96.5% of cases, respectively. Similarly, recipients of HCTs from donors generating CMVpp65CTLs restricted by HLA-A*01:01, HLA-B*08:01, or A*24:02 consistently cleared viremia without CMV disease. Those whose CMVpp65CTLs were specific for epitopes presented by 13 other class I HLA alleles also cleared. However, of 8 recipients of HCT from donors with dominant CMVpp65CTLs specific for epitopes presented by HLA B*35 alleles, 3 (37.5%) developed disease and failed all therapies (P = .048). In addition, of 14 recipients of HCT from donors with dominant CMVpp65CTLs specific for epitopes presented by HLA class II alleles, 7 (50%) developed CMV disease and 5 (35%) failed therapy (P = .01). Of note, 4 of these 14 donor/recipient pairs also shared a B*35 allele, of whom 3 failed therapy. In contrast, of 9 whose donors inherited an HLA B*35 allele but whose CMVpp65CTLs were specific for dominant epitopes presented by a coinherited HLA A*02:01 (n = 5), B*07:02 (n = 1), or other class I HLA allele (n = 3), none developed CMV disease. These data, although limited, provide evidence that the level of protection provided by CMV-CTLs is influenced by the epitope eliciting the donor’s immunodominant T-cell response and its presenting HLA allele.
Evaluation of CMV infections in seropositive recipients of HCT from donors in the bank of CMVpp65CTLs
| HLA restriction of immunodominant CMVpp65CTL from HCT donor . | N . | CMV reactivation . | Level Of CMV reactivation . | Disease . | Failed therapy . | |||
|---|---|---|---|---|---|---|---|---|
| Yes . | No . | No . | Low (2-13/slide; <10 000) . | High >100 >10 000 . | ||||
| B0702 | 25 | 19 (75%) | 6 | 6 | 10 | 9 | 3 (15%) | 2 (8%) |
| A 0201 DOM | 28 | 21 (75%) | 7 | 7 | 16 | 5 | 2 (9%) | 1 (3.5%) |
| A 1101 DOM | 1 | 1 (100%) | 0 | 0 | 1 | 0 | 0 (0%) | 0 (0%) |
| A 0101 DOM | 4 | 4 (100%) | 0 | 0 | 2 | 2 | 0 (0%) | 0 (0%) |
| A 2402 DOM | 4 | 4 (100%) | 0 | 0 | 2 | 2 | 0 (0%) | 0 (0%) |
| A 2601 DOM | 4 | 2 (50%) | 2 | 2 | 1 | 1 | 1 (25%) | 1 (25%) |
| B 0801 DOM | 3 | 3 (100%) | 0 | 0 | 1 | 2 | 0 (0%) | 0 (0%) |
| B 40, 42, 44 DOM HERN Specific | 9 | 6 (66%) | 3 | 3 | 2 | 4 | 2 (24%) | 1 (11%) |
| B 35 DOM | 8 | 7 (87.5%) | 1 | 1 | 3 | 4 | 3 (37.5%) | 3 (37.5%) *P = .048 |
| Other HLA A,B,C alleles DOM | 13 | 11 (84%) | 2 | 2 | 4 | 7 | 2 (15%) | 0 (0%) |
| HLA DR alleles DOM | 14 | 13 (92%) | 1 | 1 | 4 | 9 | 7 (50%) | 5 (35%) |
| HLA restriction of immunodominant CMVpp65CTL from HCT donor . | N . | CMV reactivation . | Level Of CMV reactivation . | Disease . | Failed therapy . | |||
|---|---|---|---|---|---|---|---|---|
| Yes . | No . | No . | Low (2-13/slide; <10 000) . | High >100 >10 000 . | ||||
| B0702 | 25 | 19 (75%) | 6 | 6 | 10 | 9 | 3 (15%) | 2 (8%) |
| A 0201 DOM | 28 | 21 (75%) | 7 | 7 | 16 | 5 | 2 (9%) | 1 (3.5%) |
| A 1101 DOM | 1 | 1 (100%) | 0 | 0 | 1 | 0 | 0 (0%) | 0 (0%) |
| A 0101 DOM | 4 | 4 (100%) | 0 | 0 | 2 | 2 | 0 (0%) | 0 (0%) |
| A 2402 DOM | 4 | 4 (100%) | 0 | 0 | 2 | 2 | 0 (0%) | 0 (0%) |
| A 2601 DOM | 4 | 2 (50%) | 2 | 2 | 1 | 1 | 1 (25%) | 1 (25%) |
| B 0801 DOM | 3 | 3 (100%) | 0 | 0 | 1 | 2 | 0 (0%) | 0 (0%) |
| B 40, 42, 44 DOM HERN Specific | 9 | 6 (66%) | 3 | 3 | 2 | 4 | 2 (24%) | 1 (11%) |
| B 35 DOM | 8 | 7 (87.5%) | 1 | 1 | 3 | 4 | 3 (37.5%) | 3 (37.5%) *P = .048 |
| Other HLA A,B,C alleles DOM | 13 | 11 (84%) | 2 | 2 | 4 | 7 | 2 (15%) | 0 (0%) |
| HLA DR alleles DOM | 14 | 13 (92%) | 1 | 1 | 4 | 9 | 7 (50%) | 5 (35%) |
Because of these findings, we reexamined our bank to estimate the proportion of patients inheriting a HLA B35 allele for whom a CMVpp65CTL restricted by a different HLA class I allele could be identified. Of 540 searches requested, we had identified a CMV65CTL line restricted by a shared class I HLA allele for 512 (94.8%). Of these, we had selected 19 CMVpp65CTL lines restricted by an HLA B35 allele. If use of HLA B35–restricted CMV65CTLs were excluded, lines restricted by an alternate HLA class I allele shared by the patient are still available for 13 of these 19. Only 6 of these patients would not have an alternative CMVpp65CTL line for therapy. This would reduce overall coverage from 512 to 506 of 540 patients (93.7%).
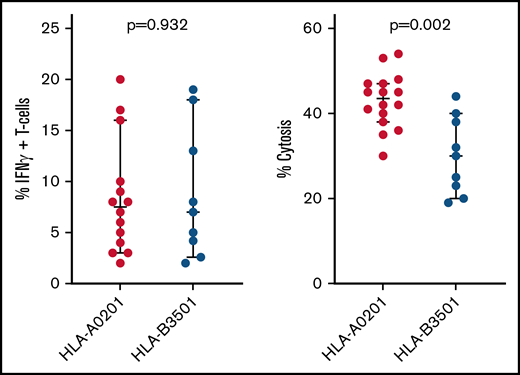
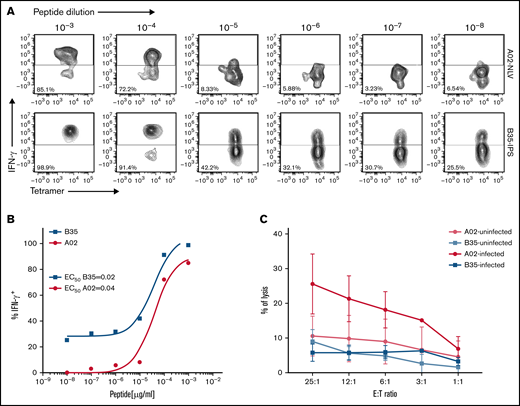
To identify a basis for failures of adoptively transferred CMVpp 65CTLs specific for peptides presented by HLA B*35 alleles, we compared HLA B*35:01–restricted CMVpp65CTLs specific for the IPSI peptide vs HLA A*02:01–restricted T cells specific for the NLV peptide. HLA B*35:01–restricted and HLA A*02:01–restricted CMVpp65CTL lines contained equivalent numbers of peptide-specific IFN-γ+ T cells (P = .932) (Figure 4). Both lysed peptide-loaded targets expressing their restricting HLA alleles. However, the HLA A*02:01–restricted CMVpp65CTLs exhibited higher cytotoxicity (P < .002). Using IPSI HLA B*35:01 and NLV-HLA A*02:01 tetramers, we isolated epitope-specific CMVpp65CTLs from separate donors and evaluated each population for avidity, as quantified by IFN-γ+ T-cell responses to CMVpp65 peptide subpools at concentrations ranging from 1 µg to 10 pg/mL. As shown in Figure 5A-B, the avidity of IPSI HLA B*35:01 tetramer+ T cells was greater than that of the NLV-HLA A*02:01 tetramer+ T cells. However, when tetramer+ T cells were tested for cytotoxic activity against autologous DCs infected with the TB40e endotheliotropic strain of CMV,33 the NLV-specific T cells lysed their targets while the IPSI-specific T cells did not (Figure 5C). Thus, although IPSI-specific HLA B*35:01–restricted T cells exhibited greater avidity and lysed peptide-loaded targets, they were ineffective against infected targets. Because CMV-derived evasins can downregulate HLA expression, we examined the effect of CMV infection on the expression of HLA alleles by DCs, including DCs inheriting both HLA A*0201 and HLA B3501. Expression of both alleles was indeed reduced but to a similar degree (data not shown). Thus, reductions in HLA expression induced by infection do not explain the difference in lysis of infected cells. Studies of CMV-infected DCs expressing other HLA B35 alleles are in progress.
Function of HLA A*0201 vs HLA B*3501 restricted CMVpp65CTLs. Comparison of NLV-specific HLA A*0201 restricted vs IPSI-specific HLA B*3501 restricted CMVpp65CTL lines in the bank, as to proportions of IFN-γ+ peptide-specific T cells in each line and the cytotoxic activity of each line against autologous phytohemagglutinin-stimulated blasts loaded with the targeted CMVpp65 peptide.
Function of HLA A*0201 vs HLA B*3501 restricted CMVpp65CTLs. Comparison of NLV-specific HLA A*0201 restricted vs IPSI-specific HLA B*3501 restricted CMVpp65CTL lines in the bank, as to proportions of IFN-γ+ peptide-specific T cells in each line and the cytotoxic activity of each line against autologous phytohemagglutinin-stimulated blasts loaded with the targeted CMVpp65 peptide.
Comparisons of HLA-B*3501–restricted, IPSI peptide–specific vs HLA A*0201– restricted, NLV peptide–specific T cells as to their avidity for cognate peptide loaded on autologous DCs and their capacity to lyse autologous CMV-infected DCs. CMV-specific CTLs were incubated for 16 hours with autologous DCs loaded with10-fold serial dilutions of CMV-pp65 subpools containing the HLA A0201 peptide epitope NLVPMVATV or the HLA B3501 epitope IPSINVHHY. IFN-γ expression was then assessed by using an intracellular cytokine assay: (A) Representative flow cytometric analysis after stimulation of NLV or IPS-specific T cells with serial dilutions of cognate peptide. (B) Peptide dose–response curves for the experiment shown in panel A. (C) Chromium-51 release assay showing specific lysis by HLA-B35–restricted CTLs and HLA-A02–restricted CTLs of autologous DCs infected or not with the TB40E endotheliotropic clinical strain of HCMV. E:T, effector-to-target ratio.
Comparisons of HLA-B*3501–restricted, IPSI peptide–specific vs HLA A*0201– restricted, NLV peptide–specific T cells as to their avidity for cognate peptide loaded on autologous DCs and their capacity to lyse autologous CMV-infected DCs. CMV-specific CTLs were incubated for 16 hours with autologous DCs loaded with10-fold serial dilutions of CMV-pp65 subpools containing the HLA A0201 peptide epitope NLVPMVATV or the HLA B3501 epitope IPSINVHHY. IFN-γ expression was then assessed by using an intracellular cytokine assay: (A) Representative flow cytometric analysis after stimulation of NLV or IPS-specific T cells with serial dilutions of cognate peptide. (B) Peptide dose–response curves for the experiment shown in panel A. (C) Chromium-51 release assay showing specific lysis by HLA-B35–restricted CTLs and HLA-A02–restricted CTLs of autologous DCs infected or not with the TB40E endotheliotropic clinical strain of HCMV. E:T, effector-to-target ratio.
Discussion
Characterization of the epitope specificities and HLA restrictions of these 138 CMVpp65CTL lines has permitted rapid selection of CMVpp65CTLs restricted by HLA alleles shared by both transplant donor and recipient. Such selection is particularly important for patients developing CMV infections after HLA partially matched transplants as infection can affect both transplanted cells and the host.34,35
Sensitization with the CMVpp65 peptide pool permits generation of CD8+ and CD4+ CMVpp65CTLs specific for epitopes presented by a broad range of HLA alleles.28,29 Unlike other approaches,36-38 neither the presenting HLA allele nor an epitope identified by an HLA-binding algorithm is used to bias the expansion of any specific CMVpp65CTL. Despite this and the fact that 188 HLA A, B, C, and DR alleles are represented in the bank, 76% of the CMVpp65CTL lines recognized epitopes presented by only 8 of the 10 most prevalent HLA A or B alleles in the bank. Presentation of immunodominant epitopes by these and other prevalent HLA alleles permitted selection of CMVpp65CTLs restricted by an HLA allele shared by HCT donor and recipient in >90% of cases.32 This feature of CMVpp65CTLs generated from healthy donors may, to a significant degree, also explain the high response rates in certain trials of third-party CMV-specific T cells in which CTLs generated from other relatives or healthy donors in the same region have been selected solely on the basis of level of HLA matching, as such matches would most likely be for the same prevalent HLA alleles, such as HLA A0201, that most often present the dominant CMVpp65 epitopes. Thus, this finding also provides evidence supporting broad applicability of more limited banks generated by recruitment of donors inheriting prevalent alleles, as described by Withers et al39 and Vickers et al40 or the “mini” bank of Tzannou et al.41
Certain immunogenic epitopes induced CMVpp65CTLs when presented by multiple alleles, including alleles other than those previously identified.36,42-44 For HERNGFTVL and QMWQARLTV, the HLA B40 and HLA B44 alleles belong to an HLA supertype.45,46 Predicted percentile ranks for all but one of these peptides for binding to the presenting HLA alleles indicate strong binding (Table 1). The exception, presentation of HERNGFTVL by HLA-A*26:01, is higher than the 2 percentile threshold usually considered for binders by the prediction algorithm. Still, multiple reported epitopes that have scored equally high using the netMHC algorithm have been proven to elicit T-cell responses similar to those detected in our study.47
We also identified pentadecapeptides containing overlapping 11 to 12 mers presented by class II and nonamers presented by class I HLA alleles. Individuals inheriting both the class II and class I alleles presenting these epitopes generated more CD3+CMVpp65CTLs than individuals responding to the presenting class I allele and a non-overlapping epitope presented by a different class II allele. These overlapping peptides might prove useful in vaccines as the class I and II alleles presenting certain overlapping epitopes (eg, HLA-A*02:01 and DRB*1 04:01) are in positive linkage disequilibrium in specific ethnogeographic groups.
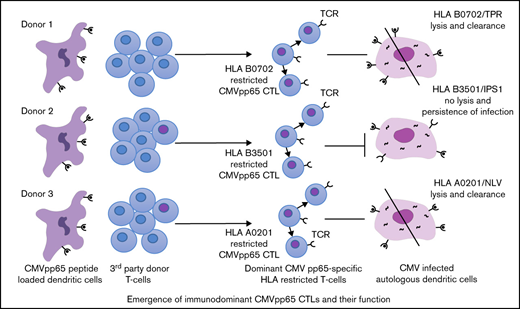
Previously, Lacey et al48 and Lidehall et al49 reported that HLA B*07:02–restricted CMVpp65-specific T cells were immunodominant in donors coinheriting HLA A*02:01 and HLA B*07:02. In this large bank, CMVpp65CTLs specific for the TPR or RPHER peptides presented by HLA B*07:02 were consistently dominant in all seropositive donors inheriting this allele irrespective of other HLA alleles coinherited, whereas HLA A*02:01–restricted CMVpp65CTLs were dominant when coinherited with any other HLA allele except HLA B*07:02 (Table 3). Immunodominance of human T-cell responses to epitopes presented by specific HLA alleles has been described for influenza A50 and several other viruses.51-54 Immunodominant responses may be associated with enhanced or impaired T cell–mediated infection control depending on the virus, the viral epitopes, and their presenting HLA alleles. For example, T-cell responses to dengue virus restricted by HLA B3501 have been found to be immunodominant in certain populations and correlated with decreased risk of severe disease.54 Conversely, immunodominant HLA B3501–restricted T-cell responses to the immunogenic GAG protein of HIV may fail to recognize a GAG mutant in B-clad HIV, thereby contributing to rapid disease progression.53 Thus, although many factors contribute to selection of immunodominant T-cell responses in vivo,55-57 and these T cells usually exhibit characteristics associated with effective immunity, they can be thwarted by viral alterations and immune evasins.
We here provide evidence that immunodominant responses to epitopes presented by certain HLA alleles can also affect clearance of CMV infections after adoptive transfer. Specifically, although treatment with CMVpp65CTLs restricted by HLA B*07:02 or HLA A*02:01 cleared drug-refractory CMV infections in a high proportion of cases, and HCTs from such donors were associated with a low incidence of drug-refractory CMV disease, adoptive therapy with immunodominant HLA B*35 allele–restricted CMVpp65CTLs was ineffective. Furthermore, recipients of HCTs from donors whose immunodominant CMVpp65CTLs were specific for epitopes presented by a shared HLA B*35 allele or class II HLA allele had a significantly greater risk of refractory CMV disease compared with recipients of HCT from HLA B*35+ donors whose dominant CMVpp65CTLs were specific for epitopes presented by HLA class I alleles other than HLA B35.
The basis for poor responses in HCT recipients treated with CMVpp65CTLs specific for epitopes presented by HLA B*3501, B*3502, and B*3508 alleles is not clear. In lines for which HLA B*3501–, B*3502–, and B*3508–restricted CMVpp65CTLs were immunodominant, proportions of IFN-γ+ HLA-B*35–restricted CMVpp65CTLs were similar to those of HLA A*02:01–restricted CMVpp65CTLs, although their cytotoxic activity against epitope-loaded targets was lower.
The T-cell receptor avidity of tetramer+ IPSI peptide–specific, HLA B*35:01–restricted CMVpp65CTLs was somewhat higher than that of tetramer+ NLV-specific, HLA A*02:01–restricted CMVpp65CTLs. Smith et al58 reported similar avidities for IPSI-specific HLA B*35–restricted compared with NLV-specific HLA A*02:01–restricted T cells. Given these findings, the basis for the selective failure of IPSI-specific, HLA B*35-01–restricted CMVpp65CTLs to lyse autologous CMV–infected cells in vitro or clear CMV infections in vivo, is more consistent with virus-induced impairments of HLA expression or epitope presentation in infected cells.
CMV invokes several mechanisms to evade virus-specific T cells, including evasins that can disrupt antigen processing, transporter associated (TAP)-mediated transfer of antigens to the endoplasmic reticulum for epitope editing and binding to HLA alleles, as well as expression of HLA alleles presenting epitopes on infected cells.58-60 Furthermore, specific evasins can act in an HLA allele–selective manner.61-63 Ameres et al63 evaluated CMVpp65CTL recognition of target cells infected with the TB40E clinical strain of CMV, either wild type or mutated to delete specific evasins US6, US3, US2, or US11. Each evasin inhibited, to different degrees, T-cell recognition of CMVpp65 epitopes presented by HLA A*02:01 (NLV), HLA B*07:02 (TPR or RPHER), and HLA B*35:01 (IPSI). However, for cells infected with the strain in which all evasins were intact, T-cell recognition of the IPSI NVHHY peptide presented by HLA B*3501 was the most markedly impaired. Whether these evasins or other mechanisms are the basis for the ineffectiveness of adoptively transferred HLA B*35–restricted CMVpp65CTLs we observed remains to be determined.
In conclusion, characterization of Good Manufacturing Practice– grade CMVpp65CTLs in our bank for epitope specificity and HLA restriction has shown a hierarchy of T-cell responses to epitopes of CMVpp65. T cells specific for immunodominant epitopes presented by specific HLA alleles may differ in their capacity to control CMV infection in recipients of alloHCT. Further analysis of specific attributes of virus-specific T-cell populations that are predictive of disease control in vivo should enhance accuracy of CTL selection algorithms and the clinical effectiveness of adoptive immunotherapy.
Acknowledgments
The bank of CMVpp65CTLs was established as part of our phase 1 (#NCT00674646) and phase 2 (#NCT01646645 and #NCT02136797) trials. The trials were approved by the Institutional Review Board/Privacy Board at MSKCC, the NMDP, and the US Food and Drug Administration. The authors thank members of the Center for Immune Cellular Therapy Laboratory, Bone Marrow Transplantation Programs in Pediatrics and Medicine, MSKCC, for their technical support.
This work was supported through National Institutes of Health, National Cancer Institute grants (P01 CA023766 and R21 CA162002) and by a National Cancer Institute Cancer Center support grant (P30 CA008748), the Aubrey Fund, The Claire Tow Foundation, Major Family Foundation, the Max Cure Foundation, Richard “Rick” J. Eisemann Pediatric Research Fund, Banbury Foundation, Edith Robertson Foundation, Larry Smead Foundation, and the German Research Foundation (KL3118/1-1).
Authorship
Contribution: E.D. and R.J.O. generated the CMVpp65CTLs; A.N.H., E.D., A.S., and L.B. characterized the CMVpp65CTLs, their phenotype, restrictions, epitope specificities, and function; S.P., A.N.H., and R.J.O. evaluated treated patients; R.S. and K.C.H. analyzed the function of HLA B3501 and HLA A0201 CMVpp65CTLs against infected targets; M.G.K. analyzed epitope specification; G.H. contributed to the overall design and biostatistical analysis of the data; R.J.O. was principal investigator for establishment of the bank; A.N.H., E.D., and R.J.O. analyzed the data and wrote the manuscript; and all authors critically reviewed the manuscript for intellectual content, accuracy, and integrity and have given final approval of the report submitted.
Conflict-of-interest disclosure: A.N.H. owns stock in Johnson & Johnson. E.D. declares consultancy, research support, and royalties from Atara Biotherapeutics. S.P. received support for the conduct of sponsored trials through MSK from Atara Biotherapeutics and Jasper Therapeutics; and honoraria from ADMA and Neovii. M.G.K. is a consultant at Artigen. R.J.O. declares consultancy, research support, and royalties from Atara Biotherapeutics. The remaining authors declare no competing financial interests.
The IND and license to develop the Third Party CMV-CTL program was transferred to Atara Biotherapeutics in July 2015 and transferred back to MSKCC in July 2019. The data related to these studies are solely the responsibility of MSKCC. Atara Biotherapeutics has no financial interest in and has not seen or reviewed the manuscript prior to submission.
The current affiliation for A.N.H. is Johnson & Johnson, New Brunswick, NJ.
The current affiliation for E.D. is Rutgers Cancer Institute of New Jersey, New Brunswick, NJ.
The current affiliation for S.P. is Boston Children’s Hospital/Dana Farber Cancer Institute, Boston, MA.
Correspondence: Richard J. O’Reilly, Memorial Sloan Kettering Cancer Center, 1275 York Ave, New York, NY 10065; e-mail: oreillyr@mskcc.org.
References
Author notes
A.N.H. and E.D. contributed equally to this study.
Requests for original data may be submitted to the corresponding author (e-mail: oreillyr@mskcc.org).
The full-text version of this article contains a data supplement.