Key Points
Patients with higher-grade CRS or ICANS have a lower likelihood of recovering blood counts at 1 month.
An increase in chemokines and growth factors may be a contributory factor toward count recovery.
Abstract
Factors contributing to hematopoietic recovery following chimeric antigen receptor (CAR) T-cell therapy have not been well studied. In an analysis of 83 patients with hematologic malignancies treated with CAR T-cell therapy, we describe patterns of hematopoietic recovery and evaluate potentially associated factors. We included patients who received axicabtagene ciloleucel (n = 30) or tisagenlecleucel (n = 10) for B-cell lymphoma, CD19-28z CAR T therapy for B-cell acute lymphoblastic leukemia (NCT01044069; n = 37), or B-cell maturation antigen targeting CAR T cells for multiple myeloma (NCT03070327; n = 6). Patients treated with CAR T cells who had not progressed, died, or received additional chemotherapy had “recovered” (per definition in Materials and methods section) hemoglobin, platelet, neutrophil, and white blood cell counts at rates of 61%, 51%, 33%, and 28% at month 1 postinfusion and 93%, 90%, 80%, and 59% at month 3 postinfusion, respectively. Univariate analysis showed that increasing grade of immune effector cell–associated neurological syndrome (ICANS), baseline cytopenias, CAR construct, and higher peak C-reactive protein or ferritin levels were statistically significantly associated with a lower likelihood of complete count recovery at 1 month; a similar trend was seen for cytokine release syndrome (CRS). After adjustment for baseline cytopenia and CAR construct, grade ≥3 CRS or ICANS remained significantly associated with the absence of complete count recovery at 1 month. Higher levels of vascular endothelial growth factor and macrophage-derived chemokines, although not statistically significant, were seen patients without complete count recovery at 1 month. This remains to be studied further in larger prospective studies.
Introduction
Chimeric antigen receptor (CAR) T-cell therapy has introduced a novel era of therapeutic options for hematological malignancies. Two CAR T-cell therapies targeting CD19 are now approved by regulatory agencies in various countries: (1) tisagenlecleucel (KYMRIAH, Novartis Pharmaceuticals) for relapsed/refractory B-cell acute lymphoblastic leukemia (ALL) in children and young adults (age <26 years) and relapsed/refractory B-cell lymphomas in adults and (2) axicabtagene ciloleucel (YESCARTA, Kite Pharmaceuticals, a Gilead company) for relapsed/refractory B-cell lymphomas in adults.1-3 Additionally, other CD19 CAR T-cell products have been studied in early clinical trials.4-7 CAR T-cell therapy directed against B-cell maturation antigen (BCMA) for relapsed/refractory multiple myeloma (MM) has shown promise and is being considered for regulatory approval.8-15
Some unique and commonly encountered toxicities of cytokine release syndrome (CRS), immune effector cell–associated neurotoxicity syndrome (ICANS), and hypogammaglobulinemia have been well-described with CAR T-cell therapy.16-21 However, there is limited understanding on the frequency or severity of cytopenias after CAR T-cell therapy, as well as hematopoietic recovery and its underlying mechanism. Hence, we aimed to comprehensively study the pattern of hematopoietic recovery and associated factors in these patients.
Materials and methods
Patient selection
We reviewed patients older than 18 years of age who received US Food and Drug Administration–approved CAR T-cell therapy (axicabtagene ciloleucel or tisagenlecleucel) for relapsed/refractory non-Hodgkin lymphoma (NHL) between May 2018 and June 2019 or who were on clinical trials for relapsed/refractory B-cell ALL (NCT01044069) between June 2010 and October 2016 or relapsed/refractory MM (NCT03070327) between May 2017 and March 2019 at Memorial Sloan Kettering Cancer Center. To eliminate confounding variables that may contribute to delayed hematopoietic recovery, patients were included in the analysis if they were alive without progression of disease or additional cytotoxic therapy for >30 days after CAR T-cell infusion. We obtained baseline patient, disease, and treatment details prior to lymphodepletion chemotherapy by retrospective chart review. Peripheral blood counts were collected for 12 months following CAR T-cell infusion or until patients were censored. Censoring events included relapse or progression of disease following CAR T-cell treatment, initiation of cytotoxic chemotherapy for maintenance, preparative conditioning for a subsequent autologous or allogeneic hematopoietic cell transplantation (HCT), subsequent treatment with additional CAR T cells, and last follow-up. The study was approved by the Institutional Review Board at Memorial Sloan Kettering Cancer Center.
CAR T-cell products and treatment details
Patients included in this study received 1 of 4 second-generation CAR T-cell constructs. For NHL, commercially available CD19-directed CAR T cells (ie, axicabtagene ciloleucel [CD28 costimulation] or tisagenlecleucel [4-1BB costimulation]) were administered.2,3 For B-cell ALL, 19-28z CAR T cells were used that target CD19, include CD28 and CD3z coactivating receptors, and they express single chain fragment-length derived from murine CD19–specific monoclonal antibody (NCT01044069).4,6,22 For MM, CAR T cells were directed against BCMA (anti-BCMA), including 4-1BB costimulation with a human-derived single chain variable fragment (NCT03070327).9,23,24 The latter two CAR T products (for B-cell ALL and MM) were manufactured at Memorial Sloan Kettering Cancer Center.
Cytokine profiling
We obtained cytokine profiles prior to lymphodepletion (baseline), prior to CAR T-cell infusion (preinfusion), and serially after CAR T-cell infusion at 10 time points postinfusion (1 hour, 1 day, 2 days, 3 days, 4 days, 5 days, 1 week, 2 weeks, 3 weeks, and 4 weeks). These serial serum samples were obtained from patients who were on clinical trials for relapsed/refractory B-cell ALL (NCT01044069) or relapsed/refractory MM (NCT03070327). Cytokine profiles for these patients were analyzed using a Luminex FLEXMAP 3D System and commercially available 38-plex cytokine detection assays.6,22,25
Definitions
Hematological count “recovery” for the respective blood count was defined as hemoglobin >8 g/dL without red cell transfusion in 2 weeks, platelets >50 × 103/μL without transfusions in 1 week, absolute neutrophil count (ANC) >1 × 103/μL, and white blood cell (WBC) count >3 × 103/μL without growth factor support in 2 weeks. Hematological count “normalization” was defined as counts in the normal range for the laboratory (ie, hemoglobin >11.2 g/dL in women and 12.5 g/dL in men, platelets >160 × 103/μL, ANC >1.5 × 103/μL, and WBC count >3 × 103/μL with the same transfusion support timeline). “Complete count recovery” was defined as recovery per above criteria in all 4 cell counts (ie, hemoglobin, platelets, ANC, and WBCs).
CRS and ICANS were graded according to the consensus grading criteria defined by American Society of Transplantation and Cellular Therapy (ASTCT).16,26 Although calculating the ICANS grade can be challenging in a retrospective setting, we were able to obtain those successfully because the Memorial Sloan Kettering Cancer Center’s practice included Mini-Mental Status Examination for all patients at that time. These included a comprehensive evaluation of orientation, speech, naming, attention, and ability to follow commands. The Immune Effector Cell-associated Encephalopathy score and ICANS grade were calculated retrospectively using these detailed neurological assessments, as we described previously.26 For patients treated after the adoption of ASTCT consensus grading, data were collected prospectively and reviewed by an internal consensus group.
Common Terminology Criteria for Adverse Events v5.0 was used for grading the severity of cytopenias.
Statistical methods
Patients with ≥1 month of follow-up were evaluated for complete count, hemoglobin, platelet, ANC, and WBC count recovery and normalization at 1 to 3, 6, 9, and 12 months post–CAR T-cell infusion. All of these patients were evaluated at 1 month, whereas fewer patients were evaluable at subsequent time points due to the occurrence of censoring events.
We evaluated the association between complete count recovery at 1 month and patient and treatment characteristics, including age, disease, prior lines of therapy, baseline cytopenia, CAR construct, lymphodepleting therapy, CRS grade, ICANS grade, grade ≥3 CRS or ICANS, prior autologous or allogeneic HCT, peak C-reactive protein (CRP) levels, and peak ferritin. Variables were compared between recovered and not recovered groups using the Fisher’s exact test for categorical variables and the Wilcoxon rank-sum test for continuous variables. Complete count recovery at 1 month was evaluated by grade ≥3 CRS or ICANS, controlling for baseline cytopenia and CAR construct, using the Cochran-Mantel-Haenszel test.
For cytokine markers measured serially, peak values were compared using the Wilcoxon rank-sum test, and comparison was done with the remaining 38 cytokines using the Benjamini-Hochberg correction. Cytokine markers were measured at 12 time points: preconditioning, preinfusion, and 1 hour, 1 day, 2 days, 3 days, 4 days, 5 days, 1 week, 2 weeks, 3 weeks, and 4 weeks postinfusion. These were collected prospectively for patients treated with CD19-28z on NCT01044069 or with BCMA-directed CAR T cells on NCT03070327. The trend of cytokine levels over time is presented graphically by recovery groups for group median, and differences were tested using the Vardi 2-sample permutation test with the clinfun package in R.27 The Wilcoxon and Vardi test results are reported with the Benjamini-Hochberg correction for 38 comparisons.
Sensitivity analysis.
Of all patients treated with CAR T-cell therapy, 32 were not included in the primary analysis because of relapse, progression, or death in the first 30 days post–CAR T-cell infusion. Hence, a sensitivity analysis was performed for complete count recovery at 1 month; these 32 patients were included in the “count not recovered” group.
Results
A total of 115 patients who were treated with CAR T-cell therapy as a commercially approved therapy for relapsed/refractory NHL or on the respective clinical trials for relapsed/refractory B-cell ALL or MM, at Memorial Sloan Kettering Cancer Center, were reviewed for this study. We excluded 32 patients who progressed or died within 30 days of receiving CAR T-cell therapy, resulting in the inclusion of 83 patients in the analysis (Table 1). Thirty (36%) patients were treated with axicabtagene ciloleucel for NHL, 10 (12%) were treated with tisagenlecleucel for NHL, 37 (45%) were treated with 19-28z CAR T cells for B-cell ALL, and 6 (7%) were treated with anti-BCMA CAR T cells for MM. A median of 4 lines of therapy (range, 1-9) were received prior to CAR T cells. Patients with NHL received a median of 4 lines of treatment (range, 2-9), patients with ALL received a median of 3 lines of treatment (range, 1-8), and patients with MM received a median of 4.5 lines of treatment (range, 4-9) prior to the administration of CAR T cells. Fourteen (17%) patients had received an autologous HCT and 15 (18%) had received an allogeneic HCT as a treatment prior to CAR T-cell therapy.4,28 One additional patient with diffuse large B-cell lymphoma had received an autologous HCT, as well as an allogeneic HCT, prior to CAR T-cell therapy.
Patient and disease characteristics (N = 83)
| Characteristics . | Data . |
|---|---|
| Age, median (range), y | 58 (19-85) |
| Males | 56 (67) |
| Disease | |
| B-cell lymphoma | 40 (48) |
| B-cell acute lymphoblastic leukemia | 37 (45) |
| Multiple myeloma | 6 (7) |
| >3 prior lines of therapy | 45 (54) |
| Baseline cytopenia | 73 (88) |
| CAR construct* | |
| Axicabtagene ciloleucel | 30 (36) |
| Tisagenlecleucel | 10 (12) |
| 19-28z CAR T cells | 37 (45) |
| Anti-BCMA CAR T cells | 6 (7) |
| Lymphodepleting chemotherapy | |
| Fludarabine and cyclophosphamide | 51 (61) |
| High-dose cyclophosphamide | 31 (37) |
| Bendamustine | 1 (1) |
| CRS grade | |
| 0 | 13 (16) |
| 1-2 | 51 (61) |
| 3-4 | 19 (23) |
| ICANS grade | |
| 0 | 36 (43) |
| 1-2 | 17 (21) |
| 3-4 | 30 (36) |
| Prior autologous or allogeneic stem cell transplantation | 30 (37) |
| Characteristics . | Data . |
|---|---|
| Age, median (range), y | 58 (19-85) |
| Males | 56 (67) |
| Disease | |
| B-cell lymphoma | 40 (48) |
| B-cell acute lymphoblastic leukemia | 37 (45) |
| Multiple myeloma | 6 (7) |
| >3 prior lines of therapy | 45 (54) |
| Baseline cytopenia | 73 (88) |
| CAR construct* | |
| Axicabtagene ciloleucel | 30 (36) |
| Tisagenlecleucel | 10 (12) |
| 19-28z CAR T cells | 37 (45) |
| Anti-BCMA CAR T cells | 6 (7) |
| Lymphodepleting chemotherapy | |
| Fludarabine and cyclophosphamide | 51 (61) |
| High-dose cyclophosphamide | 31 (37) |
| Bendamustine | 1 (1) |
| CRS grade | |
| 0 | 13 (16) |
| 1-2 | 51 (61) |
| 3-4 | 19 (23) |
| ICANS grade | |
| 0 | 36 (43) |
| 1-2 | 17 (21) |
| 3-4 | 30 (36) |
| Prior autologous or allogeneic stem cell transplantation | 30 (37) |
Unless otherwise noted, data are n (%).
Patients with B-cell lymphoma received axicabtagene ciloleucel or tisagenlecleucel, patients with B-cell ALL received 19-28z CAR T cells, and patients with MM received anti-BCMA CAR T cells.
Thirty-nine of 40 patients treated with axicabtagene ciloleucel or tisagenlecleucel were given fludarabine and cyclophosphamide for lymphodepletion. One patient received bendamustine with tisagenlecleucel, per the package label. Among the patients treated on the 2 clinical trials, 31 received high-dose cyclophosphamide, and the remaining 12 received a combination of fludarabine and cyclophosphamide.
Severity of cytopenias
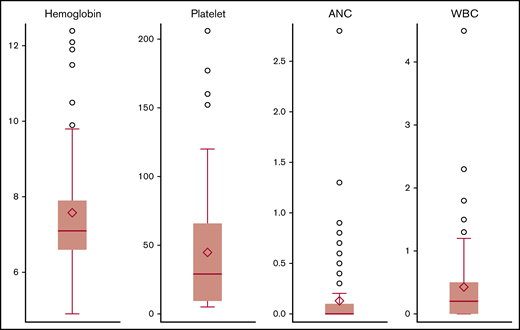
Complete blood counts were available for all evaluable patients at the described time points of 1, 2, 3, 6, 9, and 12 months. All or almost all patients had anemia (83/83), thrombocytopenia (79/83), neutropenia (82/83), and/or leucopenia (82/83) following CAR T-cell infusion. A majority of these were grade 3-4: 64 of 83 (77%) cases of anemia, 54 of 83 (65%) cases of thrombocytopenia, 79 of 83 (95%) cases of neutropenia, and 83 of 83 (100%) cases of leukopenia (supplemental Figure 1). The median nadir reported for hemoglobin was 7.1 g/dL (range, 4.9-12.4), the median nadir for platelets was 29.5 × 103/μL (range, 5-206 × 103/μL), the median nadir for ANC was 0 × 103/μL (range, 0-2.8 × 103/μL), and the median nadir for WBC count was 0.2 × 103/μL (range, 0-4.5 × 103/μL) (Figure 1); these were noted at a median of 6 (range, 1-63), 7 (range, 1-37), 4 (range, 1-13), and 5 (range, 1-36) days, respectively, following CAR T-cell infusion. With a median follow-up of 6 months (range, 1.3-92), 55 of 83 (66%) patients received ≥1 packed red cell transfusion, 43 of 83 (52%) received platelet transfusions, and 51 of 83 (62%) were given granulocyte-colony stimulating factor. One patient in this cohort died from an influenza infection during the follow-up and had a complete count recovery at that time. Additional details of infections were reported by us for patients with B-cell ALL and B-cell lymphoma.25,29 One patient was diagnosed with myelodysplastic syndrome at 10 months following CAR T-cell therapy. This patient had relapsed followed CAR T-cell therapy and had received 6 lines of therapy by this time for relapsed/refractory diffuse large B-cell lymphoma. Three of these therapies were prior to CAR T-cell therapy and included an autologous HCT, whereas 2 additional therapies were administered upon relapse following CAR T-cell therapy.
Nadir counts following CAR T-cell therapy. Circles denote outliers, error bar is median nadir value, and diamonds indicate the mean.
Nadir counts following CAR T-cell therapy. Circles denote outliers, error bar is median nadir value, and diamonds indicate the mean.
Recovery and normalization of blood counts
Recovery and normalization of each blood count and overall count recovery over time (per definitions in Methods) following CAR T-cell infusion are depicted in Figure 2.
Hematopoietic recovery and normalization of blood counts following CAR T-cell therapy. (A) Count recovery. (B) Count normalization.
Hematopoietic recovery and normalization of blood counts following CAR T-cell therapy. (A) Count recovery. (B) Count normalization.
Recovery of counts.
At 1 month, of the 83 patients included, a majority had recovered hemoglobin (51/83, 61%) and platelet counts (42/83, 51%). However, smaller percentages of patients had recovered ANC (27/83, 33%) or WBC counts (23/83, 28%) at 1 month. Hemoglobin and platelet recovery were noted in all evaluable patients by 6 months (n = 21), whereas ANC recovery was noted in all patients at 9 months (n = 14) following CAR T-cell infusion.
Normalization of counts.
Normalization was seen in few patients at 1 month for hemoglobin (6/83, 7%), platelet count (19/83, 23%), ANC (25/83, 30%), and WBC count (11/83, 13%). The rates of normalization were 6/9 (67%) for hemoglobin, 7/9 (78%) for platelet count, and 8/9 (89%) for ANC and WBC count at 12 months post–CAR T-cell infusion.
Factors associated with count recovery: univariate and multivariate analysis
We first evaluated potential patient and treatment characteristics that could be associated with recovery of complete blood counts in the univariate model (Table 2). At 1 month, complete count recovery was seen in 20 of 83 (24%) patients and was statistically significantly associated with absence of baseline cytopenias, tisagenlecleucel CAR construct, lower peak of CRP, and lower peak ferritin levels following the infusion. Additionally, with an increasing grade of ICANS, the likelihood of recovery of complete blood counts at 1 month decreased (44% with no ICANS, 18% in patients with grade 1-2 ICANS, and 3% in patients with grade 3-4 ICANS; P < .001). A similar trend was seen with the increasing grade of CRS: 31% of patients with no CRS, 29% of patients with grade 1-2 CRS, and 5% of patients with grade 3-4 CRS had recovery of complete counts at 1 month (P = .08). Among 33 patients with grade ≥3 CRS/ICANS, only 2 (6%) had complete count recovery at 1 month, whereas 31 (94%) did not (P = .002).
Univariate analysis of complete count recovery at 1 month from CAR T-cell infusion
| . | Complete count recovery (n = 20) . | No complete count recovery (n = 63) . | P . |
|---|---|---|---|
| Age, median (range), y | 64 (23-86) | 55 (20-76) | .18 |
| Disease | .52 | ||
| B-cell lymphoma (n = 40) | 12 (30) | 28 (70) | |
| B-cell acute lymphoblastic leukemia (n = 37) | 7 (19) | 30 (81) | |
| Multiple myeloma (n = 6) | 1 (17) | 5 (83) | |
| >3 prior lines of therapy | 7 (16) | 38 (84) | .07 |
| Baseline cytopenia | 14 (19) | 59 (81) | .01 |
| CAR construct | .008 | ||
| Axicabtagene ciloleucel (n = 30) | 5 (17) | 25 (83) | |
| Tisagenlecleucel (n = 10) | 7 (70) | 3 (30) | |
| 19-28z CAR T cells (n = 37) | 7 (19) | 30 (81) | |
| anti-BCMA CAR T cells (n = 6) | 1 (17) | 5 (83) | |
| Lymphodepleting chemotherapy | .32 | ||
| Fludarabine and cyclophosphamide (n = 51) | 12 (24) | 39 (76) | |
| High-dose cyclophosphamide* (n = 31) | 7 (23) | 24 (77) | |
| Bendamustine (n = 1) | 1 (100) | 0 (0) | |
| CRS grade† | .08 | ||
| 0 (n = 13) | 4 (31) | 9 (69) | |
| 1-2 (n = 51) | 15 (29) | 36 (71) | |
| 3-4 (n = 19) | 1 (5) | 18 (95) | |
| ICANS grade† | <.001 | ||
| 0 (n = 36) | 16 (44) | 20 (56) | |
| 1-2 (n = 17) | 3 (18) | 14 (82) | |
| 3-4 (n = 30) | 1 (3) | 29 (97) | |
| Grade ≥3 CRS/ICANS | 2 (6) | 31 (94) | .002 |
| Prior autologous or allogeneic stem cell transplantation | 4 (13) | 26 (87) | .11 |
| Peak CRP, median (range), mg/L | 9.6 (1.2-29.1), n = 15 | 13.7 (0.3-21 024), n = 57 | .03 |
| Peak ferritin, median (range), ng/mL | 312 (56-4923), n = 16 | 1 899 (22-546 080), n = 53 | .004 |
| . | Complete count recovery (n = 20) . | No complete count recovery (n = 63) . | P . |
|---|---|---|---|
| Age, median (range), y | 64 (23-86) | 55 (20-76) | .18 |
| Disease | .52 | ||
| B-cell lymphoma (n = 40) | 12 (30) | 28 (70) | |
| B-cell acute lymphoblastic leukemia (n = 37) | 7 (19) | 30 (81) | |
| Multiple myeloma (n = 6) | 1 (17) | 5 (83) | |
| >3 prior lines of therapy | 7 (16) | 38 (84) | .07 |
| Baseline cytopenia | 14 (19) | 59 (81) | .01 |
| CAR construct | .008 | ||
| Axicabtagene ciloleucel (n = 30) | 5 (17) | 25 (83) | |
| Tisagenlecleucel (n = 10) | 7 (70) | 3 (30) | |
| 19-28z CAR T cells (n = 37) | 7 (19) | 30 (81) | |
| anti-BCMA CAR T cells (n = 6) | 1 (17) | 5 (83) | |
| Lymphodepleting chemotherapy | .32 | ||
| Fludarabine and cyclophosphamide (n = 51) | 12 (24) | 39 (76) | |
| High-dose cyclophosphamide* (n = 31) | 7 (23) | 24 (77) | |
| Bendamustine (n = 1) | 1 (100) | 0 (0) | |
| CRS grade† | .08 | ||
| 0 (n = 13) | 4 (31) | 9 (69) | |
| 1-2 (n = 51) | 15 (29) | 36 (71) | |
| 3-4 (n = 19) | 1 (5) | 18 (95) | |
| ICANS grade† | <.001 | ||
| 0 (n = 36) | 16 (44) | 20 (56) | |
| 1-2 (n = 17) | 3 (18) | 14 (82) | |
| 3-4 (n = 30) | 1 (3) | 29 (97) | |
| Grade ≥3 CRS/ICANS | 2 (6) | 31 (94) | .002 |
| Prior autologous or allogeneic stem cell transplantation | 4 (13) | 26 (87) | .11 |
| Peak CRP, median (range), mg/L | 9.6 (1.2-29.1), n = 15 | 13.7 (0.3-21 024), n = 57 | .03 |
| Peak ferritin, median (range), ng/mL | 312 (56-4923), n = 16 | 1 899 (22-546 080), n = 53 | .004 |
Unless otherwise noted, data are n (%).
High-dose cyclophosphamide was single dose 3 g/m2 used in NCT01044069.
Per ASTCT grading criteria.
To further analyze the association between grade ≥3 CRS or ICANS and complete count recovery at 1 month, we adjusted for baseline cytopenia and CAR construct, separately (Table 3). This model was used to allow for accuracy in the presence of a low number of patients in each group and the low number of events in this study. After adjusting for baseline cytopenia and the CAR construct, absence of recovery of complete counts at 1 month remained statistically significantly associated with grade ≥3 CRS or ICANS (P = .002 and P = .01, respectively).
Association of complete count recovery at 1 month from CAR T-cell infusion and grade ≥3 CRS or ICANS with adjustment for baseline cytopenia and CAR construct
| . | ≤Grade 2 CRS or ICANS (n = 50) . | Grade 3-4 CRS or ICANS (n = 33) . | P* . |
|---|---|---|---|
| Baseline cytopenia | .002 | ||
| No (n = 10) | 5/7 (71) | 1/3 (33) | |
| Yes (n = 73) | 13/43 (30) | 1/30 (3) | |
| CAR construct | .01 | ||
| Axicabtagene ciloleucel (n = 30) | 5/19 (26) | 0/11 (0) | |
| Tisagenlecleucel (n = 10) | 7/10 (70) | 0/0 | |
| 19-28z CAR T cells (n = 37) | 6/16 (38) | 1/21 (5) | |
| anti-BCMA CAR T cells (n = 6) | 0/5 (0) | 1/1 (100) |
| . | ≤Grade 2 CRS or ICANS (n = 50) . | Grade 3-4 CRS or ICANS (n = 33) . | P* . |
|---|---|---|---|
| Baseline cytopenia | .002 | ||
| No (n = 10) | 5/7 (71) | 1/3 (33) | |
| Yes (n = 73) | 13/43 (30) | 1/30 (3) | |
| CAR construct | .01 | ||
| Axicabtagene ciloleucel (n = 30) | 5/19 (26) | 0/11 (0) | |
| Tisagenlecleucel (n = 10) | 7/10 (70) | 0/0 | |
| 19-28z CAR T cells (n = 37) | 6/16 (38) | 1/21 (5) | |
| anti-BCMA CAR T cells (n = 6) | 0/5 (0) | 1/1 (100) |
Data are number with complete count recovery at 1 month/total number of patients (% recovered).
Cochran-Mantel-Haenszel test.
Additional analyses identified factors associated with recovery of individual counts (ie, hemoglobin, platelets, and ANC) at 1 month. Grade of CRS or ICANS and peak levels of CRP and ferritin consistently remained significantly associated with recovery of each of the individual counts at 1 month (supplemental Tables 1-3). Additionally, higher median age at the time of CAR T-cell therapy was significantly associated with hemoglobin recovery at 1 month (supplemental Table 1). For platelet count recovery at 1 month, prior autologous or allogeneic HCT was also statistically significantly associated with absence of platelet count recovery (36% recovered vs 63% no recovery; P = .04) (supplemental Table 2). Prior autologous or allogeneic HCT (13% recovered vs 87% no recovery, P = .004) and >3 prior lines of therapy (22% recovered vs 78% no recovery; P = .04) were also associated with absence of ANC recovery at 1 month (supplemental Table 3).
A univariate analysis was also done for complete count recovery at 3 months in the 41 evaluable patients. Only the CAR construct (42% recovery with axicabtagene ciloleucel vs 100% with tisagenlecleucel vs 55% with 19-28z CAR T cells vs 50% with anti-BCMA CAR T cells; P = .05) had a statistically significant association with complete count recovery (supplemental Table 4).
Sensitivity analysis
The results of the sensitivity analysis for complete count recovery at 1 month, including all 115 patients, were not significantly different from the original analysis (supplemental Table 5).
Cytokine profile
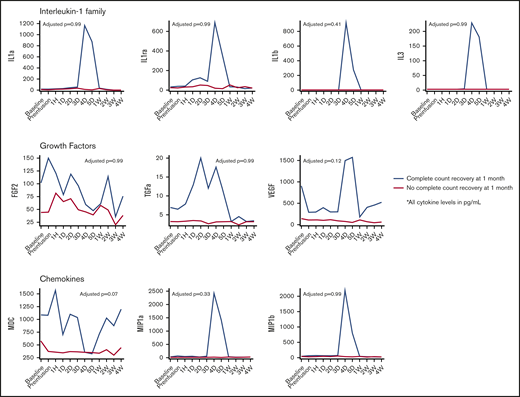
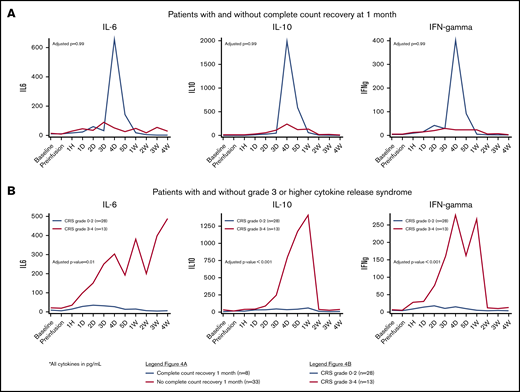
We evaluated serial cytokine profiles available for 41 patients treated with 19-28z CAR T cells (n = 35) or anti-BCMA CAR T cells (n = 6), with corresponding complete count recovery data, at 1 month. Clinical characteristics of these 41 patients are presented in supplemental Table 6. Patients with complete count recovery at 1 month had higher peak levels of macrophage-derived chemokine (MDC), with a median of 1636.0 pg/mL (range, 1202.2-5056.6; vs 840.2 pg/ML [range, 153.8-4339.7], adjusted P = .06) (Table 4). Patients with complete count recovery also had higher levels of growth factors (fibroblast growth factor-2 [FGF-2], transforming growth factor-α [TGF-α], and vascular endothelial growth factor) as well as chemokines (MDC, macrophage inflammatory protein-1a [MIP-1a], and MIP-1b) when median values were studied over time, but they were not statistically significant (Figure 3). The core cytokines associated with grade 3-4 CRS (interleukin-6 [IL-6], interleukin-10, and interferon-γ [IFN-γ]) were not significantly different in the 2 cohorts (Figure 4).
Peak cytokine levels in the first month postinfusion in patients with and without count recovery at 1 month from CAR T-cell infusion
| Cytokine . | Complete count recovery (n = 8)* . | No complete count recovery (n = 33)* . | Adjusted P† . |
|---|---|---|---|
| IL-1 family | |||
| IL-1a | 86.5 (3.2-2 299.3) | 60.9 (3.2-627.8) | .96 |
| IL-1ra | 157.2 (3.2-1 446.5) | 149.7 (21.2-4 534.5) | .96 |
| IL-1b | 15.4 (1.4-1 864.9) | 3.4 (1.6-114.3) | .96 |
| Hematopoietin (class I cytokine) family | |||
| IL-2 | 17.7 (3.2-53.0) | 18.1 (0.9-188.1) | .96 |
| IL-3 | 4.4 (3.2-567.6) | 10.6 (1.9-206.0) | .96 |
| IL-4 | 22.9 (3.2-38.6) | 3.2 (2.7-148.0) | .96 |
| IL-5 | 6.4 (1.9-182.3) | 21.7 (1.0-257.1) | .96 |
| IL-6 | 72.3 (21.5-10 000.0) | 278.3 (3.2-13 538.0) | .96 |
| IL-7 | 21.6 (6.2-16 317.9) | 18.7 (3.2-323.0) | .96 |
| IL-9 | 3.9 (2.3-105.3) | 3.2 (1.1-53.1) | .96 |
| IL-12p40 | 82.5 (3.2-167.4) | 46.6 (3.2-256.0) | .96 |
| IL-12p70 | 12.2 (3.2- 38.2) | 7.0 (3.2-67.6) | .96 |
| IL-13 | 12.5 (3.2-51.1) | 12.5 (3.2-131.9) | .96 |
| IL-15 | 42.5 (18.6-280.3) | 100.5 (7.9-413.4) | .96 |
| IFN (class II cytokine) family | |||
| IFN-α3 | 71.3 (3.2-146.8) | 61.9 (2.8-343.3) | .96 |
| IFN-γ | 31.0 (3.2-817.7) | 69.6 (2.6-5 261.5) | .96 |
| IL-10 | 135.9 (9.7-4 676.5) | 337.2 (5.4-12 376.4) | .96 |
| Growth factors | |||
| GM-CSF | 89.6 (3.2-688.4) | 105.4 (1.8-1 541.5) | .96 |
| G-CSF | 1 287.5 (219.7-45 344.7) | 4 823.9 (51.3-125 780.0) | .96 |
| EGF | 609.6 (253.3-1 483.3) | 303.6 (67.3-1 459.5) | .96 |
| FGF-2 | 188.3 (79.8-681.0) | 120.3 (39.2-670.4) | .96 |
| TGF-α | 24.2 (3.2-52.3) | 8.7 (3.2-67.2) | .96 |
| VEGF | 1 182.4 (139.1-3 795.0) | 249.5 (3.2-3 123.0) | .18 |
| TNF family | |||
| TNF-α | 41.3 (3.2-358.2) | 54.5 (17.1-450.5) | .96 |
| TNF-β | 26.7 (8.2-291.0) | 12.3 (3.2-434.2) | .96 |
| sCD40L | 20 117.5 (149.9-1 702 924.4) | 13 716.2 (994.3-9 087 239.4) | .96 |
| IL-17 family | |||
| IL-17a | 9.4 (3.2-51.9) | 7.1 (3.0-105.0) | .96 |
| Chemokines | |||
| Eotaxin | 339.5 (215.7-505.9) | 298.5 (91.4-748.3) | .96 |
| MCP-1 | 1827.0 (364.5-4932100.3) | 6 496.7 (698.1-128 465.0) | .96 |
| MCP-3 | 117.0 (8.4-4816.5) | 63.3 (3.2-826.1) | .96 |
| MDC | 1636.0 (1202.2-5056.6) | 840.2 (153.8-4 339.7) | .06 |
| MIP-1a | 95.0 (24.1-10000.0) | 80.1 (13.1-430.6) | .96 |
| MIP-1b | 100.7 (72.6-9520.2) | 94.8 (28.4-499.6) | .96 |
| IP-10 | 1479.6 (61.2-33242.5) | 6 476.3 (553.7-70 391.5) | .96 |
| IL-8 | 907.3 (5.6-260142.8) | 1287.1 (43.6-40 534.0) | .96 |
| GRO | 1543.8 (478.3-14175.4) | 2482.8 (821.3-17 728.9) | .96 |
| Fractalkine | 307.9 (51.4-4 223.9) | 499.2 (7.2-70 519.7) | .96 |
| TK receptor ligand | |||
| FLT-3L | 515.4 (8.6-431.1) | 1223.9 (8.1-3 087.9) | .96 |
| Cytokine . | Complete count recovery (n = 8)* . | No complete count recovery (n = 33)* . | Adjusted P† . |
|---|---|---|---|
| IL-1 family | |||
| IL-1a | 86.5 (3.2-2 299.3) | 60.9 (3.2-627.8) | .96 |
| IL-1ra | 157.2 (3.2-1 446.5) | 149.7 (21.2-4 534.5) | .96 |
| IL-1b | 15.4 (1.4-1 864.9) | 3.4 (1.6-114.3) | .96 |
| Hematopoietin (class I cytokine) family | |||
| IL-2 | 17.7 (3.2-53.0) | 18.1 (0.9-188.1) | .96 |
| IL-3 | 4.4 (3.2-567.6) | 10.6 (1.9-206.0) | .96 |
| IL-4 | 22.9 (3.2-38.6) | 3.2 (2.7-148.0) | .96 |
| IL-5 | 6.4 (1.9-182.3) | 21.7 (1.0-257.1) | .96 |
| IL-6 | 72.3 (21.5-10 000.0) | 278.3 (3.2-13 538.0) | .96 |
| IL-7 | 21.6 (6.2-16 317.9) | 18.7 (3.2-323.0) | .96 |
| IL-9 | 3.9 (2.3-105.3) | 3.2 (1.1-53.1) | .96 |
| IL-12p40 | 82.5 (3.2-167.4) | 46.6 (3.2-256.0) | .96 |
| IL-12p70 | 12.2 (3.2- 38.2) | 7.0 (3.2-67.6) | .96 |
| IL-13 | 12.5 (3.2-51.1) | 12.5 (3.2-131.9) | .96 |
| IL-15 | 42.5 (18.6-280.3) | 100.5 (7.9-413.4) | .96 |
| IFN (class II cytokine) family | |||
| IFN-α3 | 71.3 (3.2-146.8) | 61.9 (2.8-343.3) | .96 |
| IFN-γ | 31.0 (3.2-817.7) | 69.6 (2.6-5 261.5) | .96 |
| IL-10 | 135.9 (9.7-4 676.5) | 337.2 (5.4-12 376.4) | .96 |
| Growth factors | |||
| GM-CSF | 89.6 (3.2-688.4) | 105.4 (1.8-1 541.5) | .96 |
| G-CSF | 1 287.5 (219.7-45 344.7) | 4 823.9 (51.3-125 780.0) | .96 |
| EGF | 609.6 (253.3-1 483.3) | 303.6 (67.3-1 459.5) | .96 |
| FGF-2 | 188.3 (79.8-681.0) | 120.3 (39.2-670.4) | .96 |
| TGF-α | 24.2 (3.2-52.3) | 8.7 (3.2-67.2) | .96 |
| VEGF | 1 182.4 (139.1-3 795.0) | 249.5 (3.2-3 123.0) | .18 |
| TNF family | |||
| TNF-α | 41.3 (3.2-358.2) | 54.5 (17.1-450.5) | .96 |
| TNF-β | 26.7 (8.2-291.0) | 12.3 (3.2-434.2) | .96 |
| sCD40L | 20 117.5 (149.9-1 702 924.4) | 13 716.2 (994.3-9 087 239.4) | .96 |
| IL-17 family | |||
| IL-17a | 9.4 (3.2-51.9) | 7.1 (3.0-105.0) | .96 |
| Chemokines | |||
| Eotaxin | 339.5 (215.7-505.9) | 298.5 (91.4-748.3) | .96 |
| MCP-1 | 1827.0 (364.5-4932100.3) | 6 496.7 (698.1-128 465.0) | .96 |
| MCP-3 | 117.0 (8.4-4816.5) | 63.3 (3.2-826.1) | .96 |
| MDC | 1636.0 (1202.2-5056.6) | 840.2 (153.8-4 339.7) | .06 |
| MIP-1a | 95.0 (24.1-10000.0) | 80.1 (13.1-430.6) | .96 |
| MIP-1b | 100.7 (72.6-9520.2) | 94.8 (28.4-499.6) | .96 |
| IP-10 | 1479.6 (61.2-33242.5) | 6 476.3 (553.7-70 391.5) | .96 |
| IL-8 | 907.3 (5.6-260142.8) | 1287.1 (43.6-40 534.0) | .96 |
| GRO | 1543.8 (478.3-14175.4) | 2482.8 (821.3-17 728.9) | .96 |
| Fractalkine | 307.9 (51.4-4 223.9) | 499.2 (7.2-70 519.7) | .96 |
| TK receptor ligand | |||
| FLT-3L | 515.4 (8.6-431.1) | 1223.9 (8.1-3 087.9) | .96 |
EGF, epidermal growth factor; FLT-3L, FMS-like tyrosine kinase 3 ligand; G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; GRO, growth regulatory oncogene; IP-10, IFN-γ-inducible protein 10; MCP, monocyte chemoattractant protein; sCD40L, soluble CD40 ligand; TK, tyrosine kinase; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor.
Data are median (range) reported in pg/mL.
Wilcoxon rank-sum test P values were adjusted for 38 comparisons using the Benjamini-Hochberg method.
Cytokine trends in patients with and without count recovery at 1 month from CAR T-cell infusion. D, day; H, hour; VEGF, vascular endothelial growth factor; W, week.
Cytokine trends in patients with and without count recovery at 1 month from CAR T-cell infusion. D, day; H, hour; VEGF, vascular endothelial growth factor; W, week.
Cytokine patterns by count recovery and toxicity. Cytokines associated with CRS in patients with and without complete count recovery at 1 month from CAR T-cell infusion (A) and in patients with or without grade 3-4 CRS (B).
Cytokine patterns by count recovery and toxicity. Cytokines associated with CRS in patients with and without complete count recovery at 1 month from CAR T-cell infusion (A) and in patients with or without grade 3-4 CRS (B).
Discussion
In this retrospective analysis, we studied hematopoietic recovery following CAR T-cell therapy for hematological malignances in a cohort of patients who were not confounded by disease relapse or concomitant cytotoxic therapy. Although most patients developed cytopenias following CAR T-cell infusion, a majority (>80% for hemoglobin, platelets, and ANC and >50% for WBC and complete counts) of them recovered to adequate levels by 3 months after infusion. However, normalization of counts takes a longer time and was achieved in only ∼15% patients by 3 months and ∼60% by 9 months following the infusion. These findings are consistent with reports of cytopenias from prior trials with CAR T-cell therapy. In ZUMA-1, 17% of patients continued to have grade ≥3 cytopenias for up to 3 months after infusion, whereas the JULIET trial reported 38% of patients with grade ≥3 thrombocytopenia at 3 months.2,30 Unlike our current analysis, reports from other studies may not be for selected patients who had an ongoing response. Hence, there is a possibility of bias of disease relapse or subsequent cytotoxic therapy as a probable impediment to normal hematopoiesis in these studies.
Among factors affecting hematopoietic recovery, there was a consistent and significant association between high-grade CRS or ICANS, as well as markers of acute inflammation (ie, CRP and ferritin) with overall counts and with each individual count in the univariate analysis. Despite adjustment for other statistically significant factors in univariate analysis, grade ≥3 CRS or ICANS remained significantly associated with absence of complete count recovery at 1 month. The absence of association with CRS and ICANS at 3 months further corroborates that inflammation, which occurs early following CAR T-cell therapy, is associated with a delay in hematopoietic recovery only in the early post–CAR T-cell period. A similar association between severity of cytopenias and grade 4 CRS has also been reported with JCAR017 (a CD19 CAR T-cell therapy not included in our analysis) when used with fludarabine and cyclophosphamide lymphodepletion, for treatment of ALL, NHL, and chronic lymphocytic leukemia.31 At 3 months, only the CAR construct was significantly associated with count recovery in our study, suggesting that differences in peak, expansion, and persistence of various CAR constructs may play a role in this difference.
To understand the underlying mechanism, we conducted a more elaborate cytokine panel on a subset of patients for whom longitudinal samples were available. We found that, although the cytokines commonly associated with CRS were significantly elevated in patients with grade ≥3 CRS in our cohort, they were not significantly increased in patients with no complete count recovery at 1 month.32 Additionally, we found that MDC levels and growth factors were numerically higher in patients with complete count recovery at 1 month (although not statistically significant with adjusted P values in this small sample). This may indicate that some cytokines promote hematopoiesis and that marrow microenvironment changes may contribute to the recovery of hematopoietic progenitor cells.
Various other factors have been hypothesized to contribute to the development of cytopenias, such as lymphodepletion chemotherapy, extensive prior cytotoxic treatments, or prior autologous or allogeneic HCT, with potential impacts on hematopoietic recovery.31,33,34 Another possible reason for the development of cytopenias can be emergence of clonal hematopoiesis or development of myelodysplastic syndrome, especially in a setting of multiple cytotoxic lines of therapy preceding CAR T cells.30,34,35 No cytopenia in our study was attributed to myelodysplastic syndrome. One patient did develop myelodysplastic syndrome but that was following relapse after CAR T-cell therapy, after having received subsequent therapies and not during the count monitoring in the study. When interpreting myelodysplastic syndrome following CAR T-cell therapy, it is important to be mindful that these patients have received multiple therapies, several of which can be implicated in the development of myelodysplastic syndrome.
The cytopenias occurred frequently and were relatively prolonged, lasting several weeks following CAR T-cell infusion. One patient died from a viral infection (influenza A) in our study. Other reports from Memorial Sloan Kettering Cancer Center have shown that, of the 53 patients who received 19-28z CAR T cells for B-cell ALL, 22 (42%) patients had 26 episodes of infections within 30 days of infusion: 17 were bacterial, 4 were fungal, and 5 were viral.25 Among the B-cell lymphoma patients treated with axicabtagene ciloleucel or tisagenlecleucel at Memorial Sloan Kettering Cancer Center, 95 infection events were described in 35 of 60 (58%) treated patients.29 In another retrospective study in pediatric patients treated with CD19 CAR T cells, 37 infections were reported in 33 of 83 (40%) patients.36 Among the pivotal trials, 41 of 108 (38%) patients in ZUMA-1, 38 of 111 (34%) patients in JULIET, 32 of 75 (43%) patients in the ELIANA trial, and 14 of 33 (42%) patients treated with bb2121 developed an infection.1-3,8,30 The prolonged immunosuppression seen in our study, along with the reported high rates of infections, underscores the need to consider antimicrobial prophylaxis, although no standard guidelines are available at this time. The financial implications of these complications also warrant further investigation.
Our study is limited by being a retrospective study that includes patients with heterogeneous diagnoses. Although disease biology is expected to play some role in count recovery, we included patients with disease response only to obviate confounding from poor count recovery due to disease relapse. We also evaluated the 3 diagnoses in the univariate model to determine a difference among them, but no significant difference was noted. Second, lack of bone marrow or peripheral blood samples from patients with persistent cytopenias precludes us from studying other mechanistic reasons for delayed count recovery. This will need to be studied further in a prospective manner. Additionally, because of the diminution in sample size in subgroups and over time, association of count recovery with CAR construct may be of limited value, because specific CAR constructs were used for specific diagnoses.
Despite these limitations, we demonstrate the patterns of hematopoietic recovery in a large series of patients receiving CAR T-cell therapy who have ongoing disease response in the absence of additional chemotherapy or death. Because the utilization of CAR T-cell therapy will expand, we anticipate that our data will inform treating physicians on these important aspects of long-term adverse events. We plan to further study the underlying mechanisms and pathobiology of delayed hematopoietic recovery following CAR T-cell therapy.
Data sharing requests should be sent to Sham Mailankody (mailanks@mskcc.org).
Acknowledgment
This work was supported by National Institutes of Health, National Cancer Institute Cancer Center Support Grant P30 CA008748 (to Memorial Sloan Kettering Cancer Center).
Authorship
Contribution: T.J. and S.M. designed research, performed research, interpreted data, and wrote the manuscript; A.K. and S.M.D. conducted statistical analyses and wrote the manuscript; M.P., Y.C., T.J.P., E.S., and J.H.P. performed research and interpreted data; J.D.R. and M.A.M. performed research and collected data; and M. Smith, G.L.S., E.H., C.D., M. Scordo, C.S.S., E.M., B.D.S., M.L.P., C.W.B., S.G., R.B., and M.-A.P. interpreted data.
Conflict-of-interest disclosures: T.J. has received consulting fees from Takeda Oncology and has served on an advisory board for CareDx. G.L.S. has received research funding from Amgen and Janssen Pharmaceuticals. M. Scordo has served as a paid consultant for McKinsey & Company, Angiocrine Bioscience, Inc., and Omeros Corporation; has received research funds from Angiocrine Bioscience, Inc.; and has served on an ad hoc advisory board for Kite, a Gilead Company. C.S.S. has served as a paid consultant on advisory boards for Juno Therapeutics, Sanofi-Genzyme, Spectrum Pharmaceuticals, Novartis, Genmab, Precision Biosciences, Kite, a Gilead Company, Celgene, Gamida Cell, and GSK and has received research funds for clinical trials from Juno Therapeutics, Celgene, Precision Biosciences, and Sanofi-Genzyme. B.D.S. has acted as a consultant for Kite/Gilead, Juno/Celgene, and Novartis. M.L.P. has acted as a consultant for Noble Insights and served on advisory board for Phamacyclics. An immediate family member has served as consultant for Merck & Co., Inc.; has served on advisory boards for STRAXIMM Therapeutics, Kite Pharmaceuticals, and Seres Therapeutics; has served as a member of the Speakers Bureau for Hemedicus; has equity ownership in Seres Therapeutics and Evelo Biosciences; and holds patents and royalties for Memorial Sloan Kettering Cancer Center (intellectual property for Juno and Seres Therapeutics). C.W.B. has acted as a consultant for and served on advisory boards for Juno Therapeutics. S.G. has acted as a consultant for and received research funding from Amgen, Actinium, Celgene, Johnson & Johnson, and Takeda; has acted as a consultant for Jazz Pharmaceuticals, Novartis, Kite, and Spectrum Pharmaceuticals; and has received research funding from Miltenyi Biotec. R.B. has acted as a consultant for and has patents, royalties, and research funding from Juno Therapeutics and has acted as a consultant for Celgene. E.S. has acted as a consultant for and has patents, royalties, and research funding from Celgene and has acted as a consultant for Fate Therapeutics and Precision Biosciences. J.H.P. has acted as a consultant for Allogene Therapeutics, Amgen, AstraZeneca, Autolus Therapeutics, GSK, Incyte, Kite Pharma, Novartis, and Takeda. M.-A.P. has served on advisory boards for MolMed, NexImmune, Medigene, and Servier; has received honoraria from and served on advisory boards for AbbVie, Bellicum Pharmaceuticals, Bristol-Meyers Squibb, Nektar Therapeutics, Novartis, Omeros, and Takeda; has acted as a consultant for and received honoraria from Merck; and has received research funding from Kite/Gilead, Incyte, and Miltenyi Biotec. S.M. has received research funding from Juno Therapeutics, Celgene, Janssen Pharmaceuticals, Allogene Therapeutics, and Takeda Oncology and has received honoraria for Continuing Medical Education activity from Physician Education Resource and PleXus Education. The remaining authors declare no competing financial interests.
The current affiliation for T.J. is Hematologic Malignancies and Bone Marrow Transplantation Program, Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University School of Medicine, Baltimore, MD.
Correspondence: Sham Mailankody, Memorial Sloan Kettering Cancer Center, 1275 York Ave, New York, NY 10065; e-mail: mailanks@mskcc.org.
References
Author notes
The full-text version of this article contains a data supplement.