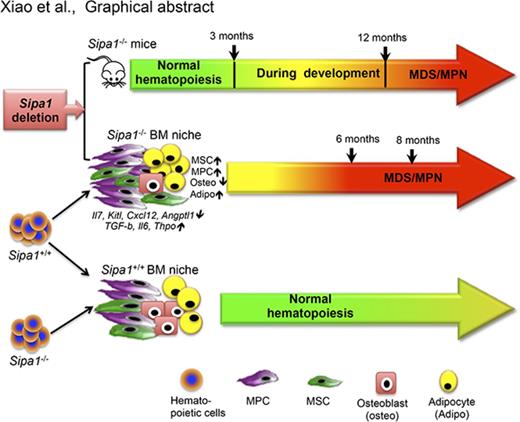
Key Points
Sipa1 loss leads to BM niche alterations prior to the initiation of MPN.
Sipa1-deficient BM niche induces lethal MPN from normal hematopoietic cells.
Abstract
Mutations of signal-induced proliferation-associated gene 1 (SIPA1), a RAP1 GTPase-activating protein, were reported in patients with juvenile myelomonocytic leukemia, a childhood myelodysplastic/myeloproliferative neoplasm (MDS/MPN). Sipa1 deficiency in mice leads to the development of age-dependent MPN. However, Sipa1 expression in bone marrow (BM) microenvironment and its effect on the pathogenesis of MPN remain unclear. We here report that Sipa1 is expressed in human and mouse BM stromal cells and downregulated in these cells from patients with MPN or MDS/MPN at diagnosis. By using the Sipa1−/− MPN mouse model, we find that Sipa1 deletion causes phenotypic and functional alterations of BM mesenchymal stem and progenitor cells prior to the initiation of the MPN. Importantly, the altered Sipa1−/− BM niche is required for the development of MDS/MPN following transplantation of normal hematopoietic cells. RNA sequencing reveals an enhanced inflammatory cytokine signaling and dysregulated Dicer1, Kitl, Angptl1, Cxcl12, and Thpo in the Sipa1−/− BM cellular niches. Our data suggest that Sipa1 expression in the BM niche is critical for maintaining BM niche homeostasis. Moreover, Sipa1 loss–induced BM niche alterations likely enable evolution of clonal hematopoiesis to the hematological malignancies. Therefore, restoring Sipa1 expression or modulating the altered signaling pathways involved might offer therapeutic potential for MPN.
Introduction
Normal hematopoiesis is well maintained by a rare population of hematopoietic stem cells (HSCs) residing in a specific bone marrow (BM) microenvironment, called niche.1 The HSC fates are determined by both the extrinsic cues emanating from their niche and the intrinsic signals triggered by interactions with the niche cells via direct cell adhesion and secreted factors.2 The BM HSC niche is composed of various types of stromal cells, including osteoblasts, adipocytes, macrophages, megakaryocytes (MKs), perivascular cells, endothelial cells, and mesenchymal stem cells (MSCs).2,3 MSCs are considered the precursor of osteoblasts, adipocytes, and chondrocytes.4 They can be functionally estimated by their ability to generate colony-forming unit-fibroblast (CFU-F) in vitro and are proposed to give rise to mesenchymal progenitors (MPCs) with single- or bi-lineage potential, but with no/little CFU-F activity.3,5
There is increasing evidence that BM niche alterations lead to the development of myeloid malignancies.6,7 Mice deficient for retinoic acid receptor γ developed myeloproliferative neoplasm (MPN)-like disease, which was induced solely by the gene loss in the microenvironment.8 Deletion of Dicer1 from mouse BM osteoblast progenitors caused myelodysplasia (MDS) that could evolve to acute myeloid leukemia (AML).9 In addition, loss of Notch signaling in the BM niche led to lethal MPN-like disease.10 A recent study revealed the critical contribution of Ptpn11 mutations in BM MPCs to leukemogenesis.11
Signal-induced proliferation-associated gene 1 (Sipa1), a principal RAP1 GTPase-activating protein, regulates signaling of integrins, growth factors, and cytokines by inactivating RAP1.12-14 Sipa1 is expressed in mouse hematopoietic stem and progenitor cells (HSPCs) and human lymphocytes.15,16 Loss of Sipa1 leads to constitutive hyperactivation of RAP1, cell proliferation, and development of malignancy.14,17,18 Mutations or abnormal expression of SIPA1 have been reported in hematopoietic malignancies and solid cancer in humans.19-21 SIPA1 gene point mutations were identified in patient mononuclear cells with juvenile myelomonocytic leukemia,22 a childhood MDS/MPN,23 and AML.24 Sipa1−/− mice show normal hematopoiesis at a younger age (before 5 months), but develop MPN, resembling human chronic myeloid leukemia (CML) or MDS, from 1 year of age.15,25 However, it is unclear whether the MPN is caused by Sipa1 loss in hematopoietic cells or in BM stromal cells.
We here report that Sipa1 is expressed in BM stromal cells and downregulated in these cells from patients with MPN or MDS/MPN. Sipa1 deficiency in mice induces significant alterations in the BM niche prior to the initiation of MDS/MPN. Importantly, the altered Sipa1−/− BM microenvironment is absolutely required for the MDS/MPN development in the Sipa1−/− mice. Sipa1 loss confers greater capacity on BM MSCs and MPCs to promote myelopoiesis. The dysregulated cytokine signaling in the Sipa1−/− BM niche may be involved in the pathogenesis of the disease.
Materials and methods
Mice
Sipa1−/− C57BL/6 mice15 were backcrossed to C57BL/6 for >15 generations. The Sipa1−/− mice at 8 to 12 weeks and 16 to 17 months were used for experiments. Age- and sex-matched Sipa1+/+ C57BL/6 mice were used as controls. CD45.1 B6.SJL-Ptprca Pepcb/BoyJ (Jackson Laboratory, Bar Harbor, ME) mice were used as recipients or donors for transplantations. All the mice were maintained in a specific pathogen-free condition in the animal facility at Karolinska Institute. Animal procedures were performed with approval from the local ethics committee (ethical number S40-14) at Karolinska Institute (Stockholm, Sweden).
Human sample collection
BM samples were collected from patients (49-78 years old) with CML, chronic neutrophilic leukemia (CNL), or chronic myelomonocytic leukemia (CMML), classified as MPN and MDS/MPN,23,26 at diagnosis and from the age-matched (45-86 years old) healthy donors. The experiments were approved by the local Ethical Committee at Stockholm (2012/4:10 and 2013/3:1) and informed consent was obtained from the patients and healthy donors.
Multicolor fluorescence activated cell sorting (FACS) of MSCs
Human and mouse MSCs were isolated as described.27,28 See supplemental Data for details.
CFU-F assay
CFU-F assay of freshly sorted BM MSCs or unfractionated cells was performed as described.27,28
Transplantations
BM cells from 7- to 10-week-old Sipa1+/+ (CD45.1+ or CD45.2+) or Sipa1−/− (CD45.2+) mice were enriched by magnetic-activated cell sorting using CD45 microbeads (Miltenyi Biotec). Three million of the cells per mouse were injected via tail vein into sublethally (6 Gy) or lethally (9.5 Gy) irradiated young (8- to 10-week-old) CD45.2 Sipa1−/− and Sipa1+/+ recipient mice. When transplanting the Sipa1−/− cells, 8- to 10-week-old CD45.1 Sipa1+/+ mice (lethally irradiated) were used as recipients. At the endpoints (7-9 months) of the primary transplantations, 3 million spleen cells from the primary Sipa1−/− recipients (CD45.2), which had developed MDS/MPN, were transplanted into sublethally irradiated Sipa1+/+ recipients (CD45.2). The analysis of hematopoiesis after transplantation is described in supplemental Data.
RNA sequencing
Total RNA was isolated from sorted cells, and libraries were constructed using NuGEN’s Ovation Ultralow Library systems (NuGEN Technologies, San Carlos, CA) and were subsequently subjected to 50 cycles of HiSequation 2000 or 76 cycles of NextSeq500 sequencing (Illumina, San Diego, CA). See supplemental Data for more details and data analysis.
Statistics
The unpaired Student t test or Mann-Whitney U test, Welch’s correction, and Kolmogorov-Smirnov test were used to compare the differences based on the data distribution. The Kaplan-Meier survival curve of the mice was generated by Prism 6.0. All reported P values were obtained using Prism 5.0 or 6.0, and P < .05 was considered statistically significant.
See additional methods in supplemental Data.
Results
Sipa1 is expressed in normal BM stromal cells and downregulated in patients with MPN
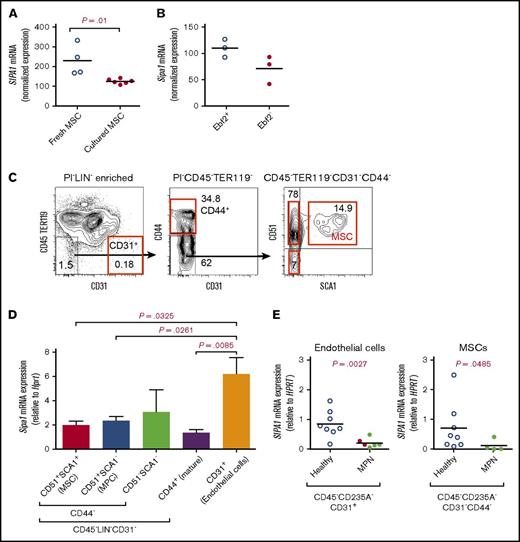
Previous studies have shown that Sipa1 was expressed in hematopoietic progenitors and lymphoid cells.15,16 Sipa1 expression in BM nonhematopoietic cells is unclear. Analysis of the microarray data from our previous studies28,29 revealed that SIPA1 was also expressed in human BM MSCs, and mouse BM MSCs expressing early B-cell factor 2 (Ebf2)27 (Figure 1A-B), a recently identified MSC population27 that is partly overlapping with the Nestin+ MSCs.30 To further determine the Sipa1 gene expression in different mouse BM stromal cell fractions, we performed quantitative real-time polymerase chain reaction (qPCR) analysis on FACS-sorted BM endothelial cells (CD45−LIN−CD31+), MSCs (CD45−LIN−CD31−CD44−CD51+SCA1+),28,29 and MPCs (CD45−LIN−CD31−CD44−CD51+SCA1−), which contain most of the CXCL12-abundant cells.5,31 We detected Sipa1 gene expression in all the stromal cell subsets, with the highest Sipa1 expression in the endothelial cells (Figure 1C-D). Interestingly, SIPA1 expression was significantly reduced in BM endothelial cells (P = .0027) of patients with CML, CNL, or CMML compared with age-matched controls, and to a lesser extent reduced in the MSCs (Figure 1E).
Sipa1 is expressed in BM mesenchymal cells and downregulated in the stromal cells from patients with MPN. (A-B) Microarray analysis showed SIPA1 gene expression in native and culture-expanded BM MSCs of healthy donors (A) and mice (B). The data on SIPA1 expression in human MSCs were extracted from 2 independent experiments previously done on the freshly sorted CD45−CD235A−CD31−CD44− cells and the culture-expanded MSCs. The data on Sipa1 expression in the Ebf2+ MSCs were from 3 independent experiments. The expression in mouse cells was normalized to 4 housekeeping genes, including Gapdh, β-Actin, Transferrin Receptor, Pyruvate Carboxylase, in mouse cells and to 3 housekeeping genes, including GAPDH, β-Actin, ISGF-3 (STAT1), in human cells by DNA-Chip analyzer (dChip) analysis, as described.27,28 (C) FACS profiles showing the gating strategy for sorting of SCA1+CD51+ MSCs, SCA1−CD51+ MPCs, and the more mature SCA1−CD51− stromal cells from young adult mouse BM. The cells were first gated within CD45−TER119−CD31−CD44− cells, and then the CD31+ endothelial cells and the CD44+ mature stromal cells were gated within the CD45−TER119− cells as indicated. (D) qPCR analysis of Sipa1 messenger RNA (mRNA) expression in the BM stromal cell subsets. Data are mean ± standard error of the mean (SEM), from 5 independent experiments. Hprt was used to normalize the expression. P values were calculated by unpaired Student t test. (E) qPCR analysis revealed downregulation of SIPA1 expression in the BM endothelial cells and MSCs of newly diagnosed patients with CML, CNL, and CMML. P values between the patients and the age-matched healthy controls were tested by unpaired Mann-Whitney U test. HPRT was used to normalize the expression. Red dot indicates CMML and CNL samples.
Sipa1 is expressed in BM mesenchymal cells and downregulated in the stromal cells from patients with MPN. (A-B) Microarray analysis showed SIPA1 gene expression in native and culture-expanded BM MSCs of healthy donors (A) and mice (B). The data on SIPA1 expression in human MSCs were extracted from 2 independent experiments previously done on the freshly sorted CD45−CD235A−CD31−CD44− cells and the culture-expanded MSCs. The data on Sipa1 expression in the Ebf2+ MSCs were from 3 independent experiments. The expression in mouse cells was normalized to 4 housekeeping genes, including Gapdh, β-Actin, Transferrin Receptor, Pyruvate Carboxylase, in mouse cells and to 3 housekeeping genes, including GAPDH, β-Actin, ISGF-3 (STAT1), in human cells by DNA-Chip analyzer (dChip) analysis, as described.27,28 (C) FACS profiles showing the gating strategy for sorting of SCA1+CD51+ MSCs, SCA1−CD51+ MPCs, and the more mature SCA1−CD51− stromal cells from young adult mouse BM. The cells were first gated within CD45−TER119−CD31−CD44− cells, and then the CD31+ endothelial cells and the CD44+ mature stromal cells were gated within the CD45−TER119− cells as indicated. (D) qPCR analysis of Sipa1 messenger RNA (mRNA) expression in the BM stromal cell subsets. Data are mean ± standard error of the mean (SEM), from 5 independent experiments. Hprt was used to normalize the expression. P values were calculated by unpaired Student t test. (E) qPCR analysis revealed downregulation of SIPA1 expression in the BM endothelial cells and MSCs of newly diagnosed patients with CML, CNL, and CMML. P values between the patients and the age-matched healthy controls were tested by unpaired Mann-Whitney U test. HPRT was used to normalize the expression. Red dot indicates CMML and CNL samples.
Altered BM niche in aged Sipa1−/− mice after the development of MDS/MPN
To investigate the contribution of the BM niche to the development of MDS/MPN, we characterized the BM niche in the 16-month-old Sipa1−/− mice. Consistent with the previous report,15 the majority of the Sipa1−/− mice developed MDS-like MPN, which can be categorized as MDS/MPN.32 The disease was manifested by anemia, thrombocytopenia, increased granulocytes, pronounced B-lymphopenia, and splenomegaly (occurring in ∼28.6% of the female mice) (Figure 2A-C; supplemental Figure 1), macrocytic erythrocytes, and increased neutrophils in the peripheral blood (PB) (supplemental Figure 1D-E). We also observed an increased leukocyte infiltration concomitant with MK hyperplasia in the Sipa1−/− BM (Figure 2D-E). The hyperplastic MK formed many loose clusters and showed smaller hypolobated, pyknotic, or fragmented nuclei and abnormal cytoplasmic morphology. There is a gender bias of the disorders toward the female Sipa1−/− mice (Figure 2B; supplemental Figure 1A-C).
Myeloproliferation and altered BM niches in aged Sipa1−/− MPN mice. The PB, BM, and spleen from the age- and sex-matched aged (16-17 months old) Sipa1+/+ and Sipa1−/− mice were collected for analyzing both hematopoiesis and BM niches. Statistical analysis was performed by unpaired Mann-Whitney U test. (A) Total white blood cells (WBCs) in 16-month-old Sipa1+/+ and Sipa1−/− mice. (B) Myeloid cells in the PB of the Sipa1+/+ and Sipa1−/− male and female mice. (C) A representative splenomegaly (right) of the aged Sipa1−/− mice. (D) Hematoxylin and eosin (H&E) staining of dysplastic MKs in the Sipa1−/− mouse BM. Scale bars represent 50 μm. (E) The increased numbers of MKs in the Sipa1−/− mouse BM. The data are expressed as numbers per squared millimeters. (F) FACS profiles for phenotypic analysis of BM stromal cells in the Sipa1+/+ and Sipa1−/− mice. The CD44− cells were first gated within CD45−TER119−CD31− cells and then subdivided into SCA1+CD51+ MSCs, SCA1−CD51+ MPCs, and SCA1−CD51− mature stromal cells. (G) Altered stromal cell composition in the BM of 16-month-old Sipa1−/− mice. Data are percent of the cells within total CD45−TER119−CD31− cells from 8 independent experiments. (H) CFU-F frequencies in whole BM cells. The right panel shows the difference in the frequencies of CFU-Fs observed between the female or male Sipa1+/+ and Sipa1−/− mice. (I) Multilineage differentiation potentials of the Sipa1−/− BM MSCs. Scale bars represent 250 μm (left), 500 μm (middle), and 100 μm (right). n = 3 independent sorting experiments. (J) μCT images of femurs in the aged Sipa1+/+ and Sipa1−/− female mice. Scale bars represent 1.0 mm. (K) Femoral bone volumes of Sipa1+/+ and Sipa1−/− mice. The statistical difference was determined by Mann-Whitney U test (A) or unpaired Student t test (B-E,G-H). See also supplemental Figure 1. Cont, control.
Myeloproliferation and altered BM niches in aged Sipa1−/− MPN mice. The PB, BM, and spleen from the age- and sex-matched aged (16-17 months old) Sipa1+/+ and Sipa1−/− mice were collected for analyzing both hematopoiesis and BM niches. Statistical analysis was performed by unpaired Mann-Whitney U test. (A) Total white blood cells (WBCs) in 16-month-old Sipa1+/+ and Sipa1−/− mice. (B) Myeloid cells in the PB of the Sipa1+/+ and Sipa1−/− male and female mice. (C) A representative splenomegaly (right) of the aged Sipa1−/− mice. (D) Hematoxylin and eosin (H&E) staining of dysplastic MKs in the Sipa1−/− mouse BM. Scale bars represent 50 μm. (E) The increased numbers of MKs in the Sipa1−/− mouse BM. The data are expressed as numbers per squared millimeters. (F) FACS profiles for phenotypic analysis of BM stromal cells in the Sipa1+/+ and Sipa1−/− mice. The CD44− cells were first gated within CD45−TER119−CD31− cells and then subdivided into SCA1+CD51+ MSCs, SCA1−CD51+ MPCs, and SCA1−CD51− mature stromal cells. (G) Altered stromal cell composition in the BM of 16-month-old Sipa1−/− mice. Data are percent of the cells within total CD45−TER119−CD31− cells from 8 independent experiments. (H) CFU-F frequencies in whole BM cells. The right panel shows the difference in the frequencies of CFU-Fs observed between the female or male Sipa1+/+ and Sipa1−/− mice. (I) Multilineage differentiation potentials of the Sipa1−/− BM MSCs. Scale bars represent 250 μm (left), 500 μm (middle), and 100 μm (right). n = 3 independent sorting experiments. (J) μCT images of femurs in the aged Sipa1+/+ and Sipa1−/− female mice. Scale bars represent 1.0 mm. (K) Femoral bone volumes of Sipa1+/+ and Sipa1−/− mice. The statistical difference was determined by Mann-Whitney U test (A) or unpaired Student t test (B-E,G-H). See also supplemental Figure 1. Cont, control.
To explore any potential BM stromal cell alterations in these mice, we dissected the BM stromal cell compartment by FACS. The frequencies of the MSCs and the mature stromal cells were significantly reduced, whereas the MPCs were increased in the aged Sipa1−/− mice (Figure 2F-G). The reduction of the MSCs was confirmed by the decreased number of CFU-Fs, reflecting the functionally defined MSCs, in Sipa1−/− mouse BM (Figure 2H left). Consistent with the observation of a female bias of the MDS/MPN, the reduced CFU-F activities were mainly detected in the MSCs from the Sipa1−/− female mice (Figure 2H right). The frequencies of the endothelial cells remained unchanged (data not shown). Moreover, the Sipa1−/− MSCs showed increased adipogenic and chondrogenic, but impaired osteogenic differentiation potential (Figure 2I). Micro-computed tomography (μCT) showed slightly reduced femoral bone volume in the aged Sipa1−/− mice (Figure 2J-K). These data suggest phenotypic and functional alterations of BM cellular niches in the aged Sipa1−/− mice with MDS/MPN.
The development of MPN in aged Sipa1−/− mice is not dependent on intrinsic loss of Sipa1 in hematopoietic cells
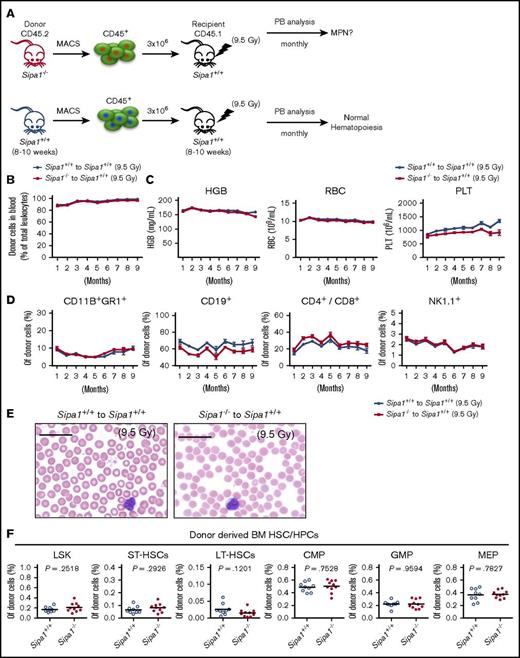
The discovery of Sipa1 expression in the BM stromal cells and the BM niche alterations in the aged Sipa1−/− mice raised a question of whether the MDS/MPN in the aged Sipa1−/− mice was attributable to the Sipa1 deletion from the BM stromal cells. To answer this question, we transplanted BM hematopoietic cells from young adult Sipa1−/− mice into lethally irradiated wild-type mice (Figure 3A). Surprisingly, we could not detect any signs of hematological disorders (Figure 3). These data clearly suggest that the loss of Sipa1 in the hematopoietic cells is not sufficient to initiate the MPN.
Sipa1−/− hematopoietic cells failed to develop any hematological disorders after transplantation into young Sipa1+/+mice. (A) Transplantation setup. The CD45.2+ cells from 8- to 10-week-old Sipa1+/+ or Sipa1−/− mouse BM were transplanted into lethally irradiated CD45.1 Sipa1+/+ recipient mice (8-10 weeks old). Donor-derived lineages in the PB were analyzed by FACS monthly after transplantation. (B) Total donor engraftment in the recipient PB. (C) Red blood cells (RBC), hemoglobin (HGB), and platelets (PLT) in the recipient PB. (D) FACS analysis of blood lineage reconstitution after transplantation. The data are mean ± SEM, from 2 independent experiments, n = 9 to 10 per group. (E) H&E staining of PB smears of the Sipa1+/+ recipients 9 months after transplantation of donor Sipa1−/− or Sipa1+/+ BM CD45.2+ cells. Scale bars represent 25 μm. (F) Reconstitution of HSPCs in the recipient BM 9 months after transplantation. CMP, common myeloid progenitor; LT-HSCs, long-term HSCs; ST-HSCs, short-term HSCs.
Sipa1−/− hematopoietic cells failed to develop any hematological disorders after transplantation into young Sipa1+/+mice. (A) Transplantation setup. The CD45.2+ cells from 8- to 10-week-old Sipa1+/+ or Sipa1−/− mouse BM were transplanted into lethally irradiated CD45.1 Sipa1+/+ recipient mice (8-10 weeks old). Donor-derived lineages in the PB were analyzed by FACS monthly after transplantation. (B) Total donor engraftment in the recipient PB. (C) Red blood cells (RBC), hemoglobin (HGB), and platelets (PLT) in the recipient PB. (D) FACS analysis of blood lineage reconstitution after transplantation. The data are mean ± SEM, from 2 independent experiments, n = 9 to 10 per group. (E) H&E staining of PB smears of the Sipa1+/+ recipients 9 months after transplantation of donor Sipa1−/− or Sipa1+/+ BM CD45.2+ cells. Scale bars represent 25 μm. (F) Reconstitution of HSPCs in the recipient BM 9 months after transplantation. CMP, common myeloid progenitor; LT-HSCs, long-term HSCs; ST-HSCs, short-term HSCs.
Loss of Sipa1 results in alterations of BM niche prior to the initiation of MDS/MPN
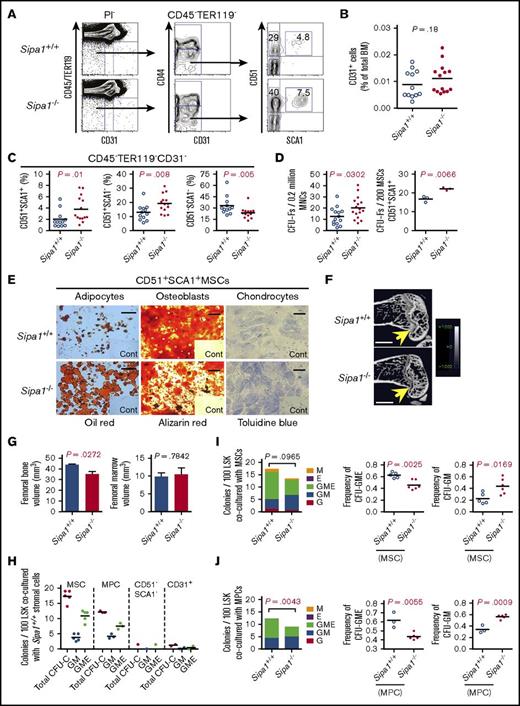
The absence of MPN after transplantation of Sipa1−/− hematopoietic cells into a wild-type environment pointed to the potential critical contribution of the Sipa1−/− BM niche to the MPN. To test this, we analyzed the BM cellular niche components in young (2-3 months old) Sipa1−/− mice where no abnormal hematopoiesis was observed (supplemental Figure 2). FACS analysis indicated that both MSCs and MPCs, but not CD31+ cells, were significantly expanded in the young Sipa1−/− BM (Figure 4A-C). Correspondingly, the CFU-F frequencies in the unfractionated BM cells and the sorted MSCs from the Sipa1−/− mice were increased (Figure 4D). The Sipa1−/− MSCs displayed increased adipogenic, but reduced osteogenic differentiation potential (Figure 4E). μCT analysis revealed a reduced femoral bone volume (Figure 4F-G left) in the young Sipa1−/− mice compared with that in the age-matched Sipa1+/+ mice, supporting the finding of impaired osteoblast differentiation capacity of the Sipa1−/− MSCs.
Phenotypic and functional alterations of BM mesenchymal cells in the Sipa1−/− mice prior to the initiation of MPN. (A) Representative FACS profiles of the analysis of BM stromal cells subsets in 3-month-old Sipa1+/+ and Sipa1−/− mice. The CD45−TER119−CD31−PI− cells were first divided into the CD44− and CD44+ cells. The SCA1+CD51+ MSCs, SCA1+CD51− MPCs, and SCA1−CD51− cells were subsequently gated within the CD44− cells. (B) The frequency of CD31+ cells in the Sipa1+/+ and Sipa1−/− mouse BM. (C) The percent of the MSCs, MPCs, and the SCA1−CD51− cells within total CD45−TER119−CD31− stromal cells. The data are from 3 independent experiments. (D) CFU-F frequencies in Sipa1+/+ and Sipa1−/− mouse BM MNCs and FACS-sorted MSCs. (E) Multilineage differentiation potentials of MSCs from Sipa1+/+ and Sipa1−/− BM. Scale bars represent 250 μm (left), 500 μm (middle), and 50 μm (right). n = 3 independent sorting experiments. (F) Representative μCT images of the longitudinal femoral section indicating reduced bone mass of Sipa1−/− mouse femurs. Scale bars represent 1.0 mm. (G) Femoral bone (left) and marrow (right) volumes of Sipa1+/+ and Sipa1−/− mice. n = 3 per group of each genotype. (H) Colony-forming unit in culture (CFU-C) colonies derived from 100 LSK cells cocultured with Sipa1+/+ BM MSC, MPC, CD51−SCA1− mature stromal cells and endothelial cells. Total CFU-C, colonies with GM, G, M, erythrocytes (E), and GME lineages were counted. (I-J) The numbers of CFU-C colonies per 100 LSK cells after coculture with Sipa1+/+ and Sipa1−/− MSC (I) and MPC (J). Data were collected from 2 to 3 independent experiments. The statistical difference was determined by unpaired Student t test. See also in supplemental Figure 2.
Phenotypic and functional alterations of BM mesenchymal cells in the Sipa1−/− mice prior to the initiation of MPN. (A) Representative FACS profiles of the analysis of BM stromal cells subsets in 3-month-old Sipa1+/+ and Sipa1−/− mice. The CD45−TER119−CD31−PI− cells were first divided into the CD44− and CD44+ cells. The SCA1+CD51+ MSCs, SCA1+CD51− MPCs, and SCA1−CD51− cells were subsequently gated within the CD44− cells. (B) The frequency of CD31+ cells in the Sipa1+/+ and Sipa1−/− mouse BM. (C) The percent of the MSCs, MPCs, and the SCA1−CD51− cells within total CD45−TER119−CD31− stromal cells. The data are from 3 independent experiments. (D) CFU-F frequencies in Sipa1+/+ and Sipa1−/− mouse BM MNCs and FACS-sorted MSCs. (E) Multilineage differentiation potentials of MSCs from Sipa1+/+ and Sipa1−/− BM. Scale bars represent 250 μm (left), 500 μm (middle), and 50 μm (right). n = 3 independent sorting experiments. (F) Representative μCT images of the longitudinal femoral section indicating reduced bone mass of Sipa1−/− mouse femurs. Scale bars represent 1.0 mm. (G) Femoral bone (left) and marrow (right) volumes of Sipa1+/+ and Sipa1−/− mice. n = 3 per group of each genotype. (H) Colony-forming unit in culture (CFU-C) colonies derived from 100 LSK cells cocultured with Sipa1+/+ BM MSC, MPC, CD51−SCA1− mature stromal cells and endothelial cells. Total CFU-C, colonies with GM, G, M, erythrocytes (E), and GME lineages were counted. (I-J) The numbers of CFU-C colonies per 100 LSK cells after coculture with Sipa1+/+ and Sipa1−/− MSC (I) and MPC (J). Data were collected from 2 to 3 independent experiments. The statistical difference was determined by unpaired Student t test. See also in supplemental Figure 2.
To evaluate the hematopoiesis-supportive function of different Sipa1−/− MSCs, MPCs, and endothelial cells, we cocultured these sorted cells with normal Lin−SCA1highKIThigh (LSK) HSPCs (Figure 4H-J). Sipa1−/− MSCs and MPCs displayed significantly stronger capacity in promoting myeloid cell differentiation, indicated by more granulocyte-macrophage (GM) colonies, but less multilineage (GM and erythrocyte, GME) colonies generated in the cultures with the Sipa1−/− stromal cells compared with that with Sipa1+/+ stromal cell counterparts (Figure 4I-J).
Taken together, these data demonstrated the dramatic alterations in composition and function of the BM niche in young Sipa1−/− mice prior to the onset of the MDS/MPN.
Sipa1-deficient BM niche induces myeloproliferation and erythrocyte dysplasia after transplantation
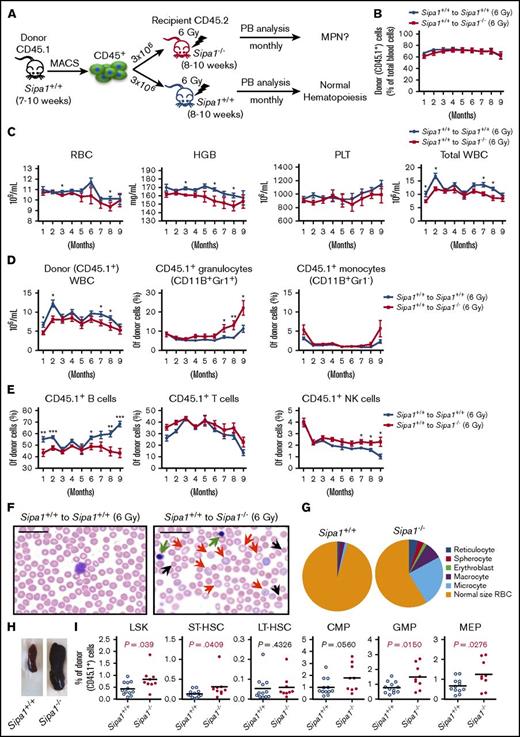
To determine the role of the altered BM niche in the young Sipa1−/− mice for the initiation of the MPN, we next transplanted the Sipa1+/+ BM hematopoietic cells into sublethally (6 Gy) irradiated Sipa1+/+ and Sipa1−/− recipients (Figure 5A). To avoid introducing donor BM stromal cells, we purified the CD45.1+ hematopoietic cells for the transplantation. About 60% to 70% donor-cell engraftment in the PB was achieved in both groups after transplantation (Figure 5B). However, we detected lower WBC, RBC, and hemoglobin counts in the Sipa1−/− recipient PB compared with that in the Sipa1+/+ recipients (Figure 5C), indicating ineffective hematopoiesis of the donor cells in the Sipa1−/− niche. The donor-derived CD11B+GR1+ granulocytes were increased at ∼7 months after transplantation, whereas the CD19+ B cells, but not T cells, were reduced in the Sipa1−/− recipients compared with that in the Sipa1+/+ recipients (Figure 5D-E). The natural killer cells were also significantly increased after transplantation in the Sipa1−/− recipient PB (Figure 5E). Moreover, PB smears of the Sipa1−/− recipients showed the presence of erythroblasts, reticular erythrocytes, spherocytes, macrocytes, and microcytes in the Sipa1−/− recipients (Figure 5F-G), supporting the notion of the dyserythropoiesis. In addition, we observed splenomegaly in 10% of the Sipa1−/− recipients 9 months after transplantation (Figure 5H). The increased myeloid cells, but decreased B cells, were also detected in the Sipa1−/− recipient BM (supplemental Figure 3A-B). Importantly, we did not observe any significant difference in host-derived hematopoietic lineage distribution between the Sipa1+/+ and Sipa1−/− recipients (supplemental Figure 3B), emphasizing little contribution of the host Sipa1−/− hematopoietic cells to the MDS/MPN.
Sipa1−/− niche induces MDS/MPN from normal hematopoietic cells after transplantation following sublethal irradiation. (A) Experimental design, normal hematopoietic cells. Three million normal BM CD45.1+ cells from a 7- to 10-week-old Sipa1+/+ mouse were sorted by magnetic-activated cell sorting (MACS) and transplanted into sublethally irradiated CD45.2+ young (8-10 week old) Sipa1+/+ and Sipa1−/− recipient mice. The PB of the recipients was monitored monthly after transplantation. (B) Total donor-derived blood reconstitution in the Sipa1+/+ and Sipa1−/− recipients after transplantation. Data are mean ± SEM, from 2 independent experiments, n = 12 Sipa1+/+ recipients and n = 10 Sipa1−/− recipients. (C) RBCs, HGB, PLTs, and total WBCs in the recipient PB. The PB was analyzed monthly for monitoring the development of the disorders in the PB of the sublethally irradiated Sipa1+/+ and Sipa1−/− recipients after transplantation. (D) Donor-derived WBC and myeloid cells in Sipa1+/+ and Sipa1−/− mice after transplantation. (E) Donor-derived B cells, T cells, and natural killer cells in the Sipa1+/+ and Sipa1−/− mice after transplantation. (F) H&E staining of PB smears of the Sipa1+/+ and Sipa1−/− recipients 9 months after transplantation. Scale bars represent 25 μm. Black arrows indicate microcytes; red arrows indicate macrocytes, and green arrows indicate erythroblasts, respectively. (G) Distribution of reticulocytes, macrocytes, microcytes, spherocytes, erythroblasts, and normal size RBC. (H) Splenomegaly in the Sipa1−/− recipients 6 to 9 months after transplantation. (I) Enhanced expansion of donor-derived HSPCs in the recipient Sipa1−/− BM 9 months after transplantation. The donor-derived (CD45.1+) HSCs and HPCs were calculated in total BM Lineage (LIN)− cells. *P < .05; **P < .01; ***P < .001, analyzed by unpaired Mann-Whitney U test. See also in supplemental Figure 3.
Sipa1−/− niche induces MDS/MPN from normal hematopoietic cells after transplantation following sublethal irradiation. (A) Experimental design, normal hematopoietic cells. Three million normal BM CD45.1+ cells from a 7- to 10-week-old Sipa1+/+ mouse were sorted by magnetic-activated cell sorting (MACS) and transplanted into sublethally irradiated CD45.2+ young (8-10 week old) Sipa1+/+ and Sipa1−/− recipient mice. The PB of the recipients was monitored monthly after transplantation. (B) Total donor-derived blood reconstitution in the Sipa1+/+ and Sipa1−/− recipients after transplantation. Data are mean ± SEM, from 2 independent experiments, n = 12 Sipa1+/+ recipients and n = 10 Sipa1−/− recipients. (C) RBCs, HGB, PLTs, and total WBCs in the recipient PB. The PB was analyzed monthly for monitoring the development of the disorders in the PB of the sublethally irradiated Sipa1+/+ and Sipa1−/− recipients after transplantation. (D) Donor-derived WBC and myeloid cells in Sipa1+/+ and Sipa1−/− mice after transplantation. (E) Donor-derived B cells, T cells, and natural killer cells in the Sipa1+/+ and Sipa1−/− mice after transplantation. (F) H&E staining of PB smears of the Sipa1+/+ and Sipa1−/− recipients 9 months after transplantation. Scale bars represent 25 μm. Black arrows indicate microcytes; red arrows indicate macrocytes, and green arrows indicate erythroblasts, respectively. (G) Distribution of reticulocytes, macrocytes, microcytes, spherocytes, erythroblasts, and normal size RBC. (H) Splenomegaly in the Sipa1−/− recipients 6 to 9 months after transplantation. (I) Enhanced expansion of donor-derived HSPCs in the recipient Sipa1−/− BM 9 months after transplantation. The donor-derived (CD45.1+) HSCs and HPCs were calculated in total BM Lineage (LIN)− cells. *P < .05; **P < .01; ***P < .001, analyzed by unpaired Mann-Whitney U test. See also in supplemental Figure 3.
Expansion of HSPCs is a common phenotype of MPN.33,34 The frequencies of donor-derived LSK, short-term–HSC (LSKCD34+FLT3−), granulocyte-macrophage progenitor (GMP; LIN−SCA1−KIT+CD34highFcγRhigh), and MK-erythrocyte progenitor (MEP; LIN−SCA1-KIT+CD34−FcγR−) were increased in the Sipa1−/− recipient BM 9 months after transplantation (Figure 5I). Meanwhile, the accumulations of CMPs and GMPs were also detected in the Sipa1−/− recipient spleen (supplemental Figure 3C). However, the frequency of host-derived HSPCs in the Sipa1−/− recipient spleen remained similar to that in the Sipa1+/+ recipients (supplemental Figure 3D).
Taken together, transplantation of normal hematopoietic cells into the Sipa1−/− niche resulted in myeloproliferation along with dysplastic erythropoiesis and leukopenia, which resembles the clinic features of the overlapping syndromes of the unclassifiable MDS/MPN35 and is consistent with MDS/MPN in mice according to the Bethesda criteria32 and the World Health Organization classification.36
Neoplastic transformation of normal hematopoietic cells after transplantation into Sipa1−/− niche
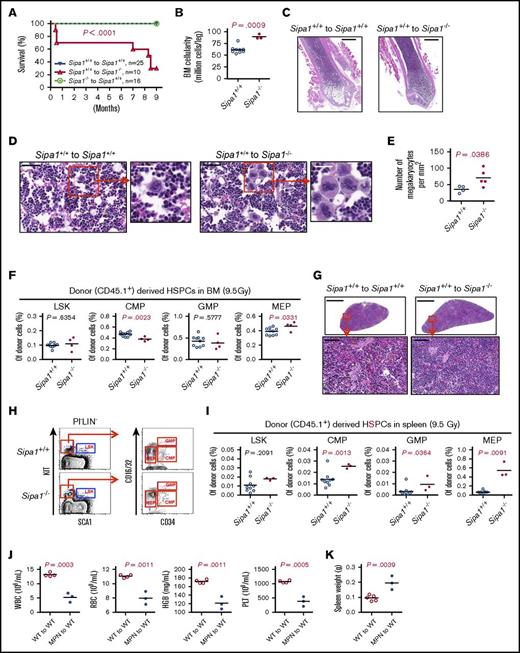
To further determine the contribution of the altered Sipa1−/− niche to the initiation of MDS/MPN and the underlying molecular mechanisms, we examined the hematopoietic activity of normal BM CD45.1+ cells after transplantation into lethally irradiated (9.5 Gy) Sipa1−/− CD45.2+ mice. About 98% donor-cell engraftment was obtained in the recipients after transplantation (supplemental Figure 4A). We observed even more severe phenotype of MDS/MPN in these Sipa1−/− recipients compared with that in the sublethally irradiated recipients, which might be due to the stronger inflammatory response following lethal irradiation. The Sipa1−/− recipients exhibited reduced survival (Figure 6A), myeloproliferation accompanied with B-cell reduction (supplemental Figure 4B-C), BM hypercellularity (Figure 6B-C), and MK hyperplasia (Figure 6D-E), a characteristic feature of malignant or clonal hematological disorders.37 The frequency of the donor-derived MEPs was increased in the Sipa1−/− recipient BM compared with that in the Sipa1+/+ BM (Figure 6F). One of the Sipa1−/− recipients displayed enlarged lymph nodes (supplemental Figure 4E). Similar to what we observed in the aged Sipa1−/− mice (Figure 2C), ∼28.6% (2/7) of the Sipa1−/− recipients developed splenomegaly 7 to 9 months after transplantation. H&E staining illustrated reduced lymphocytic component of white pulp in the Sipa1−/− recipient spleen (Figure 6G). This was associated with the increased frequencies of CMP, GMP, and MEP in the Sipa1−/− recipient spleen (Figure 6H-I). The malignant transformation of the normal donor cells in the Sipa1−/− recipients was indicated by the development of MDS in ∼75% of the secondary recipients after receiving the spleen cells from the primary Sipa1−/− recipients with MDS/MPN. The secondary recipients exhibited increased mortality, anemia, thrombocytopenia, and splenomegaly 6 months after the transplantation (Figure 6J-K). To explore the molecular mechanisms underlying the pathogenesis of the disease, we performed whole-exome sequencing of the donor cells sorted from Sipa1−/− and Sipa1+/+ primary recipients. However, we only detected a few genetic variants in the donor cells from Sipa1−/− BM and spleen in 1 of the experiments (data are not shown).
Development of lethal MDS/MPN in the lethally irradiated Sipa1−/− recipients. Three million normal BM CD45.1+ cells from 8- to 9-week-old Sipa1+/+ mice were transplanted into lethally irradiated CD45.2+ young (8-10 week old) Sipa1+/+ and Sipa1−/− recipient mice. (A) Kaplan-Meier survival curves of the lethally irradiated Sipa1+/+ and Sipa1−/− recipients after transplantation. The statistic difference was determined by Logrank Mantel Cox test. (B) The total BM cellularity 9 months after the transplantation. (C) Representative H&E-stained femoral sections showed increased leukocytes infiltration in the BM from the Sipa1−/− recipients. Scale bars represent 1.0 mm. (D) Representative H&E-stained femoral sections showed increased MKs in the BM of the Sipa1−/− recipient mice. Scale bars represent 50 μm (black) and 25 μm (white). (E) The increased numbers of MKs in the Sipa1−/− recipient bone sections. The data are expressed as numbers per squared millimeters. (F) The frequencies of HSPCs in the recipient BM at the endpoint of the experiments. (G) H&E-stained spleen sections of Sipa1+/+ and Sipa1−/− recipients 9 months after transplantation. Scale bars represent 1.0 mm for the upper panels and 50 μm for the lower panels. (H) Representative FACS profile showing gating strategy for HSPCs in spleen of Sipa1+/+ and Sipa1−/− recipients 7 to 9 months after transplantation. (I) Frequencies of HSPCs in spleens of the Sipa1+/+ and Sipa1−/− recipients. (J) Reduced mature blood cells in the PB of secondary recipients 6 months after transplantation of the spleen cells from a primary Sipa1−/− recipient with MPN. (K) The spleen weight of the secondary recipient mice 6 months after transplantation. The statistical differences in panels B-K were determined by nonparametric Mann-Whitney U test or parametric Student t test with Welch’s correction according the data distribution. See also in supplemental Figure 4. KIT, CD117 or Proto-Oncogene C-Kit; WT, wild type.
Development of lethal MDS/MPN in the lethally irradiated Sipa1−/− recipients. Three million normal BM CD45.1+ cells from 8- to 9-week-old Sipa1+/+ mice were transplanted into lethally irradiated CD45.2+ young (8-10 week old) Sipa1+/+ and Sipa1−/− recipient mice. (A) Kaplan-Meier survival curves of the lethally irradiated Sipa1+/+ and Sipa1−/− recipients after transplantation. The statistic difference was determined by Logrank Mantel Cox test. (B) The total BM cellularity 9 months after the transplantation. (C) Representative H&E-stained femoral sections showed increased leukocytes infiltration in the BM from the Sipa1−/− recipients. Scale bars represent 1.0 mm. (D) Representative H&E-stained femoral sections showed increased MKs in the BM of the Sipa1−/− recipient mice. Scale bars represent 50 μm (black) and 25 μm (white). (E) The increased numbers of MKs in the Sipa1−/− recipient bone sections. The data are expressed as numbers per squared millimeters. (F) The frequencies of HSPCs in the recipient BM at the endpoint of the experiments. (G) H&E-stained spleen sections of Sipa1+/+ and Sipa1−/− recipients 9 months after transplantation. Scale bars represent 1.0 mm for the upper panels and 50 μm for the lower panels. (H) Representative FACS profile showing gating strategy for HSPCs in spleen of Sipa1+/+ and Sipa1−/− recipients 7 to 9 months after transplantation. (I) Frequencies of HSPCs in spleens of the Sipa1+/+ and Sipa1−/− recipients. (J) Reduced mature blood cells in the PB of secondary recipients 6 months after transplantation of the spleen cells from a primary Sipa1−/− recipient with MPN. (K) The spleen weight of the secondary recipient mice 6 months after transplantation. The statistical differences in panels B-K were determined by nonparametric Mann-Whitney U test or parametric Student t test with Welch’s correction according the data distribution. See also in supplemental Figure 4. KIT, CD117 or Proto-Oncogene C-Kit; WT, wild type.
Dysregulated inflammatory cytokines and growth factors in the Sipa1−/− BM stromal cells
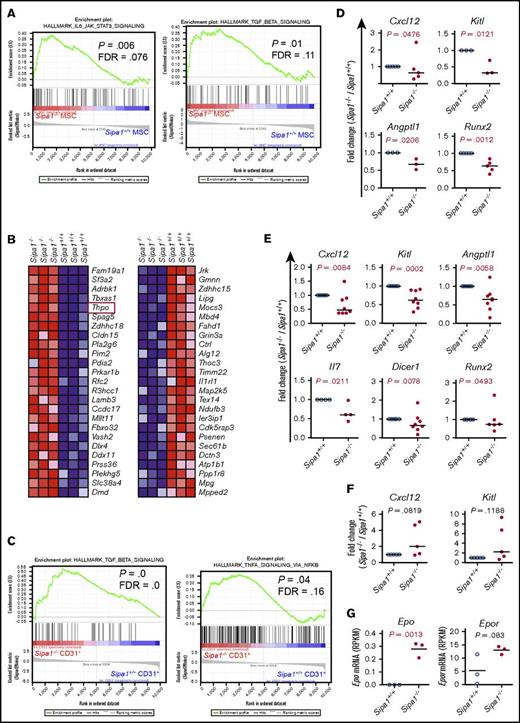
To investigate the molecular mechanisms underlying the niche-induced MDS/MPN, we performed RNA sequencing on the native BM MSCs, MPCs, and endothelial cells from young (8-10 week old) Sipa1−/− and Sipa1+/+ mice. As expected, gene set enrichment analysis showed that G-protein signal pathways, including Ras and Rap1 signaling, were elevated in the Sipa1−/− MSCs and endothelial cells (supplemental Figures 5A-B and 6). Proinflammatory cytokines, including transforming growth factor-β (TGF-β) and the IL6/JAK/STAT3 signaling pathways, were significantly increased in the Sipa1−/− MSCs (Figure 7A). Similarly, Il4 and Mapk in the FcεRI-mediated signaling pathway were also elevated in the Sipa1−/− MPCs (supplemental Figure 5C). Within the top 25 changed genes in the Sipa1−/− MPCs, thrombopoietin (Thpo), a growth factor critical for HSC maintenance and the development of MPN, was increased in the Sipa1−/− MPCs (Figure 7B). The TGF-β and tumor necrosis factor-α (TNF-α) signaling pathways were also enhanced in the Sipa1−/− endothelial cells (Figure 7C). In addition, the extracellular matrix receptor interactions, wingless-type MMTV integration site family β (WNT-β) catenin, interleukin-2 (IL-2), β-catenin (Ctnnb1), and Notch signaling were enriched in the Sipa1−/− endothelial cells (supplemental Figure 5D-E).
Altered molecular profiles of BM stromal cell subsets in Sipa1−/−young adult mice. Gene set enrichment analysis was carried out on the RNA-sequencing data to identify differentially expressed genes in the Sipa1−/− stromal cells. The RNA sequencing was performed on FACS-sorted BM MSCs, MPCs, and endothelial cells from 2 to 3-month-old mice. Data were from 3 independent experiments. False discovery rate-q value represents the false discovery rate of the P value. (A) Upregulated IL-6/JAK2/STAT3 and TGF-β signaling pathways in the Sipa1−/− MSCs vs Sipa1+/+ MSCs. (B) The top 25 altered genes in the Sipa1−/− MPCs relative to that in the Sipa1+/+ mice. The red frame highlights Thpo gene. Red indicates high expression, and blue indicates low expression. (C) Enhanced TGF-β and TNF-α signaling in the Sipa1−/− endothelial cells. (D) qPCR analysis of Kitl, Angptl1, Cxcl12, and Runx2 expressions in Sipa1+/+ and Sipa1−/− MSCs. (E) qPCR analysis of Kitl, Angptl1, Il7, Cxcl12, Dicer1, and Runx2 expressions in Sipa1+/+ and Sipa1−/− MPCs. (F) qPCR analysis of Kitl and Cxcl12 expressions in Sipa1+/+ and Sipa1−/− endothelial cells. The statistical differences in panels D-F were analyzed by unpaired Mann-Whitney U test or Kolmogorov-Smirnov test. (G) RNA sequencing revealed upregulation of Epo and Epor in the Sipa1−/− endothelial cells. P values were calculated by unpaired Student t test. See also in supplemental Figures 5 and 6.
Altered molecular profiles of BM stromal cell subsets in Sipa1−/−young adult mice. Gene set enrichment analysis was carried out on the RNA-sequencing data to identify differentially expressed genes in the Sipa1−/− stromal cells. The RNA sequencing was performed on FACS-sorted BM MSCs, MPCs, and endothelial cells from 2 to 3-month-old mice. Data were from 3 independent experiments. False discovery rate-q value represents the false discovery rate of the P value. (A) Upregulated IL-6/JAK2/STAT3 and TGF-β signaling pathways in the Sipa1−/− MSCs vs Sipa1+/+ MSCs. (B) The top 25 altered genes in the Sipa1−/− MPCs relative to that in the Sipa1+/+ mice. The red frame highlights Thpo gene. Red indicates high expression, and blue indicates low expression. (C) Enhanced TGF-β and TNF-α signaling in the Sipa1−/− endothelial cells. (D) qPCR analysis of Kitl, Angptl1, Cxcl12, and Runx2 expressions in Sipa1+/+ and Sipa1−/− MSCs. (E) qPCR analysis of Kitl, Angptl1, Il7, Cxcl12, Dicer1, and Runx2 expressions in Sipa1+/+ and Sipa1−/− MPCs. (F) qPCR analysis of Kitl and Cxcl12 expressions in Sipa1+/+ and Sipa1−/− endothelial cells. The statistical differences in panels D-F were analyzed by unpaired Mann-Whitney U test or Kolmogorov-Smirnov test. (G) RNA sequencing revealed upregulation of Epo and Epor in the Sipa1−/− endothelial cells. P values were calculated by unpaired Student t test. See also in supplemental Figures 5 and 6.
Consistent with the impaired osteogenic differentiation potential of the Sipa1−/− MSCs, the expression of runt-related transcription factor 2 (Runx2), a master gene for osteoblast differentiation, was reduced in the Sipa1−/− MSCs and MPCs (Figure 7D-E). Furthermore, expression of the lymphopoiesis-promoting cytokine Il7 and Dicer1, a microRNA-processing gene, was reduced in the Sipa1−/− MPCs (Figure 7E).
Cxcl12, Kitl, and Angptl1 are known to be critical for maintaining HSC quiescence.38-42 The significant downregulations of Cxcl12, Kitl, and Angptl1 were observed in Sipa1−/− BM MSCs and MPCs, but not in endothelial cells (Figure 7D-F) compared with the Sipa1+/+ cell counterparts. However, erythropoietin (Epo) expression in the Sipa1−/− endothelial cells was increased (Figure 7G). Together, these data indicate that Sipa1 loss in BM stromal cells causes molecular alteration of the niche factors that may contribute to the pathogenesis of MPN.
Discussion
Recent studies have reported that genetic alterations of BM stromal cells can be involved in the pathogenesis of MPN and AML.2,11 However, the cellular and molecular mechanisms underlying the microenvironment-induced leukemogenesis remain poorly understood. We here demonstrate that Sipa1 deletion results in BM niche alterations leading to the development of MDS/MPN.32,35 The initiation of the MDS/MPN in this model is dependent on the altered BM niche resulting from the Sipa1 deficiency in BM microenvironment, not in hematopoietic cells. The dysregulated inflammatory cytokines in Sipa1-deficient niche might contribute to the pathogenesis of MDS/MPN.
SIPA1 is a G-protein signaling component of many growth factors and cytokine, including IL-3, granulocyte-macrophage colony-stimulating factor, and CXCL12 in hematopoietic cells.12-14 SIPA1 point mutations were detected in mononuclear cells of patients with juvenile myelomonocytic leukemia but lacking detectable mutations of other G-protein signaling molecules KRAS, NRAS, and PTPN11.22 However, the cell origin of the mutation remains unclear. Previous studies showed that the majority of the Sipa1−/− mice and 15% Sipa1+/− mice developed CML-like MPN.15,25 We here observed MDS-like MPN mainly in the aged Sipa1−/− female mice. This discrepancy in the observed disease phenotype might be due to different genetic background of the mice. The female-biased development of the MDS/MPN could be attributed to the declined bone-forming ability in the aged female mice,43,44 which could deteriorate the phenotype of the impaired osteoblast differentiation induced by Sipa1 deletion. Nevertheless, the impact of Sipa1 deletion in BM microenvironment on the MDS/MPN remains unexplored.
We here for the first time report SIPA1 expression in human and mouse native BM stromal cells. The importance of Sipa1 expression in BM niche is supported by the abnormal expansion and differentiation of MSCs and MPCs in young Sipa1−/− mouse BM prior to the initiation of the MDS/MPN, which could contribute to the pathogenesis of the disease. Furthermore, the SIPA1 gene is downregulated in BM MSCs and endothelial cells from patients with CML, CMML, and CNL. This finding merits future study on the impact of SIPA1 gene alteration in BM niche on the treatment outcome in patients with MPN, particularly in the patients who undergo allogenic stem cell transplantation.
The absolute requirement of Sipa1−/− BM niche for the generation of the MPN in the Sipa1−/− aged mice is demonstrated by the reciprocal transplantation of normal Sipa1+/+ hematopoietic cells into irradiated Sipa1−/− and Sipa1+/+ mice, or vice versa. The sustained normal hematopoiesis after transplantation of Sipa1−/− hematopoietic cells in normal microenvironment suggests that the intrinsic loss of Sipa1 gene in hematopoietic cells is not sufficient to induce the MPN. In contrast, the Sipa1−/− recipients developed lethal MDS/MPN after transplantation of Sipa1+/+ hematopoietic cells. The features of the disease in the Sipa1−/− recipients almost completely recapitulated the MDS/MPN in the aged Sipa1−/− mice. Our data suggest that the MDS/MPN in the aged Sipa1−/− mice is actually driven by the BM niche, not the Sipa1 deletion in hematopoietic cells. This finding is in contrast to the previous report that Sipa1−/− hematopoietic cells from the mice with established MPN continued developing CML-like disease after transplantation.15 This discrepancy could be mainly due to the fact that the donor Sipa1−/− cells in the previous study were already transformed to be leukemic, and thus, might have developed niche independence, as reported.45 Therefore, it is critical that we transplanted Sipa1−/− hematopoietic cells from young adult mice where hematopoiesis remained normal. The reoccurrence of the MDS-like phenotype in the secondary wild-type recipients supports the notion of the niche-induced malignant transformation of the donor cells. The disease phenotype is likely due to potential epigenetic alterations or selection of clonal hematopoiesis in the donor hematopoietic cells. However, the exact underlying mechanisms require further investigation in the future.
Understanding the molecular mechanisms mediating the niche-induced MPN is fundamental for identifying predicting factors for leukemogenesis. The increased expression of proinflammatory cytokines like TGF-β and TNF-α is a common feature of mouse models with MPN.2 This was also observed in patients with MPN.46,47 IL6/JAK/STAT3 signaling has been implicated in MPN progression.48-52 The elevated IL-6, TGF-β, and TNF-α signaling pathways in the Sipa1−/− stromal cells may contribute to myeloproliferation possibly via altering HSC lineage fate decision in the Sipa1−/− mice, as previously suggested.50,53
THPO-MPL signaling is critical for maintaining HSCs in adult BM54 and is involved in the pathogenesis of MPN.55,56 The upregulation of Thpo in the Sipa1−/− BM stromal cells may be associated with the enhanced granulopoiesis and the overproduction of MEPs and MK hyperplasia in the aged Sipa1−/− mice under steady state and after transplantation. The increased number of MKs in BM might in turn promote the progression of the MDS/MPN via platelet factor secretion, as described recently.57 In addition, it has been shown that EPO can direct multipotent HSPCs toward committed erythrocyte progenitors.58 The increased Epo gene expression in Sipa1−/− endothelial cells might also contribute to the increase of MEPs.57
Il7 is critical for maintaining normal lymphopoiesis in adult mice.59 The reduced Il7 expression in the Sipa1−/− MPCs could indirectly lead to myeloproliferation in the Sipa1−/− recipients after transplantation. Furthermore, Cxcl12, Kitl, and Angptl1 are critical to maintain normal hematopoiesis through keeping HSC quiescence,5,40,60,61 and the reduction of these genes in BM niche cells were detected in previous MPN mouse models.29,62 The downregulation of these genes in the Sipa1−/− MSCs and MPCs could affect HSC retention and quiescence and eventually promote the myelopoiesis. Deletion of Dicer1 from osteoprogenitors leads to the development of MDS and AML.9 Thus, the downregulation of Dicer1 in the Sipa1−/− MPCs, enriched with osteoblast progenitors,31 might contribute to the niche alteration and initiation of the MPN in the Sipa1−/− mice.
In conclusion, we have demonstrated that Sipa1 loss in the BM stromal cells results in BM niche alterations, which are absolutely required for the development of MDS/MPN. The enhanced inflammatory cytokine signaling and myelopoiesis-promoting activities in the Sipa1−/− BM niche may have contributed to the disease pathogenesis. Our data provide novel evidence for niche-driven MPN and the underlying cellular and molecular mechanisms.
The full-text version of this article contains a data supplement.
Acknowledgments
The authors are grateful to Minna Taipale and Andranik Durgaryan at Karolinska Institute and Liseotte Lenner at Linköping University for their technical assistance. All major computations were performed on resources provided by the Swedish National Infrastructure for Computing through Uppsala Multidisciplinary Center for Advanced Computational Science under Project b2014299. The authors acknowledge the MedH Core Flow Cytometry facility (Karolinska Institute) for providing cell-sorting/analysis services.
This study was supported by Swedish Research Council (K2013-99X-22241-01-5), Swedish Childhood Society (TJ2013-0048, PR2015-0142, and PROJ12/081), Swedish Cancer Society (CAN 2009/1589 and CAN 2012/891), Åke Olsson Foundation, Radiumhemmets Forskningsfonder, and Karolinska Institute Wallenberg Institute for Regenerative Medicine (H.Q.), and Knut and Alice Wallenbergs Foundation (M.S.).
Authorship
Contribution: H.Q. and P.X. designed, performed, analyzed data and wrote the manuscript; M. Dolinska, L.S., M.K., A.-S.J., M. Dimitriou, and T.B. assisted part of the study and manuscript review; Y.Z. performed μCT analysis and wrote the corresponding method in the manuscript; X.L. analyzed RNA sequencing data and wrote the corresponding method in the manuscript; N.M. provided the Sipa1−/− mice; G.Z.R. provided the morphologic assessment of histologic and cytologic preparations; D.T.S., E.H.-L., and J.W. provided scientific input on the study and manuscript review; M.S. performed the RNA and exome sequencing experiments and provided scientific input, and all authors have read and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hong Qian, Center for Hematology and Regenerative Medicine, Department of Medicine, Karolinska Institute, Karolinska University Hospital, SE-141 86 Stockholm, Sweden; e-mail: hong.qian@ki.se.