GATA2 immunohistochemistry predicts myeloid dysplasia, complex cytogenetic abnormalities, and poor prognosis in adult MDS.
GATA2 immunohistochemistry is variable in adult AML and predicts neutropenia and complex cytogenetics but does not correlate with survival.
Visual Abstract
GATA binding protein 2 (GATA2) is a conserved zinc finger transcription factor that regulates the emergence and maintenance of complex genetic programs driving development and function of hematopoietic stem and progenitor cells (HSPCs). Patients born with monoallelic GATA2 mutations develop myelodysplastic neoplasm (MDS) and acute myeloid leukemia (AML), whereas acquired GATA2 mutations are reported in 3% to 5% of sporadic AML cases. The mechanisms by which aberrant GATA2 activity promotes MDS and AML are incompletely understood. Efforts to understand GATA2 in basic biology and disease will be facilitated by the development of broadly efficacious antibodies recognizing physiologic levels of GATA2 in diverse tissue types and assays. Here, we purified a polyclonal anti-GATA2 antibody and generated multiple highly specific anti-GATA2 monoclonal antibodies, optimized them for immunohistochemistry on patient bone marrow bioosy samples, and analyzed GATA2 expression in adults with healthy bone marrow, MDS, and acute leukemia. In healthy bone marrow, GATA2 was detected in mast cells, subsets of CD34+ HSPCs, E-cadherin–positive erythroid progenitors, and megakaryocytes. In MDS, GATA2 expression correlates with bone marrow blast percentage, positively correlates with myeloid dysplasia and complex cytogenetics, and is a nonindependent negative predictor of overall survival. In acute leukemia, the percent of GATA2+ blasts closely associates with myeloid lineage, whereas a subset of lymphoblastic and undifferentiated leukemias with myeloid features also express GATA2. However, the percent of GATA2+ blasts in AML is highly variable. Elevated GATA2 expression in AML blasts correlates with peripheral neutropenia and complex AML cytogenetics but, unlike in MDS, does not predict survival.
Introduction
Myelodysplastic neoplasm (MDS) and acute myeloid leukemia (AML) are aggressive hematologic malignancies derived from hematopoietic stem and progenitor cells (HSPCs; clinically referred to as blasts).1,2 Both are caused by genomic alterations including cytogenetic structural variants and sequence-level molecular variants that accumulate within HSPCs. In MDS, these variants lead to ineffective hematopoiesis with peripheral cytopenias and morphologic dysplasia. In AML, mutations trigger a clonal expansion of bone marrow blasts that ablates healthy hematopoietic progenitors. In both disorders, curative treatment involves cytotoxic therapy often coupled to allogeneic hematopoietic stem cell transplantation.3,4 However, even when patients tolerate therapy, morbidity and mortality remain high.5
GATA binding protein 2 (GATA2) is a conserved zinc finger transcription factor belonging to a family of GATA factors (GATA1-6) that bind genomic GATA motifs to promote complex genetic programs.6,7 GATA2 is critical for development and function of the hematopoietic system, lymphatics, central nervous system, uterus, prostate, and other organs.8-12 Mice with homozygous Gata2 deletion die early in embryogenesis because of an inability to form blood,13 whereas conditional loss of Gata2 in the adult bone marrow causes marrow failure.14 Although significant progress has been made in elucidating GATA2-dependent mechanisms,14-19 many aspects of GATA2 biology and linked pathologies remain poorly understood. For instance, the human bone marrow subsets that normally express GATA2 protein remain incompletely defined, although recent findings in GATA2 reporter mouse systems have succeeded in partially mapping GATA2 protein expression in embryonic and adult murine hematopoietic subpopulations.20-22 These and other studies suggest that GATA2 is expressed homogeneously at a low level in long-term HSPCs, whereas its expression is variable and sometimes highly dynamic in more differentiated multipotent progenitors.
GATA2 also has significant clinical importance. Persons born with monoallelic mutations in GATA2 or in enhancers23-25 regulating GATA2 expression develop GATA2 deficiency syndrome (GDS).26-29 Patients with GDS may experience a range of symptoms, the most devastating of which are bone marrow failure, MDS, and AML. The mechanisms by which germ line GATA2 mutations promote MDS and AML are unresolved.17 Acquired GATA2 mutations also are found in 3% to 5% of sporadic MDS and AML cases, and their contributions to leukemogenesis are only beginning to be elucidated.30-34 Finally, select studies have suggested that elevated GATA2 messenger RNA (mRNA) transcript levels may predict AML disease severity and poor clinical outcome,35,36 indicating that elevated GATA2 activity also could play a role in leukemogenesis. In support of this concept, GATA2 behaves as an oncogene in androgen-refractory prostate cancer.37 In contrast, reduced GATA2 expression underlies MDS and AML predisposition in GDS.23,24 Furthermore, GATA2 functions as a tumor suppressor in acute promyelocytic leukemia.30
Progress in elucidating GATA2 mechanisms has been hindered by the lack of clinical assays using anti-GATA2 antibodies to detect GATA2 in primary hematopoietic cells. However, recently, a commercial anti-GATA2 polyclonal antibody enabled single-cell analysis of human GATA2 levels,38 whereas a well-established anti-GATA2 polyclonal antibody39 enabled GATA2 immunohistochemistry (IHC) in murine bone marrow.40 Here, we optimized the well-established anti-GATA2 polyclonal antibody to perform IHC on decalcified formalin-fixed paraffin-embedded bone marrow biopsies from human patients and evaluated GATA2 expression in benign adult bone marrow and adult MDS. We optimized the antibody for dual IHC alongside a panel of hematopoietic lineage markers, and quantified GATA2 expression in blasts, erythroid precursors, mast cells, and megakaryocytes. We generated 3 anti-GATA2 mouse monoclonal antibodies (mAbs) that are sensitive and specific for detecting mouse and human GATA2. We then scored GATA2 expression in the blast compartment of 106 acute leukemias. We compared GATA2 expression with clinicopathologic metrics to identify relationships between GATA2 expression, disease pathobiology, and patient outcome.
Materials, patients, and methods
Case selection
This work was approved by the University of Wisconsin-Madison institutional review board under protocol no. 2018-1510. All samples used in this study constituted residual material and, per protocol, patient consent was not required. Cases were identified by searching the electronic medical record. Benign bone marrows were selected from patients with no known disorder involving the bone marrow, whose bone marrow had been evaluated by a board-certified hematopathologist and determined to be without morphologic or cytogenetic abnormality; when ancillary testing was documented, they reported normal findings. For MDS and AML cases, diagnoses were rendered per the World Health Organization (WHO) Classification of Tumors of Hematopoietic and Lymphoid Tissues, revised Fourth edition.41 Whenever possible, the University of Wisconsin-Madison uses criteria similar to that of Nordic Guidelines42 to screen for germ line predisposition mutations. Within this framework, none of the cases harbored known germ line mutations. Pediatric patients and Veterans Affairs patients were excluded per our institutional review board protocol.
Isolation of anti-GATA2 polyclonal antibodies
Generation of anti-GATA2 mAbs
Amino acids 1 to 204 of human GATA2 were codon optimized for Escherichia coli and cloned into the pET28a vector (Novagen/MilliporeSigma, Burlington, MA) with C-terminal 6-His tag and transformed into BL21 RIL E coli. Bacteria were induced at an optical density of at 600 nm of 0.6 with 1 mM isopropyl β-D-1-thiogalactopyranoside for 5 hours at 37°C. Bacteria were lysed in 20 mL lysis buffer (5 mM imidazole, 250 mM NaCl, and 20 mM tris(hydroxymethyl)aminomethane [Tris] at pH 7.9, 0.5% Nonidet P-40, and 20 mg lysozyme) per liter of culture, then sonicated and centrifuged for 1 hour at 27 000g at 4°C, after which the soluble fraction was incubated with 1 mL nickel-nitrilotriacetic acid beads (New England Biolabs, Ipswich, MA) overnight at 4°C. Beads were loaded into a column and washed with 200 mL wash buffer (30 mM imidazole, 250 mM NaCl, and 20 mM Tris at pH 7.9), then eluted with elution buffer (300 mM imidazole, 500 mM NaCl, and 20 mM Tris at pH 7.9) in 1 mL fractions. Peak fractions were dialyzed into phosphate-buffered saline. BALB/cJ mice were immunized by Green Mountain Antibodies (Burlington, VT) using the standard complete/incomplete Freund adjuvant protocol. Sera were screened against a hGATA2(1-204) fragment lacking 6-His tag after tobacco etch virus protease cleavage. The spleens from mice with the highest serum titers were collected to generate hybridomas and screened via enzyme-linked immunosorbent assay and western blot. Selected lines were subcloned and screened again, followed by production and protein G purification.
Anti-GATA2 polyclonal IHC and dual IHC
Anti-GATA2 IHC with rabbit polyclonal antibody was performed as described previously.40 For scoring, high magnification images of random fields were generated, and ImageJ (National Institutes of Health) was used to quantify all mononuclear cells and GATA2+ mononuclear cells. At least 3 independent fields and 500 cells were examined. Dual IHC experiments were performed with polyclonal anti-GATA2 antibody and anti-CD117 no. ab32363 Abcam (Cambridge, UK), anti-CD34 790-2927 Ventana-Roche (Roche Diagnostics, Indianapolis, IN), or anti–E-cadherin 790-4497 Ventana-Roche. Dual IHC scoring was performed similarly to aforementioned description, except cells positive for 1 marker were quantified followed by dual-positive cells. At least 100 positive cells were quantified, except in 6 cases in which only between 28 and 99 total positive cells were present on the biopsy. See supplemental Materials for dual IHC protocols.
Anti-GATA2 monoclonal (15D2 clone) IHC
IHC was performed on a Ventana Discovery Ultra. All reagents were Roche-Ventana proprietary. Deparaffinization and heat-induced epitope retrieval were performed on the instrument with CC1 buffer (no. 950-500) and incubated in 15D2 antibody diluted 1:20 in casein diluent (no. 760-219) for 60 minutes at 37°C. Anti-mouse hapten (no. 760-4814) was applied for 28 minutes at 37°C with anti hapten-horseradish peroxidase (no. 760-4311), followed by a rinse in reaction buffer (no. 950-300) and incubation in ChromoMap DAB detection (no. 950-300) for a preset time. Subsequently, the slide was removed from the instrument and washed with dish soap and warm tap water, then rinsed with distilled water, counterstained in hematoxylin, rinsed with distilled water, dehydrated in a 60°C oven, dipped in xylene, and coverslipped.
Microscopy
Brightfield images were gathered on an Olympus BX43 microscope and 40× air objective lens with 0.65 numerical aperture at room temperature using an Lumenera Infinity 5 camera and Lumenera Infinity Capture software. All images were white balanced in Adobe Photoshop.
Statistical analyses
All authors had access to the data, which were analyzed by M.L. and D.R.M. Percentage GATA2+ cells was compared between groups using nonparametric methods: Jonckheere test was used when the explanatory factor was ordinal; Wilcoxon rank-sum test was used for comparisons across 2 groups; and Spearman test of rank correlation was used for continuous explanatory variables. Kendall τ testing was used for correlation of percent GATA2+ blasts with flow cytometry immunophenotype. Overall survival (OS) was calculated as time from diagnosis to death, with surviving patients censored at point of last follow-up. Kaplan-Meier analysis was used to estimate median OS with supporting 95% confidence interval. Cox proportional hazard models used to determine whether OS associated with GATA2 percentage. Statistical significance was P < .05 with no adjustment for multiple testing. Analyses were performed using R (version 4.2.1).
Results
GATA2 IHC in benign bone marrow
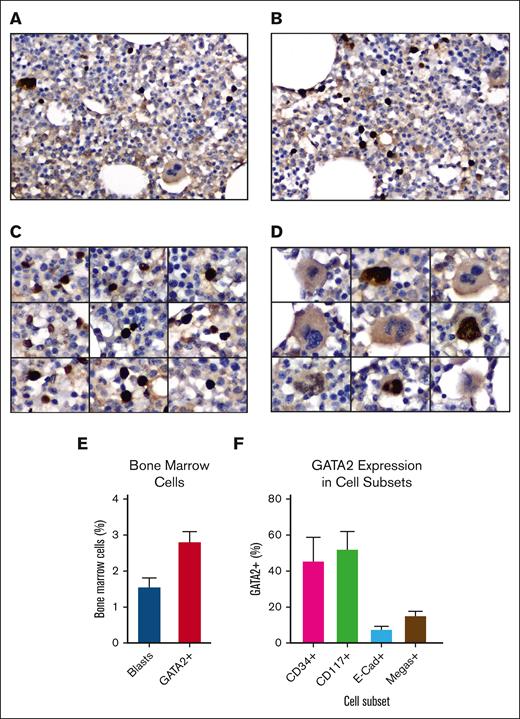
To identify and characterize GATA2+ cells in normal human bone marrow, GATA2 IHC was performed on 10 adult bone marrow biopsies (supplemental Tables 1 and 2) using a rabbit anti-GATA2 polyclonal antibody.39,40 These images revealed strong nuclear labeling in 3% (range, 2%-4%) of mononuclear cells and a subset of megakaryocytes (Figure 1A-D). The bone marrow blast percentages measured according to WHO guidelines was 1.5% (range 1%-3%), suggesting that GATA2 expression extended beyond the blast compartment (Figure 1E).
GATA2 IHC in benign adult bone marrow. (A-B) Representative fields of GATA2 IHC in adult bone marrow (DAB and hematoxylin; original magnification ×400). (C) GATA2+ mononuclear cells (DAB and hematoxylin; original magnification ×400). (D) Representative megakaryocytes, showing both GATA2+ and GATA2− examples (DAB and hematoxylin; original magnification ×400). (E) Percent bone marrow blasts and percent GATA2+ bone marrow mononuclear cells (n = 10). (F) Percent of representative cells positive for indicated marker, which are also GATA2+ by dual IHC (n = 5). Error bars = standard error of the mean (SEM).
GATA2 IHC in benign adult bone marrow. (A-B) Representative fields of GATA2 IHC in adult bone marrow (DAB and hematoxylin; original magnification ×400). (C) GATA2+ mononuclear cells (DAB and hematoxylin; original magnification ×400). (D) Representative megakaryocytes, showing both GATA2+ and GATA2− examples (DAB and hematoxylin; original magnification ×400). (E) Percent bone marrow blasts and percent GATA2+ bone marrow mononuclear cells (n = 10). (F) Percent of representative cells positive for indicated marker, which are also GATA2+ by dual IHC (n = 5). Error bars = standard error of the mean (SEM).
To better classify the GATA2+ cells, we performed dual IHC for GATA2 and CD34 (blasts), CD117 (myeloid blasts, immature erythroids, and mast cells), and E-cadherin (immature erythroids) in 5 of the biopsies (supplemental Figure 1A-D). We also quantified GATA2+ megakaryocytes, which were easily recognized by hematoxylin counterstain (Figure 1D). Earlier published data support critical roles for GATA2 in HSPCs,43 early myeloid precursors,13,44 and mast cells,45,46 and a requirement for GATA2 downregulation during erythroblast maturation.47
We first analyzed the CD34+, CD117+, and E-cadherin–positive compartments and quantified the percent of GATA2+ cells within each group (Figure 1F). GATA2 was coexpressed in 46% of CD34+ cells, but there was variability between cases (range, 18%-92%). Similarly, GATA2 was coexpressed in 52% of CD117+ cells, but coexpression ranged from 22% to 82% across cases. CD117bright mast cells were universally GATA2+. Within the E-cadherin–positive population, 8% of cells were GATA2+ (range, 4%-12%). Finally, 15% of megakaryocytes were GATA2+ (range, 2%-26%).
We also scored GATA2 expression by focusing specifically on the GATA2+ population and scoring GATA2+ cells for marker coexpression (supplemental Figure 1E). Using this approach, 36% of GATA2+ cells were CD34+ (range, 17%-60%), 27% were CD117+ (range, 4%-44%), and 3% were E-cadherin–positive (range, 1%-5%).
GATA2 IHC in MDS
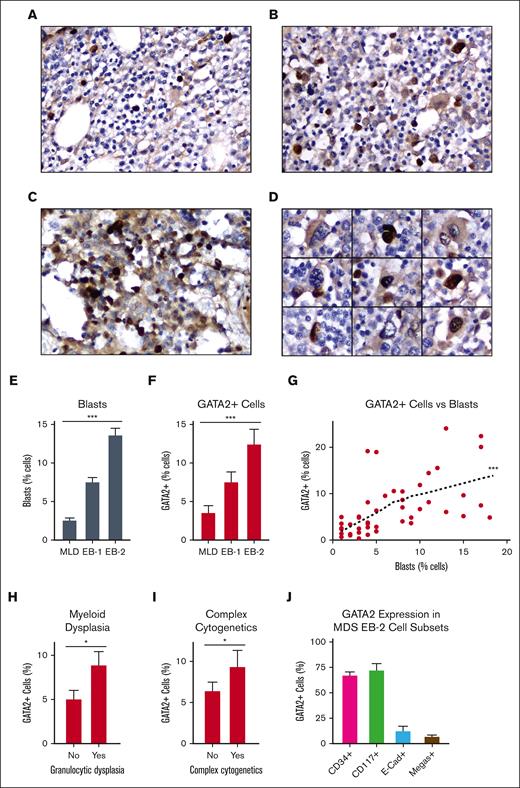
Acquired GATA2 mutations are found in a subset of sporadic MDS cases,31 whereas patients with germ line GATA2 mutations are predisposed to develop MDS.48,49 We applied our optimized GATA2 IHC protocol to a cohort a 47 MDS cases, consisting of pretreatment diagnostic bone marrow biopsies from adult patients with 1% to 18% bone marrow blasts (supplemental Table 3). Similar to normal bone marrow, GATA2+ cells in MDS appeared primarily as mononuclear cells and a subset of megakaryocytes (Figure 2A-D). Because blast percentage increased with MDS severity (Figure 2E) we found a strong positive correlation with the percent of GATA2+ cells (P < .001) (Table 1, Figure 2F-G). Percent GATA2+ cells also correlated with the presence of morphologic dysplasia within the myeloid lineage (P = .038) (Figure 2H) and with complex cytogenetics as defined by the WHO (P = .048; Figure 2I). Within this cohort of 10 MDS cases with complex cytogenetics, the most common recurrent abnormalities were events leading to 5q loss (9 cases) or loss of chromosome 7 (4 cases). Targeted myeloid malignancy mutation panels had been performed in 26 cases (supplemental Figure 2). Within this cohort, no significant relationship between GATA2+ cells and specific genetic mutations or total mutational burden was identified.
GATA2 IHC in adult MDS. (A-C) Representative fields of GATA2 IHC in adult MDS bone marrow involved by (A) MDS-multilineage dysplasia (MLD), (B) MDS-EB1, and (C) MDS-EB2 (DAB and hematoxylin; original magnification ×400). (C) GATA2+ mononuclear cells in MDS-EB2 (DAB and hematoxylin; original magnification ×400). (D) Representative megakaryocytes in MDS, showing both GATA2+ and GATA2− examples (DAB and hematoxylin; original magnification ×400). (E-G) Percent bone marrow blasts and percent GATA2+ bone marrow mononuclear cells (n = 47). (H-I) Relationship between GATA2+ cells and (H) myeloid dysplasia (n = 46) and (I) complex cytogenetics (n = 47) in MDS. (J) Percent of representative cells positive for indicated marker, which are also GATA2+ by dual IHC in MDS-EB2 (n = 5). Error bars = SEM; ∗P < .05 and ∗∗∗P < .005.
GATA2 IHC in adult MDS. (A-C) Representative fields of GATA2 IHC in adult MDS bone marrow involved by (A) MDS-multilineage dysplasia (MLD), (B) MDS-EB1, and (C) MDS-EB2 (DAB and hematoxylin; original magnification ×400). (C) GATA2+ mononuclear cells in MDS-EB2 (DAB and hematoxylin; original magnification ×400). (D) Representative megakaryocytes in MDS, showing both GATA2+ and GATA2− examples (DAB and hematoxylin; original magnification ×400). (E-G) Percent bone marrow blasts and percent GATA2+ bone marrow mononuclear cells (n = 47). (H-I) Relationship between GATA2+ cells and (H) myeloid dysplasia (n = 46) and (I) complex cytogenetics (n = 47) in MDS. (J) Percent of representative cells positive for indicated marker, which are also GATA2+ by dual IHC in MDS-EB2 (n = 5). Error bars = SEM; ∗P < .05 and ∗∗∗P < .005.
The close correlation between GATA2+ cells and blasts suggested that MDS blasts represented the predominant GATA2+ cell population, and we applied our dual IHC approach to better classify these cells. We chose to specifically analyze cases of MDS with excess blasts 2 (EB-2), which harbor 10% to 19% bone marrow blasts and clinically are characterized by poor survival and high rates of AML transformation (Figure 2J; supplemental Figure 3A-D).50 GATA2 was coexpressed in 68% of CD34+ cells (range, 59%-71%), 73% of CD117+ cells (range, 59%-86%), and 13% of E-cadherin–positive cells (range, 4%-25%). These values were elevated compared with those of our benign bone marrow cohort but the increases were not statistically significant. Quantification of CD34, CD117, and E-cadherin coexpression within the GATA2+ cell subset yielded similar findings (supplemental Figure 3E). Quantification of megakaryocytes was challenging, secondary to dysplastic changes. However, we found 8% of megakaryocytes to be GATA2+ (range, 0%-22%), which was significantly less than that of our benign cohort (P < .001; Figure 2J).
We compared GATA2 expression levels to OS in patients with MDS. The size of our MDS study cohort precluded extensive stratification by disease or treatment type. However, we analyzed the relationship between GATA2 and survival within cases of MDS with multilineage dysplasia, EB-1, and EB-2 (Table 2). Within these diagnostic categories, only patients with MDS–multilineage dysplasia showed a robust association between survival and GATA2+ cells: each 1% increase in GATA2+ cells was associated with a 20% increase in immediate risk of death (P = .015). The effect was less pronounced for EB-1 and EB-2 cases, however further testing revealed that the association between mortality and GATA2+ cells did not significantly differ between groups (P = .323). This implies that a single hazard ratio is sufficient to summarize the relationship across diagnostic subtypes and implies an 11% increase in immediate risk of death per 1% increase in GATA2+ cells (P = .009).
We performed a similar analysis after stratifying patients by their International Prognostic Scoring System Revised (IPSS-R) score, which is a common risk stratification tool that is used for therapeutic decision making in patients with MDS.51,52 The association between percent GATA2+ cells and mortality differed according to IPSS-R score (P = .041). Increased GATA2+ cells within the low IPSS-R score category correlated with mortality (P = .016), but this was not true for intermediate and high IPSS-R score categories.
In conclusion, the percent GATA2+ cells negatively correlates with survival in MDS, and this relationship is most significant in low grade MDS and in patients who fall into the low IPSS-R category.
Anti-GATA2 mAbs
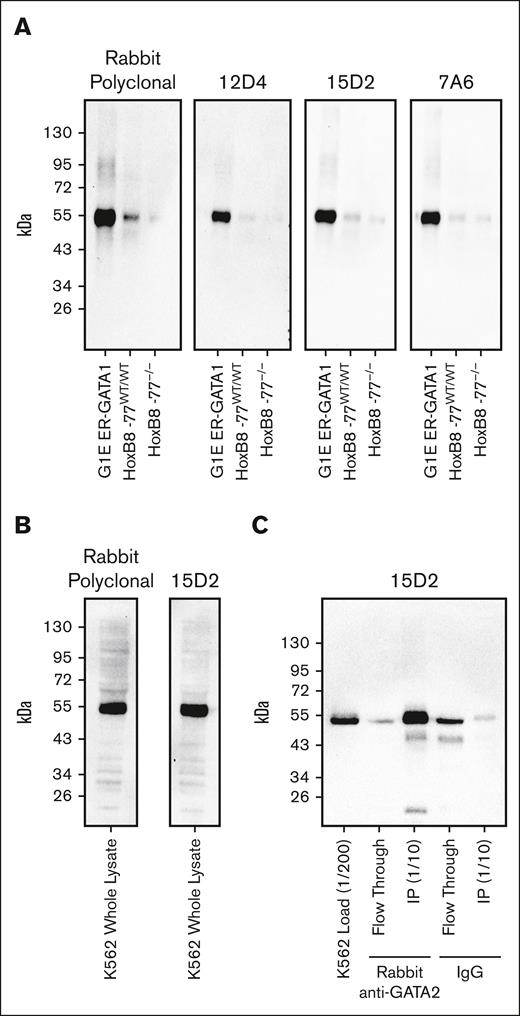
Because the rabbit anti-GATA2 polyclonal antibody represented a finite resource, we generated 3 unique anti-GATA2 mAbs. Each mAb recognized a single band corresponding to GATA2 on western blots loaded with whole-cell lysate from mouse HoxB8-immortalized primary myeloid progenitor cells53 or human K562 immortalized human chronic myelogenous leukemia cells with primitive erythroblast attributes54 (Figure 3A-B). To confirm that this single immunoreactive band was GATA2, we used our validated rabbit polyclonal anti-GATA2 antibody to immunoprecipitate GATA2 from K562 whole-cell lysates. Probing of the immunoprecipitated material with the 15D2 mAb confirmed specific recognition of GATA2 (Figure 3C). The 15D2 mAb then yielded ideal IHC staining using standard automated IHC workflows. Ten AML bone marrow core biopsies were evaluated by anti-GATA2 IHC performed with either the validated anti-GATA2 polyclonal antibody or the 15D2 mAb, and the antibodies yielded identical staining patterns (supplemental Figure 4).
Validation of anti-GATA2 mAbs. (A) Anti-GATA2 western blots of whole-cell lysates from mouse G1E ER-GATA1 cells, mouse HoxB8-immortalized myeloid progenitor cells, and mouse HoxB8-immortalized myeloid progenitors with homozygous deletion of the −77 Gata2 enhancer element resulting in reduced GATA2 expression. (B) Anti-GATA2 western blots of K562 whole-cell lysate. (C) Anti-GATA2 IP using rabbit polyclonal anti-GATA2 antibody or rabbit immunoglobulin G (IgG) control, and western blot with 15D2 anti-GATA2 mAb.
Validation of anti-GATA2 mAbs. (A) Anti-GATA2 western blots of whole-cell lysates from mouse G1E ER-GATA1 cells, mouse HoxB8-immortalized myeloid progenitor cells, and mouse HoxB8-immortalized myeloid progenitors with homozygous deletion of the −77 Gata2 enhancer element resulting in reduced GATA2 expression. (B) Anti-GATA2 western blots of K562 whole-cell lysate. (C) Anti-GATA2 IP using rabbit polyclonal anti-GATA2 antibody or rabbit immunoglobulin G (IgG) control, and western blot with 15D2 anti-GATA2 mAb.
GATA2 IHC in acute leukemia
We applied the 15D2 anti-GATA2 mAb to 106 bone marrow biopsies reflecting initial diagnostic samples from patients with new diagnoses of acute leukemia, including 75 AMLs, 26 acute lymphoblastic leukemias (ALLs) representing 19 B-cell ALLs (B-ALLs) and 7 T-cell ALLs (T-ALLs), and 5 acute undifferentiated leukemias (AULs; supplemental Table 4). Cases were then scored for percent GATA2+ leukemic blasts.
The percent GATA2+ blasts was significantly higher in AMLs than in ALLs and AULs (Figure 4A-E; supplemental Figure 5). However, GATA2 expression across AML cases ranged from 0% to 95% GATA2+ blasts (Figure 4F). Within the ALL cohort, 85% of ALLs had no detectable GATA2 expression. A single B-ALL case (1 of 19 B-ALL cases, 5% of B-ALLs) contained 10% GATA2+ blasts, and, interestingly, also harbored a KMT2A rearrangement. We also identified 3 T-ALL cases (3 of 7 T-ALL cases; 43%) in which 10% to 90% of blasts were GATA2+. Two T-ALL cases represented early T-cell precursor ALLs. One AUL also showed GATA2 expression in 10% of blasts and, whereas it met diagnostic criteria for AUL, it harbored pathogenic mutations in CALR, DNMT3A, and TET2, which is a pattern suggestive of myeloid derivation. Interesting, this AUL also contained a GATA2 variant of uncertain significance corresponding to an insertion within the spacer region between GATA2 zinc finger domains (c.975_1010dup; p.Asn326_Arg337dup). A 9 amino acid GATA2 spacer insertion has been identified in a family affected by GDS.55,56 Unfortunately, the patient is deceased, and germ line evaluation was not conducted.
GATA2 IHC in adult AML. (A-D) Representative fields of GATA2 IHC in 3 different adult AML cases (3,3′-Diaminobenzidine [DAB] and hematoxylin; original magnification ×400). (E-F) Percent GATA2+ bone marrow blasts in acute leukemia subtypes (AMLs, n = 75; ALLs, n = 26; AULs, and n = 5). (G-H) Correlation between percent GATA2+ blasts and (G) neutropenia (n = 75) and (H) complex cytogenetics (n=74)) in AML. Error bars = SEM; ∗P < .05 and ∗∗∗P < .005.
GATA2 IHC in adult AML. (A-D) Representative fields of GATA2 IHC in 3 different adult AML cases (3,3′-Diaminobenzidine [DAB] and hematoxylin; original magnification ×400). (E-F) Percent GATA2+ bone marrow blasts in acute leukemia subtypes (AMLs, n = 75; ALLs, n = 26; AULs, and n = 5). (G-H) Correlation between percent GATA2+ blasts and (G) neutropenia (n = 75) and (H) complex cytogenetics (n=74)) in AML. Error bars = SEM; ∗P < .05 and ∗∗∗P < .005.
AMLs with higher percentages of GATA2+ blasts showed higher rates of peripheral neutropenia and complex cytogenetics (Figure 4G-H). Within the cohort of 14 AML cases with complex cytogenetics, recurrent abnormalities included events leading to 5q loss (9 cases), trisomy 8 (8 cases), whole or partial loss of chromosome 7 (7 cases), and whole or partial loss of chromosome 17 (7 cases). However, unlike in MDS, there was no relationship between percent GATA2+ blasts and number of AML blasts in the bone marrow or peripheral blood. We compared GATA2 expression across AML diagnostic categories, and sorted AML cases into 4 categories based on whether they qualified as therapy-related AML, AML with myelodysplasia-related changes, AML with a defining cytogenetic abnormality, or AML not otherwise specified. We found no difference in percent GATA2+ blasts across these subcategories (Table 3).
Next, we compared percent GATA2+ AML blasts with blast immunophenotype as measured by flow cytometry at initial diagnosis (supplemental Figure 6). This revealed a significant negative correlation between GATA2+ blasts and blast expression of HLA-DR, CD11b, and CD14. Although AML blasts typically show dim expression of CD45, we found a significant positive correlation between GATA2+ blasts and bright expression of CD45.
Of the 75 AML cases, 46 had been subjected to a myeloid malignancy mutation panel (supplemental Figure 7). Within this cohort, we found no association between GATA2+ blasts and mutations in the 57 genes examined. When the test was decomposed to individual elements, tyrosine kinase domain mutations in FLT3 (P = .058) and mutations in ASXL2 (P = .062) approached, but did not qualify for, statistically significant associations with percent GATA2+ blasts.
We evaluated the relationship between GATA2 expression and disease-free survival in AML. Treatment and outcome data were available for 68 AML cases and 47 had experienced either disease relapse or death during the follow-up period (Table 4). We found no relationship between GATA2 and disease-free survival, even when patients were stratified by intention to cure or based on whether they received an allogeneic hematopoietic stem cell transplantation.
Discussion
Our study represents, to our knowledge, the first to document GATA2 protein expression by routine IHC in decalcified formalin-fixed paraffin-embedded bone marrow biopsies, the most common type of archived patient bone marrow sample, using both a polyclonal anti-GATA2 antibody and a new anti-GATA2 mAb compatible with automated clinical workflows. This enabled assessment of GATA2 protein expression in healthy adult bone marrow and in the setting of adult MDS and acute leukemia.
Our findings using human bone marrow biopsies build on published animal studies to improve our understanding of GATA2 in biology and disease. Tight control of GATA2 levels is critical for embryonic (primitive) and adult (definitive) hematopoiesis. HSPCs manipulated to have low or high GATA2 levels have reduced bone marrow reconstitution activity.18,57,58 In more differentiated cell states, consistent GATA2 expression is critical for mast cell formation,45,59 whereas GATA2 downregulation is required for formation of megakaryocyte and erythroid progenitors.60,61 The expression level of GATA2 protein in lymphoid progenitors is less clear. Patients with GDS often suffer from life-threatening immunodeficiency because of reduced numbers of B cells, B-cell progenitors, monocytes, dendritic cells, and/or natural killer cells.62 However, GATA2 mRNA levels in these mature cell types are low, and it is reasonable to assume that reduced GATA2 levels in HSPCs underlies the cytopenias in these patients, although one cannot rule out defects in lineage-specific GATA2 functions.63
In healthy bone marrow, we measured GATA2 protein expression in 1% to 3% of total mononuclear cells and confirmed that these included a mixture of HSPCs, erythroid progenitors, and mast cells. Dual IHC revealed that many CD34+ cells have undetectable GATA2 expression. Given that CD34 is an HSPC marker64,65 and that GATA2 plays critical roles in HSPC biology, this may seem surprising. However, it is likely to reflect the heterogeneous nature of the HSPC population. In routine clinical practice, CD34 is typically considered a marker of bone marrow blasts, which correspond to HSPCs. However, although blasts typically appear as a homogeneous population by Wright staining and are quantified as such in clinical bone marrow differentials, they are a highly diverse population with divergent immunophenotypes, gene network activities, and biological functions.66,67 Mapping GATA2 expression across this landscape of progenitor cell types has gained momentum with the application of single-cell proteomics and the generation and characterization of GATA2 reporter mice.20-22,38 Our analysis of GATA2 expression is broadly in line with these reports and support a prevailing model wherein GATA2 exerts its activities in a highly cell- and context-specific manner.
Our finding that GATA2 is expressed in a subset of megakaryocytes coincides with active investigations into the biology and cellular origins of these cells.68-70 Bone marrow megakaryocytes can be separated into 3 distinct subpopulations based on ploidy and gene expression patterns.71 One subpopulation harbors high-ploidy and elevated GATA2 mRNA levels, whereas a second subpopulation displays a gene expression signature enriched for GATA2 and RUNX1 regulatory sites. Other studies have implicated GATA2 with GATA1, RUNX1, FLI1, and TAL1 in forming a core megakaryocyte transcriptional regulatory network.72,73 Future studies will clarify the identity of this GATA2+ megakaryocyte subpopulation and enable more detailed mechanistic studies on the role of GATA2 in these cells.
We found that GATA2 expression in MDS shows a positive, linear correlation with MDS blast percentage, and a majority of GATA2+ cells in MDS coexpress CD34. As MDS EB-2 cases show increased CD34+ blasts, GATA2+ cells, and similar ratios of CD34+GATA2+ cells to that of healthy bone marrows, the increase in GATA2 expression seen in MDS is primarily because of increased myeloblasts. Thus, our findings that elevated GATA2 expression is a nonindependent predictor of worse outcome in MDS is concordant with the established negative prognostic value of elevated blasts.74
We demonstrate that elevated GATA2 expression was predictive of myeloid dysplasia and complex cytogenetics in MDS. Because GATA2 correlates with MDS blast percentage, the link between GATA2 IHC, myeloid dysplasia, and complex cytogenetics could simply be a reflection of elevated blasts and, thus, more advanced disease. However, we see a similar relationship between GATA2 and complex cytogenetics in AML, in which the relationship between GATA2 IHC and blasts is decoupled. Moreover, although morphologic dysplasia is challenging to score in the setting of AML, we found that neutropenia, which is expected in the setting of myeloid dysplasia, is positively associated with GATA2 expression in AML. Earlier findings seeking to link GATA2 mRNA expression to cytogenetic abnormalities have been confounding, with 1 study showing that elevated GATA2 transcripts correlate with normal karyotype but only in intermediate-risk AML.36 We demonstrate that elevated GATA2 expression in the blast compartment is linked to complex cytogenetics in both MDS and AML, in a manner that is not completely dependent on blast percentage.
The wide variation in GATA2+ blasts across AML cases is intriguing, given the tight correlation between GATA2 and blast percentage in benign and MDS bone marrows. Notably, this variation in GATA2+ blasts exists even in AMLs with myelodysplasia-related changes, which are considered to represent leukemic transformation of preexisting MDS.75 The variation in GATA2+ blasts across AML cases suggests that processes that normally regulate levels of GATA2 expression16,76,77 become defective upon AML transformation. In addition, the significant negative correlation between GATA2+ blasts and monocytic markers CD11b and CD14 suggests that reduced GATA2 levels in AML may promote AML monocytic phenotypes. Of note, GATA2-deficient fetal murine progenitor cells exhibit a skewed differentiation potential toward monocytic at the expense of granulocytic lineage.53,78,79 It may have implications in ongoing efforts to clarify the relationship between MDS with high blast counts (10%-19% blasts) and AML. Our findings clearly indicate a difference in GATA2 regulation between these 2 entities.
GATA2 expression in select cases of B-ALL, T-ALL, and AUL was surprising. Interestingly, all positive cases harbor evidence of an underlying myeloid signature. In the AUL case with GATA2+ blasts, we found mutations in CALR, DNMT3A, and TET2, which are characteristic of myeloid malignancies. This case also harbored a GATA2 variant that was classified as a variant of uncertain significance but is very likely to disrupt GATA2 function.55 The 2 T-ALLs with GATA2+ blasts represented early T-cell precursor ALLs, a WHO-defined variant of T-ALL with an overlapping myeloid expression profile.80,81 Finally, the B-ALL case with GATA2+ blasts harbored a KMT2A translocation representing a B-ALL subtype that often expresses myeloid markers82 and is prone to lineage switching.83
Our study has some significant drawbacks. Our case cohorts are relatively small, and future studies incorporating larger cohorts will enable more detailed analyses. Our cases also include only adult cases of MDS and AML, which predominate among new diagnoses. Future studies should examine GATA2 expression in the pediatric setting, especially among ALL cases, which are more common in this age group. Our study also did not include patients with known germ line GATA2 mutations.84 Recent studies suggest that when MDS or AML arise in patients with GDS, they can be accompanied by epigenetic repression of the remaining wild-type GATA2 allele.85 Future studies will analyze GATA2 bone marrow expression in patients with GDS with and without associated myeloid malignancies and determine whether pathologic GATA2 repression can be routinely detected by GATA2 IHC. If so, it would potentially be a straightforward and inexpensive assay to detect early leukemic transformation in this patient population.
Acknowledgments
The authors acknowledge the intellectual and technical contributions of the Biostatistics and Epidemiology Research Design Core and the Biostatistics Shared Resource to the development of this manuscript.
This work was funded by Institute of Clinical and Translational Sciences award UL1 TR002373 and a University of Wisconsin Carbone Cancer Center support grant P30 CA014520. Z.J.G. acknowledges support from University of Wisconsin Carbone Cancer Center support grant P30 CA014520. The authors thank the University of Wisconsin Translational Research Initiatives in Pathology laboratory, supported by the University of Wisconsin Department of Pathology and Laboratory Medicine and University of Wisconsin Carbone Cancer Center (P30 CA014520), for use of its facilities and services. D.R.M. received support from K08DK127244 and T32HL007899. E.H.B. received support from the National Institutes of Health (DK68634) and the Edward P. Evans Foundation. Additional funding to D.R.M. and D.J.R. was provided by the Department of Pathology and Laboratory Medicine at the University of Wisconsin-Madison.
Authorship
Contribution: D.J.R. and D.R.M. identified the bone marrow biopsy cases; E.H.B. provided the polyclonal anti-GATA2 antibody, which was affinity purified by D.R.M.; A.T.P., E.H.B., and D.R.M. generated the anti-GATA2 mAbs; D.J.R., Z.J.G., and D.R.M. tabulated clinicopathologic data from the medical record; D.J.R., T.S.P., A.T.P., D.P., U.S.P., and D.R.M. scored the GATA2 expression; M.L. performed the bulk of the statistical comparisons with more limited contribution from D.R.M.; and D.R.M. generated the figures and drafted the initial manuscript with critical discussions, recommendations, and contributions from E.H.B., Z.J.G., and M.L.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Daniel R. Matson, Department of Pathology and Laboratory Medicine, University of Wisconsin-Madison, Room 1770 WIMR, 1111 Highland Ave, Madison, WI 53705; email: drmatson@wisc.edu.
References
Author notes
Deidentified original data are available on request from the corresponding author, Daniel R. Matson (drmatson@wisc.edu).
The full-text version of this article contains a data supplement.

![GATA2 IHC in adult AML. (A-D) Representative fields of GATA2 IHC in 3 different adult AML cases (3,3′-Diaminobenzidine [DAB] and hematoxylin; original magnification ×400). (E-F) Percent GATA2+ bone marrow blasts in acute leukemia subtypes (AMLs, n = 75; ALLs, n = 26; AULs, and n = 5). (G-H) Correlation between percent GATA2+ blasts and (G) neutropenia (n = 75) and (H) complex cytogenetics (n=74)) in AML. Error bars = SEM; ∗P < .05 and ∗∗∗P < .005.](https://ash.silverchair-cdn.com/ash/content_public/journal/bloodadvances/8/1/10.1182_bloodadvances.2023011554/2/m_blooda_adv-2023-011554-gr4.jpeg?Expires=1763488046&Signature=wToel-1SqK8zVrYzrKIpSJzerJh3tu17P14fGuunl3vTQqVRwFQmaa1WBUefIPQ7oqRP~B1mVCOiLY38leS2umtzsPAE1w5VrOX7yS~tCBl8Wl76hOZqNC0uyfaDcWpSyTJDm~Aw0~DVNwDmsxdNAqapbJyjHKowuAX3VrHQl8MsvgfVMGN611XbmDCuZyMU9lPrhREL3YXP0d8bw7qu1OjqrTpErE4gvDVb4rCVJxyRn3Sul-LS9vT7mKCvwkK8g6yp3BF6ShGv~0ztCwcnhF8K2YwY~USODUAhmH-wIcXzJ5VU23pOwikrVUE7BjBOOuNyB5PdUWGlM0eWJRNu9Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)