Abstract
Extravascular coagulation leading to fibrin deposition accompanies many immune and inflammatory responses. Although recognized by pathologists for decades, and probably pathologic under certain conditions, the physiologic functions of extravascular coagulation remain to be fully defined. This study demonstrates that thrombin can activate macrophage adhesion and prompt interleukin-6 (IL-6) and monocyte chemoattractant protein-1 (MCP-1) production in vivo. Peritoneal macrophages were elicited with thioglycollate (TG) and then activated in situ, either by intraperitoneal injection of lipopolysaccharide (LPS) or by injection of antigen into mice bearing antigen-primed T cells. Others previously established that such treatments stimulate macrophage adhesion to the mesothelial lining of the peritoneal cavity. The present study demonstrates that thrombin functions in this process, as macrophage adhesion was suppressed by Refludan, a highly specific thrombin antagonist, and induced by direct peritoneal administration of purified thrombin. Although recent studies established that protease activated receptor 1 (PAR-1) mediates some of thrombin's proinflammatory activities macrophage adhesion occurred normally in PAR-1–deficient mice. However, adhesion was suppressed in fibrin(ogen)-deficient mice, suggesting that fibrin formation stimulates macrophage adhesion in vivo. This study also suggests that fibrin regulates chemokine/cytokine production in vivo, as direct injection of thrombin stimulated peritoneal accumulation of IL-6 and MCP-1 in a fibrin(ogen)-dependent manner. Given that prior studies have clearly established inflammatory roles for PAR-1, thrombin probably has pleiotropic functions during inflammation, stimulating vasodilation and mast cell degranulation via PAR-1, and activating cytokine/chemokine production and macrophage adhesion via fibrin(ogen).
Introduction
Vasodilation and increased vascular permeability are among the earliest signs of inflammation. These events stimulate the extravasation of inactive coagulant precursors, which become activated upon exposure to extravascular tissues. The ensuing coagulation cascade culminates with the generation of thrombin, a protease that cleaves extravasated fibrinogen, prompting its polymerization and deposition as fibrin. Accordingly, localized extravascular fibrin deposition accompanies many type 1 T helper cell (Th1)–associated responses, including autoimmune neuropathologies,1-4 glomerulonephritis,5,6rheumatoid arthritis,7-9 Crohn's disease,10,11 allograft rejection,12,13delayed-type hypersensitivity,14-19 and viral infections.20,21 For some time, it has been appreciated that such Th1-associated coagulation has physiologic consequences, as the swelling that accompanies delayed-type hypersensitivity responses is suppressed in anticoagulated or fibrinogen-deficient subjects.14-19 However, the full significance of immune-associated extravascular coagulation remains to be defined.
Recent studies suggest that thrombin is a physiologic mediator of inflammatory events. Administration of recombinant hirudin, a highly specific thrombin antagonist, reduces pathology and leukocyte infiltration in a mouse glomerulonephritis model.22Hirudin analogs also suppress mast cell degranulation and vasodilation in a carrageenin-induced inflammation model,23 and prevent onset and ameliorate established disease in mouse arthritis models.24 25 Together, these studies strongly suggest that thrombin has physiologic functions during immunity/inflammation.
The vasodilatory activities of thrombin likely result from its capacity to stimulate PAR-1, a 7-transmembrane–spanning, G-protein–coupled receptor activated upon cleavage by thrombin.26 Consistent with the aforementioned hirudin studies, PAR-1–deficient mice27 exhibit diminished inflammation in glomerulonephritis and carrageenin models.22,23 In addition, cutaneous injection of a PAR-1–activating peptide stimulates mast cell degranulation and vasodilation in wild-type mice,23 and vascular permeability is suppressed in PAR-1–deficient mice.28 Thus, thrombin-stimulated activation of PAR-1 may constitute one mechanism by which extravascular coagulation influences inflammation.
Thrombin may also influence inflammation through its ability to stimulate fibrin deposition. Indeed, fibrin is a ligand for CD54 (ICAM-1),29,30 CD11b/CD18 (CR3, Mac-1),31-33and CD11c/CD18 (CR4, p150/95),34,35 adhesion-promoting receptors expressed by endothelial cells, neutrophils, monocytes/macrophages, as well as subsets of dendritic, natural killer, and T cells. Studies using blocking peptides and specific monoclonal antibodies suggest that CD11b/CD18-fibrin interactions regulate leukocyte adherence to vascular clots36 and implanted biomaterials.37 38 Thus, extravascular fibrin may act as a provisional adhesion matrix for leukocyte accumulation at sites of inflammation.
Extravascular fibrin may also directly stimulate leukocyte activities. Fibrin(ogen) reportedly stimulates tumor necrosis factor alpha (TNFα) and IL-1β expression by macrophages39,40 and chemokine secretion by endothelial cells,41,42fibroblasts,43 and neutrophils.44 We recently demonstrated that fibrinogen also stimulates macrophage chemokine expression, apparently via Toll-like receptor 4 (TLR4).45As prior studies suggest that TLR4 signals “danger” in response to bacterial lipopolysaccharide (LPS),46-48 we propose that TLR4 may likewise signal danger in response to extravascular coagulation, thereby stimulating the production of chemokines, attracting leukocytes, and enhancing immune surveillance at sites of inflammation.
Until recently, few studies had convincingly evaluated inflammatory roles for fibrin in vivo, in part due to a lack of suitable agents for experimental depletion or antagonism of fibrinogen. Ancrod, a proteolytic enzyme derived from snake venom, proteolyzes fibrinogen, transiently generating a fibrinogen-deficient state. Experimental administration of ancrod produced data consistent with roles for fibrin in experimental encephalomyelitis,1,4glomerulonephritis,49,50 arthritis,51transplant rejection,52 and the containment of bacterial infections.53,54 Recently, gene-targeted fibrinogen-deficient mice were generated55 and used to confirm roles for fibrin(ogen) in wound healing56 and the control of bacterial infections.57 Detailed studies of the immune and inflammatory responses in fibrinogen-deficient mice have yet to be reported.
Here, we define and distinguish roles for thrombin, PAR-1, and fibrinogen in a mouse peritonitis model. We demonstrate that thrombin plays an important role in stimulating the adhesion of inflammatory peritoneal macrophages in vivo. Taking advantage of PAR-1–deficient and fibrinogen-deficient mice, we demonstrate that thrombin-stimulated macrophage adhesion is PAR-1 independent, but fibrinogen dependent. We also demonstrate that thrombin stimulates the peritoneal accumulation of cytokines and chemokines in a fibrinogen-dependent manner. Whereas others have clearly established inflammatory roles for PAR-1,22,23 28 our data indicate that extravascular coagulation leading to thrombin production can also regulate inflammation through fibrinogen, presumably via thrombin-stimulated production of fibrin.
Materials and methods
Animals
Mice aged 6 to 10 weeks old were used for these experiments. C57BL/6 mice were purchased from Taconic (Germantown, NY). Transgenic mice were bred at the Trudeau Institute Animal Breeding Facility. All experimental mice were age- and sex-matched. PAR-1–deficient mice27 (backcrossed 6 generations to C57BL/6 mice) were originally obtained from the Jackson Laboratory (Bar Harbor, ME). Fibrinogen-deficient mice55 (backcrossed 7 generations to C57BL/6 mice) and OT-II T-cell receptor transgenic mice58 were generously supplied by Jay Degen (Children's Hospital Medical Center, Cincinnati, OH) and Francis Carbone (University of Melbourne, Parkville, Australia)/William Heath (The Walter and Eliza Hall Institute, Parkville, Australia), respectively. Animals were housed in a specific pathogen-free facility and cared for according to the Trudeau Institute Animal Care and Use Committee guidelines.
Generation of antigen-specific Th1 cells
Naı̈ve CD4+ T cells were enriched from spleens and lymph nodes of OT-II T-cell receptor transgenic mice58 using CD4 monoclonal antibody (mAb)–based magnetic cell sorting (Miltenyi Biotec, Auburn, CA) followed by density gradient enrichment of resting naı̈ve cells (interface of 80%/62% percoll). Successful purification was confirmed by flow cytometry (> 85% CD4+Vα2+Vβ5+). We then cultured the OT-II cells (2.5 × 105/mL) with mitomycin C–treated splenic C57BL/6 antigen-presenting cells (1 × 106/mL) in RPMI media containing 7.5% fetal bovine serum, 2 mM glutamine, 50 U/mL penicillin, 50 μg/mL streptomycin, and 50 μM 2-mercaptoethanol. To generate Th1 cells, we supplemented cultures with ovalbumin peptide ISQAVHAAHAEINEAGR (Ova) (10 μM, New England Peptide, Fitchburg, MA), IL-2 (20 U/mL, Roche Molecular Biochemicals, Indianapolis, IN), IL-12 (5 ng/mL, BD Pharmingen, San Diego, CA) and anti–IL-4 (clone 11B11, 10 μg/mL, Trudeau Institute Core Antibody Facility). After 6 days, the differentiated cells were routinely > 90% CD4+Vα2+Vβ5+ by flow cytometry. After washing with phosphate-buffered saline (PBS), we adoptively transferred 10 × 106 of these Th1 effectors to naı̈ve mice by intravenous injection. We also confirmed Th1 differentiation by restimulating cells in vitro on CD3 monoclonal antibody-precoated (10 μg/mL, clone 2C11, BD Pharmingen) plates, collecting supernatants at 48 hours, and measuring interferon gamma production by sandwich enzyme-linked immunosorbent assay (ELISA) using an OptEIA kit (BD Pharmingen).
In vivo assays for peritoneal macrophage adhesion and IL-6/monocyte chemoattractant protein-1 production
To elicit inflammatory macrophages, mice received intraperitoneal injections of 3 mL sterile thioglycollate (TG) broth (Becton Dickinson Microbiology Systems, Cockeysville, MD). Assays were performed 4 days later, when macrophage recruitment was maximal (not shown). For antigen-specific assays, mice received adoptive transfers of OT-II Th1 cells 18 to 24 hours prior to initiation of macrophage activation by intraperitoneal injection of 50 μg Ova in 200 μL sterile PBS (Life Technologies, Rockville, MD). Alternatively, TG-primed mice that had not received Th1 cells were given intraperitoneal injections of Escherichia coli serotype 0111:B4 LPS (Sigma Chemical, St Louis, MO) or human alpha thrombin (Enzyme Research Laboratories, South Bend, IN). The thrombin used in these experiments was found to contain less than 0.1 units/mL endotoxin, as determined by Pyrochrome Limulus Amebocyte Lysate Assay (Associates of Cape Cod, Falmouth, MA). At the indicated times, mice were killed and peritoneal cells and fluid were harvested by washing the cavity with 7 mL PBS. Total cell numbers were determined using a hemocytometer and the percentages of macrophages were assessed by evaluation of Wright-Giemsa–stained cytospin smears (HEMA 3; Fisher Scientific, Pittsburgh, PA). Macrophages were identified as large cells with abundant cytoplasm and a single nucleus containing pale diffuse chromatin. Flow cytometry confirmed similar frequencies of macrophages (forward/side scatterhighMac-1+Gr-1−, not shown). IL-6 and monocyte chemoattractant protein-1 (MCP-1) protein levels in the harvested exudate fluid were determined by sandwich ELISA using OptEIA kits (BD Pharmingen).
Anticoagulation with Refludan
Refludan59 (16 000 antithrombin units/mg) was reconstituted as directed by the manufacturer (Hoechst Marion Roussel, Kansas City, MO), diluted in sterile PBS, and injected along with the activating stimuli. In pilot studies, we established that 2 mg/kg Refludan efficiently antagonized coagulation in mice, as reported for recombinant hirudin.22 The short half-life of Refludan led us to evaluate dosages up to 20 mg/kg, which also promoted effective anticoagulation without any apparent toxicity (not shown).
Statistics
Statistical significance was evaluated by Student ttest using the program Instat 2.01 (GraphPad Software, San Diego, CA).
Results
Injection of inflammatory stimuli into the peritoneal cavity of mice prompts an initial recruitment of neutrophils, followed by an accumulation of macrophages. Upon subsequent activation in situ, these inflammation-elicited macrophages adhere to the mesothelial lining of the peritoneal cavity,60,61 resulting in a dramatic decrease in the number of macrophages that can be recovered by peritoneal lavage.60 61 This assay provides a quantitative means to monitor the activation of macrophage adhesion in vivo.
Prior studies using this assay established that macrophage adhesion can be triggered by specific antigen in mice bearing antigen-sensitized T cells.60-63 In our version of this model, we inject mice with TG to recruit inflammatory macrophages, adoptively transfer OT-II transgenic T-cell receptor Th1 cells, and then inject Ova, the antigen recognized by OT-II T cells. After 5 hours, we harvest the peritoneal cells and enumerate macrophages by performing differential cell counts. As shown in Figure 1A, administration of TG recruits macrophages to the peritoneal cavity, and adoptive transfer of OT-II TCRtg Th1 cells followed by injection of Ova greatly decreases numbers of recoverable macrophages. This response is T cell and antigen dependent, as neither Th1 cells nor Ova stimulate macrophage adhesion when injected alone (Figure 1A). As previously reported,62this model can also be used to assay macrophage adhesion stimulated by LPS (Figure 1B).
The activation of inflammation-elicited peritoneal macrophages is thrombin dependent in vivo.
(A) The activation-induced adhesion of peritoneal macrophages by antigen-specific Th1 cells is thrombin dependent. OT-II transgenic T-cell receptor Th1 effector cells were adoptively transferred to C57Bl/6 mice that had been primed with TG (3 mL intraperitoneally) 3 days earlier. The next day, Ova peptide (50 μg intraperitoneally) or PBS vehicle (200 μL) were administered, and 5 hours later peritoneal exudate cells were harvested and macrophages were enumerated. Where indicated, mice received Refludan (2 mg/kg intraperitoneally) at the time of Ova administration. The data depicts the averages and standard deviations of groups of 3 mice. Th1 cells induced antigen-specific macrophage activation (P < .01), which was suppressed by Refludan (P = .02). This experiment was replicated twice. (B) The activation-induced adhesion of peritoneal macrophages by LPS is thrombin dependent. Peritoneal macrophages were elicited with TG (3 mL intraperitoneally). Four days later, LPS (1 μg intraperitoneally) or vehicle control (200 μL PBS) were administered, and macrophage numbers in peritoneal exudates were determined 5 hours later. Where indicated, mice also received Relfudan (20 mg/kg intraperitoneally) at the time of LPS administration. The data depicts the averages and standard deviations of groups of 5 mice. LPS induced macrophage activation (P < .0001) that was suppressed by Refludan (P < .0001). This experiment has been replicated 4 times.
The activation of inflammation-elicited peritoneal macrophages is thrombin dependent in vivo.
(A) The activation-induced adhesion of peritoneal macrophages by antigen-specific Th1 cells is thrombin dependent. OT-II transgenic T-cell receptor Th1 effector cells were adoptively transferred to C57Bl/6 mice that had been primed with TG (3 mL intraperitoneally) 3 days earlier. The next day, Ova peptide (50 μg intraperitoneally) or PBS vehicle (200 μL) were administered, and 5 hours later peritoneal exudate cells were harvested and macrophages were enumerated. Where indicated, mice received Refludan (2 mg/kg intraperitoneally) at the time of Ova administration. The data depicts the averages and standard deviations of groups of 3 mice. Th1 cells induced antigen-specific macrophage activation (P < .01), which was suppressed by Refludan (P = .02). This experiment was replicated twice. (B) The activation-induced adhesion of peritoneal macrophages by LPS is thrombin dependent. Peritoneal macrophages were elicited with TG (3 mL intraperitoneally). Four days later, LPS (1 μg intraperitoneally) or vehicle control (200 μL PBS) were administered, and macrophage numbers in peritoneal exudates were determined 5 hours later. Where indicated, mice also received Relfudan (20 mg/kg intraperitoneally) at the time of LPS administration. The data depicts the averages and standard deviations of groups of 5 mice. LPS induced macrophage activation (P < .0001) that was suppressed by Refludan (P < .0001). This experiment has been replicated 4 times.
Roles for thrombin in macrophage adhesion in vivo
As the procoagulant enzyme thrombin has been implicated in a variety of inflammatory responses,22-25,28 and as the nonspecific anticoagulants heparin and warfarin reportedly block macrophage adhesion in peritoneal models,60 we sought to evaluate roles for thrombin in the activation of macrophage adhesion in vivo. To specifically evaluate thrombin, we performed peritoneal macrophage adhesion assays in the presence of Refludan, a commercially available hirudin analog.59 As discussed in the “Introduction,” hirudin analogs were previously used to establish inflammatory roles for thrombin in mouse models.22-25
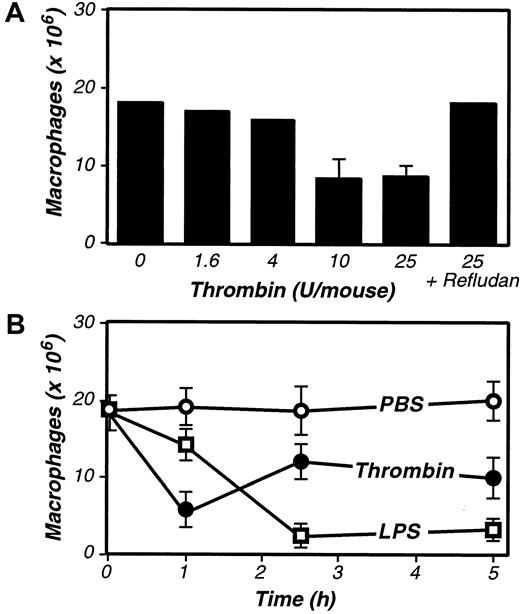
We found that Refludan significantly inhibited the activation of peritoneal macrophages in vivo. Both OT-II/Ova- and LPS-stimulated macrophage adhesion was suppressed by administration of Refludan (Figure 1A-B), suggesting that thrombin has critical adhesion-promoting functions in these peritoneal models. Notably, further studies confirmed an earlier report64 that intraperitoneal injection of purified thrombin itself can activate macrophage adhesion (Figure 2A).
Purified thrombin activates inflammation-elicited peritoneal macrophages in vivo.
(A) Dose response to thrombin. Four days after TG-stimulated macrophage elicitation (3 mL intraperitoneally), the indicated dosages of purified thrombin were administered, and macrophage numbers in peritoneal exudates were determined after 2.5 hours. (B) Kinetics of macrophage activation in response to thrombin and LPS. Four days after TG-stimulated macrophage elicitation, mice received intraperitoneal injections of thrombin (20 U, ●), LPS (1 μg, ■), or vehicle control (200 μL PBS, ○), and macrophage numbers in peritoneal exudates were determined at the indicated times. For A and B, the data represent the averages and standard deviations of 4 animals per group.
Purified thrombin activates inflammation-elicited peritoneal macrophages in vivo.
(A) Dose response to thrombin. Four days after TG-stimulated macrophage elicitation (3 mL intraperitoneally), the indicated dosages of purified thrombin were administered, and macrophage numbers in peritoneal exudates were determined after 2.5 hours. (B) Kinetics of macrophage activation in response to thrombin and LPS. Four days after TG-stimulated macrophage elicitation, mice received intraperitoneal injections of thrombin (20 U, ●), LPS (1 μg, ■), or vehicle control (200 μL PBS, ○), and macrophage numbers in peritoneal exudates were determined at the indicated times. For A and B, the data represent the averages and standard deviations of 4 animals per group.
As thrombin appeared to be an important mediator of macrophage adhesion, it had to be generated during the course of our peritoneal assays. Indeed, treatment with LPS is well known to up-regulate expression of tissue factor,65 66 an initiator of the coagulation cascade, thereby stimulating thrombin production. Consistent with LPS functioning via the induction of coagulant activities that prompt thrombin production, kinetic analyses revealed that thrombin stimulated macrophage adhesion more rapidly than did LPS (Figure 2B). However, the LPS-stimulated macrophage adhesion was more complete, even at saturating thrombin doses, suggesting that LPS may activate macrophage adhesion via both thrombin-dependent and -independent mechanisms.
Thrombin-stimulated macrophage adhesion is PAR-1 independent and fibrinogen dependent
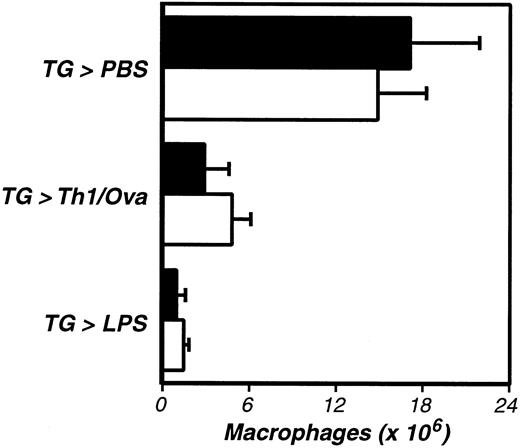
Having established roles for thrombin in the activation of macrophage adhesion in this model, we next explored its mechanism of action. Recent studies of thrombin's inflammatory activities have implicated PAR-1 in transmitting thrombin-mediated signals.22,23,28 Thus, we evaluated the activation of macrophage adhesion in PAR-1–deficient mice.27 We first established that TG could stimulate recruitment of inflammatory macrophages to the peritoneal cavity in PAR-1–deficient mice (Figure3). We then evaluated macrophage adhesion and found no defects in antigen- or LPS-stimulated adhesion in PAR-1–deficient mice (Figure 3). Thus, thrombin stimulates the adhesion of peritoneal macrophages independent of PAR-1.
The activation of inflammation-elicited peritoneal macrophages is not PAR-1 dependent.
PAR-1–deficient (−/−, ■) and littermate control (+/−, ▪) mice were compared, using the assays described in Figure 1. The data represent the averages and standard deviations of 5 animals per group. Neither TG-induced macrophage recruitment nor macrophage activation were significantly impaired in PAR-1–deficient mice.
The activation of inflammation-elicited peritoneal macrophages is not PAR-1 dependent.
PAR-1–deficient (−/−, ■) and littermate control (+/−, ▪) mice were compared, using the assays described in Figure 1. The data represent the averages and standard deviations of 5 animals per group. Neither TG-induced macrophage recruitment nor macrophage activation were significantly impaired in PAR-1–deficient mice.
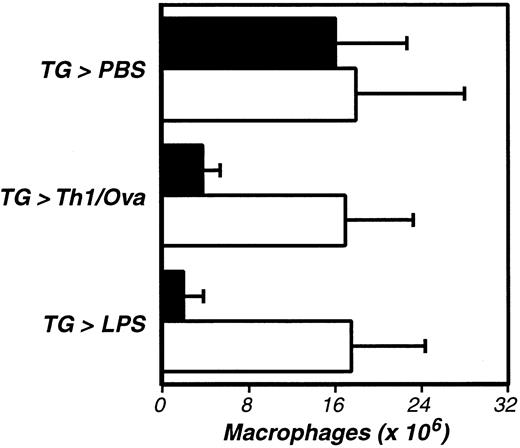
Having ruled out PAR-1, we next examined whether thrombin-stimulated fibrin formation accounts for thrombin's role in macrophage adhesion. Indeed, prior studies had established that peritoneal macrophages harvested soon after antigen stimulation are coated with fibrin(ogen).67 To explore functional roles for fibrin, we evaluated macrophage adhesion in fibrinogen-deficient mice.55 Again, we began by demonstrating that fibrinogen deficiency does not suppress the TG-stimulated elicitation of macrophages to the peritoneal cavity (Figure4). Subsequent analyses revealed that antigen-, LPS-, and thrombin-stimulated macrophage adhesion were all fibrinogen dependent, each being significantly suppressed in fibrinogen-deficient mice (Figure 4 and Figure5). As antigen and LPS are known to stimulate macrophage procoagulant activity leading to thrombin production, and as thrombin stimulates the conversion of fibrinogen to fibrin, the simplest interpretation of our data is that thrombin-mediated fibrin formation functions in the adhesion of inflammatory macrophages.
The activation of inflammation-elicited peritoneal macrophages is fibrinogen dependent.
Fibrinogen-deficient (−/−, ■) and littermate control (+/−, ▪) mice were compared, using the assays described in Figure 1. The data represent the averages and standard deviations of 5 animals per group. TG-induced recruitment of inflammatory macrophages was not significantly impaired in fibrinogen-deficient mice, but macrophage activation was significantly diminished in the absence of fibrinogen (Th1/Ova, P < .002; LPS, P < .002). This experiment was repeated 3 times.
The activation of inflammation-elicited peritoneal macrophages is fibrinogen dependent.
Fibrinogen-deficient (−/−, ■) and littermate control (+/−, ▪) mice were compared, using the assays described in Figure 1. The data represent the averages and standard deviations of 5 animals per group. TG-induced recruitment of inflammatory macrophages was not significantly impaired in fibrinogen-deficient mice, but macrophage activation was significantly diminished in the absence of fibrinogen (Th1/Ova, P < .002; LPS, P < .002). This experiment was repeated 3 times.
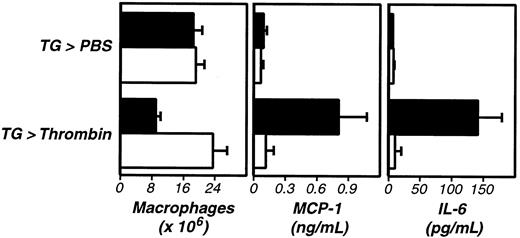
Thrombin stimulates fibrinogen-dependent cytokine and chemokine production in vivo.
Four days after TG administration, wild-type (+/+, ▪) or fibrinogen-deficient (−/−, ■) mice received intraperitoneal injections of thrombin (20 U). Macrophage numbers in peritoneal exudates were determined after 5 hours (left panel). Thrombin-stimulated macrophage activation was fibrinogen dependent (P < .001). Examination of the exudate fluid by ELISA revealed that thrombin also stimulated increases in peritoneal levels of MCP-1 (middle panel, P < .005) and IL-6 (right panel,P < .001) that were fibrinogen dependent (P < .005 and P < .001, respectively). The data represent averages and standard deviations of 4 animals per group. We repeated this experiment twice.
Thrombin stimulates fibrinogen-dependent cytokine and chemokine production in vivo.
Four days after TG administration, wild-type (+/+, ▪) or fibrinogen-deficient (−/−, ■) mice received intraperitoneal injections of thrombin (20 U). Macrophage numbers in peritoneal exudates were determined after 5 hours (left panel). Thrombin-stimulated macrophage activation was fibrinogen dependent (P < .001). Examination of the exudate fluid by ELISA revealed that thrombin also stimulated increases in peritoneal levels of MCP-1 (middle panel, P < .005) and IL-6 (right panel,P < .001) that were fibrinogen dependent (P < .005 and P < .001, respectively). The data represent averages and standard deviations of 4 animals per group. We repeated this experiment twice.
Thrombin stimulates fibrinogen-dependent IL-6 and MCP-1 production in vivo
During the course of these studies, we discovered that levels of the cytokine IL-6 (Figure 5, right panel) and the chemokine MCP-1 (Figure 5, middle panel) were significantly elevated in peritoneal fluid harvested after administration of thrombin. This thrombin-stimulated cytokine/chemokine production was suppressed by Refludan (not shown) and failed to occur in fibrinogen-deficient mice (Figure 5). As with macrophage adhesion, thrombin-stimulated cytokine/chemokine production proceeded normally in PAR-1–deficient mice (not shown). Stimulation by OT-II Th1 cells/Ova or LPS also prompted cytokine/chemokine production, but only the thrombin-stimulated cytokine/chemokine production was suppressed by Refludan and failed to occur in fibrinogen-deficient mice (not shown). Thus, although thrombin is not absolutely required for cytokine/chemokine production in response to antigen or LPS, thrombin clearly has the capacity to stimulate secretion of inflammatory mediators in vivo in a fibrin(ogen)-dependent manner.
Discussion
In vitro, thrombin reportedly stimulates leukocyte chemotaxis68-70 and proliferation,71-73 and activates mast cell degranulation.74 In theory, gene-targeting techniques could provide an unambiguous means to study inflammatory roles for thrombin in vivo. However, targeted deletion of thrombin or earlier components of the coagulation cascade (ie, tissue factor, factor VII, factor V, or factor X)75-81 results in embryonic or perinatal lethality. Thus, it has not yet been possible to produce adult animals genetically lacking the capacity to generate thrombin.
Recent studies using analogs of recombinant hirudin suggest that thrombin is a physiologic mediator of inflammatory events. These highly specific thrombin antagonists reduce pathology in murine glomerulonephritis,22 arthritis,24,25 and carrageenin-induced inflammation models.23Mechanistically, some proinflammatory activities of thrombin apparently result from its capacity to stimulate PAR-1, as PAR-1–deficient mice exhibit diminished inflammation in the glomerulonephritis and carrageenin models.22 23
In this report we demonstrated additional inflammatory roles for thrombin in vivo using a mouse peritonitis model. Specifically, we found that Refludan, a hirudin-based pharmacologic thrombin antagonist, suppressed antigen- or LPS-stimulated activation of macrophage adhesion. We also demonstrated that intraperitoneal injection of purified thrombin activates macrophage adhesion, and simultaneously stimulates the peritoneal accumulation of IL-6 and MCP-1. Despite the aforementioned studies implicating PAR-1 in thrombin-stimulated inflammation, we found that the proinflammatory activities of thrombin in these peritoneal models were PAR-1 independent. Rather, both thrombin-stimulated cytokine/chemokine production and macrophage adhesion required fibrinogen, as each was suppressed in fibrinogen-deficient mice.
Mechanistically, the simplest interpretation of our data are that (1) antigen-specific T cells and LPS elicit expression of procoagulant activity stimulating thrombin production, (2) thrombin cleaves fibrinogen, prompting fibrin formation, and (3) fibrin then functions in macrophage adhesion.
Although others have clearly shown that activation of inflammatory peritoneal macrophages stimulates their adhesion to the mesothelial lining of the peritoneal cavity,61 we are presently unable to distinguish between several potential models of fibrin-stimulated macrophage adhesion. One possibility is that fibrin directly mediates macrophage adhesion by simultaneously binding CD11b/CD18 and ICAM-1, fibrin-binding receptors expressed by macrophages and mesothelial cells, respectively. Indeed, an analogous fibrin-mediated bridging model probably accounts for leukocyte adhesion to endothelial cells.29,30 However, as neutrophils also express high levels of CD11b/CD18, but are not depleted from the peritoneal exudates upon macrophage activation62 64 (not shown), we consider a simple bridging model to be an unlikely explanation for thrombin/fibrin(ogen)-stimulated macrophage adhesion.
Alternatively, fibrin could stimulate macrophage adhesion by activating mesothelial cell expression of ligands for macrophage adhesion molecules. Numerous recent studies have established that fibrin(ogen) can activate expression of molecules by endothelial cells, fibroblasts, and leukocytes.39-45 However, if fibrin stimulates mesothelial cell expression of adhesion-promoting ligands, those ligands would need to be macrophage-specific, since neutrophils were not depleted during our assays.
We favor a third model, in which fibrin directly binds and cross-links receptors on macrophages, thereby transmitting signals that stimulate adhesion. Indeed, peritoneal macrophages harvested shortly after stimulation are coated with fibrin(ogen),67 and fibrin(ogen) can directly stimulate macrophage secretion of cytokines and chemokines in vitro.40 45 Here, we demonstrated that injection of thrombin activates fibrin(ogen)-dependent cytokine/chemokine secretion in vivo. Thus, we believe that thrombin-stimulated fibrin formation directly stimulates peritoneal macrophages, prompting adhesion and secretion of inflammatory mediators. Although we cannot exclude contributions by other cell types, our preliminary data strongly suggest that macrophages are the source of cytokine/chemokine production in our model, as plastic adherent peritoneal cells harvested shortly after the injection of thrombin secreted elevated levels of IL-6 and MCP-1 in vitro without any further stimulation (not shown).
Notably, our studies cannot distinguish between activities of fibrin(ogen) and those of fibrin(ogen)-degradation products (FDPs). FDPs reportedly stimulate vascular permeability,82endothelial cell retraction,83,84 monocyte/macrophage IL-1 and IL-6 production,85,86 and leukocyte chemotaxis.87-89 FDPs can be generated via plasmin-mediated proteolysis of fibrin, and mice with reduced or no plasmin have been generated by gene-targeted deletion of plasminogen activators or plasminogen, respectively.90,91 Such plasmin-deficient mice display increased pathology in glomerulonephritis92 and arthritis51 93 models, suggesting that plasmin-generated FDPs may well function in inflammation.
Regardless of the precise mechanism, our data are relevant to a number of human pathologies. Extravascular coagulation accompanies many Th1-associated diseases, including autoimmune neuropathologies,1-4 glomerulonephritis,5,6rheumatoid arthritis,7-9 Crohn's disease,10,11 and allograft rejection.12 13As our studies indicate that thrombin and fibrin(ogen) function to stimulate cytokine/chemokine production and macrophage adhesion in vivo, extravascular coagulation likely exacerbates Th1-associated chronic inflammation. Thus, treatment modalities that specifically block inflammation-associated thrombin formation, fibrin deposition, and/or fibrin degradation may constitute novel approaches for controlling pathologic Th1 responses associated with autoimmunity and transplantation. They may likewise provide novel means to attenuate acute inflammation resulting from trauma, burns, or infections.
Our finding that thrombin/fibrin(ogen) can regulate cytokine/chemokine production and macrophage adhesion may also be relevant to septic shock. Bacterial endotoxins prompt expression of procoagulant activities,65,66 and recent studies indicate that therapeutic administration of physiologic vascular anticoagulants reduces septic mortality,94-97 suggesting that procoagulants and/or their products (eg, fibrin, FDPs) play pathologic roles in sepsis. Future studies will be required to clarify roles for thrombin/fibrin(ogen)-stimulated cytokine/chemokine production and/or macrophage adhesion during septic shock.
Increased vascular permeability leading to plasma exudation is among the earliest signs of inflammation. As plasma contains coagulant precursors that become activated upon exposure to extravascular cells, inflammation prompts extravascular thrombin and fibrin formation. Accumulating evidence suggests that this extravascular coagulation has pleiotropic immune and inflammatory functions. Thrombin clearly plays multiple roles, evidenced by the diminished inflammatory responses of both PAR-1– and fibrinogen-deficient mice. Notably, PAR-3 and PAR-4 are also activated by thrombin,98 though we have yet to assess inflammatory functions of those thrombin receptors. Our data suggest that extravascular fibrin also has multiple inflammatory roles, including the regulation of macrophage adhesion and the stimulation of cytokine/chemokine production. Given these pleiotropic activities, we hypothesize that extravascular coagulation may function as nature's adjuvant, both signaling “danger” at sites of inflammation and providing a provisional fibrin matrix that spatially localizes the ensuing host response.
We are indebted to the employees of the Trudeau Institute Animal Breeding and Experimental Animal Maintenance Facilities for dedicated care of the mice used for these studies. We also wish to thank Gail Huston for sharing expertise with the OT-II model, Jean Brennan for assistance with differential cell analyses, and Sachin Mani for technical assistance.
Supported by funds from Trudeau Institute.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Stephen T. Smiley, Trudeau Institute, 100 Algonquin Ave, Saranac Lake, NY 12983; e-mail:ssmiley@trudeauinstitute.org.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal