Abstract
This study investigated the feasibility of allogeneic (alloSCT) and autologous stem cell transplantation (ASCT) as postconsolidation therapy for patients with myelodysplastic syndromes (MDSs) or acute myeloid leukemia after MDS. Patients with a histocompatible sibling were candidates for alloSCT and the remaining patients for ASCT. Remission-induction therapy consisted of 1 or 2 courses with idarubicin, cytarabine, and etoposide, followed by one intensive consolidation course with cytarabine and mitoxantrone. Initially, bone marrow cells were used for ASCT. Subsequently, mobilized blood stem cells were used in an attempt to shorten posttransplantation hypoplasia. With a median follow-up of 3.6 years the 184 evaluable patients showed a 4-year survival rate of 26% and a median survival of 13 months. The remission-induction chemotherapy induced complete remission (CR) in 100 patients (54%). The 4-year disease-free survival (DFS) rate was 29% and the median DFS was 12 months. Twenty-eight of 39 patients (72%) with a donor were allografted in CR-1, including 2 patients who underwent transplantation in CR-1 without a consolidation course. Thirty-six of 59 patients (61%) without a donor received ASCT in CR-1. The 4-year DFS rates in the group of patients with or without a donor were 31% and 27%, respectively. The 4-year survival rates from CR were 36% and 33%, respectively. This large prospective study shows the feasibility of both alloSCT and ASCT. This treatment approach leads to a relatively high remission rate, and the majority of patients in remission received the SCT in CR-1. The ongoing study investigates whether this approach is better than treatment with chemotherapy only.
Introduction
The natural history of the myelodysplastic syndromes (MDSs) ranges from a chronic course that may span years to a rapid course of leukemia progression. Refractory anemia (RA) and RA with ringsideroblasts (RARS) are characterized by a low risk of transformation to acute myeloid leukemia (AML) and a median survival usually in excess of 30 months.1 In contrast the median survival of patients with RA and excess of blasts (RAEB) or RAEB in transformation (RAEBt) is less than 12 months.2,3 The karyotype is an additional prognostic factor for survival in MDS.4,5 An international workshop has proposed a scoring system based on cytogenetic abnormalities, marrow blasts, and peripheral blood cytopenias that identifies patients in whom median survival is less than 1 year.6 Allogeneic bone marrow transplantation (BMT) is usually considered the treatment of choice for the limited group of young patients with histocompatible siblings.7-9 Treatment of patients with MDS with chemotherapy only results in a few long-term survivors.10-12
Therefore, we initiated a clinical trial to assess the feasibility and the efficacy of intensive remission-induction and consolidation chemotherapy, followed by autologous stem cell transplantation (ASCT) or allogeneic stem cell transplantation (alloSCT). Patients with an HLA-identical family donor were candidates for alloSCT either in first complete remission (CR-1) or as salvage therapy if no CR was achieved. The remaining patients in remission were candidates for ASCT in CR-1. In the initial phase of the study autologous bone marrow cells were infused after the bone marrow ablative therapy. The protocol was adapted because of the very prolonged hematopoietic recovery, and the autologous bone marrow rescue was replaced by a rescue with peripheral blood stem cells mobilized with filgrastim during the recovery phase of the consolidation course.
Patients, materials, and methods
Patient selection criteria
Patients were eligible for the study if they had (1) RA, RARS, or RAEB with less than 10% blasts in the marrow and multiple chromosomal abnormalities; (2) RAEB with more than 10% blasts in the bone marrow; (3) RAEBt; (4) chronic myelomonocytic leukemia (CMML) with a neutrophil count of more than 16 × 109/L or a monocyte count of more than 2.6 × 109/L in the blood or 5% blasts in the bone marrow; or (5) secondary AML supervening on overt MDS (AML-MDS) of more than 6 months duration.
Exclusion criteria were the following: (1) age less than 16 years or more than 60 years; (2) previous intensive chemotherapy, and/or radiotherapy for MDS or AML; (3) treatment with biological response modifiers and/or low-dose cytarabine within 2 months prior to entry; (4) no informed consent; (5) performance status World Health Organization scale of 3 or 4; or (6) life expectancy of less than 3 months.
Informed consent of each patient has been obtained in accordance with the Helsinki protocol.
Study design
The remission-induction course consisted of idarubicin 10 mg/m2 per day as a 5-minute intravenous injection on days 1, 3, and 5 combined with a continuous intravenous infusion of cytarabine of 100 mg/m2 per day on days 1 through 10, and 1-hour intravenous infusions of etoposide of 100 mg/m2/d on days 1 through 5. In case of partial response a second identical remission-induction course was scheduled. The remaining patients were advised to receive as salvage therapy either an allogeneic transplantation with HLA-identical donor, if available, or a chemotherapy regimen containing high-dose cytarabine. Patients entering a CR after 1 or 2 courses of remission therapy received 1 consolidation course, starting 4 weeks after the beginning of the (last) remission-induction course. The consolidation course consisted of 2-hour intravenous infusions of cytarabine 500 mg/m,2every 12 hours for 6 days, combined with 30-minute intravenous infusions of mitoxantrone 12 mg/m2 per day on days 4 through 6. Patients who were treated after amendment of the study received Filgrastim 300 μg/d subcutaneously from day 20 after the start of the consolidation course until completion of the stem cell aphereses.
HLA typing of patients, parents, and siblings was initiated at the onset of induction therapy in all patients younger than 50 years (or younger than 55-60 years, according to the policy of the center). The patient was proposed for allografting in case of an HLA-A, -B, -DR identical, mixed lymphocyte culture nonreactive sibling, a 1-locus class-I mismatched sibling, or a phenotypically identical parent. The alloSCT was planned as soon as possible after recovery from the consolidation course. Patients not eligible for allogeneic BMT were scheduled to receive ASCT.
The transplantation conditioning regimens used throughout this study were based on total body irradiation (TBI) or busulphan. T-cell depletion of the allografts may be performed according to the ongoing protocols of the centers.
Required clinical investigations
The MDS section of the pathology review committee of the European Organization for Research and Treatment of Cancer (EORTC) Leukemia Cooperative Group centrally assessed pretreatment bone marrow and blood smears (coordinator, Dr H. Zwierzina). The classification of MDS and AML was performed according to the criteria of the French-American-British (FAB) working group. One smear of bone marrow and blood was also reviewed centrally at the time of CR or first relapse. Apart from the other standard investigations, it was mandatory to perform cytogenetic analysis with banding techniques prior to the start of chemotherapy.
Definitions
AML evolved from myelodysplasia is defined as secondary AML (AML-MDS). AML after chemotherapy or radiotherapy is defined as therapy-related AML (t-AML).
CR is defined as absence of clinical manifestations of leukemia and less than 5% blasts in a normocellular marrow with normal morphology. The peripheral blood neutrophil count should be at least 1.5 × 109/L, and the platelet count should be more than 100 × 109/L. Normalization of cytogenetic abnormalities has not been included in the definition of CR.
Partial remission (PR) is characterized by bone marrow containing less than 25% blasts, and more than 50% decrease of blast percentage from pretherapeutic levels, normal, or near-normal blood counts (granulocytes > 1.5 × 109/L, platelets > 70 × 109/L, hemoglobin > 6 mmol/L) and no circulating blast cells. These findings should last for at least 8 weeks, except for changes induced by subsequent treatment.
Statistical analysis
All patients were registered prospectively at the EORTC Data Center in Brussels. The duration of survival was calculated from the date of start of treatment until death from whatever cause. For patients who achieved CR after induction, the disease-free survival (DFS) was calculated from the date of CR-1 until the date of first relapse or until death in CR. Patients who died in C-1 were censored at that moment for the time-to-relapse analysis, and patients who relapsed were censored at that moment for the “treatment-related mortality.” The duration of survival of remitters corresponds to the time from CR-1 to the date of death. The actuarial curves were computed by using the Kaplan-Meier technique,13 and the standard errors (SE) of the estimates were obtained by the Greenwood formula.13The estimates of the incidence of relapse and of death in CR were obtained by using the cumulative incidence method13 in which the risks of death in CR and of relapse were considered as competing risks. The Cox proportional hazard model has been used to determine the prognostic importance of several factors regarding the DFS and survival from CR and to obtain estimates of the hazard ratio (HR) and the corresponding 95% confidence interval (CI). All analyses were performed according to the intent-to-treat principle.
Results
Description of patient population
A total of 197 patients from 35 institutions was registered between November 1992 and April 1997. The database was frozen in October 1999. Six patients were not eligible for the study because of wrong diagnosis (1 patient, 44% marrow blasts), incomplete information to secure diagnosis (2 patients), age of 64 years (1 patient), and hyperbilirubinemia (2 patients, > 1.5 normal values). Among the remaining 191 patients, 2 patients were not evaluable because treatment was never started (1 patient) or a wrong schedule was used (1 patient). No data were available for 5 patients.
The median age of the 184 evaluable patients was 47 years (range, 16-60). The classification according to the FAB criteria and the cytogenetic data are summarized in Table1. Cytogenetic data were evaluable in 151 of the 184 patients (82%). The majority of the unevaluable patients had insufficient number of normal metaphases (< 10 metaphases). Eighty (53%) were classified into the intermediate or poor prognostic cytogenetic groups according to International Prognostic Scoring System (IPSS).6 In addition the 115 MDS patients with an evaluable karyotype (83% of the patients with MDS) have been characterized according to the IPSS (Table 1). On the basis of this scoring system only 12 patients qualified for good risk MDS or intermediate-1 MDS. The remainder of the patients had either intermediate-2 and high-risk MDS or AML-MDS and CMML. Twenty patients had therapy-related MDS. The remaining patients had primary MDS.
Morphologic and cytogenetic characteristics of 184 evaluable patients and prognostic classification according to the IPSS number for 115 patients with MDS with evaluable karyotype
| Characteristics . | No. (%) . |
|---|---|
| FAB classification | |
| RA/RARS | 8 (4.3) |
| RAEB less than 10% blasts | 11 (6.0) |
| RAEB 10% blasts or more | 43 (23.4) |
| RAEBt | 60 (32.6) |
| CMML | 16 (8.7) |
| AML after MDS | 46 (25.0) |
| Cytogenetic characteristics* | |
| No sufficient cytogenetic data† | 33 (18) |
| Successful cytogenetic examination | 151 (82) |
| Good | 71 (47.0) |
| Intermediate | 34 (22.5) |
| Poor | 46 (30.5) |
| IPSS classification | |
| Low-risk group (0 points) | 1 (0.9) |
| Intermediate-1 group (0.5-1.0 points) | 11 (9.6) |
| Intermediate-2 group (1.5-2.0 points) | 48 (41.7) |
| High-risk group (> 2 points) | 55 (47.8) |
| Characteristics . | No. (%) . |
|---|---|
| FAB classification | |
| RA/RARS | 8 (4.3) |
| RAEB less than 10% blasts | 11 (6.0) |
| RAEB 10% blasts or more | 43 (23.4) |
| RAEBt | 60 (32.6) |
| CMML | 16 (8.7) |
| AML after MDS | 46 (25.0) |
| Cytogenetic characteristics* | |
| No sufficient cytogenetic data† | 33 (18) |
| Successful cytogenetic examination | 151 (82) |
| Good | 71 (47.0) |
| Intermediate | 34 (22.5) |
| Poor | 46 (30.5) |
| IPSS classification | |
| Low-risk group (0 points) | 1 (0.9) |
| Intermediate-1 group (0.5-1.0 points) | 11 (9.6) |
| Intermediate-2 group (1.5-2.0 points) | 48 (41.7) |
| High-risk group (> 2 points) | 55 (47.8) |
Cytogenetic risk groups according to IPSS.
Less than 10 normal metaphases, less than 3 abnormal metaphases, or no data at all.
Remission induction and salvage therapy
Eighty-eight patients (48%) entered CR after 1 remission-induction course. Thirty-six patients received a second remission-induction course, and 12 of those 36 patients entered CR. Altogether 100 patients (54%) entered CR after 1 or 2 remission-induction courses. Twenty-nine patients (16%) died during the remission-induction therapy. The remaining 55 patients had either persisting disease or a prolonged hypoplasia after chemotherapy (Table2). Seventeen patients who failed to enter CR had an HLA-identical sibling donor available. Seven of those 17 patients received an allogeneic BMT as salvage therapy (Figure1). Six additional patients have received transplants with alternative donors, and 4 patients received ASCT (Figure 1). Twelve patients entered CR after salvage therapy, and 6 patients are still in CR at the time of last reporting. Ten additional patients are alive with evidence of disease. A schematic overview of the treatment administered to all patients is presented in Figure 1.
Results of remission-induction therapy
| . | Courses . | Percent . | |
|---|---|---|---|
| I . | I + II . | ||
| Complete remission | 88 | 100 | 54 |
| Partial remission | 37 | 19 | 10 |
| Resistant | 29 | 26 | 13 |
| Persisting hypoplasia | 6 | 10 | 5 |
| Death in hypoplasia | 24 | 29 | 16 |
| . | Courses . | Percent . | |
|---|---|---|---|
| I . | I + II . | ||
| Complete remission | 88 | 100 | 54 |
| Partial remission | 37 | 19 | 10 |
| Resistant | 29 | 26 | 13 |
| Persisting hypoplasia | 6 | 10 | 5 |
| Death in hypoplasia | 24 | 29 | 16 |
Flow chart of treatment administered according to the availability of an HLA-identical sibling donor.
* indicates 1 alternative donor alloSCT in CR-1. # indicates 6 alternative donor alloSCT as salvage. CR indicates complete remission; no cons, no consolidation course received; alloSCT, allogeneic stem cell transplantation; ASCT, autologous stem cell transplantation; alt AlloSCT, alternative donor (mismatched family donors or unrelated donors) alloSCT; salv, salvage therapy; CCR, patients alive in continuous complete remission; between brackets, CCR after transplantation.
Flow chart of treatment administered according to the availability of an HLA-identical sibling donor.
* indicates 1 alternative donor alloSCT in CR-1. # indicates 6 alternative donor alloSCT as salvage. CR indicates complete remission; no cons, no consolidation course received; alloSCT, allogeneic stem cell transplantation; ASCT, autologous stem cell transplantation; alt AlloSCT, alternative donor (mismatched family donors or unrelated donors) alloSCT; salv, salvage therapy; CCR, patients alive in continuous complete remission; between brackets, CCR after transplantation.
Consolidation and postconsolidation therapy
Ten patients in CR after remission-induction chemotherapy did not receive the consolidation course: 6 patients had an HLA-identical sibling (Figure 1). Two patients were allografted in CR-1 with marrow from an HLA-identical sibling, and 1 patient received an ASCT. Seven patients relapsed before the consolidation course was given. Two patients received alloSCT as salvage therapy after relapse: 1 patient received a transplant of stem cells from a sibling donor and 1 patient from an alternative donor (Figure 1). One of those 10 patients was alive with evidence of disease.
Ninety patients received the consolidation course (Table3). Two patients died because of toxicity of the consolidation course, 19 patients relapsed before transplantation, 7 went off the study because of toxicity/treatment refusal, and 1 patient was allografted with an unrelated donor (Figure 1, Table 3). Sixty-one patients (61% of the patients who achieved CR) received the planned transplant in first remission after the consolidation course. An HLA-identical sibling was identified for 33 patients who received the consolidation course (Figure 1). Twenty-six of the 33 patients (79%) with a donor were allografted in CR-1. The transplantation conditioning included TBI and cyclophosphamide in 85% of the patients. T-cell depletion was performed in 50% of the allografted patients. Ten patients died because of complications of the procedure, 4 patients relapsed, and 12 remained in remission. One of the 7 patients who did not receive transplants remained in CR-1.
Patients' characteristics and outcomes according to the availability of a HLA-identical sibling in 100 patients who reached complete remission
| . | Donor No. (%) . | No Donor No. (%) . | P3-150 . |
|---|---|---|---|
| Total | 39 | 61 | |
| Disease | |||
| MDS | 30 (77) | 47 (77) | .82 |
| AML-MDS | 9 (23) | 14 (23) | |
| Age at start | |||
| Younger than 50 years | 30 (77) | 36 (59) | |
| 50-60 years | 9 (23) | 25 (41) | .10 |
| Median, range | 43, 21-58 | 48, 20-60 | |
| IPSS cytogenetic risk group | |||
| Unknown | 4 (10) | 13 (21) | |
| Good | 17 (44) | 26 (43) | |
| Intermediate | 10 (26) | 11 (18) | .76 |
| Poor | 8 (21) | 11 (18) | |
| Response after first induction | |||
| CR | 32 (82) | 56 (92) | |
| PR | 5 (13) | 4 (7) | .14 |
| Resistance | 2 (5) | 1 (2) |
| . | Donor No. (%) . | No Donor No. (%) . | P3-150 . |
|---|---|---|---|
| Total | 39 | 61 | |
| Disease | |||
| MDS | 30 (77) | 47 (77) | .82 |
| AML-MDS | 9 (23) | 14 (23) | |
| Age at start | |||
| Younger than 50 years | 30 (77) | 36 (59) | |
| 50-60 years | 9 (23) | 25 (41) | .10 |
| Median, range | 43, 21-58 | 48, 20-60 | |
| IPSS cytogenetic risk group | |||
| Unknown | 4 (10) | 13 (21) | |
| Good | 17 (44) | 26 (43) | |
| Intermediate | 10 (26) | 11 (18) | .76 |
| Poor | 8 (21) | 11 (18) | |
| Response after first induction | |||
| CR | 32 (82) | 56 (92) | |
| PR | 5 (13) | 4 (7) | .14 |
| Resistance | 2 (5) | 1 (2) |
Patient characteristics have been compared, using the usual χ2 test or the χ2 test for linear trend in case of ordered variables (cytogenetic risk group, response after first induction course).
Thirty-five of the 57 patients (61%) without a donor received ASCT in CR-1. The transplantation conditioning included TBI and cyclophosphamide in 51% of the patients. The other regimens contained busulphan and cyclophosphamide in 29% of the patients or other non-TBI protocols in 20% of the cases. Four patients died because of complications, 19 patients relapsed, and 12 patients were in continuing CR. Six of the 22 patients who did not receive transplants remained in remission (Figure 1).
Overall results
The median survival of the 184 evaluable patients was 13 months, and the actuarial survival rate at 4 years was 26% (SE = 3.5) (Figure 2A). The median actuarial follow-up was 3.6 years. Median duration of DFS was 12 months. The DFS rate at 4 years from CR was 29% (SE = 4.8%) (Figure 2B). Relapse occurred in 52 of the 100 patients who entered remission, and 16 patients died in CR-1. At 4 years, the estimated cumulative incidence of relapse was 54.5%, and the cumulative incidence of death in CR was 16.6%. Thirty-eight patients were alive and in remission: 6 patients after salvage therapy and 32 patients after remission-induction and consolidation chemotherapy.
Survival curves.
Survival of 184 patients (A) and disease-free survival (DFS) of 100 patients who achieved complete remission (B). N indicates number of patients; O, number of events (death or relapse).
Survival curves.
Survival of 184 patients (A) and disease-free survival (DFS) of 100 patients who achieved complete remission (B). N indicates number of patients; O, number of events (death or relapse).
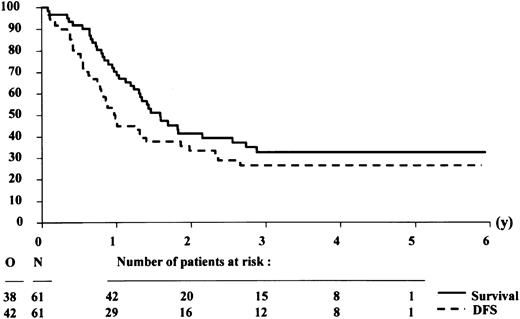
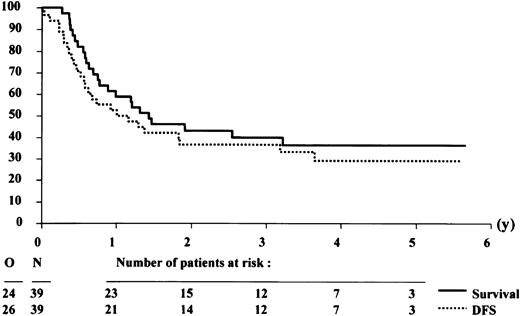
Effect of donor availability
The patient characteristics according to the availability of a donor among the 100 patients who reached CR are presented in Table 3. The characteristics of both groups did not differ significantly, but relatively more patients with a donor were younger than 50 years. Twenty-eight of 39 patients with a donor were allografted in CR-1, including 2 patients without a consolidation course. Thirty-five of 61 patients without a donor were autografted in CR-1. The 4-year DFS rates in the group of patients with or without a donor were 30.8% (SE = 7.9%) and 27.3% (SE = 6.1%), respectively (Figures3 and 4). The estimated hazard ratio (HR) of the donor versus no donor group was 0.93 with a 95% CI of 0.57-1.52. With the use of the Cox model, the estimated HR of older versus younger patients was 1.57 (P = .07). The IPSS cytogenetic risk groups appeared to be of major prognostic importance (estimated HR = 1.75, Wald testP = .0006). The donor versus no donor comparison adjusted by age and IPSS cytogenetic risk groups yielded an estimated HR = 0.77 with a 95% CI of 0.45-1.33. This finding indicates that the estimated daily risk of having an event (relapse or death) in the “donor” group is 23% lower than in patients without a donor.
Survival and disease-free survival (DFS) from complete remission of patients with an HLA-identical sibling donor.
The analysis was performed on an intention-to-treat basis, assuming that all patients with a donor would receive an allogeneic stem cell transplantation after the consolidation course.
Survival and disease-free survival (DFS) from complete remission of patients with an HLA-identical sibling donor.
The analysis was performed on an intention-to-treat basis, assuming that all patients with a donor would receive an allogeneic stem cell transplantation after the consolidation course.
Survival and disease-free survival (DFS) from complete remission of patients without an HLA-identical sibling donor.
The analysis was performed on an intention-to-treat basis, assuming that all patients without a donor would receive an autologous stem cell transplantation after the consolidation course.
Survival and disease-free survival (DFS) from complete remission of patients without an HLA-identical sibling donor.
The analysis was performed on an intention-to-treat basis, assuming that all patients without a donor would receive an autologous stem cell transplantation after the consolidation course.
The 4-year survival rates from CR in the group of patients with or without a donor were 36.4% (SE = 8.1%) and 32.7% (SE = 6.5%), respectively (Figures 3 and 4). The estimated HR of the donor versus no donor group was 1.04 with a 95% CI of 0.62-1.74. The donor versus no donor comparison adjusted by age and cytogenetics yielded an estimated HR = 0.79 with a 95% CI of 0.45-1.38.
Reconstitution after ASCT in CR-1
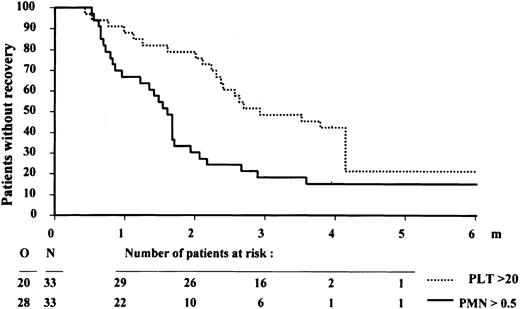
One of the main goals of this study was to test the feasibility to harvest autologous stem cells and to perform ASCT after one consolidation course. Thirty-five of the 57 patients without a donor (61%) received ASCT in CR-1 (Figure 1). The main reasons for not performing the planned transplantation were prolonged hypoplasia and/or insufficient stem cell harvest and early relapse. Seventeen patients received autologous bone marrow cells, 13 received mobilized stem cells, and 5 patients received both bone marrow and mobilized blood stem cells. The median number of infused granulocyte-macrophage colony-forming units (CFU-GM) was 15 × 104/kg body weight. The median time to achieve a granulocyte level of 0.5 × 109/L was 48 days after stem cell infusion, and the median time to reach a level of 20 × 109/L platelets was 85 days. The time to reach a platelet count of 20 × 109/L and a neutrophil count of 0.5 × 109/L is presented in Figure5. The median number of infused CFU-GM (23 × 104/kg body weight) in the mobilized blood stem cell grafts was higher compared with the infused number of bone marrow CFU-GM of 5 × 104/kg body weight. This finding resulted in a more rapid hematopoietic recovery after ASCT (data not shown).
Hematopoietic recovery after autologous stem cell transplantation.
Time to recovery of a platelet count (PLT) of 20 × 109/L and a neutrophil count (PMN) of 0.5 × 109/L of 33 patients who received transplants of autologous stem cells.
Hematopoietic recovery after autologous stem cell transplantation.
Time to recovery of a platelet count (PLT) of 20 × 109/L and a neutrophil count (PMN) of 0.5 × 109/L of 33 patients who received transplants of autologous stem cells.
Discussion
Multidrug chemotherapy, such as that applied to induce CR in de novo AML, is effective in MDS with CR rates averaging from 50% to 64%.10-12,14 In the present prospective, multicenter study 100 of 184 evaluable patients (54%) achieved CR after 1 or 2 courses of remission-induction chemotherapy. Twenty-nine patients died because of complications of therapy, and 55 patients had either persistent disease or have remained hypoplastic. Twelve additional patients entered CR after salvage chemotherapy or after salvage alloSCT, resulting in an overall CR rate of 61%. This CR rate is similar to the CR rate of several trials using high-dose cytarabine, fludarabine, or idarubicin.12 15
The remission duration of MDS treated with AML-type consolidation and/or maintenance chemotherapy is usually short and shorter than similarly treated de novo AML.10,11,14 Estey et al15 suggested that patients with MDS, when compared with de novo AML patients, were more likely to have poor prognostic characteristics, in particular complex cytogenetic abnormalities involving chromosomes 5 and/or 7.
To improve the long-term results of treatment of this high-risk patient group, we developed this study to assess the feasibility of ASCT after 1 course of intensive consolidation chemotherapy for those patients who were not eligible for allotransplantation with an HLA-identical sibling donor. MDSs are clonal stem cell disorders. This fact may raise concern about the presence of sufficient numbers of residual normal stem cells to perform ASCT. However, consolidation therapy that includes high-dose chemotherapy with ASCT seems to be an attractive therapeutic option for the following reasons. Chemotherapy induces cytogenetically normal CRs in the majority of patients with MDS.11 In addition the peripheral stem cell harvests of patients with MDS are usually polyclonal, when assessed by polymerase chain reaction techniques based on X-chromosome inactivation patterns.16 Preliminary data indicate that repopulation after transplantation with mobilized peripheral stem cells is much faster compared with repopulation after autologous BMT.17,18 The experience with ASCT in patients with MDS is limited, and the follow-up is usually short.19-21 An analysis of 79 patients from the European Group for Blood and Marrow Transplant registry showed a 2-year DFS of 34%.22 This finding was significantly inferior to the DFS that was achieved for an age-matched group of patients who underwent transplantation in first remission for de novo AML.22 The 4-year survival and DFS rate of the 61 patients without a donor in CR was 33% and 27%, respectively. Thirty-six of those patients received an ASCT in CR-1 and 1 patient was salvaged after relapse with alloSCT and stem cells from an alternative donor. A similar percentage (62%) of 39 patients with MDS in CR-1 could receive ASCT in a French study.21 The results obtained after ASCT compare favorably to the long-term results obtained with chemotherapy only.10,12 15
AlloSCT with histocompatible siblings is the treatment approach with the highest curative potential for patients with MDS. The issue as to whether patients with MDS should receive remission-induction therapy prior to the transplantation is controversial. Some patient categories may be identified that indicate a low likelihood to enter CR after intensive chemotherapy. Those patients are characterized by a prolonged history of MDS, older age, hypocellular marrow, or multiple chromosomal abnormalities.15 In those cases allogeneic BMT may be considered as first-line therapy.23 An increase in the proportion of marrow blasts to more than 5% has a negative effect on DFS after transplantation, mainly because of an increased risk of relapse.23 One of the analyses from Appelbaum et al7 showed a cumulative relapse risk of 45% in 30 patients with RAEB or RAEBt who received transplants, irrespective of whether patients were treated with immediate transplantation or after remission-induction therapy.24 However, the numbers were limited, and only 6 of the 20 patients treated with chemotherapy prior to the transplantation received the transplant in CR-1.24The event-free survival was 8% when patients had more than 19% marrow blasts at the time of transplantation in a French analysis.25 The cumulative 5-year DFS of 28 patients with RAEBt who had been allografted without a prior attempt to induce remission was 19% in an analysis of the European Group for Blood and Marrow Transplantation (EBMT).23 The 4-year survival and DFS rate of the 39 patients with a donor in CR was 36% and 31%, respectively. Twenty-eight of these patients received an alloSCT in CR-1, and 1 patient was salvaged after relapse. The 4-year DFS rate of the 26 patients who actually underwent transplantation in CR-1 according to the protocol was 42% (data not shown) similar to the DFS of 44% of 230 patients allografted in CR-1 observed in a recent analysis of the EBMT.26
The postconsolidation therapy differed according to the availability of a donor. A true comparison of autologous and allogeneic transplantation was not possible because the percentage of patients who received transplants differed in the 2 groups. Twenty-six of 39 (67%) patients with a donor were allografted, and 35 of 61 (57%) without a donor were autografted. This finding indicates that one should not perform a value comparison according to the treatment actually given but rather according to treatment planned: donor (patients intended to receive an alloSCT) versus no donor (planned ASCT). The 4-year survival and DFS rates in the group of patients with or without a donor were not significantly different. With the use of the Cox model, the adjustment by age and by the cytogenetic risk groups did not influence the comparison of donor versus no donor. The analyses of this study show that it is allowed to draw conclusions regarding the comparison donor versus no donor. However, it was not the intention of this study to prove that one approach was better than the other. Most clinicians in this cooperative group consider alloSCT as the preferred treatment. Therefore, the presence of a donor is likely to introduce a selection bias, which may have been only partly corrected by the Cox model statistics. The IPSS cytogenetic risk groups appeared to be of major prognostic importance both in the group with and without a donor. This shows that cytogenetic features are of utmost importance to compare the outcome, particularly from one study to another.
The actuarial survival at 4 years was 26% in this study. This finding compares favorably with the survival of patients who are treated with supportive care or nonintensive treatment approaches such as described in the study used for the development of the International MDS Risk Classification (IPSS).6 Forty-six patients younger than 60 years were classified in the intermediate-2 risk group of the IPSS study. The actuarial survival rate at 4 years was 10% in the IPSS study. Fifty-six patients were classified in the high-risk group of the IPSS study, and none of these patients was surviving at 4 years. In contrast, 38 patients of our study were in remission after a median of 4 years following initiation of intensive antileukemic therapy. The overall IPSS score did not influence significantly the survival in this study (data not shown). This finding is not surprising because 89% of the patients with MDS fall in the IPSS intermediate-2 and high-risk group. It also confirms the observation of Estey et al15 that the FAB classification (RAEB, RAEBt, or AML-MDS) does not affect the outcome after intensive chemotherapy. Both the cytogenetic risk groups and the duration of antecedent hematologic disorder were of prognostic significance.15
This study shows that the majority of patients younger than 60 years with high-risk MDS or AML-MDS can reach a CR after intensive chemotherapy. Furthermore, stem cell transplantation is feasible in these patients. The majority of patients in remission reach the transplantation step. Intensive consolidation therapy with stem cell transplantation leads to prolonged survival and potential cure in a substantial number of patients.
This paper reports on a prospective study of the European Organization for Research and Treatment of Cancer (EORTC), the European Group for Blood and Marrow Transplantation (EBMT), the Swiss Study Group for treatment of Cancer (SAKK), and the Gruppo Italiano Malattie Ematologiche Maligne dell'Adulto (GIMEMA) Leukemia Cooperative Groups (EORTC study 06921).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Theo de Witte, Department of Hematology, University Medical Center St Radboud, Nijmegen, The Netherlands; e-mail: t.dewitte@hemat.azn.nl.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal