Abstract
The behavior of the 2 sialidase forms present in the erythrocyte membrane was investigated in 117 subjects with type 2 diabetes mellitus versus 95 healthy controls. A significant increase of the acidic form of sialidase, which is anchored to the membrane by a glycosylphosphatidylinositol bridge, was observed in erythrocyte resealed membranes. On the contrary, the neutral form of the enzyme, the only one capable of removing lipid- and protein-bound sialic acid from endogenous and exogenous sialoderivatives, was significantly reduced with a consequent increase of erythrocyte membrane total sialic acid content. Disease duration, therapy, glycemia, parameters of metabolic control, and presence of complications, except nephropathies, had no influence on the tested enzyme activities. Diabetic subjects showed a different erythrocyte age distribution, with an almost double proportion of young red cells and only one quarter of senescent ones compared with controls. In young erythrocytes, diabetic and control subjects had the same distribution of the 2 enzymes, while in senescent cells the acidic enzyme was increased 3.5-fold and the neutral form was reduced by half in the diabetic subjects. The increase of both acidic sialidase and total membrane-bound sialic acid, together with an overpresence of young red cells in diabetics, suggests that in this pathological condition there might be an altered aging process with a diminished expression of the neutral form of the enzyme and an increase of bound sialic acid. It has been suggested that the expression of the neutral enzyme requires some activation mechanism that is impaired in diabetes.
Introduction
Alterations in the sialic acid content of different tissues in patients with both type 1 and 2 of diabetes mellitus have been reported.1-3 However, while an increase in the sialic acid content of plasma has been consistently observed,4-6conflicting results have been obtained with regard to different tissues from diabetic patients: most investigators have observed a decrease,1,7,8 while others have observed an increase9 or no difference at all.10 As to the erythrocyte membrane, where sialic acid plays an important role in the maintenance of red cell structure, permeability, integrity, viability, and survival in circulating blood11-13 and where it is physiologically decreased during aging, the effect of the diabetic condition is yet unclear.14
To date, little information is available on the possible involvement of sialidase in diabetic disease.1,15-17 This enzyme is present in the erythrocytes, as opposed to other cell types, only at the plasma membrane level together with different sialoderivatives. Recent studies have shown that the erythrocyte plasma membrane is characterized by the presence of 2 different forms of sialidase having optimal pH in the acidic and neutral range, respectively, and also differing both in terms of membrane anchoring modalities and interaction with endogenous substrates.18 19
Because of recent reports on the involvement of several erythrocyte membrane glycohydrolases in the diabetic pathological process,20 and in an attempt to contribute to the understanding of this very important and controversial issue, we performed several experiments in subjects with type 2 diabetes with the following objectives: (a) to detect a possible relationship between erythrocyte membrane sialic acid content and the variation in activity of the 2 sialidase forms; (b) to verify the possible impact of metabolic control, disease duration, therapy, and complications on the sialic acid–sialidase system; (c)given the important role of sialic acid in erythrocyte aging, to investigate if mean erythrocyte age is modified by the diabetic condition and, secondarily, to verify in both healthy and diabetic subjects the content of the 2 sialidases in erythrocytes of different ages; and (d) considering the important biophysical and biochemical modifications occurring in the erythrocyte membrane in diabetes,21-23 to evaluate possible changes affecting the 2 sialidase forms of the erythrocyte membrane.
Patients, materials, and methods
Materials
Commercial chemicals were of the highest grade available. Recombinant phosphatidylinositol-specific phospholipase C (PIPLC) fromBacillus thuringiensis (2000 U/mg) was purchased from Oxford Glyco System (Abingdon, United Kingdom); 4-methylumbelliferone (MU), 4-methylumbelliferyl α-N-acetyl-D-neuraminic acid (MU-NeuAc), N-acetyl-D-neuraminic acid (NeuAc), creatine hydrate, crystalline bovine serum albumin (BSA), N-2-hydroxyethylpiperazine-N-2-ethane sulfonic acid (HEPES), and Percoll were from Sigma Chemical (St Louis, MO). Dowex 2X8 resin (200 to 400 mesh) was obtained from Bio-Rad Laboratories (Richmond, VA). Water doubly distilled in a glass apparatus was used to prepare the different solutions.
Subjects
A total of 117 type 2 diabetic patients were selected from the diabetes outpatient clinic of the Bassini Hospital of Cinisello Balsamo (Milan, Italy) and from the Department of Diabetology, Italian National Research Center on Aging (Ancona, Italy). The disease was diagnosed according to World Health Organization criteria.24 Some patients with good metabolic control (glycated hemoglobin [HbA1c] ≤ 7.0%) had received no pharmacologic treatment according to the American Diabetes Association guidelines for diabetes treatment 25; others had been treated with oral hypoglycemic drugs and/or insulin. All patients were on an appropriate diet. Thyroid, renal, and liver function tests were normal. Diabetic complications were evaluated through medical history, clinical examination, fundoscopy, electrocardiogram, large-vessel Doppler ultrasonography, proteinuria and microalbuminuria, electromyography, and tests for the detection of autonomic neuropathy. Supine blood pressure was recorded and hypertension defined as systolic blood pressure above 160 mm Hg and/or diastolic blood pressure above 90 mm Hg and/or if patients were on antihypertensive treatment. Height and weight were measured and body mass index calculated. Ninety-five controls were selected from apparently healthy adult subjects who had entered the Laboratory of Clinical Chemistry and Microbiology of the Bassini Hospital for scheduled hematochemical follow-up evaluations. None of these subjects had signs of metabolic, endocrinologic, or hemolytic diseases; hemoglobinopathies; major organ diseases; or a positive family history for diabetes. Although appropriate for comparison with diabetic subjects, the control group was significantly different in terms of mean age. All the subjects gave their informed consent for blood drawing. The main characteristics of the study population are shown in Table 1.
Main characteristics of the subjects considered in the study
| . | No. of subjects . | Age, y . | Duration of disease, y . | Body mass index, kg/m2 . | Fasting blood glucose, mM/L . | HbA1c, % . | Fructosamine, μM/100 mL . |
|---|---|---|---|---|---|---|---|
| Controls | 95 | 49 ± 13 | 26.12 ± 0.05 | 5.0 ± 0.6 | 4.5 ± 0.9 | 215 ± 30 | |
| Total diabetic patients | 117 | 63 ± 9 | 11.7 ± 8.2 | 30.8 ± 5.6 | 9.9 ± 3.3 | 7.6 ± 1.5 | 340 ± 60 |
| Diabetics with complications | 60 | 63 ± 7 | 14.1 ± 7.6 | 31.1 ± 5.4 | 10.9 ± 3.7 | 7.9 ± 1.6 | 345 ± 65 |
| Diabetics without complications | 57 | 62 ± 8 | 9.8 ± 6.8 | 30.2 ± 6.0 | 8.7 ± 1.3 | 7.1 ± 1.1 | 325 ± 55 |
| Untreated diabetics | 14 | 62 ± 7 | 5.2 ± 4.1 | 28.8 ± 4.0 | 7.8 ± 1.1 | 6.0 ± 0.4 | 274 ± 29 |
| Diabetics treated with oral hypoglycemic agents only | 68 | 62 ± 8 | 10.8 ± 7.5 | 31.3 ± 6.2 | 9.7 ± 2.9 | 7.2 ± 1.6 | 329 ± 44 |
| Diabetics treated with oral hypoglycemic agents and insulin | 35 | 64 ± 6 | 17.3 ± 7.1 | 29.7 ± 3.9 | 10.8 ± 3.9 | 8.4 ± 1.4 | 389 ± 83 |
| . | No. of subjects . | Age, y . | Duration of disease, y . | Body mass index, kg/m2 . | Fasting blood glucose, mM/L . | HbA1c, % . | Fructosamine, μM/100 mL . |
|---|---|---|---|---|---|---|---|
| Controls | 95 | 49 ± 13 | 26.12 ± 0.05 | 5.0 ± 0.6 | 4.5 ± 0.9 | 215 ± 30 | |
| Total diabetic patients | 117 | 63 ± 9 | 11.7 ± 8.2 | 30.8 ± 5.6 | 9.9 ± 3.3 | 7.6 ± 1.5 | 340 ± 60 |
| Diabetics with complications | 60 | 63 ± 7 | 14.1 ± 7.6 | 31.1 ± 5.4 | 10.9 ± 3.7 | 7.9 ± 1.6 | 345 ± 65 |
| Diabetics without complications | 57 | 62 ± 8 | 9.8 ± 6.8 | 30.2 ± 6.0 | 8.7 ± 1.3 | 7.1 ± 1.1 | 325 ± 55 |
| Untreated diabetics | 14 | 62 ± 7 | 5.2 ± 4.1 | 28.8 ± 4.0 | 7.8 ± 1.1 | 6.0 ± 0.4 | 274 ± 29 |
| Diabetics treated with oral hypoglycemic agents only | 68 | 62 ± 8 | 10.8 ± 7.5 | 31.3 ± 6.2 | 9.7 ± 2.9 | 7.2 ± 1.6 | 329 ± 44 |
| Diabetics treated with oral hypoglycemic agents and insulin | 35 | 64 ± 6 | 17.3 ± 7.1 | 29.7 ± 3.9 | 10.8 ± 3.9 | 8.4 ± 1.4 | 389 ± 83 |
Values are expressed as mean ± SD.
HbA1c reference interval: 4.0% to 5.8%; fructosamine reference interval: 100 to 278 μM/100 mL.
Blood collection and red cell separation
Blood samples were obtained by vein puncture from both healthy adult donors and diabetic patients and processed immediately. Heparin was used as anticoagulant. Erythrocytes from controls and diabetic patients were separated from leukocytes and platelets by filtration through a column of α-cellulose and microcrystalline cellulose (2:1 by weight) according to the method of Beutler26 in HEPES-buffered isotonic saline: 133 mM NaCl, 4.5 mM KCl, 10 mM HEPES, pH 7.4. The hematocrit of the filtered blood was adjusted to approximately 30% and the suspensions stored at 4°C until used. Cell counts were performed in a Burker chamber (PBI International, Milan, Italy).
Preparation of discontinuous Percoll gradients
Human erythrocytes of different biological age, from both controls and diabetic patients, were separated as described by Salvo et al27 and following the modifications suggested by Mosca et al.28 Briefly, the following solutions were prepared at 26°C: HEPES-buffered stock solution (HBS stock): 2.66 M NaCl, 0.09 M KCl, 0.2 M HEPES, pH 7.4; solution A (BSA-HEPES-buffered solution, pH 7.4): 19 volumes of 5.25% (w/v) BSA in water solution added to 1 volume of HBS-stock solution; solution B (BSA-Percoll-HEPES-buffered solution, pH 7.4): 19 volumes of 5.25% (w/v) BSA in Percoll added to 1 volume of HBS-stock solution. Solutions A and B were filtered through 0.45 μm cellulose acetate filters and mixed to form 5 solutions at final “Percoll” concentrations of 60%, 66%, 70%, 74%, and 80% (vol/vol; density 1.087-1.098 g/mL, pH 7.4). Discontinuous 5-step gradients were prepared by overlayering 1.0 mL of 80%, 8.0 mL of 74%, 8.0 mL of 70%, 8.0 mL of 66%, and 4.0 mL of 60% of Percoll concentration using a peristalting pump in 36 mL tubes. The same discontinuous Percoll gradient was successfully applied for diabetic patient erythrocyte separation.
Fractionation of erythrocytes according to biological age
This study was performed on 10 healthy subjects (mean age 46 ± 9 years) and 10 diabetic patients (mean age 47 ± 13 years; fasting blood glucose 10.11 ± 1.1 mM/L; HbA1c7.9% ± 2.6%) who were part of the study population. Four tubes containing 4.0 mL each of filtered blood (hematocrit 30%, corresponding to 20 × 109-24 × 109 cells per tube) in 10 mM HEPES buffer, pH 7.4, containing 133 mM NaCl, 4.5 mM KCl, were layered on the discontinuous gradient. In parallel, the fractionation was performed using a buffer with high content of KCl (133 mM) and low content of NaCl (4.5 mM), according to the indications of Sorette.29 Centrifugation was carried out in a swing-out rotor at 2000g for 15 minutes at 20°C. The centrifuge was slowly decelerated over 3 minutes to prevent gradient disturbance. The plasma (about 1.5 mL) remaining above the gradient was removed, and all cell fractions were collected manually over the liquid interface by aspiration from the top of the gradient using a Pasteur pipette attached to a peristalting pump (about 26 fractions, usually 1.2 mL for tube). The following age-dependent red cell parameters were characterized in each fraction: mean cellular volume (MCV), mean cellular hemoglobin (MCH), and mean cellular hemoglobin concentration (MCHC), determined with a Cell Counter (Coulter STKR, Coulter Scientifics, Milan, Italy); HbA1c, determined by immunoenzymatic assay (Roche, Basel, Switzerland); and red cell creatine, measured by the method described by Griffiths et al30 using recrystallized creatine as standard. Manual reticulocyte counts were made according to Brecher.31 The fractions containing the young cells concentrated in the upper layer (over 60%), the average age cells layered over 66% and 70%, and the fractions containing the senescent cells layered over 74% were pooled and washed 3 times with HEPES-buffered isotonic saline at 4°C to remove Percoll.
Preparation of unsealed and resealed membranes from different erythrocyte populations
Unsealed ghost membranes obtained from young, average aged, and old control and diabetic erythrocytes were prepared at 4°C according to Steck et al,32 and resealed ghosts were obtained according to Venerando et al.19 Assessment of the resealed ghosts and verification of efficacy of the treatment was performed by determining the activity of acetylcholinesterase (Ach-esterase)33 and NADH-cytocrome c oxidoreductase (NADH-cyt C ox/red).32
Release of sialidase from resealed membranes, obtained from control and diabetic erythrocytes, by treatment with PIPLC
Resealed membranes obtained from controls and diabetic erythrocytes were treated with PIPLC according to Venerando et al19 using 2.5 mg protein. Preliminary experiments on resealed membranes obtained from diabetic erythrocytes have confirmed that the methodology described above can be also employed for this purpose.
Total sialic acid content in resealed membranes obtained from control and diabetic erythrocytes
The total sialic acid content of resealed membranes obtained from control and diabetic erythrocytes was determined by the chromatographic micro procedure of Caimi et al34 on samples digested for 90 minutes in 0.1 N sulfuric acid at 80°C, from which insoluble material was removed by centrifugation. The total ganglioside-bound and protein-bound NeuAc present in young and senescent resealed membranes was determined according to Tettamanti et al.35 Ganglioside-bound NeuAc was determined according to Svennerholm36 after exhaustive dialysis of the sample against distilled water (3 changes a day for at least 3 days). Glycoprotein-bound NeuAc was determined by measuring free NeuAc, liberated after hydrolysis at 80°C in 0.1 N sulfuric acid for 90 minutes, by the chromatographic micro procedure of Caimi et al.34
Action of neutral sialidase on endogenous substrates
Resealed membranes obtained from controls and diabetic erythrocytes were incubated at 37°C for 18 hours in mixtures containing, in a final volume of 1 mL, of 50 mM/L citric acid–sodium phosphate buffer, pH 7.2, containing 0.15 M NaCl and 1 mg protein. At the end of the incubation, the mixtures were centrifuged at 28 000g for 20 minutes, and the supernatant was collected. The pellet was washed 3 times with 0.2 mL of 0.15 M NaCl. At the end, the supernatants were recentrifuged at 4°C at 150 000g for 10 minutes. The final supernatants and the final pellets were analyzed as described above. Blank mixtures containing protein inactivated at 100°C for 10 minutes were run concomitantly.
Enzyme assays
The assays of resealed membrane sialidases obtained from control and diabetic erythrocytes were routinely determined by fluorimetric methods.37 The assay mixtures, containing in a final volume of 0.1 mL, 50 mM citric acid–sodium phosphate buffer (at established optimal pHs), 0.15 M NaCl, 0.6 mM MU-NeuAc (optimum concentration), 10 to 60 μg protein of enzyme preparation, and 0.6 mg albumin, were incubated for up to 30 minutes at 37°C. The blank mixtures consisted of the incubation mixtures lacking the enzyme preparation. Enzyme activities were expressed as units (U), ie, the amount of enzyme that liberates 1 μM of product per minute at 37°C under optimal conditions.
Other methods
Protein content was determined by the method of Lowry et al38 using crystalline BSA as the standard; when HEPES buffer was present, the Coomassie brilliant blue Bradford method39 was used. Ach-esterase activity was determined by the method of Ellman et al,33 and NADH-cyt C ox/red was determined by the method reported by Steck and Kant.32Total phospholipids were extracted according to Ladisch and Gillard40 and determined as phosphorus by the method of Barlett.41 Fasting venous plasma glucose and fructosamine (as 1 deoxy-1-morpholino-D-fructose) were measured by colorimetric assay (Roche).
Statistical analysis
Tests of skewness and kurtosis showed significant differences from normal distribution, and nonparametric analysis techniques were used. The data were transformed into ranges, the means being compared by the Mann-Whitney and Wilcoxon tests. Multiple comparisons of the means were done by the Kruskal-Wallis test and, when necessary, adjusted with the Bonferroni correction; the correlations were done by the Spearman method. For all the analyses, the SPSS/PC package (SPSS, Chicago, IL) was used.42
Results
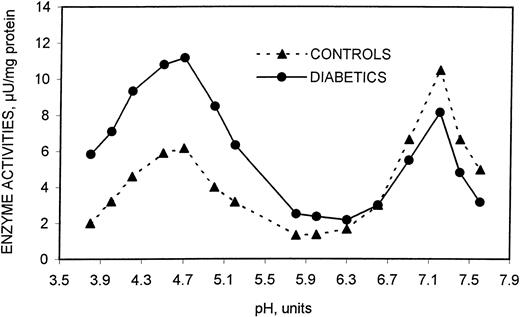
In a preliminary phase of this investigation using the methodology optimized by Venerando et al,19 we verified by the specific enzymatic markers—Ach-esterase and NADH-cyt C ox/red—that the resealed membranes from diabetics had the same conformational characteristics as those from healthy subjects (data not shown). The optimal conditions for the assay of both acidic and neutral sialidase forms as well as their main characteristics and kinetic properties were defined using these membranes. For the 2 enzyme forms, optimum pH (Figure 1) and Kmvalues were the same as those observed in previously investigated healthy subjects (25.5 × 10−6 and 3.05 × 10−6 for acidic and neutral sialidase, respectively).19 On the contrary, the apparentVmax values were markedly different in the 2 erythrocyte populations, the acidic form having higher values and the neutral form lower values in diabetic patient erythrocytes compared with healthy subjects.
Effect of pH on the sialidase activity contained in human erythrocyte resealed vesicles from healthy and diabetic subjects.
For details, see “Patients, materials, and methods.”
Effect of pH on the sialidase activity contained in human erythrocyte resealed vesicles from healthy and diabetic subjects.
For details, see “Patients, materials, and methods.”
Acid and neutral sialidase content in human erythrocytes from controls and diabetic patients
The subsequent study of 117 diabetic patients (Table2) showed that the acidic form of the enzyme is significantly increased when compared with controls (1.8-fold), as opposed to the neutral form, which is significantly decreased (1.3-fold). As a consequence, the total bound sialic acid is substantially increased in diabetic patients and, obviously, a significantly different acidic–neutral form ratio was also found (Table 2). No significant difference of acidic or neutral sialidase activity has been shown relative to the duration of the disease and the type of therapy administered. No correlation with glycemia and parameters of metabolic control either in the whole patient population or within selected subgroups (Table 1) was observed. The presence or absence of complications did not seem to influence the enzymatic activities, with the only exception being nephropathies, for which a significant correlation between the acidic–neutral sialidase ratio and the severity of the disease (R = 0.365,P < .006) was observed.
Sialidase activity and total sialic acid content in erythrocyte resealed vesicles from controls and type 2 diabetic patients
| . | Controls (95 subjects) . | Diabetic patients (117 subjects) . |
|---|---|---|
| Sialidase activity, μU/mg protein | ||
| Acidic sialidase | 6.2 ± 2.0 | 11.2 ± 3.7† |
| (2.7-11.7) | (3.3-27.3) | |
| Neutral sialidase | 10.5 ± 3.5 | 8.2 ± 4.0† |
| (3.0-23) | (1.7-24.8) | |
| Acidic–neutral sialidase ratio | 0.6 ± 0.2 | 1.5 ± 0.5† |
| (0.2-1.2) | (0.35-2.71) | |
| Total sialic acid content,* μg/mg protein | 48.9 ± 10.6 | 81.3 ± 19.3† |
| (30.7-69.1) | (44.9-13.4) | |
| . | Controls (95 subjects) . | Diabetic patients (117 subjects) . |
|---|---|---|
| Sialidase activity, μU/mg protein | ||
| Acidic sialidase | 6.2 ± 2.0 | 11.2 ± 3.7† |
| (2.7-11.7) | (3.3-27.3) | |
| Neutral sialidase | 10.5 ± 3.5 | 8.2 ± 4.0† |
| (3.0-23) | (1.7-24.8) | |
| Acidic–neutral sialidase ratio | 0.6 ± 0.2 | 1.5 ± 0.5† |
| (0.2-1.2) | (0.35-2.71) | |
| Total sialic acid content,* μg/mg protein | 48.9 ± 10.6 | 81.3 ± 19.3† |
| (30.7-69.1) | (44.9-13.4) | |
The data are expressed as mean ± SD. Ranges are reported in parentheses.
Total sialic acid includes lipid- and protein-bound content.
P < .001, diabetic patients versus controls.
Acidic sialidase release from erythrocyte resealed membranes of controls and diabetic patients after exhaustive treatment with PIPLC
Because the acidic sialidase is bound to the erythrocyte membrane through the glycosylphosphatidylinositol (GPI) anchor, which is sensitive to the hydrolytic action of the specific bacterial PIPLC,18 we studied the effect of the B thuringiensis PIPLC on the pools of resealed membranes obtained from healthy and diabetic subjects. The results are reported in Table3. Following the optimized assay conditions (see “Patients, materials, and methods”), we found a different behavior of the acidic sialidase in the 2 populations: in the controls the enzyme is completely released in the supernatant (88%) while in the diabetic patients the release occurs only partially (26%). From Table 3 it can also be seen that neither in the controls nor in diabetic subjects is the neutral form of the enzyme releasable.
Sialidase release from erythrocyte resealed membranes of controls and type 2 diabetic patients after exhaustive treatment with specific bacterial phospholipase C
| . | Controls . | Diabetic patients . | ||||
|---|---|---|---|---|---|---|
| Resealed membranes . | Supernatant . | Pellet . | Resealed membranes . | Supernatant . | Pellet . | |
| Total protein, mg | 2.5 | 0.30 ± 0.01 | 2.0 ± 0.1 | 2.5 | 0.50 ± 0.02 | 2.0 ± 0.1 |
| % | 100 | 12 | 80 | 100 | 19.5 | 79.5 |
| Acidic sialidase | ||||||
| Specific activity | 6.3 ± 2.1 | 46.2 ± 3.7 | ND | 11.7 ± 3.9 | 15.2 ± 1.7 | 8.5 ± 0.8 |
| Total activity | 15.8 ± 5.3 | 13.9 ± 1.1 | ND | 29.2 ± 9.3 | 7.6 ± 0.8 | 17.0 ± 1.7 |
| % | 100 | 88 | 100 | 26 | 58.2 | |
| Neutral sialidase | ||||||
| Specific activity | 9.8 ± 3.2 | ND | 12.2 ± 1.8 | 8.17 ± 3.90 | ND | 9.83 ± 2.02 |
| Total activity | 24.5 ± 8.6 | ND | 24.4 ± 3.7 | 20.4 ± 10.0 | ND | 19.7 ± 4.0 |
| % | 100 | 99.6 | 100 | 96.6 | ||
| . | Controls . | Diabetic patients . | ||||
|---|---|---|---|---|---|---|
| Resealed membranes . | Supernatant . | Pellet . | Resealed membranes . | Supernatant . | Pellet . | |
| Total protein, mg | 2.5 | 0.30 ± 0.01 | 2.0 ± 0.1 | 2.5 | 0.50 ± 0.02 | 2.0 ± 0.1 |
| % | 100 | 12 | 80 | 100 | 19.5 | 79.5 |
| Acidic sialidase | ||||||
| Specific activity | 6.3 ± 2.1 | 46.2 ± 3.7 | ND | 11.7 ± 3.9 | 15.2 ± 1.7 | 8.5 ± 0.8 |
| Total activity | 15.8 ± 5.3 | 13.9 ± 1.1 | ND | 29.2 ± 9.3 | 7.6 ± 0.8 | 17.0 ± 1.7 |
| % | 100 | 88 | 100 | 26 | 58.2 | |
| Neutral sialidase | ||||||
| Specific activity | 9.8 ± 3.2 | ND | 12.2 ± 1.8 | 8.17 ± 3.90 | ND | 9.83 ± 2.02 |
| Total activity | 24.5 ± 8.6 | ND | 24.4 ± 3.7 | 20.4 ± 10.0 | ND | 19.7 ± 4.0 |
| % | 100 | 99.6 | 100 | 96.6 | ||
Sialidase specific and total activities are expressed as μU/mg protein and μU/total protein, respectively.
The data are expressed as mean ± SD of 5 experiments.
ND indicates not detectable.
Sialic acid release from erythrocyte resealed membranes of controls and diabetic patients after neutral sialidase action on endogenous substrates
The neutral sialidase action on the sialoderivatives present at the level of the erythrocyte membrane itself has been also verified in both populations, and in Table 4 the variations of the sialic acid content are reported. The enzyme action is significantly diminished in the diabetic population in which the sialic acid release decreases as compared with controls (6.3% and 21.2% in diabetic and control subjects, respectively). The observed variation is independent from the chemical nature of the endogenous substrates.
Release of sialic acid from erythrocyte resealed membranes after neutral sialidase action on endogenous substrates
| . | Membrane-bound sialic acid before treatment . | Membrane-bound sialic acid after treatment . | Sialic acid released . | % of release . |
|---|---|---|---|---|
| Controls | ||||
| Total | 48.9 ± 10.6 | 38.5 ± 6.3 | 10.4 ± 0.8 | 21.2 |
| Protein-bound | 43.0 ± 1.8 | 34.7 ± 2.8 | 8.3 ± 0.8 | 19.3 |
| Lipid-bound | 3.2 ± 0.3 | 2.9 ± 0.1 | 2.0 ± 0.1 | 64.0 |
| Diabetics | ||||
| Total | 81.3 ± 19.3 | 76.2 ± 9.1 | 5.1 ± 0.7 | 6.3 |
| Protein-bound | 71.6 ± 12.5 | 68.1 ± 6.6 | 3.5 ± 0.4 | 4.9 |
| Lipid-bound | 8.0 ± 1.5 | 6.3 ± 1.0 | 1.6 ± 0.1 | 20.4 |
| . | Membrane-bound sialic acid before treatment . | Membrane-bound sialic acid after treatment . | Sialic acid released . | % of release . |
|---|---|---|---|---|
| Controls | ||||
| Total | 48.9 ± 10.6 | 38.5 ± 6.3 | 10.4 ± 0.8 | 21.2 |
| Protein-bound | 43.0 ± 1.8 | 34.7 ± 2.8 | 8.3 ± 0.8 | 19.3 |
| Lipid-bound | 3.2 ± 0.3 | 2.9 ± 0.1 | 2.0 ± 0.1 | 64.0 |
| Diabetics | ||||
| Total | 81.3 ± 19.3 | 76.2 ± 9.1 | 5.1 ± 0.7 | 6.3 |
| Protein-bound | 71.6 ± 12.5 | 68.1 ± 6.6 | 3.5 ± 0.4 | 4.9 |
| Lipid-bound | 8.0 ± 1.5 | 6.3 ± 1.0 | 1.6 ± 0.1 | 20.4 |
Sialic acid is expressed as μg/mg of erythrocyte membrane protein.
The data reported are mean ± SD of 7 experiments.
Characterization and identification of erythrocytes from controls and diabetic patients fractionated according to biological age
Four cell layers were identified after the fractionation of erythrocytes obtained from the studied subjects (see “Patients, materials, and methods”). Different parameters were used for characterization: creatine, HbA1c, MCH, MCHC, and MCV27,28 43 (Table 5). Creatine and MCV decreased and MCHC and HbA1c increased going from the first to the last layer, while MCH content remained virtually unchanged throughout the gradient.
Characterization according to biological age of the different erythrocyte density fractions obtained from controls and diabetic patients
| Parameter . | Fraction . | Controls . | Diabetic patients . |
|---|---|---|---|
| Creatine, mg/100 cells | R | 4.31 ± 0.86 | 4.84 ± 0.91 |
| 1 | 3.93 ± 0.74 | 4.20 ± 0.78 | |
| 2 | 1.62 ± 0.345-151 | 1.73 ± 0.325-151 | |
| 3 | 1.55 ± 0.295-151 | 1.61 ± 0.345-151 | |
| 4 | 0.54 ± 0.115-151 | 0.63 ± 0.095-151 | |
| HbA1c, % | R | 3.51 ± 0.39 | 3.84 ± 0.45 |
| 1 | 3.93 ± 0.425-150 | 4.96 ± 0.385-151,5-152 | |
| 2 | 4.25 ± 0.515-151 | 6.23 ± 0.535-151,5-152 | |
| 3 | 4.56 ± 0.585-151 | 7.11 ± 0.685-151,5-152 | |
| 4 | 5.12 ± 0.495-151 | 8.23 ± 0.775-151,5-152 | |
| MCH, pg | R | 28.2 ± 2.0 | 27.5 ± 1.7 |
| 1 | 28.4 ± 1.7 | 28.8 ± 1.9 | |
| 2 | 28.6 ± 1.9 | 28.8 ± 2.1 | |
| 3 | 29.1 ± 2.1 | 29.0 ± 2.1 | |
| 4 | 29.5 ± 1.8 | 28.9 ± 2.0 | |
| MCHC, g/L | R | 289 ± 24 | 275 ± 28 |
| 1 | 301 ± 19 | 299 ± 23 | |
| 2 | 316 ± 235-150 | 310 ± 295-150 | |
| 3 | 329 ± 205-151 | 323 ± 285-151 | |
| 4 | 338 ± 215-151 | 333 ± 315-151 | |
| MCV, fL | R | 97.4 ± 11.9 | 98.6 ± 11.3 |
| 1 | 94.2 ± 10.9 | 96.1 ± 10.8 | |
| 2 | 90.8 ± 9.4 | 92.9 ± 9.7 | |
| 3 | 88.6 ± 8.8 | 90.7 ± 8.1 | |
| 4 | 84.6 ± 9.55-150 | 86.7 ± 7.95-150 |
| Parameter . | Fraction . | Controls . | Diabetic patients . |
|---|---|---|---|
| Creatine, mg/100 cells | R | 4.31 ± 0.86 | 4.84 ± 0.91 |
| 1 | 3.93 ± 0.74 | 4.20 ± 0.78 | |
| 2 | 1.62 ± 0.345-151 | 1.73 ± 0.325-151 | |
| 3 | 1.55 ± 0.295-151 | 1.61 ± 0.345-151 | |
| 4 | 0.54 ± 0.115-151 | 0.63 ± 0.095-151 | |
| HbA1c, % | R | 3.51 ± 0.39 | 3.84 ± 0.45 |
| 1 | 3.93 ± 0.425-150 | 4.96 ± 0.385-151,5-152 | |
| 2 | 4.25 ± 0.515-151 | 6.23 ± 0.535-151,5-152 | |
| 3 | 4.56 ± 0.585-151 | 7.11 ± 0.685-151,5-152 | |
| 4 | 5.12 ± 0.495-151 | 8.23 ± 0.775-151,5-152 | |
| MCH, pg | R | 28.2 ± 2.0 | 27.5 ± 1.7 |
| 1 | 28.4 ± 1.7 | 28.8 ± 1.9 | |
| 2 | 28.6 ± 1.9 | 28.8 ± 2.1 | |
| 3 | 29.1 ± 2.1 | 29.0 ± 2.1 | |
| 4 | 29.5 ± 1.8 | 28.9 ± 2.0 | |
| MCHC, g/L | R | 289 ± 24 | 275 ± 28 |
| 1 | 301 ± 19 | 299 ± 23 | |
| 2 | 316 ± 235-150 | 310 ± 295-150 | |
| 3 | 329 ± 205-151 | 323 ± 285-151 | |
| 4 | 338 ± 215-151 | 333 ± 315-151 | |
| MCV, fL | R | 97.4 ± 11.9 | 98.6 ± 11.3 |
| 1 | 94.2 ± 10.9 | 96.1 ± 10.8 | |
| 2 | 90.8 ± 9.4 | 92.9 ± 9.7 | |
| 3 | 88.6 ± 8.8 | 90.7 ± 8.1 | |
| 4 | 84.6 ± 9.55-150 | 86.7 ± 7.95-150 |
All data are mean ± SD of 10 different experiments.
P < .05.
P < .01, “R” versus fractions.
P < .01, diabetic patients versus controls.
On the basis of the above markers (Table 5) and according to reported criteria,27,28 43 the different fractions were identified as follows: (a) fraction 1, at 66% Percoll, largely composed of “young erythrocytes” (creatine content 3.93 ± 0.74 mg for 100 cells); (b) fractions 2 and 3 at 70% and 74% Percoll, composed of “average age erythrocytes” (creatine content 1.62 ± 0.34 and 1.55 ± 0.29 mg for 100 cells, respectively); and (c) fraction 4 at 80% Percoll, prevalently made of “senescent erythrocytes” (creatine content 0.54 ± 0.11 mg for 100 cells). Reticulocytes were mostly confined, 75% of total, to a very thin film at 60% Percoll (“fraction R” [fraction enriched in reticulocytes]; creatine content 4.31 ± 0.86 mg for 100 cells); the remaining 25% were present in the fraction 1 (“young erythrocytes”), while the other fractions had no reticulocytes.
Also in diabetics the erythrocyte fractionation by age showed 4 cell layers corresponding to those obtained in the controls, with the same specific markers distribution (Table 5). However, compared with controls, fraction 1 appeared larger and contaminated by 20% of all reticulocytes; fractions 2 and 3 are somewhat thinner, while fraction 4 was markedly reduced. All fractions (2, 3, and 4), as in controls, were completely devoid of reticulocytes. Fraction R appeared larger and thicker with 80% of the reticulocytes present in the samples (Table5). Fractions R (from controls and diabetics) were not considered in the sialidase study.
To assess possible changes in red blood cell hydration due to membrane fluxes via KCl cotrasport, according to the indications by Sorette,29 the Percoll gradient was performed also with an isotonic buffer with high KCl and low NaCl concentration (133 mM and 4.5 mM, respectively). In our experimental conditions, we found no significant differences, with the exception of a nonsignificant increase (about 12%) of reticulocytes in fraction R.
Distribution of differently aged erythrocytes in controls and diabetic patients
On the basis of the evidence that the erythrocyte turnover is accelerated in diabetic subjects compared with controls,23we evaluated the percentage of young, average aged, and senescent erythrocytes in the 2 populations. The erythrocyte age distribution data in controls and diabetics, reported in Table6, are expressed as micromoles of phosphorus content of membrane phospholipids and as number of cells. It is evident that in diabetic patients the young erythrocytes are almost doubled relative to controls, the senescent ones are reduced by a quarter, while the average aged erythrocytes are equally represented in the 2 populations.
Erythrocyte age distribution in controls and diabetics
| . | Controls . | Diabetics . | ||
|---|---|---|---|---|
| μM phosphorus (%) . | No. of cells, × 109(%) . | μM phosphorus (%) . | No. of cells, × 109 (%) . | |
| Total erythrocytes | 83.1 ± 3.7 (100) | 99.1 ± 6.9 (100) | 75.5 ± 4.2 (100) | 96.6 ± 4.9 (100) |
| Young erythrocytes | 11.6 ± 0.5 (14.6) | 13.4 ± 0.7 (14.6) | 24.1 ± 1.3 (34.9) | 34.3 ± 2.0 (38.2) |
| Average aged erythrocytes | 52.4 ± 2.8 (65.7) | 59.8 ± 2.7 (64.9) | 41.5 ± 2.6 (60.2) | 49.5 ± 3.5 (55.2) |
| Senescent erythrocytes | 15.6 ± 0.9 (19.7) | 18.9 ± 1.3 (20.5) | 3.41 ± 0.19 (4.9) | 5.9 ± 0.4 (6.6) |
| . | Controls . | Diabetics . | ||
|---|---|---|---|---|
| μM phosphorus (%) . | No. of cells, × 109(%) . | μM phosphorus (%) . | No. of cells, × 109 (%) . | |
| Total erythrocytes | 83.1 ± 3.7 (100) | 99.1 ± 6.9 (100) | 75.5 ± 4.2 (100) | 96.6 ± 4.9 (100) |
| Young erythrocytes | 11.6 ± 0.5 (14.6) | 13.4 ± 0.7 (14.6) | 24.1 ± 1.3 (34.9) | 34.3 ± 2.0 (38.2) |
| Average aged erythrocytes | 52.4 ± 2.8 (65.7) | 59.8 ± 2.7 (64.9) | 41.5 ± 2.6 (60.2) | 49.5 ± 3.5 (55.2) |
| Senescent erythrocytes | 15.6 ± 0.9 (19.7) | 18.9 ± 1.3 (20.5) | 3.41 ± 0.19 (4.9) | 5.9 ± 0.4 (6.6) |
Separation was performed on Percoll discontinuous gradient. Twenty-one milliliters of blood (5.83 × 106 and 6.44 × 106 erythrocytes per microliter for controls and diabetics, respectively), after elimination of contaminant leukocytes and platelets, were resuspended in 25 mL; 4.0 mL (20 × 109-22 × 109 erythrocytes) were loaded on the gradient.
The data are expressed as mean ± SD of 5 experiments.
Behavior of acidic and neutral sialidases in controls and diabetic patients in differently aged erythrocytes
The assay of the 2 sialidase forms performed in erythrocytes of different ages (Figure 2) showed that the distribution of the acidic and neutral sialidase changes in both control and diabetic subjects in relation to erythrocyte age. Histograms in Figure 3 show that, in controls, the acidic sialidase is the prevalent form of the enzyme in young cells (75%), while the neutral form prevails in senescent ones (84%). In diabetic patients the acidic sialidase is the prevalent form of the enzyme, but the neutral sialidase does not increase with erythrocyte aging and in senescent red cells the levels of the latter enzyme are markedly diminished.
Effect of pH on the sialidase activity contained in resealed vesicles obtained from human erythrocytes of different ages.
Effect of pH on the sialidase activity contained in resealed vesicles obtained from human erythrocytes of different ages.
Behavior of acidic and neutral sialidase in differently aged human erythrocyte resealed vesicles obtained from control and diabetic subjects.
Behavior of acidic and neutral sialidase in differently aged human erythrocyte resealed vesicles obtained from control and diabetic subjects.
Discussion
The evidence that in the diabetic condition the erythrocyte membrane presents structural and functional modifications,21-23,44 including altered levels of sialic acid,14,45 together with the demonstration in the same membrane of 2 sialidase forms,19 prompted us to investigate the behavior of the erythrocyte plasma membrane sialidases in diabetes.
This report provides evidence for the involvement of both sialidases in the diabetic condition: in fact, a significant increase (45%) of the acidic form and a significant decrease (22%) of the neutral form of the enzyme assayed on the exogenous substrate (MU-NeuAc) were observed in the erythrocyte resealed membranes from diabetic subjects. The lack of correlation with glycemic levels, metabolic compensation status, and disease duration suggests that the metabolic abnormalities may be related to insulin action impairment. In fact, enzyme level variations occur regardless of the presence of complications, with the only exception observed being nephropatic diabetic patients, in whom a correlation between the acidic–neutral sialidase ratio and the severity of the disease was demonstrated. The marked decrease observed in diabetic patients of the neutral sialidase, the only form of enzyme responsible for the release of sialic acid from endogenous sialoderivatives (present either at the erythrocyte membrane or in the plasma4,6), is likely responsible for the higher sialic acid content (40%) found in erythrocyte membranes of these subjects. The accumulation of sialoglycoproteins in the glomerular capillary wall, previously demonstrated in nephropatic patients,16 46 is in agreement with these observations.
At this point it is important to remember that while the acidic sialidase is normally available to assay, the neutral sialidase is present in a “cryptic” form.19 Therefore, it may be suggested that its activity could be unmasked when major changes of the membrane enzymes and proteins occur, especially as a consequence of specific physiologic events involving certain domains of the erythrocyte membrane such as vesiculation processes.47,48It cannot be excluded that the neutral sialidase is masked or negatively influenced in its expression by additional nonspecific alterations in the physicochemical and dynamic properties of erythrocyte membrane, which are known to occur in diabetes.21-23,44 In this condition, protein glycosylation and lipid peroxidation processes might contribute to the reduced lifespan and deformability of erythrocytes49 as well as to the altered content of specific membrane enzymes, ie, Ca/Mg-, Na/K-ATPase,50,51 Ach-esterase,23,52 and several glycohydrolases,20 with also an interference on the physiologic unmasking process of neutral sialidase.
In fact, on the basis of our more recent experience, we know that in vitro vesiculation of erythrocyte membrane, which mimics the physiologic process of vesiculation, results in the release of vesicles enriched in acidic sialidase and Ach-esterase, with the concurrent unmasking of neutral sialidase.53 Therefore, it is conceivable that an impairment of this process, possibly caused by membrane alterations, could account in diabetic patients for the less pronounced decrease of acidic sialidase and Ach-esterase23with their consequent apparent increase. Both above enzymes are associated to the membrane through a GPI anchor.18Interestingly, the erythrocyte membrane of diabetic subjects was less sensitive to the action of a specific PIPLC: in fact, after treatment with B thuringiensis phospholipase C, diabetic erythrocyte resealed membranes liberated a much lower amount (only 26% vs 88%) of acidic sialidase than those obtained from healthy subjects. Probably, the characteristic changes occurring at the red cell membrane level,49,54 the first one being the increased level of sialic acid due to neutral sialidase activity reduction, could interfere with GPI-anchored proteins. Thus, diabetic erythrocytes could become less susceptible to PIPLC action. A similar behavior has been shown in diabetes for Ach-esterase,23 a specific marker of the outer surface for GPI-anchored proteins.
The increased content of sialic acid occurring in diabetics, as a consequence of the altered ratio between the 2 forms of erythrocyte sialidases, is particularly important in the light of its role in the maintenance of erythrocyte viability and survival in circulating blood.55 On this basis, we investigated whether the erythrocyte population age in diabetics was different compared with healthy subjects. The method used to separate differently aged erythrocytes allowed us to obtain young, average aged, and senescent elements clearly identified by specific characteristic markers (Table5). In our experimental conditions, reticulocytes are present mainly in fraction R and in negligible amounts in fraction 1, while being completely absent in the other fractions. Notably, no changes were observed in the erythrocyte distribution, from both controls and diabetic subjects, along the gradient used to separate red cells according to their age, due to the use of high Na+/low K+- or low Na+/high K+-containing buffers.29 This evidence reasonably excludes that the experimental conditions used to separate erythrocytes may cause per se modifications of the erythrocyte density affecting their distribution along the gradient. These data suggest that in diabetic patients the red cell population is qualitatively different from healthy subjects, with a net prevalence of younger cells. Interestingly, while young erythrocytes from controls and diabetics have the same content of the 2 sialidases, in diabetics senescent red cells have a markedly decreased expression of neutral sialidase. This suggests that in this pathological condition the erythrocyte does not undergo a normal maturation process. These data are consistent with the notion reported by Mazzanti et al45 56 regarding the accelerated erythrocyte aging in human diabetes mellitus. Presumably, the higher negative charge at the erythrocyte surface due to sialic acid increase results in a premature sequestration of diabetic red cells by macrophages and proves the importance of the neutral sialidase for the physiologic death of erythrocytes.
On the basis of the overall evidence provided in this report, we believe that the diabetic condition induces functional alterations of the sialic acid–sialidase system of the erythrocyte membrane. The finding that the membrane perturbations occurring in diabetes alter the activity of the only enzyme able to reduce the sialic acid content of erythrocyte surface, with concomitant decrease of the lifespan of red cells, focuses the attention on the relevance of the molecular events involving the expression of sialidases in the process of erythrocyte aging.
Supported by grants from MURST PRIN 2000 (B.V.) and from the University of Milan (60%-1999) (A.L.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Bruno Venerando, Dipartimento di Chimica e Biochimica Medica—Facoltà di Medicina e Chirurgia—Università di Milano, LITA, Via Fratelli Cervi 93-20090 Segrate, Milano, Italia; e-mail: bruno.venerando@unimi.it.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal