TO THE EDITOR:
Histologicaltransformation (HT) to diffuse large B-cell lymphoma (DLBCL) is a rare event in Waldenström macroglobulinemia (WM) and is associated with a poor prognosis.1-4 It confers an inferior outcome compared with WM patients without HT.2,3 Most transformed WM patients present with elevated serum lactate dehydrogenase (LDH) levels and extranodal disease.1 Among extranodal sites, the central nervous system (CNS) is one of the most frequently involved sites identified at diagnosis of transformed WM (ranging from 13% to 18%).1,3 However, the prognostic value of CNS involvement is unknown, and the rate of CNS involvement at relapse has not been previously reported in this setting.
In de novo DLBCL, CNS relapse is a rare complication characterized by poor outcomes.5 Fewer than 5% of patients with DLBCL experience a CNS relapse in the rituximab era. Several risk factors for CNS involvement have been identified, such as the number of extranodal sites,6 specific location(s) of extranodal sites (testis, kidney, adrenal glands, breast), and activated B-cell (ABC) subtype.7 The CNS International Prognostic Index (CNS-IPI) combines the IPI factors in addition to the involvement of kidneys and/or adrenal glands and allows identifying a higher-risk group of patients (12%) with an incidence of CNS relapse of 10% to 12%.8 More recently, 2 specific genomic subtypes (the hc-MCD subtype with MYD88L265P and CD79B mutations and a subgroup with high-grade tumors characterized by double-hit biology or TP53 mutations) were found to be associated with CNS recurrence.9
Given that high IPI, ABC subtype, and MYD88L265P mutation are common features in transformed WM,1-4 this observation prompted us to investigate CNS involvement in a large cohort of transformed WM, with the objectives of describing the rate of baseline CNS involvement, the risk of CNS involvement at relapse, and the risk factors associated with CNS relapse.
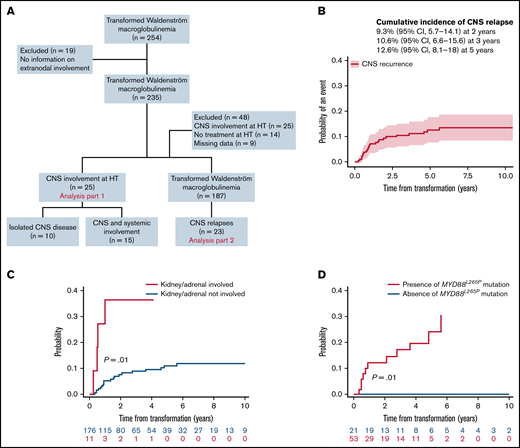
This international multicenter retrospective study included patients with a diagnosis of WM and a concurrent or sequential histological diagnosis of DLBCL. CNS disease was diagnosed by detection of DLBCL cells in the cerebrospinal fluid and/or by brain biopsy. Patients with CNS involvement by lymphoplasmacytic cells (Bing-Neel syndrome) were excluded. Of 254 identified patients with HT diagnosed between 1988 and 2020, 19 were excluded due to a lack of data on extranodal involvement (Figure 1A). The first part of the analysis (n = 235 patients) focused on the baseline CNS involvement (ie, CNS involvement at HT). Clinicobiological characteristics were compared between groups using χ-square or Fisher’s exact tests or Mann-Whitney U tests as appropriate. We analyzed CNS involvement at relapse in the second part of the study. Forty-eight additional patients were excluded due to baseline CNS involvement (n = 25), absence of treatment at HT (n = 14), and lack of details on follow-up (n = 9), leading to a final population of 187 patients. Cumulative incidence of CNS relapse was analyzed using competing-risk models that accounted for other events like systemic relapse or death from any cause, reporting subhazard ratios (SHRs). The presence of MYD88L265P mutation was tested by allele-specific polymerase chain reaction on bone marrow samples at diagnosis of WM. This study was approved by the institutional review board of each center and conducted in accordance with the Declaration of Helsinki.
Study flowchart and estimated risk of CNS relapse in transformed WM. (A) Flowchart of inclusion. Patients with transformed WM (254) were analyzed, 235 for CNS involvement at HT and 187 for CNS relapse. (B) Cumulative incidence of CNS relapses. (C) Cumulative incidence of CNS relapse stratified by kidney/adrenal involvement. (D) Cumulative incidence of CNS relapse stratified by the presence of MYD88L265P mutation.
Study flowchart and estimated risk of CNS relapse in transformed WM. (A) Flowchart of inclusion. Patients with transformed WM (254) were analyzed, 235 for CNS involvement at HT and 187 for CNS relapse. (B) Cumulative incidence of CNS relapses. (C) Cumulative incidence of CNS relapse stratified by kidney/adrenal involvement. (D) Cumulative incidence of CNS relapse stratified by the presence of MYD88L265P mutation.
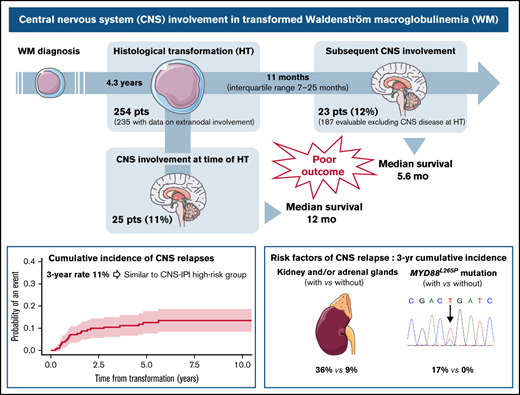
At the time of HT, CNS was involved in 25 patients (11%), including 10 (4%) with parenchymal disease, 10 (4%) with leptomeningeal, 4 (2%) with both, and 1 with unspecified CNS involvement. Characteristics associated with CNS involvement at HT were performance status 2 to 4 (P = .03) and ≥2 extranodal sites (P = .02) (supplemental Table 1). Median survival after HT was 1 year (95% confidence interval [CI], 0.7-2.5) for patients with CNS involvement at HT and 1.8 year (95% CI, 1.2-2.6) for those without CNS disease (P = .74) (supplemental Figure 1A). Patients with isolated CNS involvement (n = 10) were treated with high-dose (HD) methotrexate (MTX) (HD-MTX) and HD-cytarabine either combined (n = 5) or alone (HD-MTX, n = 2; HD-cytarabine, n = 3). Eleven out of 15 patients with concurrent systemic involvement received MTX (HD-MTX in 7, intrathecal in 4) combined with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) (n = 8) or temozolomide (n = 3). Three patients were treated with rituximab, dexamethasone, ara-C, and carboplatin (R-DHAC) and one with CHOP + radiotherapy. Twenty patients relapsed or progressed, including 7 CNS recurrence. We observed no difference in survival based on isolated CNS involvement (median, 1.1 years; 95% CI, 0.1-not reached) compared with CNS and systemic involvement (median, 1 year; 95% CI, 0.3-7.6) (P = .94) (supplemental Figure 1B).
For further analyses, the 25 patients with CNS involvement at the time of HT were excluded (Figure 1A). With a median follow-up of 7 years (95% CI, 5.8-8.1), 23 of 187 patients (12%) presented with a CNS relapse. The estimated 2- and 3-year rates of CNS relapse were 9% (95% CI, 6-14) and 11% (95% CI, 7-16), respectively (Figure 1B). The median time to relapse in the CNS was 11 months (interquartile range, 7-25). Seventy percent were isolated CNS relapses. The location was leptomeningeal in 43% of cases, parenchymal in 35%, both in 17%, and unspecified in 4%. Characteristics at HT of 23 patients with CNS relapse were 87% stage III or IV, 91% extranodal involvement (including 41% with >1 extranodal site), 60% elevated LDH, and 72% IPI 3 to 5. According to CNS-IPI risk groups (data available for 20 patients), 9 patients (45%) belonged to the high-risk group, 10 (50%) to the intermediate-risk group, and 1 (5%) to the low-risk group. All patients with CNS relapse and available data for MYD88 mutation status (n = 11) carried an MYD88L265P mutation.
Prior to CNS relapse, 87% (n = 20) of patients had received R-CHOP regimen as first-line treatment for HT, and 43% (n = 10) had received CNS prophylaxis (33% intrathecal chemotherapy, 5% HD-MTX, and 5% both). Eighteen patients (78%) achieved a complete response before progression. After CNS relapse, 96% of patients received salvage treatment: a combination of HD-MTX and HD-cytarabine (48%), HD-MTX alone (30%), or HD-cytarabine alone (9%). Four patients underwent consolidative autologous stem cell transplantation (SCT). The median survival after CNS relapse was 5.6 months (95% CI, 2.8-27.2). Among the 6 survivors, 2 underwent autologous SCT, and 2 are receiving ongoing ibrutinib therapy.
Factors associated with a 3-year cumulative incidence of CNS relapse in univariate analysis (Table 1) were involvement of kidney/adrenal glands (SHR, 4.4; P = .01) (Figure 1C) and presence of MYD88L265P mutation (P = .01) (Figure 1D). Of note, among 74 patients with data available for MYD88 mutation status, 11 CNS relapses occurred in patients with MYD88L265P mutation (n = 53; 21%), whereas no relapses were observed in the MYD88WT cohort (n = 21). A trend toward a higher risk of CNS relapse for ≥2 extranodal sites (SHR, 2.3; 95% CI 0.98-5.3; P = .06) was observed (supplemental Figure 2A). Cumulative incidence according to CNS-IPI risk groups (0% in the low-risk, 9% in the intermediate-risk, and 14% in the high-risk group) was not statistically significant (P = .47) (supplemental Figure 2B).
Risk factors for CNS relapse in transformed WM
| Variable . | Cumulative incidence at 3 y . | Univariate model . | |||||
|---|---|---|---|---|---|---|---|
| With . | Without . | ||||||
| % . | 95% CI . | % . | 95% CI . | SHR . | 95% CI . | P . | |
| Age > 60 | 13 | 8-19 | 4 | 8-13 | 1.70 | 0.59-4.87 | .32 |
| Female sex | 11 | 5-20 | 10 | 6-17 | 0.89 | 0.38-2.09 | .80 |
| Treatment prior to HT | 10 | 6-16 | 11 | 4-22 | 0.75 | 0.31-1.80 | .51 |
| ECOG PS 2-4 | 9 | 3-18 | 11 | 6-18 | 0.82 | 0.33-2.00 | .66 |
| B symptoms | 10 | 5-18 | 11 | 6-19 | 0.73 | 0.30-1.76 | .48 |
| LDH > ULN | 10 | 5-16 | 9 | 3-19 | 0.78 | 0.32-1.89 | .58 |
| β2 microglobulin >3 mg/L | 17 | 8-27 | 6 | 3-23 | 1.17 | 0.34-4.00 | .81 |
| Albumin <3.5 g/dL | 8 | 3-15 | 12 | 5-21 | 0.46 | 0.17-1.23 | .12 |
| Stage III-IV | 12 | 7-18 | 6 | 10-17 | 1.74 | 0.53-5.69 | .36 |
| ≥2 extranodal sites | 21 | 10-35 | 8 | 6-24 | 2.28 | 0.98-5.30 | .06 |
| Extranodal involvement | |||||||
| Kidney/adrenal | 36 | 10-65 | 9 | 5-14 | 4.36 | 1.39-13.72 | .01 |
| Testis | 0 | NA | 11 | 7-16 | 0.90 | 0.14-5.94 | .91 |
| Bone marrow | 17 | 9-28 | 7 | 4-13 | 1.99 | 0.88-4.49 | .10 |
| Liver | 17 | 2-43 | 10 | 6-15 | 1.48 | 0.33-6.66 | .61 |
| Skin | 13 | 2-34 | 10 | 6-16 | 1.02 | 0.24-4.30 | .98 |
| MYD88L265P mutation | 17 | 8-30 | 0 | NA | .01 | ||
| CNS-IPI | |||||||
| Low risk (0-1) | 0 | NA | ref. | .47 | |||
| Intermediate risk (2-3) | 9 | 4-16 | 2.03 | 0.28-14.6 | |||
| High risk (4-6) | 14 | 6-24 | 2.99 | 0.41-21.92 | |||
| Variable . | Cumulative incidence at 3 y . | Univariate model . | |||||
|---|---|---|---|---|---|---|---|
| With . | Without . | ||||||
| % . | 95% CI . | % . | 95% CI . | SHR . | 95% CI . | P . | |
| Age > 60 | 13 | 8-19 | 4 | 8-13 | 1.70 | 0.59-4.87 | .32 |
| Female sex | 11 | 5-20 | 10 | 6-17 | 0.89 | 0.38-2.09 | .80 |
| Treatment prior to HT | 10 | 6-16 | 11 | 4-22 | 0.75 | 0.31-1.80 | .51 |
| ECOG PS 2-4 | 9 | 3-18 | 11 | 6-18 | 0.82 | 0.33-2.00 | .66 |
| B symptoms | 10 | 5-18 | 11 | 6-19 | 0.73 | 0.30-1.76 | .48 |
| LDH > ULN | 10 | 5-16 | 9 | 3-19 | 0.78 | 0.32-1.89 | .58 |
| β2 microglobulin >3 mg/L | 17 | 8-27 | 6 | 3-23 | 1.17 | 0.34-4.00 | .81 |
| Albumin <3.5 g/dL | 8 | 3-15 | 12 | 5-21 | 0.46 | 0.17-1.23 | .12 |
| Stage III-IV | 12 | 7-18 | 6 | 10-17 | 1.74 | 0.53-5.69 | .36 |
| ≥2 extranodal sites | 21 | 10-35 | 8 | 6-24 | 2.28 | 0.98-5.30 | .06 |
| Extranodal involvement | |||||||
| Kidney/adrenal | 36 | 10-65 | 9 | 5-14 | 4.36 | 1.39-13.72 | .01 |
| Testis | 0 | NA | 11 | 7-16 | 0.90 | 0.14-5.94 | .91 |
| Bone marrow | 17 | 9-28 | 7 | 4-13 | 1.99 | 0.88-4.49 | .10 |
| Liver | 17 | 2-43 | 10 | 6-15 | 1.48 | 0.33-6.66 | .61 |
| Skin | 13 | 2-34 | 10 | 6-16 | 1.02 | 0.24-4.30 | .98 |
| MYD88L265P mutation | 17 | 8-30 | 0 | NA | .01 | ||
| CNS-IPI | |||||||
| Low risk (0-1) | 0 | NA | ref. | .47 | |||
| Intermediate risk (2-3) | 9 | 4-16 | 2.03 | 0.28-14.6 | |||
| High risk (4-6) | 14 | 6-24 | 2.99 | 0.41-21.92 | |||
CNS-IPI, central nervous system-International Prognostic Index; ECOG PS, Eastern Cooperative Oncology Group performance status; HT, histological transformation; SHR, sub-hazard ratio; ULN, upper limit of normal.
Our findings demonstrate that CNS involvement frequently occurs in transformed WM, affecting about a quarter of patients combining involvement at HT and relapses. In this population, the rate of CNS relapse seems similar to that observed in DLBCL patients belonging to the CNS-IPI high-risk group. Outcomes associated with CNS involvement in our study are poor, in line with the known prognosis of transformed WM,1-4 but markedly different from that reported with Bing-Neel syndrome (survival rate of 71% to 86% at 5 years),10-12 emphasizing the need to properly document CNS involvement in WM through accurate cytological or histological analysis. A particularly high incidence of CNS relapse is observed in patients with kidney/adrenal involvement and/or MYD88L265P mutation. We previously reported poorer survival after HT in patients with MYD88L265P mutation.4 This can possibly be explained at least partly by this high rate of CNS relapse in these patients. Limitations of this study are inherent to its retrospective design, but a prospective assessment of an infrequent complication (HT in WM) is challenging. There is room for improvement in the therapy of transformed WM, but the therapeutic approach will need to take into account the risk of CNS involvement, including more accurate identification of CNS occult disease and the use of prophylactic and/or therapeutic agents crossing the blood–brain barrier.
Acknowledgments
This work was supported by Cancer Research UK [C355/A26819], FC AECC, and AIRC under the “Accelerator Award Program” [EDITOR] to M.A. and R.G.-S.
Contribution: E.D. and A.J.D. designed the study and wrote the manuscript; E.D., L.K., and A.J.D. analyzed the data; and all authors were involved in data collection and interpretation and approved the final version of the manuscript.
Conflict-of-interest disclosure: P.M. received research funds and consulting fees from Beigene and Janssen. P.K. is a principal investigator of studies for which Mayo Clinic has received research funding from AbbVie, Sanofi, Amgen, GSK, Ichnos, Takeda, Regeneron, and Karyopharm; has received honoraria from X4 Pharmaceuticals, Beigene, Pharmacyclics, Imidex, Clinical Care Options, GSK, Oncopeptides, Cellectar, and Karyopharm. J.J.C. received research funds and consulting fees from Abbvie, AstraZeneca, Beigene, Janssen, Pharmacyclics, Polyneuron, Roche, and TG Therapeutics. The remaining authors declare no competing financial interests.
Correspondence: Eric Durot, Department of Hematology, Centre Hospitalier Universitaire de Reims, Hôpital Robert Debré, Ave du Général Koenig, 51092 Reims Cedex, France; e-mail: edurot@chu-reims.fr.
References
Author notes
Presented in abstract form at the 63rd annual meeting of the American Society of Hematology, Atlanta, GA, 12 December 2021.
Requests for data sharing may be submitted to Eric Durot (edurot@chu-reims.fr).
The full-text version of this article contains a data supplement.