Abstract
Rituximab is a chimeric monoclonal antibody directed at CD20 with significant activity in non-Hodgkin lymphoma (NHL) and chronic lymphocytic leukemia (CLL). A variety of pathways of tumor cytotoxicity different from cytotoxic chemotherapy have been proposed for this therapeutic antibody including antibody-dependent cellular cytotoxicity and complement-mediated cell lysis. This report describes that a proportion of patients with CLL receiving rituximab treatment have in vivo activation of caspase-9, caspase-3, and poly(ADP-ribose) polymerase (PARP) cleavage in blood leukemia cells immediately following infusion of rituximab. This suggests that apoptosis using a pathway similar to fludarabine and other chemotherapeutic agents is intricately involved in the blood elimination of tumor cells after rituximab treatment. Patients having caspase-3 activation and PARP cleavage in vivo had a significantly lower blood leukemia cell count after treatment as compared to those without caspase activation. Significant down-modulation of the antiapoptotic proteins XIAP and Mcl-1 was also noted, possibly explaining in part how rituximab sensitizes CLL cells to the cytotoxic effect of chemotherapy in vivo. These findings suggest that the therapeutic benefit of antibody-based therapy in vivo for patients with CLL depends in part on induction of apoptosis and provides another area of focus for studying mechanisms of antibody-resistance in neoplastic cells.
Introduction
Since the initial introduction of serotherapy with a murine antibody in 1980,1 a variety of novel therapies for the treatment of low-grade non-Hodgkin lymphoma (NHL) and B-cell chronic lymphocytic leukemia (CLL) have been introduced.2One of those is rituximab, a chimeric antibody directed against CD20. Studies have demonstrated that rituximab is quite effective in the treatment of both relapsed NHL at lower doses3-7 and CLL at higher doses.8 9 Unlike many other therapies used in both NHL and CLL, rituximab has tumor selectivity and hence is not associated with morbidity commonly experienced with standard cytotoxic chemotherapy.
One of the attractive features of immunotherapy with monoclonal antibodies, including rituximab, is a mechanism of cell clearance that is proposed to be different than that of cytotoxic chemotherapy. Specifically, it is believed that monoclonal antibodies eliminate tumor cells by both complement-mediated cell lysis and antibody-dependent cellular cytotoxicity.10 A recent report by Clynes et al11 demonstrated the importance of specific Fc receptors in mediating the cytotoxic effects of rituximab against NHL in vivo in a severe combined immunodeficiency (SCID) mouse model. Specifically, this study demonstrated that deficiency of FcγIII receptors or use of altered antibodies that prevented binding to the Fc receptor resulted in diminished in vivo response to the rituximab therapy. Such findings strongly implicate the importance of the Fc receptor in mediating the in vivo effect of rituximab. Another study demonstrated that murine anti-CD20 antibodies can inhibit cell growth but more importantly induce apoptosis on Fc receptor cross-linking in vitro in human lymphoma cell lines.12 This finding supports the importance of Fc receptor-antibody interaction in tumor cell elimination.
A major limitation of in vitro and in vivo animal model studies involving tumor cell lines for exploring mechanisms of tumor elimination by novel agents is that data derived from such sources are not reflective of what actually occurs in a patient. Specifically, it remains uncertain if apoptosis contributes to the cytotoxic effects of monoclonal antibodies used in the treatment of patients with CLL, NHL, or other related malignancies whose biology is quite different from dividing immortalized tumor cell lines. In addition, the specific pathway of apoptosis used bears great relevance to what mechanisms of resistance develop, making these therapies ineffective. Unlike most human tumors, patients with CLL have easily assessable neoplastic cells in the blood, making serial in vivo sampling during treatment quite feasible. We therefore sought to study the in vivo contribution of apoptotic pathways to the clinical response observed in patients with CLL receiving treatment with rituximab.
Patients, materials, and methods
Clinical study
The patients studied were enrolled on an institutional review board (IRB)–approved multicenter trial at the Walter Reed Army Medical Center and Johns Hopkins Oncology Center. The results of this study have been preliminarily reported.9 All patients gave written informed consent prior to participation. Patients reported herein had histologically documented CLL as defined by the modified National Cancer Institute (NCI) criteria13 and met these same criteria for requiring treatment.
Therapy was administered with close monitoring as outlined previously.9 Briefly, for the first treatment, 100 mg rituximab was administered over 4 hours. Infusions were then administered on a 3-times-a-week schedule for 4 weeks with the full dose (cohort 1, 250 mg/m2 or cohorts 2 and 3, 375 mg/m2) assigned specific to this cohort. Cohort 2 differed from cohort 3 by the rate rituximab was administered after the second infusion. Patients were assessed for disease response at the completion of therapy.
Sample processing
Blood was obtained before and immediately after infusion of rituximab (approximately 4 hours) during treatments 1 and 2 of therapy on the trial as outlined above. Following this, blood was immediately transferred at room temperature to the laboratory where mononuclear cells were separated using density gradient centrifugation (Ficoll-Paque Plus, Pharmacia Biotech, Piscataway, NJ). Whole-cell lysates were prepared by pelleting 1.25 × 108phosphate-buffered saline (PBS)–washed mononuclear cells in a microcentrifuge, aspirating the supernatant, and adding 0.5 mL cold lysis buffer as described previously.14 This cell suspension was incubated at 4°C for 40 minutes with constant agitation, then centrifuged for 15 minutes at 14 000 rpm at 4°C. The supernatant was recovered, aliquoted, and frozen at −80°C. Studies examining in vitro modulation of antiapoptosis proteins in vitro were performed from CLL cells obtained from patients with CLL as part of an IRB-approved protocol. Similar methods for lysate preparation were applied to CLL cells incubated in vitro with rituximab (10 μg/mL) and media for 4 hours.
Assessment of B-cell depletion after rituximab therapy
The CLL cells immediately before treatment with rituximab and immediately after treatment were washed with PBS and then incubated with an anti-CD19 phycoerythrin (PE)–labeled antibody (Becton Dickinson, San Jose, CA) and an anti-CD5 fluorescein isothiocyanate (FITC)–labeled antibody (Becton Dickinson) for 10 minutes. The cells were washed with PBS and were then analyzed on a FACScan (Becton Dickinson) illuminated at 488 nm and measuring green fluorescence (detecting FITC levels) at 530 nm and red fluorescence (measuring PE content of the cells) at more than 600 nm on an exponential scale. CD19- and CD5-coexpressing cells before treatment were compared to similar coexpression immediately after treatment. These studies showed the median pretreatment percentage of CD19/CD5+ cells was 86.9% (95% CI ± 5.3), whereas the posttreatment percentage was 72.3% (95% CI ± 11.8). The overlapping 95% CIs between these time points provide support for examining changes before and after therapy in whole-cell lysate protein levels.
Western blotting
Immunoblot assays were performed as described in detail elsewhere, using the multiple antigen detection (MAD) immunoblotting method that has been previously described.15 Protein was quantified in each supernatant by the bicin choninic acid (BCA) method (Pierce, Rockford, IL). Each sample was normalized for total protein content (12.5 μg/lane) and subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE; 12% gel), followed by transfer to nitrocellulose filters. The primary antibodies used represented rabbit polyclonal antisera raised either against synthetic peptides (Bcl-2, Bax, Mcl-1) or recombinant protein produced in bacteria (caspase-3, caspase-8, and caspase-9).16-20Other primary antibodies included murine monoclonal antibodies specific for β-actin (Sigma Immunochemicals), XIAP (Tranduction Laboratories, Lexington, KY), and poly(ADP-ribose) polymerase (PARP; a kind gift from N. A. Berger). Secondary antibodies consisted of horseradish peroxidase (HRP)–conjugated goat anti–rabbit IgG or goat anti–mouse IgG (Bio-Rad Laboratories, Richmond, CA). Detection was performed by an enhanced chemiluminescence (ECL) method (Amersham, Arlington Heights, IL), followed by colorimetric detection, using SG substrate (Vector Laboratories, Burlingame, CA). For verification of equivalence in protein loading, the blot was probed with anti–β-actin antibody, showing equivalence in protein loading. Protein bands were quantified by laser densitometry.
Statistical analysis
Groups of data were compared using Student paired ttest with SPSS software (version 9.0, SPSS, Chicago, IL) or Quatropro software (Novell, Orem, Utah). All P values are 2-sided.
Results
Rituximab induces caspase-3 activation in CLL cells in vivo
Based on observations noted in cell lines,12 we hypothesized that the clinical response observed in patients with CLL receiving rituximab may in part be mediated through induction of apoptosis. In the context of a clinical trial of rituximab in the treatment of CLL that included 33 patients, we performed pretreatment and posttreatment sampling (approximately 4 hours after initiation of therapy) of peripheral blood leukemia cells from 10 patients with CLL on days 1 and 3 of treatment. Patients who were not included either had a low lymphocyte count at the beginning of therapy or had rapid decrease in lymphocyte count after the first treatment. In these patients, we were unable to assess the status of caspase activation or expression of various apoptosis-regulating proteins.
Caspase-3 serves as an effector caspase, causing cleavage of a variety of important cellular proteins including PARP. Therefore, expression and proteolytic processing of caspase-3 and PARP were examined in all patients for whom pretreatment and posttreatment cellular lysates were obtained. The results of the studies performed on all 10 patients studied are summarized in Table 1, with representative examples shown in Figure1. In 3 patients, we were able to demonstrate that rituximab induces cleavage of caspase-3 with subsequent appearance of the 17-kd cleavage product corresponding to the large subunit of the catalytically active protease. Concurrent with these changes, we also detected cleavage of PARP, a known caspase substrate. These data suggest that at least part of the clinical effectiveness of rituximab in vivo may be explained by apoptosis induction in the leukemia cells.
Change in caspase and apoptosis proteins in vivo after treatment with rituximab
| Patient no. . | Clinical response . | PARP cleavage . | Caspase-3 processing . | Caspase-8 processing . | Caspase-9 processing . | ||||
|---|---|---|---|---|---|---|---|---|---|
| Day 1* . | Day 3 . | Day 1 . | Day 3 . | Day 1 . | Day 3 . | Day 1 . | Day 3 . | ||
| 1 | PR | + | + | + | +/− | − | − | + | + |
| 17 | PR | + | + | + | + | − | − | + | + |
| 25 | SD with imp | + | + | +/− | − | − | − | − | − |
| 7 | PR | − | − | ND | ND | ND | ND | − | − |
| 8 | SD | − | − | − | − | − | − | + | − |
| 9 | SD | − | − | − | − | − | − | − | − |
| 12 | SD | − | − | − | − | − | − | − | − |
| 14 | SD | − | − | − | − | − | − | − | + |
| 16 | SD | − | − | − | − | − | − | + | − |
| 28 | SD | − | − | − | − | − | − | − | − |
| Patient no. . | Clinical response . | PARP cleavage . | Caspase-3 processing . | Caspase-8 processing . | Caspase-9 processing . | ||||
|---|---|---|---|---|---|---|---|---|---|
| Day 1* . | Day 3 . | Day 1 . | Day 3 . | Day 1 . | Day 3 . | Day 1 . | Day 3 . | ||
| 1 | PR | + | + | + | +/− | − | − | + | + |
| 17 | PR | + | + | + | + | − | − | + | + |
| 25 | SD with imp | + | + | +/− | − | − | − | − | − |
| 7 | PR | − | − | ND | ND | ND | ND | − | − |
| 8 | SD | − | − | − | − | − | − | + | − |
| 9 | SD | − | − | − | − | − | − | − | − |
| 12 | SD | − | − | − | − | − | − | − | − |
| 14 | SD | − | − | − | − | − | − | − | + |
| 16 | SD | − | − | − | − | − | − | + | − |
| 28 | SD | − | − | − | − | − | − | − | − |
+ indicates present; −, absent; ND, not done; PR, partial response; SD with imp, stable disease with improvement.
Time after treatment.
Representative Western blot study from patient 17 demonstrating findings before and after therapy with rituximab.
CLL cells from the blood were recovered from patients before treatment (−) and on days 1 and 3 of treatment (+). Protein lysates were prepared, normalized for total protein content, and analyzed by SDS-PAGE/immunoblotting using antibodies specific for casepase-3, caspase-8, caspase-9, PARP, Mcl-1, and XIAP. Mcl-1 and XIAP are down-regulated. Caspase-3 was processed with formation of active p17 after treatment with rituximab on days 1 and 3 concurrent with PARP cleavage. Caspase-8 proform level remained unchanged. Caspase-9 proform level was reduced after treatment with rituximab on days 1 and 3, indicating that caspase-9 is being processed.
Representative Western blot study from patient 17 demonstrating findings before and after therapy with rituximab.
CLL cells from the blood were recovered from patients before treatment (−) and on days 1 and 3 of treatment (+). Protein lysates were prepared, normalized for total protein content, and analyzed by SDS-PAGE/immunoblotting using antibodies specific for casepase-3, caspase-8, caspase-9, PARP, Mcl-1, and XIAP. Mcl-1 and XIAP are down-regulated. Caspase-3 was processed with formation of active p17 after treatment with rituximab on days 1 and 3 concurrent with PARP cleavage. Caspase-8 proform level remained unchanged. Caspase-9 proform level was reduced after treatment with rituximab on days 1 and 3, indicating that caspase-9 is being processed.
Evidence of caspase-9 involvement in rituximab-induced apoptosis of CLL cells
Caspase-3 can be activated through a variety of mechanisms (for a review, see Reed21) including pathways involving caspase-9 (mitochondrial pathway) and caspase-8 (tumor necrosis factor [TNF]-family death receptor pathway). To further characterize the pathway of in vivo apoptosis used by rituximab, we performed additional studies to examine if levels of the proforms of either caspase-8 or caspase-9 decreased after treatment, an indication that these proteases have undergone proteolytic processing and activation. The results of these studies for each patient are shown in Table 1. In all 10 patients studied, no decrease in caspase-8 zymogen was noted. Of the 3 patients having activation of caspase-3 following rituximab treatment, 2 also had a notable decline in caspase-9 zymogen protein. Three additional patients had evidence of a decline in caspase-9 zymogen without caspase-3 activation or PARP cleavage. Figure 1 shows a representative CLL specimen, demonstrating that the proform of caspase-9 decreased after treatment, whereas no change in procaspase-8 levels was noted. These findings suggest that rituximab induces apoptosis in vivo through a caspase-9 pathway, although triggering of this pathway in vivo does not always result in demonstrable activation of effector caspases such as caspase-3 and subsequent cleavage of PARP.
Rituximab favorably modulates Mcl-1 and XIAP in vivo in human CLL cells
Relative overexpression of antiapoptotic proteins Bcl-2 and Mcl-1 and altered ratio of Bcl-2 to proapoptotic protein Bax have been reported to be associated with lower response rates to chemotherapy regimens in CLL.22-30 To date, both preclinical and clinical trials in CLL and NHL have documented that rituximab can chemosensitize neoplastic cells to cytotoxic therapy.31-33We hypothesized therefore that rituximab may alter expression of one or more of these apoptosis-regulating proteins in CLL cells, thus offering a plausible explanation for these clinically observed phenomena. Expression of Bcl-2, Bax, Mcl-1, and XIAP proteins was examined in pretreatment and posttreatment samples from CLL patients receiving rituximab therapy. These studies demonstrated that neither Bcl-2 nor Bax protein changed following treatment with rituximab (data not shown). In contrast, relative levels of both XIAP and Mcl-1 protein were diminished on day 3 after treatment with rituximab in 10 and 9 of the patients, respectively. Representative examples are presented in Figures 1 and 2 showing rituximab induces changes in Mcl-1 and XIAP in vivo in circulating CLL cells. Indeed, expression of XIAP declined significantly in all patients examined after rituximab treatment on day 1 (P = .008) and day 3 (P = .03). A similar significant decline was noted with Mcl-1 protein expression in 9 of the 10 patients examined on day 1 (P = .012) and day 3 (P = .01). These data suggest in vivo treatment with rituximab reduces expression of certain antiapoptotic proteins and may explain why rituximab appears to chemosensitize cells even in the absence of primary induction of apoptosis in vivo.
Rituximab treatment in vivo often results in favorable changes in Mcl-1 and XIAP expression.
CLL cells from the blood were recovered from patients before and on days 1 and 3 of treatment. Protein lysates were prepared, normalized for total protein content, and analyzed by SDS-PAGE/immunoblotting using antibodies specific for Mcl-1 and XIAP. Protein bands were quantified by laser densitometry. Mcl-1 and XIAP are significantly (P < .05) down-regulated relative to the pretreatment expression on days 1 and 3 in the majority of patients tested.
Rituximab treatment in vivo often results in favorable changes in Mcl-1 and XIAP expression.
CLL cells from the blood were recovered from patients before and on days 1 and 3 of treatment. Protein lysates were prepared, normalized for total protein content, and analyzed by SDS-PAGE/immunoblotting using antibodies specific for Mcl-1 and XIAP. Protein bands were quantified by laser densitometry. Mcl-1 and XIAP are significantly (P < .05) down-regulated relative to the pretreatment expression on days 1 and 3 in the majority of patients tested.
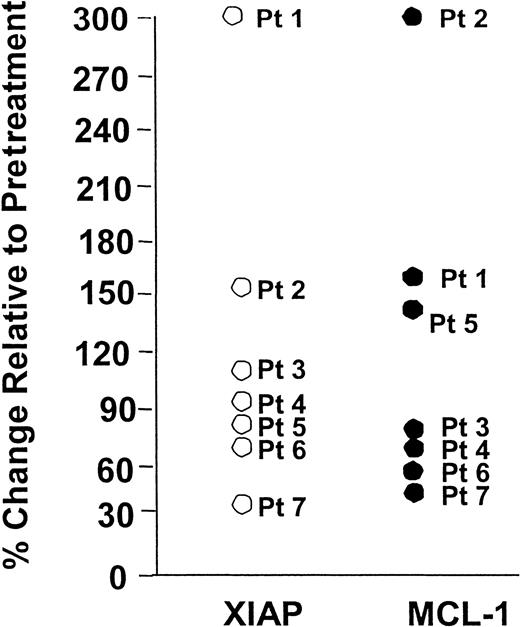
Rituximab modulates Mcl-1 and XIAP in vitro in some patients' CLL cells
Because we have demonstrated that rituximab modulates Mcl-1 and XIAP in vivo in a subset of patients treated with rituximab, we sought to determine if this also occurred in vitro following treatment with rituximab. Similar to data presented by others using B-cell lymphoma cell lines,12 rituximab as a single agent did not induce in vitro apoptosis in human CLL cells (data not shown). Figure3 demonstrates the paired in vitro evaluation of CLL cells derived from 7 patients incubated with or without rituximab (10 μg/mL). Levels of both Mcl-1 and XIAP decreased in 4 of the 7 patients. The median changes in Mcl-1 and XIAP relative to baseline were 85% (range, 56%-314%) and 98% (range, 34%-318%). These data provide further evidence that treatment with rituximab reduces expression of certain antiapoptotic proteins without concurrently inducing apoptosis.
Rituximab treatment in vitro results in favorable changes in Mcl-1 and XIAP expression in a subset of patients with CLL.
CLL cells from the blood were obtained and exposed to media or rituximab for 4 hours. Following this, protein lysates were prepared, normalized for total protein content, and analyzed by SDS-PAGE/immunoblotting using antibodies specific for Mcl-1 and XIAP. Protein bands were quantified by laser densitometry. Mcl-1 and XIAP decreased in a proportion of patients and increased in others that generally are in concordance with each other.
Rituximab treatment in vitro results in favorable changes in Mcl-1 and XIAP expression in a subset of patients with CLL.
CLL cells from the blood were obtained and exposed to media or rituximab for 4 hours. Following this, protein lysates were prepared, normalized for total protein content, and analyzed by SDS-PAGE/immunoblotting using antibodies specific for Mcl-1 and XIAP. Protein bands were quantified by laser densitometry. Mcl-1 and XIAP decreased in a proportion of patients and increased in others that generally are in concordance with each other.
Rituximab-induced apoptosis in vivo correlates with favorable clinical outcome
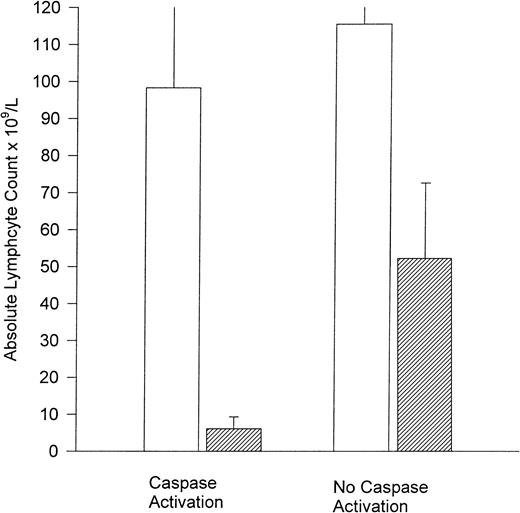
To determine if the in vivo laboratory documentation of caspase-3 activation and PARP cleavage was clinically relevant, we correlated this occurrence with clinical end points. As assessment of apoptosis activation occurred in the blood compartment, we compared both pretreatment and posttreatment absolute blood lymphocyte counts between patients with and without caspase-3 activation. As demonstrated in Figure 4, the mean lymphocyte count before treatment was similar in patients demonstrating versus not demonstrating caspase activation (mean, 98.3 × 109/L; 95% CI ± 73.6 versus 115.5 × 109/L; 95% CI ± 20.9). In contrast, the posttreatment absolute blood lymphocyte numbers from patients demonstrating caspase activation were significantly lower than those not having caspase activation (mean, 6.1 × 109/L; 95% CI ± 6.33 versus 52.2 × 109/L; 95% CI ± 40;P = .04). Furthermore, all 3 of the patients with caspase-3 and PARP cleavage experienced clinical benefit of rituximab treatment with 2 displaying partial responses (as defined by modified NCI criteria)13 and 1 having stable disease with improvement. Rituximab-induced decreases in Mcl-1 were noted in all 3 of these patients (30.7% baseline patient 1, partial response; 37.2% baseline patient 17, partial response; 61.1% baseline patient 25, stable disease with improvement). Also, the CLL cells of 2 patients with partial responses (patients 1 and 17) in whom caspase-3 proteolytic processing and PARP cleavage were observed exhibited similar decreases (64% and 86% of baseline) in XIAP, whereas the patient demonstrating stable disease with improvement had a slight increase in XIAP over baseline (26%).
Caspase activation correlates with posttreatment lymphocyte count.
Pretreatment (■) and posttreatment (▨) lymphocyte count (× 109/L) for patients with and without caspase-3 activation.
Caspase activation correlates with posttreatment lymphocyte count.
Pretreatment (■) and posttreatment (▨) lymphocyte count (× 109/L) for patients with and without caspase-3 activation.
Discussion
To our knowledge, this report represents the first study to provide evidence that a therapeutic monoclonal antibody induces apoptosis in vivo in humans. Although preliminary, these data suggest that rituximab exerts its cytotoxic effects in vivo against human CLL cells at least in part through induction of apoptosis via a caspase-9 (mitochondrial) pathway, without evidence of concomitant activation of the caspase-8 pathway. Induction of apoptosis in vivo was associated with clinical benefit as measured by a significant decline of tumor cells in the compartment measured (ie, blood). Furthermore, 2 of the patients described herein having demonstrable caspase-3 activation also attained a partial response to therapy, and the third patient demonstrated signs of clinical benefit. These data provide clinical support for the potential significance of a known in vitro mechanism for antibody cytotoxicity. In addition, our findings also point to potential pathways that might explain resistance to antibody-based therapy.
To date, the apoptotic pathway by which rituximab induces apoptosis in CLL cells or lymphoma cells in vitro has been poorly characterized. Work by Shen and colleagues12 demonstrated in lymphoma cell lines that anti-CD20 antibody-induced apoptosis appeared to be partially dependent on increases in intracellular calcium, as demonstrated by inhibition of apoptosis by intracellular and extracellular calcium chelators. A follow-up study by this group34 showed rituximab to be the most effective of several CD20 antibodies in vitro and documented caspase-3 activation concurrent with induction of apoptosis. Similar in vitro activation of caspase-3 was demonstrated by 2 other groups.35,36 Our study is the first to demonstrate that caspase-3 activation occurs in vivo in human tumor cells concurrent with cleavage of PARP, a known caspase-3 substrate. Furthermore, activation of this effector caspase appears to be associated in rituximab-treated CLL cells with activation of caspase-9. Typically, caspase-9 is activated by a mitochondria-dependent pathway involving release of cytochromec from the organelles and activation of Apaf-1, which binds and activates procaspase-9 (for a review, see Reed37) The mitochondrial pathway is commonly activated by cytotoxic agents active in CLL such as fludarabine38 and other cytotoxic anticancer drugs.39 In contrast, caspase-8 was not processed in any patient samples examined. This initiator caspase is typically activated by TNF/FAS-family cytokine receptors and is often used by immune effector cells that mediate antibody-dependent cellular cytotoxicity.40 This observation does not diminish the importance of in vivo Fc receptor interaction recently shown to be important with rituximab in NHL cell line xenograft murine experiments,11 but it remains to be determined whether the antitumor mechanisms that depend on Fc receptors also trigger a caspase-9–dependent pathway. Given the anomalous results in some patients demonstrating clinical benefit in the absence of caspase processing, it is quite likely that alternative mechanisms including antibody-mediated cellular cytotoxicity and complement-mediated cell lysis also have an impact on clinical benefit observed with rituximab.
It is of interest that some CLL specimens derived from patients on rituximab therapy demonstrated evidence of capase-9 activation in the absence of concomitant caspase-3 or PARP cleavage (Table 1). This observation suggests that some CLL cells possess a block in the caspase cascade at or between the point of activation of caspase-3 and caspase-9. Multiple mechanisms could potentially account for such impairments in this step of the mitochondrial pathway of apoptosis including phosphorylation of caspase-3 by protein kinase B (AKT), overexpression of Hsp70, and overexpression of the IAP family member proteins (for a review, see Deveraux et al41).
Several studies have reported an association of elevated levels of antiapoptotic proteins Bcl-2 and Mcl-1 or altered ratios of antiapoptotic and proapoptotic proteins (Bcl-2:Bax) of the Bcl-2 family with a lower clinical response rates to fludarabine-based regimens in CLL.22-28 Similarly, elevated levels of XIAP, an IAP family member that inhibits caspases,29,30,41 have been associated with less durable responses to cytarabine-containing regimens in acute myeloid leukemia, though its relevance to CLL has not yet been explored.42 To date, both preclinical and clinical trials in CLL and NHL have documented that rituximab chemosensitizes tumor cells to the effects of cytotoxic therapy.31-33 This formed the basis for the hypothesis that rituximab may alter expression of one or more apoptosis regulatory proteins in CLL cells and thus offer a plausible explanation for this clinically observed phenomenon. Serial studies of a variety of antiapoptotic proteins in vivo demonstrated that both Mcl-1 and XIAP were significantly modulated in virtually all patients examined. Similar findings were observed in a proportion of different patients following in vitro incubation with rituximab. Although preliminary, this finding suggests that such modulation of antiapoptosis signals in vivo may in part explain why rituximab has been observed to sensitize neoplastic cells (including CLL cells) to the effects of cytotoxic chemotherapy. Indeed, preliminary observations suggest that more complete responses are obtained when rituximab is combined with fludarabine33 in previously untreated patients with CLL. Future studies directed at serial in vivo quantification of these proteins in the context of clinical trials combining rituximab with fludarabine or other CLL therapies may provide the opportunity to test this hypothesis. In addition, studies examining resistance mechanisms of rituximab and potential cross-resistance of apoptosis between this agent and chemotherapy should be considered.
The authors wish to thank the patients who participated in this trial and to acknowledge the research team (Kathy Park, Margaret Lucas, and Amy Goodrich) that assisted in coordinating the clinical trial; Robin Howard, for statistical input; and Drs Michael Grever and Joseph Drabick, for reviewing the manuscript. Additional thanks are provided to the National Institutes of Health, the Leukemia and Lymphoma Society of America, the D. Warren Brown Foundation, and Sidney Kimmel Cancer Research Foundation for support.
The clinical trial was supported in part by a grant from Genentech and IDEC Pharmaceuticals. This work was supported by the National Cancer Institute (P01 CA81534-02 and CA98099), the Sidney Kimmel Cancer Research Foundation, the D. Warren Brown Foundation, and the Leukemia and Lymphoma Society of America.
J.C.B. and S.K. contributed equally to the production of this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
John C. Byrd, B302 Starling Loving Hall, The Ohio State University, 320 W 10th Ave, Columbus, OH 43210; e-mail:byrd-3@medctr.osu.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal