Abstract
Red blood cells (RBCs) parasitized by Plasmodium falciparum are rigid and poorly deformable and show abnormal circulatory behavior. During parasite development, knob-associated histidine-rich protein (KAHRP) and P falciparum erythrocyte membrane protein 3 (PfEMP3) are exported from the parasite and interact with the RBC membrane skeleton. Using micropipette aspiration, the membrane shear elastic modulus of RBCs infected with transgenic parasites (with kahrp or pfemp3 genes deleted) was measured to determine the contribution of these proteins to the increased rigidity of parasitized RBCs (PRBCs). In the absence of either protein, the level of membrane rigidification was significantly less than that caused by the normal parental parasite clone. KAHRP had a significantly greater effect on rigidification than PfEMP3, contributing approximately 51% of the overall increase that occurs in PRBCs compared to 15% for PfEMP3. This study provides the first quantitative information on the contribution of specific parasite proteins to altered mechanical properties of PRBCs.
Introduction
Malaria caused by Plasmodium falciparumremains the most serious and widespread parasitic disease of humans. Clinical symptoms of malaria occur during the asexual stage of the parasite's life cycle, when it multiplies within red blood cells (RBCs). The extreme virulence of P falciparum and the occurrence of severe, often fatal clinical complications is related to the ability of RBCs parasitized by mature forms of the parasite to accumulate in the microvasculature of a variety of organs.1 This abnormal circulatory behavior for RBCs appears to be directly related to parasite-induced alteration of its mechanical and adhesive properties.
During the last 2 decades, the altered adhesive properties of parasitized RBCs (PRBCs) have been studied intensively (see2,3 for recent reviews). In contrast, alterations of their mechanical properties, and the molecular mechanisms underpinning these changes, have been relatively ignored. Previous studies have clearly demonstrated that the deformability of intact PRBCs is profoundly reduced.4-6 The overall increase in red cell rigidity is due, in part, to the presence of the large, nondeformable intracellular parasite and to a number of stage-specific parasite-encoded proteins that associate with the RBC membrane skeleton.3,5,6Paulitschke and Nash6 used micropipette aspiration to measure the rigidity of RBCs parasitized by a number of unrelated parasite lines of knobby and knobless phenotypes. In general, the membranes of knobby PRBCs were more rigid than those lacking knobs; however, there was considerable variation in rigidity, particularly between knobby lines, with some knobby PRBCs only slightly more rigid than others infected with knobless parasite lines. Unfortunately, in their study, there was no characterization of the parasite genotype or immunohistochemical analysis of the PRBCs to determine precisely which parasite proteins were or were not expressed in different parasite lines. As such, though increased membrane rigidity is likely to result from the combined effect of a number of parasite proteins interacting with the RBC membrane, the lack of a transfection system for P falciparum to knock out individual genes has until now prevented assessment of the contribution of individual proteins to membrane rigidification. Although still in its infancy, a method to create single gene knockouts in P falciparum is now available, and 2 well-characterized transgenic parasite clones are available for study with specific deletions of the genes for 2 membrane skeleton–associated proteins, the knob-associated histidine rich protein (KAHRP)7 and P falciparum erythrocyte membrane protein 3 (PfEMP3).8 In mature parasites, both proteins are exported to the membrane skeleton of infected RBCs. KAHRP binds to spectrin and actin,9 but the interaction of PfEMP3 with the membrane skeleton is less well understood. Nonionic detergent solubility experiments, however, strongly support a noncovalent linkage of PfEMP3 to the RBC membrane skeleton.10 11
In the current study, we have made use of these 2 transgenic parasite lines in which the genes for KAHRP and PfEMP3 have been deliberately disrupted to determine, for the first time, the precise contribution of single, specific parasite proteins to the increased rigidity of the PRBC membrane. We demonstrate that both KAHRP and PfEMP3 contribute to the increased rigidity of PRBCs and that the contribution of KAHRP is significantly greater. Our study provides the first quantitative information on the contribution of specific parasite proteins to the altered mechanical properties of PRBCs and increases our understanding of parasite proteins that strongly influence the virulence of P falciparum.
Materials and methods
Parasitized red blood cells
Transgenic clones of P falciparum KKO (kahrp gene knockout) and EMP3KO (pfemp3 gene knockout) were generated from the well-characterized parasite clone 3D7 by transfection, as previously described.7,8 Parasites were maintained in continuous in vitro culture in human RBCs using standard procedures12 in HEPES-buffered RPMI 1640 containing AlbumaxII (Gibco BRL, Grand Island, NY).13 For the transgenic clones, culture medium was further supplemented with 0.1 μM pyrimethamine (Sigma Chemical, St Louis, MO).7 Cultures of knob-expressing parasite clones (3D7 and EMP3KO) were subjected to weekly flotation in gelatin to maintain synchrony and expression of knobs, as recently described.14 Synchronous cultures of the knobless clone (KKO) were maintained by weekly selection for ring-stage parasites using sorbitol, as previously described.15 Cultures were used for experiments when most (more than 90%) of the parasites were mature, pigmented trophozoites. Lack of expression of KAHRP or PfEMP3 in the transgenic clones was confirmed by immunofluorescence using specific antisera and the presence or absence of membrane knobs determined by transmission electron microscopy.7
Determination of membrane shear elastic modulus
The shear elastic modulus of the RBC membrane was determined by micropipette aspiration, as previously described.5,6,16Briefly, cultured PRBCs were diluted with culture medium further supplemented with 2% (wt/vol) bovine serum albumin to approximately 1 × 106 RBC/mL and introduced into a chamber (approximately 2 mm deep) located on the stage of an inverted light microscope (Leica DMIRB). Precision glass micropipettes (internal diameter, 1.1-1.4 μm) with a long (approximately 8 mm) parallel taper were fabricated, filled with phosphate-buffered saline, and connected to a hydrostatic pressure system with a resolution of 0.01 mm H2O. Pipettes were mounted on a hydraulic micromanipulator (Narishige, Japan), maneuvered into the open side of the chamber and visualized using a ×63, high numerical aperture (0.7) objective lens. Images were viewed and analyzed on a high-resolution monitor using customized digital image capture and analysis software (Total Turnkey Solutions, Coburg, Victoria, Australia) (Figure1). Under these conditions, malaria parasites could easily be visualized within RBCs. To minimize potentially confounding factors such as abnormal cell morphology, reduced availability of free membrane, and large, rigid parasites in PRBCs that could affect comparisons of the elastic modulus between uninfected RBCs and PRBCs or between different parasite clones, only PRBCs that remained approximately biconcave-discoid and contained a single mature parasite that consumed no more than one third of the RBC volume were selected for analysis. Membranes of individual RBCs were aspirated progressively into the pipette using increasingly negative suction pressures. The membrane shear elastic modulus was determined by measuring the length of a membrane tongue (L) aspirated from the RBC into the pipette for a range of aspiration pressures (P) and calculated from the linear regression of dL/dP as previously described.17 The range of aspiration pressures was 1.0 to 5.0 mm H2O for normal and uninfected RBCs or 1.0 to 10.0 mm H2O for PRBCs. Membrane tongue lengths varied from 0.53 to 2.83 μm for normal/ uninfected RBCs and from 0.40 to 2.65 μm for PRBCs. Calculation and comparison of shear elastic moduli was valid given that over these ranges, normal/uninfected RBCs and PRBCs behaved as if elastic with linear extension. Linear regression of dL/dP for all cells tested gave comparable mean Pearson correlation coefficients of 0.95 for normal/uninfected RBCs and 0.96 for PRBCs. All measurements were performed at room temperature (20°C-25°C).
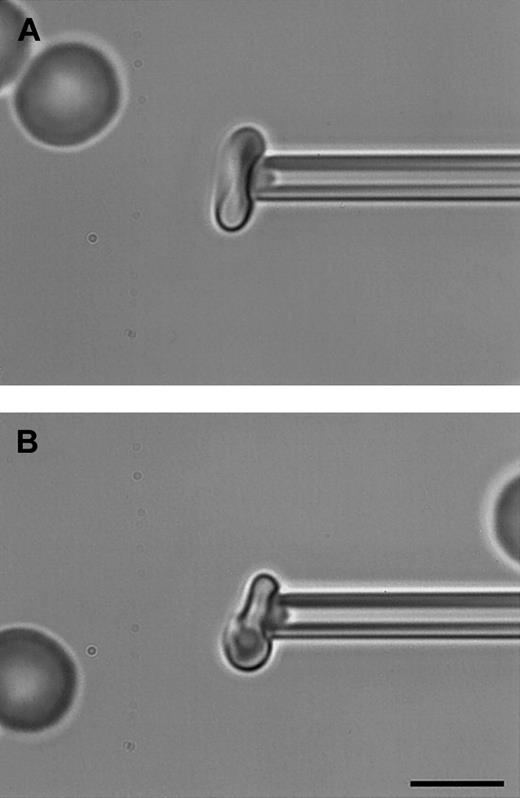
Determination of membrane shear elastic modulus by micropipette aspiration.
Bright-field digitally captured images of a typical micropipette (1.3 μm internal diameter) used for determination of the shear elastic modulus of (A) an uninfected RBC and (B) an RBC infected with a mature malaria (P falciparum) parasite as described in “Materials and methods.” A portion of the RBC membrane aspirated into the pipette can be visualized clearly, shown here at a pressure of 2.0 mm H2O (A) and 4.0 mm H2O (B). Note that in panel B, only PRBCs that remained approximately discoid and that contained relatively small but mature trophozoites were measured so that there was sufficient free membrane to aspirate away from the parasite itself. Scale bar, 5 μm.
Determination of membrane shear elastic modulus by micropipette aspiration.
Bright-field digitally captured images of a typical micropipette (1.3 μm internal diameter) used for determination of the shear elastic modulus of (A) an uninfected RBC and (B) an RBC infected with a mature malaria (P falciparum) parasite as described in “Materials and methods.” A portion of the RBC membrane aspirated into the pipette can be visualized clearly, shown here at a pressure of 2.0 mm H2O (A) and 4.0 mm H2O (B). Note that in panel B, only PRBCs that remained approximately discoid and that contained relatively small but mature trophozoites were measured so that there was sufficient free membrane to aspirate away from the parasite itself. Scale bar, 5 μm.
Results
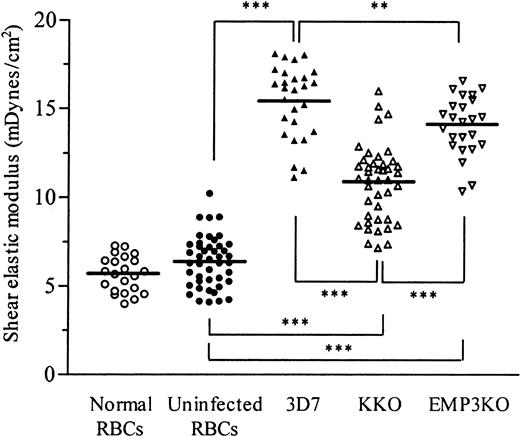
For all parasite clones, the presence of mature pigmented trophozoites within RBCs was associated with marked rigidification of the RBC membrane and with the parental clone, 3D7 showing, on average, a 137% increase in elastic modulus when compared with uninfected RBCs (Figure 2). Interstrain variability, particularly between knobby parasite lines, has been observed previously, with increases in membrane rigidity ranging from 90% to 300% for knobby strains when compared with nonparasitized RBCs.6 The increase in elastic modulus caused by parasite clone 3D7 tested in this study appears to lie at the lower end of this range. Although all cultures were stage synchronized to minimize effects on the elastic modulus caused by differences in parasite age, there is still considerable variation in the moduli between different PRBCs. The magnitude of the variance of the data, however, is similar for the different parasite lines tested and for uninfected RBCs, suggesting that this variation is unlikely to be a major confounding factor influencing comparisons of elastic modulus between the different parasite clones.
Effect of KAHRP and PfEMP3 on the membrane shear elastic modulus of red blood cells infected by mature stages of
P falciparum. Membrane shear elastic modulus of normal, uninfected, and parasitized RBCs was measured by micropipette aspiration in 5 (for normal RBCs) to 8 (for uninfected RBCs and PRBCs) separate experiments as described in “Materials and methods.” Different pipettes were used throughout the study, but in each experiment the same pipette was used to measure uninfected RBCs and PRBCs. Normal RBCs were from freshly drawn human venous blood and were suspended in fresh parasite culture medium supplemented with 2% bovine serum albumin. Uninfected RBCs were nonparasitized RBCs from each of the 3 different parasite cultures. Each point represents the shear elastic modulus for an individual RBC. Solid horizontal bars represent the mean of all data in each group. Significant differences between pairs of parasite clones are shown as **(P < .01) and ***(P < .001) by the Mann-Whitney Utest.
Effect of KAHRP and PfEMP3 on the membrane shear elastic modulus of red blood cells infected by mature stages of
P falciparum. Membrane shear elastic modulus of normal, uninfected, and parasitized RBCs was measured by micropipette aspiration in 5 (for normal RBCs) to 8 (for uninfected RBCs and PRBCs) separate experiments as described in “Materials and methods.” Different pipettes were used throughout the study, but in each experiment the same pipette was used to measure uninfected RBCs and PRBCs. Normal RBCs were from freshly drawn human venous blood and were suspended in fresh parasite culture medium supplemented with 2% bovine serum albumin. Uninfected RBCs were nonparasitized RBCs from each of the 3 different parasite cultures. Each point represents the shear elastic modulus for an individual RBC. Solid horizontal bars represent the mean of all data in each group. Significant differences between pairs of parasite clones are shown as **(P < .01) and ***(P < .001) by the Mann-Whitney Utest.
Membranes of RBCs parasitized by knobby clones (3D7 or EMP3KO) were significantly more rigid than those infected with the knoblesskahrp knockout clone (P < .001) (Figure 2). In the absence of KAHRP (and membrane knobs), the increase in membrane rigidity of PRBCs over uninfected RBCs was approximately halved compared with the increase that occurred in RBCs parasitized by the knobby parental parasite clone. Similarly, loss of PfEMP3 (without loss of KAHRP or knobs) also reduced the maximum level of membrane rigidification, though its contribution was far less pronounced than that caused by KAHRP (Figure 2).
Discussion
A number of reasons might account for the difference in the level of rigidification caused by these 2 proteins. One is that there is indirect evidence, based on the relative abundance of mRNA, that KAHRP may be more abundant in PRBCs than PfEMP3.18 Such estimates, however, are complicated by the repetitive nature of the coding sequences of these 2 genes, preventing simple conclusions being drawn based on antibody reactivity. Another is that, unlike KAHRP, PfEMP3 does not cluster in high density at knobs but assumes a more even distribution around the membrane skeleton,11 possibly influencing the magnitude of its effect. At present, we cannot rule out the possibility that the effect of loss of either of these proteins on membrane rigidity may occur as an indirect consequence of loss or altered level of expression of some other, perhaps currently unidentified, protein at the membrane skeleton.
In normal RBCs, tetramers of spectrin, actin, proteins 4.1 and 4.2, ankyrin, and adducin interact to form a complex, ordered network that underlies the RBC lipid membrane. The membrane and cytoskeleton are connected to each other through interactions between ankyrin and the RBC anion transporter band 3 and protein 4.1 with integral membrane sialoglycoproteins, predominantly glycophorin A.19,20Maintenance of the physiological deformability of normal RBCs is highly dependent on the preservation of this well-defined architecture of the membrane skeleton and a highly elastic membrane.21Abnormal molecular interactions that result in cross-linking of cytoskeletal proteins can markedly increase the rigidity of RBCs, as evidenced by the reduced deformability of RBCs treated with monoclonal antibodies to glycophorin A that spans the RBC membrane and interacts with spectrin through protein 4.1.22 Although the precise mechanisms leading to decreased deformability of PRBCs are poorly understood, the contribution of parasite proteins to increased membrane rigidity is most likely attributed to their direct or indirect interactions with proteins of the RBC membrane skeleton. KAHRP is known to associate with spectrin, actin, and ankyrin in the RBC skeleton9,23,24 and may cross-link spectrin, resulting in increased membrane rigidity. Treatment of RBCs with oxidative agents, known to cause protein–protein cross-linking, markedly decreases RBC deformability because of the formation of oxidative cross-links between individual spectrin tetramers and between spectrin and hemoglobin.23 25-27 Such cross-linking of spectrin tetramers within the skeletal network could limit the extent of extension and folding of spectrin during cell deformation and subsequently increase membrane rigidity.
The net effect of the various malaria proteins at the membrane skeleton of PRBCs appears to effectively increase the level of cross-linking of spectrin by the formation of many protein–protein interactions, resulting in a more robust RBC that is more resistant to destruction during parasite development. In addition to binding to the RBC cytoskeleton by a direct interaction with spectrin, and possibly actin,28-30 KAHRP also anchors the parasites' exported cytoadherence ligand, PfEMP1, to the RBC membrane through an interaction of KAHRP with the cytoplasmic tail of PfEMP1.28 In this way, interactions of KAHRP with spectrin and PfEMP1, which spans the RBC membrane, would cause additional increases in the rigidity of the membrane skeleton. Further study of parasite lines that do not express PfEMP1 would be useful to address the contribution of KAHRP–PfEMP1 associations to increased PRBC rigidity.
Finally, there is evidence to suggest that mature parasites release exo-antigens that may increase the rigidity of uninfected RBCs.5,31 32 Here, we have quantified and compared the elastic moduli for normal, noncultured RBCs and uninfected RBCs cultured in the presence of malaria parasites. In contrast to previous studies, we found no significant increases in the rigidity of uninfected RBCs (Figure 2).
In conclusion, our study provides the first critical assessment of the contribution and importance of individual parasite proteins to the altered mechanical properties of PRBCs. The results add significantly to our understanding of proteins that contribute to the extreme virulence of falciparum malaria and, in the broader sense, increase our knowledge of cytoskeletal interactions that maintain and regulate cellular mechanical properties.
We thank Professor Gerard Nash (The University of Birmingham, United Kingdom) for expert advice and assistance.
Supported by the National Health and Medical Research Council of Australia (NHMRC), The Wellcome Trust, and the National Institutes of Health (NIH) (DK32094-10).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Brian M. Cooke, Dept of Microbiology, PO Box 53, Monash University, Clayton, Victoria 3800, Australia; e-mail:brian.cooke@med.monash.edu.au.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal