Key Points
Patients with KMT2A-rearranged AML have post-HCT leukemia-free and overall survival rates similar to those with adverse risk AML.
Adverse-risk AML confers a 71% higher risk of relapse and a 45% higher risk of death after HCT than intermediate-risk AML.
Abstract
Little is known about whether risk classification at diagnosis predicts post-hematopoietic cell transplantation (HCT) outcomes in patients with acute myeloid leukemia (AML). We evaluated 8709 patients with AML from the CIBMTR database, and after selection and manual curation of the cytogenetics data, 3779 patients in first complete remission were included in the final analysis: 2384 with intermediate-risk, 969 with adverse-risk, and 426 with KMT2A-rearranged disease. An adjusted multivariable analysis detected an increased risk of relapse for patients with KMT2A-rearranged or adverse-risk AML as compared to those with intermediate-risk disease (hazards ratio [HR], 1.27; P = .01; HR, 1.71; P < .001, respectively). Leukemia-free survival was similar for patients with KMT2A rearrangement or adverse risk (HR, 1.26; P = .002, and HR, 1.47; P < .001), as was overall survival (HR, 1.32; P < .001, and HR, 1.45; P < .001). No differences in outcome were detected when patients were stratified by KMT2A fusion partner. This study is the largest conducted to date on post-HCT outcomes in AML, with manually curated cytogenetics used for risk stratification. Our work demonstrates that risk classification at diagnosis remains predictive of post-HCT outcomes in AML. It also highlights the critical need to develop novel treatment strategies for patients with KMT2A-rearranged and adverse-risk disease.
Introduction
Cytogenetic abnormalities are the most useful prognostic indicators for patients with acute myeloid leukemia (AML) and can provide insight into overall patient outcomes.1-7 There are few studies, and no large, AML-specific studies, that analyzed how well cytogenetics at the time of diagnosis predicts outcomes after allogeneic hematopoietic cell transplant (HCT).8,9
The 2010 AML-Medical Research Council classification system categorized patients with AML based purely on cytogenetic characteristics as either intermediate or adverse risk.2 One subset, patients with 11q23 translocations (site of the lysine (K)-specific methyltransferase 2A [KMT2A] gene), could be classified in either category, based on the translocation partner.
Translocations of 11q23 involve KMT2A, which encodes a histone 3, a lysine 4 methyltransferase that is involved in epigenetic regulation of transcriptional activation.10-12 These translocations are frequently referred to as rearrangements of MLL, the mixed-lineage leukemia gene, as this was the name before changes in the Human Genome Organization gene nomenclature. KMT2A translocations are seen in patients with de novo AML, secondary AML after myelodysplastic syndrome (MDS), or therapy-related AML (tAML) after treatment with a topoisomerase II inhibitor.13-15
KMT2A rearrangements can occur with many translocation partners; at least 135 have been described.16 Among the most common in adult AML are KMT2A-AF9 t(9;11)(p22;q23), KMT2A-ENL t(11;19)(q23; p13.3), KMT2A-AF6 t(6;11) (q27;q23), and KMT2A-AF10 t(10;11) (p12;q23). Recent prognostic classification systems for AML have made distinctions between translocation partners. Some have noted that outcomes for t(9;11), and possibly for t(11;19), are similar to intermediate-risk AML, whereas all other translocation partners are classified as poor or adverse risk.1,2
We hypothesized that risk classification at diagnosis of patients with AML would be predictive of post-HCT outcomes among patients with intermediate- and adverse-risk disease, but that outcomes for patients with KMT2A rearrangement would depend on the translocation partner present. In this report, we present an analysis from the Center for International Blood and Marrow Transplant Research (CIBMTR) database comparing adult patients who underwent HCT in the first complete remission (CR1) with those with intermediate- or adverse-risk or KMT2A-rearranged AML, with a focus on the differences in relapse, leukemia-free survival (LFS), nonrelapse mortality (NRM), and overall survival (OS). To date, this is the largest annotated cohort of post-HCT outcomes of patients with AML and is reflective of real-world outcomes based on contemporary practice.
Methods
Data sources
This was a retrospective registry study approved and conducted through the CIBMTR. The CIBMTR is a collaborative research effort between the National Marrow Donor Program/Be the Match Registry and the Medical College of Wisconsin. More than 450 centers around the world contribute detailed clinical, pathological, and outcomes data to the CIBMTR on their patients who undergo stem cell transplant.
All patients included in this study signed a written, informed consent for data capture in the CIBMTR database. The National Marrow Donor Program Institutional Review Board approved the study. As with all CIBMTR studies, this work was performed in compliance with all applicable federal regulations pertaining to the protection of human research subjects.
Study population
Adult patients with AML aged 18 to 70 years who were in CR1 and had undergone the first HCT from 2007 through 2016 were considered for additional review (n = 8709). Patients with acute promyelocytic leukemia, favorable risk or unknown cytogenetics, syngeneic transplants, mismatched unrelated donors (<7 of 8), multiple donors, ex vivo T-cell depletion, or previous allogeneic transplants were excluded. Data were not used for patients from whom consent for research was not obtained, whose data were associated with an embargoed center, or who did not have the appropriate data forms available (supplemental Table 1).
Manual curation (KM, YZ, AGA, and RMM) to define KMT2A rearrangements and the presence of additional cytogenetic abnormalities was performed on the data of 3999 patients, according to the AML-Medical Research Council 2010 classification system.2 Chromosomal data from forms submitted on each patient were used to determine their cytogenetic classification. Images of the karyotypes and fluorescence in situ hybridization data were not available for confirmation. Patients were classified as carrying KMT2A-rearranged, intermediate-risk or adverse-risk AML. An additional 86 patients with favorable cytogenetics and 9 patients with an ambiguous classification were excluded.
A total of 426 patients were identified as having an 11q23 translocation, based on available cytogenetics data from the CIBMTR forms. Translocations involving 11q23 were categorized as t(6;11), t(9;11), t(10,11), or t(11;19); the remaining rearrangements for which a partner was known were categorized as “other.” Patients with an 11q23 rearrangement and no information regarding translocation partner were categorized as “undefined.” Manual curation for complex karyotypes (CKs) was limited by the available data, as was identification of deletion or loss of chromosomes 5, 7, and 17p.
Measurable residual disease
The CIBMTR operational definition of measurable residual disease (MRD) at the time of HCT for AML was used to define the presence or absence of MRD. During the study period, participating centers may have defined MRD by flow cytometry, cytogenetics, or molecular sequencing. Additional information on the criteria used can be found in the supplemental Information.
End points and definitions
The primary outcome was OS, defined as time from HCT to death from any cause. Secondary outcomes included LFS, defined as time to disease relapse or death from any cause; relapse, defined as the reappearance of at least 5% blasts on morphological, cytogenetic, flow, or molecular evaluation in bone marrow, blood, or an extramedullary site, per the treating center; NRM, defined as time to death without evidence of disease relapse; and a composite outcome of graft-versus-host disease (GVHD)–free, relapse-free survival (GRFS), defined as time to development of grades 3 to 4 acute GVHD, systemic therapy-requiring chronic GVHD, relapse, or death within the first 12 months after HCT.17
Statistical considerations
Patient- and transplant-related variables were compared using the χ2 test for categorical variables and the Kruskal-Wallis test for continuous variables. Kaplan-Meier curves were used to estimate the probability of OS and LFS. Cumulative incidence was used to estimate the probability of relapse and NRM, with each treated as a competing risk for the other. For all 4 outcomes, patients were censored at the time of last follow-up. In addition, 95% confidence intervals (CI) were reported for all probability estimates.
A Cox proportional hazards model was built for the outcomes of relapse, NRM, LFS, and OS with the main effect of KMT2A-rearranged vs intermediate-risk vs adverse-risk disease. The model considered the covariates of age at time of HCT, sex, Karnofsky performance status score at time of HCT, HCT comorbidity index (HCT-CI) score, time from diagnosis to achieving CR1, time from CR1 to HCT, white blood cell (WBC) count at the time of diagnosis, de novo vs therapy-related vs secondary AML, MRD status at time of HCT, KMT2A subtype, year of transplant, donor type, recipient cytomegalovirus (CMV) serostatus, conditioning intensity (myeloablative [MA] with total body irradiation (TBI) vs MA with chemotherapy only vs reduced-intensity/non-MA [RIC/NMA] conditioning), use of in vivo T-cell depletion, and choice of GVHD prophylaxis. Interactions between main effect and all covariates were tested at a stringent significance level of P < .01, given the number of comparisons. Adjusted probabilities of outcomes were calculated with the final Cox models, stratified by main effect and weighted by the pooled sample proportion value for each prognostic factor. Imputation was not performed for missing data; only available data were analyzed. Analyses were performed in SAS, version 9.4.18,19
Results
Patient characteristics
Of the 8709 adult patients with AML considered for inclusion in the study, 3779 were selected as outlined in supplemental Table 1, with completeness of follow-up shown in supplemental Table 2.
Patient characteristics are shown in Table 1. A total of 426 patients with KMT2A rearrangement were studied, compared with 2384 patients without KMT2A rearrangement who had intermediate-risk disease and 969 patients without KMT2A rearrangement who had adverse-risk disease.
Patients with KMT2A rearrangement were younger, with a median age of 46, and more than half the patients presented before age 60. tAML was most frequently seen in the KMT2A-rearrangment group, 21% compared with 4% in the intermediate-risk group and 6% in the adverse-risk group. More patients with KMT2A rearrangement had been treated for breast cancer (11%, as opposed to 2% and 3% in patients with intermediate or adverse risk, respectively), and more women were diagnosed with KMT2A-rearranged AML whereas the other 2 groups had a slight male predominance.
Antecedent MDS was less common in patients with rearrangement in KMT2A, found in 6%, but was noted in 19% of the intermediate-risk group and 23% of the adverse-risk group.
Although all 3 groups achieved CR1 at similar rates, patients with adverse-risk disease more commonly required 2 inductions and were less likely to achieve CR1 within 4 weeks.
Patient characteristics by KMT2A translocation partner
Of the 426 patients found to have a KMT2A rearrangement (Table 2), 112 patients (26%) had t(9;11) and comprised the largest subtype group. Translocation t(11;19) was the next most common (62 patients, 15%), followed by t(6;11) with 41 patients (10%) and t(10;11) with 28 patients (7%). Translocation partners other than chromosomes 6, 9, 10, and 11 were detected in 47 patients (11%), designated “KMT2A other.” An additional 136 patients had 11q23 rearrangements with an unspecified translocation partner and were categorized as “KMT2A undefined.”
Patients with t(10;11) had the youngest median age, at 34; the patients classified as KMT2A other had the highest median age, at 54. For t(9;11), t(11;19), t(6;11), and t(10;11), there were nearly twice as many female as male patients per category.
tAML was found most frequently in those with t(9;11), with 38 (34%) patients having received prior therapy. Breast cancer was the most common prior malignancy. Other prior diagnoses included lymphoma (both Hodgkin and non-Hodgkin) and solid tumors.
Extramedullary disease was present at diagnosis at a single-digit frequency in all groups.
The majority of patients in all subcategories required only 1 course of induction to achieve CR1. Most patients with KMT2A rearrangement achieved MRD− status before transplant, regardless of translocation partner.
Donor type and graft type were similar in all subgroups, as were rejection and GVHD prophylaxis regimens and year of transplant. The percentage of patients who underwent MA conditioning was similar for all translocation partners, but the use of chemotherapy vs TBI varied somewhat. CMV+status was more common than CMV− status in all groups.
Relapse of AML
A univariable analysis of the risk of relapse at 1, 3, and 5 years after transplantation found that it was comparable in patients with KMT2A translocation and intermediate risk but higher at all time points in patients with adverse-risk disease (Table 3). The probability of relapse at 1 year was similar across KMT2A subtypes.
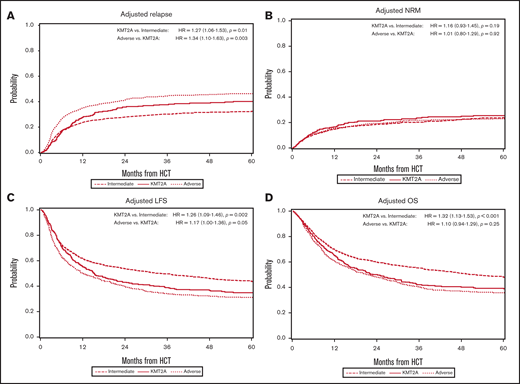
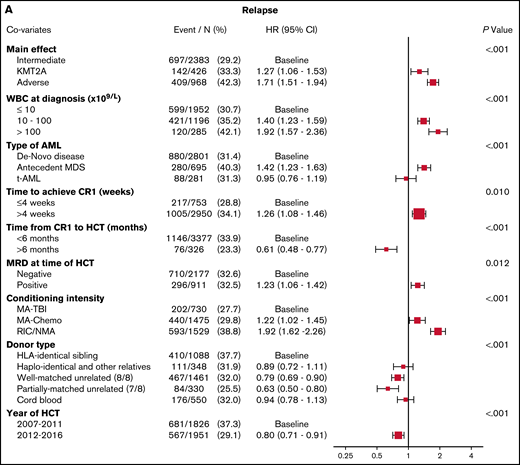
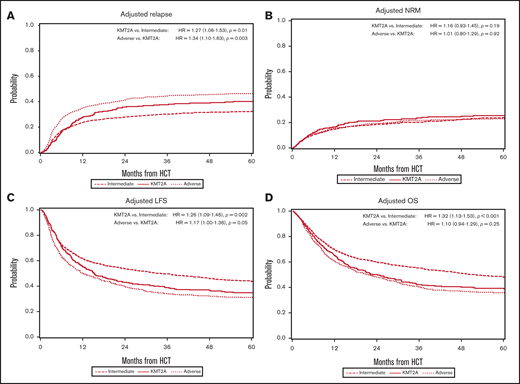
In the multivariable analysis, the KMT2A-rearranged and adverse-risk groups had an increased hazard ratio (HR) for relapse of 1.27 (95% CI, 1.06-1.53; P = .01) and 1.71 (95% CI, 1.51-1.94; P < .001), respectively, when compared with the intermediate-risk group (Figure 1A). Other predictors of relapse included higher WBC count at diagnosis, a longer time to achieve CR1, pre-HCT MRD positivity, conditioning other than TBI-based MA conditioning, and donors other than HLA-identical siblings. Relapse risk was also more apt to occur in those with a history of prior MDS, but not in those with therapy-related disease. Data on therapies used in the post-HCT relapse setting outside of the second transplant were not available for analysis.
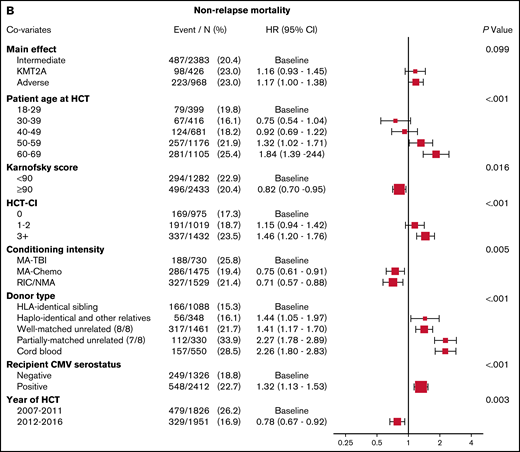
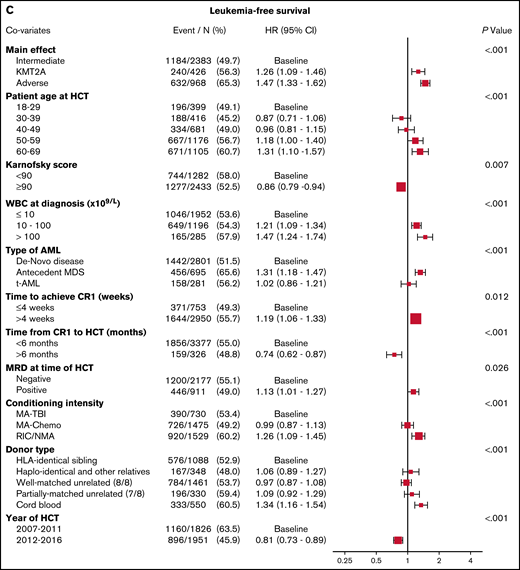
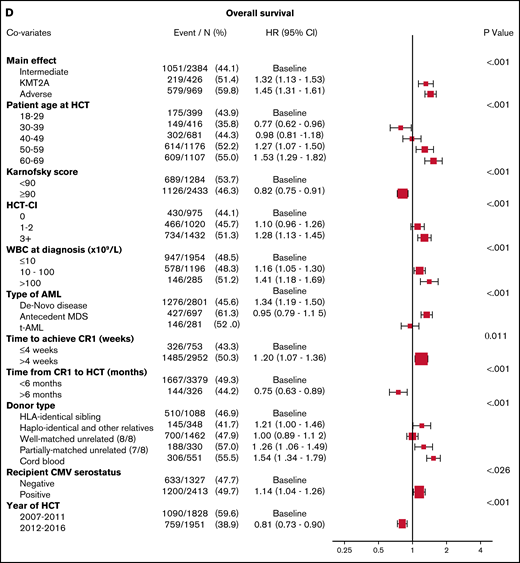
Multivariable analyses. (A) The outcome of relapse, with the main effect of cytogenetic category and including the listed covariates. The HR for relapse among those with KMT2A rearrangement was 1.27, and among those with adverse risk was 1.71. (B) The outcome of NRM, with the main effect of cytogenetic category and including the listed covariates. The HR for NRM was not significantly different among the groups. (C) The outcome of LFS, with the main effect of cytogenetic category and including the listed covariates. The HR among those with KMT2A rearrangement was 1.26, and among patients with adverse risk was 1.47. (D) The outcome of OS, with the main effect of cytogenetic category and including the listed covariates. The HR for death among those with KMT2A rearrangement was 1.32 and among patients with adverse risk was 1.45.
Multivariable analyses. (A) The outcome of relapse, with the main effect of cytogenetic category and including the listed covariates. The HR for relapse among those with KMT2A rearrangement was 1.27, and among those with adverse risk was 1.71. (B) The outcome of NRM, with the main effect of cytogenetic category and including the listed covariates. The HR for NRM was not significantly different among the groups. (C) The outcome of LFS, with the main effect of cytogenetic category and including the listed covariates. The HR among those with KMT2A rearrangement was 1.26, and among patients with adverse risk was 1.47. (D) The outcome of OS, with the main effect of cytogenetic category and including the listed covariates. The HR for death among those with KMT2A rearrangement was 1.32 and among patients with adverse risk was 1.45.
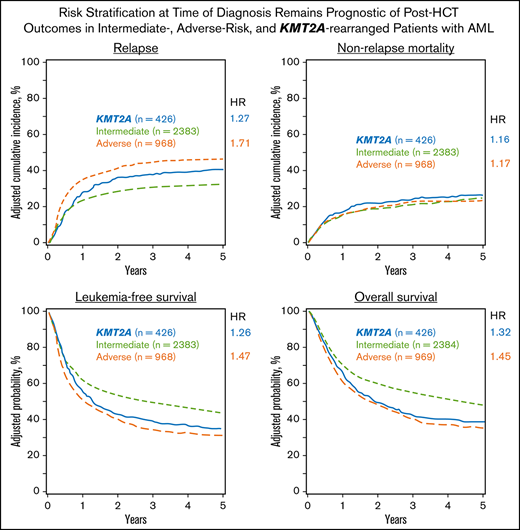
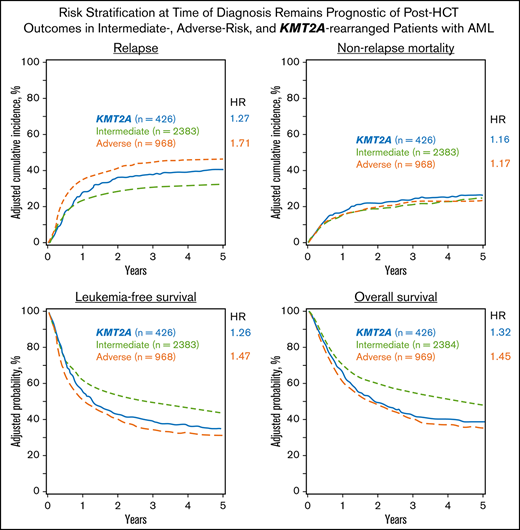
The adjusted cumulative incidence function (CIF) for relapse is shown in Figure 2A.
Adjusted multivariable analysis by cytogenetic group. (A) Curves of the CIF for the outcome of relapse, showing the highest risk of relapse was among patients with adverse risk. KMT2A-rearranged patients also had an increased risk of relapse after 12 months. (B) Curves of the CIF for the outcome of NRM, showing no significant difference among the intermediate-risk, adverse-risk, or KMT2A-rearranged groups. (C) Kaplan-Meier curves for the outcome of LFS, demonstrating that patients with KMT2A rearrangements did worse than those with intermediate risk but similarly to patients with adverse risk. (D) Kaplan-Meier curves for the outcome of OS, where the KMT2A-rearranged group’s survival closely approximated that of the adverse-risk group.
Adjusted multivariable analysis by cytogenetic group. (A) Curves of the CIF for the outcome of relapse, showing the highest risk of relapse was among patients with adverse risk. KMT2A-rearranged patients also had an increased risk of relapse after 12 months. (B) Curves of the CIF for the outcome of NRM, showing no significant difference among the intermediate-risk, adverse-risk, or KMT2A-rearranged groups. (C) Kaplan-Meier curves for the outcome of LFS, demonstrating that patients with KMT2A rearrangements did worse than those with intermediate risk but similarly to patients with adverse risk. (D) Kaplan-Meier curves for the outcome of OS, where the KMT2A-rearranged group’s survival closely approximated that of the adverse-risk group.
NRM
NRM rates were similar for patients with KMT2A-rearrangement, intermediate-risk, or adverse-risk disease at 1, 3, and 5 years after transplant by univariable analysis (Table 3). NRM risk was similar for all translocation partners in patients with KMT2A rearrangement (Table 4). The CIF curve is shown in Figure 2B.
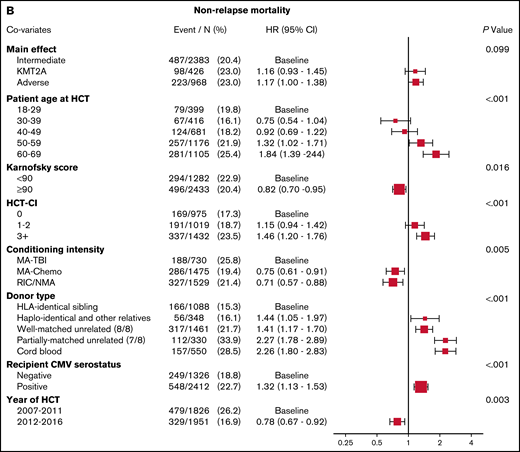
Figure 1B demonstrates that the results of multivariable analysis for NRM was not significantly different among cytogenetic groups, with HR, 1.16 (95% CI, 0.93-1.45) for KMT2A-rearranged disease and HR, 1.17 (95% CI, 1.00-1.38) for adverse-risk disease (P = .099). Predictors of NRM included older age, poor performance status, higher HCT-CI score, conditioning regimen, donor type, CMV positivity, and year of transplant.
LFS
LFS at 1, 3, and 5 years after transplantation was worse for patients with adverse-risk disease (Figure 2C). The 1-year probability of LFS overlapped for the KMT2A-rearranged and intermediate-risk groups but was worse for those in the adverse-risk group. Similar trends were seen at 3 and 5 years in the univariable analysis (Table 3). No differences in LFS according to translocation partner were noted (Table 4).
As shown in Figure 1C, the multivariable analysis detected worse LFS for the KMT2A-rearranged and adverse-risk groups, compared with the intermediate-risk group (HR, 1.26; 95% CI, 1.09-1.46; P = .002, and HR, 1.47; 95% CI, 1.33-1.62; P < .001, respectively). Increasing WBC count was a predictor of LFS, as was longer time to CR1. MRD+patients, those who underwent RIC/NMA, and those with a cord blood donor also had worse LFS. In addition, patients with prior MDS had worse LFS.
GRFS
GRFS at 1, 3, and 5 years was similarly poor for patients with KMT2A-rearranged or adverse-risk disease and was somewhat better for those with intermediate-risk disease (Table 5). Five-year GRFS could be compared only between the intermediate- and adverse-risk groups, given the number of patients available for analysis, and was significantly worse among patients with adverse risk (9% vs 15%).
A multivariable analysis for GRFS detected that it was not significantly worse in patients with KMT2A rearrangement but it was worse for patients in the adverse-risk group, compared with those in the intermediate-risk group (HR, 1.14; 95% CI, 1.01-1.28; P = .03, and HR, 1.23; 95% CI, 1.14-1.34; P < .001, respectively) (Table 6). Age was included in the model but was not statistically significant. Later transplant was associated with improved GRFS, and a history of antecedent MDS was associated with a significantly worse outcome (HR, 1.19; 95% CI, 1.08-1.30; P < .001).
OS
A univariable analysis showed a 42% 5-year OS for the KMT2A-rearranged group, compared with 48% for the intermediate-risk group and 34% for the adverse-risk group (Table 3). No difference in OS was detected according to KMT2A translocation partner (Table 4).
OS in a multivariable model (Figure 1D) was again worse for the KMT2A-rearranged and adverse-risk groups (HR, 1.32; 95% CI, 1.13-1.53; P < .001, and HR, 1.45; 95% CI, 1.31-1.61; P < .001, respectively). Poorer OS was also noted in those >50 years of age, with poor performance status, higher HCT-CI, score, higher WBC at diagnosis, and longer time to achieve CR1. Patients who underwent HCT <6 months after CR1 had worse OS, as did those with donors who were not well-matched or identical. Secondary AML was again a poor predictor, whereas tAML was not.
The Kaplan-Meier curve for OS is shown in Figure 2D.
MRD
The proportion of patients with MRD positivity was similar between the groups: 21% of patients with a KMT2A rearrangement, 30% of patients with intermediate-risk disease, and 30% of patients with adverse-risk disease. Pre-HCT MRD positivity was associated with relapse but did not meet statistical significance for LFS (HR, 1.23; 95% CI, 1.06-1.42; P = .006, and HR, 1.13; 95% CI, 1.01-1.27; P = .04, respectively). MRD was not associated with NRM or OS.
Outcomes among KMT2A translocation partners
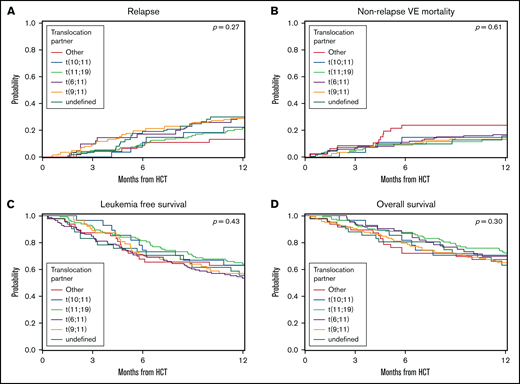
A secondary analysis was performed to determine the influence of translocation partner on outcomes for patients with KMT2A rearrangement. We excluded the patients whose translocation partner had not been defined from the secondary analysis. We then compared the 112 patients with t(9;11) against the remaining patients with KMT2A rearrangement and the 62 patients with t(11;19) with the remaining patients with KMT2A rearrangement and then combined both the t(9;11) and t(11;19) groups and compared them with the remaining 116 patients for whom a translocation partner had been defined. We looked at the risk of relapse, NRM, LFS, and OS and found no difference between the groups for any outcome (Table 4; Figure 3; multivariable analysis results in supplemental Table 3).
Univariable analysis by translocation partner. (A) CIF curves for the outcome of relapse, where patients with KMT2A rearrangement were stratified by translocation partner. No significant differences were noted between the groups. Log-rank, P = .27. (B) CIF curves for the outcome of NRM, where patients with KMT2A rearrangement were stratified by translocation partner. No significant differences were noted between the groups. Log-rank, P = .61. (C) Kaplan-Meier curves for the outcome of LFS, where patients with KMT2A rearrangement were stratified by translocation partner. No significant differences were noted between the groups. Log-rank, P = .43. (D) Kaplan-Meier curves for the outcome of OS, where patients with KMT2A rearrangement were stratified by translocation partner. No significant differences were noted between the groups. Log-rank, P = .27.
Univariable analysis by translocation partner. (A) CIF curves for the outcome of relapse, where patients with KMT2A rearrangement were stratified by translocation partner. No significant differences were noted between the groups. Log-rank, P = .27. (B) CIF curves for the outcome of NRM, where patients with KMT2A rearrangement were stratified by translocation partner. No significant differences were noted between the groups. Log-rank, P = .61. (C) Kaplan-Meier curves for the outcome of LFS, where patients with KMT2A rearrangement were stratified by translocation partner. No significant differences were noted between the groups. Log-rank, P = .43. (D) Kaplan-Meier curves for the outcome of OS, where patients with KMT2A rearrangement were stratified by translocation partner. No significant differences were noted between the groups. Log-rank, P = .27.
We also performed a multivariable analysis for our main 4 outcomes that included only patients in the KMT2A-rearranged group who had t(9;11) as compared to those with intermediate-risk disease (Table 7). In this analysis, NRM, relapse, LFS, and OS were not significantly different between patients with t(9;11) and those with intermediate risk.
Additional chromosomal abnormalities and CK analysis
Of the 426 patients with KMT2A rearrangement in our study, 145 had additional karyotypic abnormalities, and 281 had an 11q23 translocation alone. The most common additional chromosomal abnormality was trisomy 8, which occurred as an isolated abnormality in 39 patients and with other abnormalities in 23 others. Monosomy 7 was detected in 10 patients, del(7q) in 3; monosomy 5 was found in 3 patients and del(5q) in 10. In supplemental Table 4, we present exploratory multivariable analyses for patients in the intermediate- and adverse-risk groups in comparison with all the patients in the KMT2A-rearranged group, those in the KMT2A-rearranged group without additional abnormalities, and those in the group with additional abnormalities. KMT2A with additional abnormalities had outcomes similar to those of patients with intermediate risk for all 4 outcomes at our level of significance (P < .01). LFS and OS were significantly shorter for the patients in the KMT2A-rearranged group who did not have additional abnormalities.
We performed a manual review to determine cytogenetic complexity in the KMT2A-rearranged group and found 29 instances of CK among them: 7 with t(9;11), 1 with t(11;19), 3 each with t(6;11) and t(10;11), 2 with a different translocation partner, and 13 in whom the translocation partner was not defined. We performed a univariable analysis to evaluate our main outcomes in those in the KMT2A-rearranged group with CK, compared with those without CK; no significant differences were detected (supplemental Table 5).
Discussion
For most patients with AML with intermediate- or adverse-risk prognostic features, allogeneic HCT remains the only curative therapy.20-22 Until now, there have been limited quantitative data to compare post-HCT outcomes between these groups. This study is the largest presentation to date of real-world evidence on post-HCT AML outcomes using curated cytogenetics data. We used a refined stratification by diagnostic risk after expert manual curation and robust clinical and outcomes data collected from 165 centers by the CIBMTR to provide quantitative comparisons. Our results show that patients with adverse risk have a 71% increased risk of relapse, 47% increased risk of death from recurrent leukemia, and 45% increased risk of death overall, compared with those with intermediate-risk disease. Although HCT remains an essential component of curative therapy for patients with AML, it may not fully overcome the differences in disease biology detected at diagnosis.
Patients with a KMT2A rearrangement may fall into either the intermediate- or adverse-risk category at the time of diagnosis, based on translocation partner, and thus provide a compelling subgroup for further analysis. We found that LFS and OS for patients with KMT2A rearrangement are similar to those with adverse risk in the posttransplant setting. The KMT2A-rearranged group had a higher risk of relapse than the intermediate-risk group, but a lower relapse risk than the adverse-risk group. No differences in NRM were seen based on cytogenetic grouping. Patients with KMT2A rearrangement with t(9;11) were analyzed as a separate group in a multivariable model, and their outcomes were indistinguishable from those in the intermediate-risk group, suggesting that their risk classification at diagnosis remains predictive of their post-HCT outcomes. When all patients with KMT2A rearrangement were stratified by translocation partner, our study did not detect differences between the groups in any outcome.
We also provide quantitative, real-world data on the long-term outcomes of patients with AML who undergo HCT. For those with intermediate or adverse risk, our univariable analysis showed that 5-year post-HCT OS was 48% and 34%, respectively. Patients with KMT2A rearrangement had a 5-year OS of 42%. Although we could not perform a direct comparison with non-transplant treatment approaches with our available dataset, we noted that large studies of patients with AML have shown a 5-year OS for patients with KMT2A rearrangement of 20% to 25%.7
In addition, our real-world data show that KMT2A-rearranged disease was more common among women and that antecedent breast cancer had been detected in 4 to 6 times as many patients in the KMT2A-rearranged group as in the intermediate- or adverse-risk groups. These data reinforce the association between KMT2A translocations and antecedent chemotherapy and highlight the necessity of counseling women who have been treated with anthracycline-based regimens about the risks of developing tAML.23-27
Prior studies looking at whether translocation partner is predictive of outcomes in the posttransplant setting are mixed. A 2015 study of 159 patients with KMT2A-rearranged AML drew from the European Group for Blood and Marrow Transplantation database.28 They found that patients with t(11;19) had the highest 2-year OS at 73% ± 10%, followed by t(9;11) at 64% ± 6%, t(10;11) at 40% ± 13%, and t(6;11) at 24% ± 11%. The results suggested that the stratification was driven by a high relapse risk in the latter 2 groups of ∼50% at 2 years. Our study found lower rates of relapse that were similar for different translocation partners: ∼20% to 30% at 1 year. A multivariable analysis of 49 patients in the KMT2A-rearranged group who underwent allo-HCT by the German Acute Myeloid Leukemia Intergroup did not find a significant difference in post-HCT OS based on KMT2A subtype.29 Our analysis also did not detect a significant difference in post-HCT outcomes based on the KMT2A partner, despite having more patients per translocation subgroup than any other cohort in the published literature.
The relatively poor OS of patients with KMT2A rearrangement suggests that novel therapies are needed to treat this high-risk population. KMT2A rearrangements lead to the recruitment of the H3K79 histone methyltransferase DOT1L, which alters histone methylation and results in the overexpression of HoxA9 and Meis1 and a subsequent block in cellular differentiation.30 A phase 1 study of the DOT1L inhibitor pinometostat found that it is well tolerated, with modest clinical activity as a single agent.31 A combination study of pinometostat and azacitidine in patients with relapsed/refractory disease is ongoing, as is a trial of pinometostat with induction therapy in patients who were previously untreated.32,33 Preclinical studies have shown that targeting menin, a protein that binds to the N-terminus of KMT2A fusion proteins, may also provide an avenue for directed therapy in these patients.34 Clinical trials are also ongoing with menin inhibitors, and preclinical data suggest that combining DOT1L and menin inhibition may ultimately result in better outcomes for these patients than the use of either class of agent alone.35-37 The spleen tyrosine kinase inhibitor entospletinib is also in early-phase trials in this population.38 Although these studies are taking place in patients before HCT, including their use as a bridge to transplant, studies of their use in the post-HCT setting as maintenance therapies are clearly needed.
As trials of targeted therapies are ongoing, our study provides insights into transplant strategies that may be effective in this population. A subgroup analysis showed that those who underwent MA conditioning, especially with TBI, had improved LFS and lower relapse risk, compared with those who received RIC/NMA transplants. The benefits of MA conditioning, however, must be considered against the risk of NRM, which was higher in this group; on balance, conditioning intensity did not play a role in OS in our analysis. MA conditioning may be most useful for patients selected based on age, HCT-CI, and other patient- and disease-related factors, such as MRD positivity. Patients who go into transplant with MRD have been described to be more likely to relapse and have a decreased OS.39 In a recent study, Hourigan et al found that these risks may be mitigated by the use of MA conditioning in patients with molecular MRD.40 Prospective studies are needed to evaluate whether MA regimens should be preferred among patients with KMT2A rearrangement, and the role of MRD in this setting should be examined. In addition, as patients with KMT2A rearrangement do worse than those with intermediate-risk disease, use of donor lymphocyte infusions or posttransplant maintenance may also be considered as early interventions in this population.
In conclusion, our analysis is the largest to date of the posttransplant outcomes of patients with AML. Diagnostic risk classification remains predictive of transplant outcomes, but 5-year OS after HCT is robust and provides evidence of the curative potential of this strategy. Post-HCT outcomes for t(9;11) patients hew closely to those of patients with intermediate-risk disease. In aggregate, however, patients with KMT2A rearrangement have LFS and OS similar to patients with adverse-risk disease. Stratification of all patients with KMT2A rearrangement by translocation partner did not show significant differences. This may be because, despite having the largest analysis published to date, we remain underpowered to detect such a difference. We recognize that our analysis of registry data may be limited by the heterogeneity of the contributing centers, changes in MRD assessment over the study period, and missing data, as well as the lack of genomic information. However, our study highlights the need to consider whether prognostic scoring systems for patients with AML who undergo HCT should include all cytogenetic data from the time of diagnosis. Further studies are needed to evaluate the role of genomic data and clonal evolution in refining risk prediction for this population. In the case of KMT2A, we posit that targeted approaches to treatment and transplantation in this unfavorable patient subset represent a critical unmet need.
Acknowledgments
This work was funded in part by the Weinberg Family and Mortimer J. Lacher fellowships, an American Society of Clinical Oncology Young Investigator Award (ASCO YIA), a National Institutes of Health K12 Paul Calabresi Career Development Award for Clinical Oncology (K12-CA184746), and under the auspices of the Memorial Sloan Kettering Cancer Center (MSKCC) Clinical Research Methodology Curriculum (CRMC)/Weill Cornell Clinical and Translational Science Center (CTSC) (K.M.). The CIBMTR is supported primarily by National Institutes of Health grant U24CA076518 from the National Cancer Institute (NCI), the National Heart, Lung, and Blood Institute (NHLBI), and the National Institute of Allergy and Infectious Diseases (NIAID); by grant HHSH250201700006C from the Health Resources and Services Administration (HRSA); and by grants N00014-20-1-2705 and N00014-20-1-2832 from the Office of Naval Research. Support was also provided by Be the Match Foundation, the Medical College of Wisconsin, the National Marrow Donor Program, and from the following commercial entities: AbbVie, Actinium Pharmaceuticals Inc, Adaptive Biotechnologies Corporation, Adienne SA, Allovir Inc, Amgen Inc, Angiocrine Bioscience, Astellas Pharma US, Bluebird Bio Inc, Bristol Myers Squibb Co, CSL Behring, CytoSen Therapeutics Inc, Daiichi Sankyo Co Ltd, Eurofins Viracor, ExcellThera, Fate Therapeutics, Gamida-Cell Ltd, Genentech Inc, GlaxoSmithKline, Incyte Corporation, Janssen/Johnson and Johnson, Jasper Therapeutics, Jazz Pharmaceuticals Inc, Karyopharm Therapeutics, Kiadis Pharma, Kite (a Gilead company), Kyowa Kirin, Legend Biotech, Magenta Therapeutics, Medac GmbH, Merck and Co, Millennium, the Takeda Oncology Co, Miltenyi Biotec Inc, MorphoSys, Novartis Pharmaceuticals Corporation, Omeros Corporation, Oncoimmune Inc, Orca Biosystems Inc, Pfizer Inc, Pharmacyclics LLC, Sanofi Genzyme, Seagen Inc, Stemcyte, Takeda Pharmaceuticals, Tscan, Vertex, Vor Biopharma, Xenikos BV.
Authorship
Contribution: K.M., initiated the project, manually curated and analyzed the data, and drafted the manuscript; A.G.-A. manually curated and interpreted the data and edited the manuscript; R.M.-M. manually curated the data; M.-J.Z. performed the statistical analyses and interpreted the data; H.-L.W. managed the project, performed statistical analyses, curated and interpreted the data, and created the tables and figures; K.B.-S. managed the project, curated the data, performed the statistical analysis, and created the tables; J.S. managed the project, performed statistical analyses, and curated the data; C.H., B.S., M.L., P.K., and D.W. provided project oversight, interpreted the data, and reviewed the manuscript; Y.Z. initiated and designed the project, manually curated and interpreted the data, and reviewed the manuscript; M.S.T. provided project oversight, reviewed the statistical analyses, interpreted the data, and reviewed the manuscript; and all remaining authors interpreted the data and reviewed the manuscript.
Conflict-of-interest disclosure: J.C. reports conflicts with Incyte Inc, Jazz Pharmaceuticals, Daiichi-Sankyo, and Pfizer Inc, and owns stock in Bluebird Bio Inc. V.R.B. reports receiving personal fees from Agios, Takeda, Partner Therapeutics, Incyte, Omeros, AbbVie, Partnership for Health and Analytic Research LLC, Pfizer, CSL Behring, and Rigel Pharmaceuticals; grants from Jazz, National Marrow Donor Program, and Tolero Pharmaceuticals; and other support from Oncoceutics, outside the submitted work. M.R.G. reports receiving grants, personal fees, and nonfinancial support from Incyte; grants from Janssen, Genentech, and Forma Therapeutics; grants and nonfinancial support from Amgen; personal fees from AbbVie, Agios, Astellas, Daiichi Sankyo, Cardinal Health, Premier, Trovagene, Pfizer, Merck, and BMS/Celgene; and other support from Medtronic, outside the submitted work. G.H. reports other support from Pfizer, Kite Pharma, Incyte, Jazz Pharmaceuticals, Pharmacyclics, Takeda, the Falk Foundation, Astellas Pharma, outside the submitted work. C.H. reports other support from Merck and Sellas, outside the submitted work. A.A. reports grants and personal fees from Incyte Corporation, Boston Biomedical, and Null, outside the submitted work. A.K. reports conflicts with BMS, Takeda, Pfizer, Janssen, Sanofi, Karyopharm, and GSK. H.L. reports grants from BMS and Karyopharm and personal fees from Agios, outside the submitted work. T.N. reports other support from Karyopharm and Novartis, outside the submitted work. R.R. reports personal fees from Kite/Gilead, Bristol Myers, Monsanto, Incyte, Magenta, Pharmacyclics, and Atara, outside the submitted work. D.R. reports personal fees from Amgen, Kite, AROG, Pharmacyclics, Seattle Genetics, Pfizer, Novartis, Sanofi-Aventis, Incyte, Gilead, Jazz, Celegene, and AbbVie; other support from Celltron/Teva, Mustang, Bayer, Stemline, outside the submitted work. A.S. reports research collaboration with CRISPR Therapeutics/Vertex Pharmaceuticals, and Novartis Pharmaceuticals; he is the principal investigator of a clinical trial for gene therapy of sickle cell disease sponsored by Vertex Pharmaceuticals/CRISPR Therapeutics, and the sponsor provides funding for the clinical trial, which includes salary support paid to his institution; the support is not related in any way to this work product. He also has research collaboration with Novartis on their sickle cell gene therapy clinical trial for which he is not financially compensated in any way. C.U. reports other support from Jazz Pharmaceutical, outside the submitted work. D.W. reports grants from Incyte and personal fees from Fate, outside the submitted work. The remaining authors declare no competing financial interests.
Correspondence: Kamal Menghrajani, Leukemia Service, Department of Medicine, Memorial Sloan Kettering Cancer Center, 1275 York Ave, New York, NY 10065; e-mail: menghrak@mskcc.org.
References
Author notes
CIBMTR supports accessibility of research in accordance with the National Institutes of Health (NIH) Data Sharing Policy and the National Cancer Institute (NCI) Cancer Moonshot Public Access and Data Sharing Policy. The CIBMTR releases only deidentified datasets that comply with all relevant global regulations regarding privacy and confidentiality. Please send requests to the corresponding author: menghrak@mskcc.org.
The full-text version of this article contains a data supplement.