The phenotype of Bcr-Abl–transformed cells is characterized by a growth factor-independent survival and a reduced susceptibility to apoptosis. Furthermore, Bcr-Abl kinase alters adhesion features by phosphorylating cytoskeletal and/or signaling proteins important for integrin function. Integrin-mediated adhesion to extracellular matrix molecules is critical for the regulation of growth and apoptosis. However, effects of integrin signaling on regulation of apoptosis in cells expressing Bcr-Abl are largely unknown. The influence of adhesion on survival and apoptosis in Bcr-Abl+ and Bcr-Abl− BaF3 cells was investigated. p185bcr-abl–transfected BaF3 cells preadhered to immobilized fibronectin had a significant survival advantage and reduced susceptibility to apoptosis following γ-irradiation when compared with the same cells grown on laminin, on polylysin, or in suspension. Both inhibition of Bcr-Abl kinase by STI571 and inhibition of specific adhesion reversed the fibronectin-mediated antiapoptotic effect in BaF3p185. The DNA damage response of Bcr-Abl−BaF3 cells was not affected by adhesion to fibronectin. In contrast to parental BaF3 cells, BaF3p185 adherent to fibronectin did not release cytochrome c to the cytosol following irradiation. The fibronectin-mediated antiapoptotic mechanism in Bcr-Abl–active cells was not mediated by overexpression of Bcl-XL or Bcl-2 but required an active phosphatidylinositol 3-kinase (PI-3K). Kinase-active Bcr-Abl in combination with fibronectin-induced integrin signaling led to a hyperphosphorylation of AKT. Thus, cooperative activation of PI-3K/AKT by Bcr-Abl and integrins causes synergistic protection of Bcr-Abl+ cells from DNA damage–induced apoptosis.
Introduction
c-Abl is a nonreceptor protein tyrosine kinase that is ubiquitously expressed and localized both in the cytoplasm and the nucleus. Its enzymatic activity is tightly regulated in normal cells and plays an important role for the regulation of cell cycle progression and the cellular response to DNA damage.1 The constitutively active oncogenic variants of c-Abl, such as p190Bcr-Abl, p210Bcr-Abl, and v-Abl, lead to growth factor-independent survival and proliferation in various growth factor-dependent hematopoietic cells,2-4 induce a transformed phenotype in vitro and in vivo,3,5-7 and cause malignant disease in various animal models.8-11 The Bcr-Abl fusion protein is generated by reciprocal translocations that are the hallmark of distinct hematologic malignancies such as chronic myelogenous leukemia (CML) and subtypes of acute lymphoblastic leukemia. Bcr-Abl+ cells are protected from apoptosis subsequent to DNA damage and growth factor withdrawal,12-16 are capable of cell cycle progression in the absence of appropriate signals,17,18 and display defects in cell migration and adhesion to extracellular matrix (ECM).19,20 During recent years the molecular mechanisms by which Bcr-Abl causes transformation have been studied extensively. Transformation by Bcr-Abl is dependent on the tyrosine kinase activity of the Bcr-Abl protein.2,21 Accordingly, most of the abnormalities induced by Bcr-Abl are blocked by specific Abl kinase inhibitors such as STI571.12,22-24 A multitude of substrates of the Bcr-Abl tyrosine kinase have been identified, including Bcr-Abl itself and numerous proteins involved in the signal transduction of growth factors such as Shc,25,26phosphatidylinositol-3-kinase (PI-3K),27Ras,7,28 c-Myc,29 Jak/Stat,30focal adhesion kinase (Fak),31,32 the Src kinases (Hck and Lyn),33 Grb10,34 Grb4,35 and c-Jun.36 However, no single pathway has been identified that is responsible for all of the cellular effects of Bcr-Abl. Therefore, it has to be concluded that Bcr-Abl induces a cascade of redundant signals resembling endogenous signal transduction pathways of a variety of mediators such as tyrosine kinase growth factor receptors, the Jak/Stat-dependent receptors, stress response pathways, or integrins.37,38 The integrin family is a major class of receptors involved in the interaction of cells with ECM components.39 Adhesion to ECM molecules has profound effects on proliferation, migration, and apoptosis.40Binding of ECM ligands to the specific integrin chains leads to activation of intracellular messenger molecules. This includes tyrosine and serine/threonine kinase activation, as well as changes in the content of intracellular calcium and phospholipids.41The interaction of integrins with their ECM ligands, however, depends on their activation from inside the cell (inside-out signaling).42,43 Some growth factors like stem cell factor, granulocyte-macrophage colony-stimulating factor, or interleukin-3 (IL-3) have been demonstrated to activate integrins.44-46 The oncogene Bcr-Abl acts both on inside-out and outside-in integrin signaling by interacting with a number of cytoskeletal proteins such as Paxillin,47Fak,31 or Crkl.48 It has been hypothesized that these interactions are responsible for the abnormal integrin function shown in Bcr-Abl+ CML progenitor cells. CML progenitors adhere significantly less to VLA4 (integrins α4/β1) and VLA5 (integrins α5/β1) binding regions of fibronectin despite a normal expression of these proteins, indicating a defect β1-integrin function in Bcr-Abl+ cells.49 In addition CML progenitors are resistant to β1 integrin-mediated inhibition of proliferation.50 In contrast, normal hematopoietic progenitor cells are inhibited after binding to fibronectin at low concentrations of growth factors.51 Finally, Bcr-Abl–transformed cells exhibit an increased rate of spontaneous motility.20 52 Whether this crosstalk between integrins and Bcr-Abl impairs the induction of apoptosis following DNA damage in CML or normal cells has not been studied so far. In the present study, we demonstrate that integrin-mediated adhesion to fibronectin selectively protects cells with an active Bcr-Abl kinase from apoptosis following DNA damage through inhibition of cytochrome c release from mitochondria. We further show that this survival advantage is dependent on cooperative activation of the PI-3K/AKT pathway by integrins and Bcr-Abl.
Materials and methods
Reagents
The Abl-kinase inhibitor STI571 was kindly provided by E. Buchdunger (Novartis, Basel, Switzerland). A stock solution (10 mg/mL) was prepared by dissolving the compound in dimethyl sulfoxide (DMSO)/H2O (1:1) and kept at −20°C. STI571 was used at a concentration of 1 μM. The PI-3 kinase inhibitor LY294002 was obtained from Calbiochem (San Diego, CA) and used at a concentration of 5 μM. The MEK inhibitor PD980059 (Calbiochem) was dissolved in DMSO and used in a final concentration of 25 μM.
Cells and cell culture techniques
The murine wild-type (wt) BaF3 pro–B-lymphocyte cell line depends on IL-3 for growth and viability, whereas the BaF3p185 expressing p185Bcr-Abl became factor independent. Both lines were kindly provided by J. Duyster (Munich, Germany). BaF3p185 was transformed with pSLXBcr-Abl. For transfection with p185bcr-abl a BaF3 subclone was selected that was growing adherent on ECM proteins. The murine 32D myeloid cell line also grows IL-3 dependent and was obtained from the DSMZ cell culture collection (DSMZ, Braunschweig, Germany). The factor-independent 32Dp210 Bcr-Abl oligoclonal cell line was generated by infecting the parental cells with virus produced by infecting the ecotropic Phoenix cell line (Nolan G, Stanford, Stanford, CA) with the retroviral vector Mig-p21053 as previously described.54 As control, parental cells were infected with empty Mig vector. Cells were cultivated at 37°C with 5% CO2 in serum-free UltraCULTURE medium (BioWhittaker; Belgium) complemented with glutamine (2 mM) and 1 ng/mL recombinant murine IL-3 as indicated. Cells were seeded out on 6-well or 96-well plates coated with 0.1 mg/mL fibronectin (Biochrom, Berlin, Germany), 0.1 mg/mL laminin (Biochrom), and 1 μg/mL polylysin (Biochrom). To obtain nonadhesive dishes, culture dishes were treated with a solution of poly(2-hydroxyethyl methacrylate) (polyHEMA; Sigma, St Louis, MO), dissolved at 10 mg/mL in 95% ethanol, and allowed to evaporate.
In some experiments monoclonal antibodies to integrin chains were added to the cultures 1 hour before seeding out the cells on fibronectin-coated 6-well plates. Purified rat anti–mouse integrin β1 (9EG7; 5 μg/mL), rat anti–mouse integrin α4 (9C10; 10 μg/mL), and rat anti–mouse integrin α5 (5H10-27; 10 μg/mL) were obtained from Pharmingen (San Diego, CA).
Survival assay
Cells were plated into 96-well plates at 1 × 104/well in 200 μL of their respective media. After the indicated time period, the viable cells in each well were assayed to their ability to transform 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) into a purple formazan. Therefore, 10 μL of a 10 mg/mL MTT solution was added in each well. After an incubation period of 2 hours at 37°C, the purple formazan was released by adding lysis buffer (15% sodium dodecyl sulfate [SDS] in DMF/H2O 1:1, pH 4.5) and shaking overnight in the dark. The absorbance of the samples was measured in an enzyme-linked immunosorbent assay (ELISA) reader at 570 nm.
Adhesion to fibronectin
BaF3p185 expressing Bcr-Abl and wtBaF3 were suspended (1 × 106/3 mL) and incubated in 6-well plates coated with fibronectin overnight at 37°C. Nonadherent cells were gently removed, and adherent cells were recovered by trypsinization. The percentage of adherent cells was quantified by counting cells in the adherent and nonadherent fraction by using a hemocytometer.
Cell extract preparation
Extracts were prepared as follows: Cells were collected by centrifugation and dissolved in lysis buffer (50 mM Tris-HCl, pH 7.6; 250 mM NaCl; 0.1% Triton X-100; 5 mM EDTA; 1 μg/mL leupeptin; 1 mM phenylmethylsulfonyl fluoride [PMSF]; 1 mM dithiothreitol [DTT]; and 1 μg/mL aprotinin). For phospho-AKT and AKT immunoblots, cells were lysed in modified RIPA (10 mM Tris-HCl, pH 8.0; 150 mM NaCl; 0.5% sodium deoxycholate; 0.1% Triton X-100; 2 μg/mL aprotinin; 2 μg/μL leupeptin; 2 μg/μL pepstatin; 0.5 mM PMSF; 0.5 mM DTT; 25 mM NaF; 0.2 mM NaVO3; and 0.1 μM ocadaic acid). The extracts were quantified by using Bradford protein assay with bovine serum albumin (BSA) as a protein standard.
For the analysis of cytosolic cytochrome c, cells were harvested by centrifugation, washed twice, and resuspended in lysis buffer (25 mM HEPES, pH 7.5; 5 mM MgCl2; 1 mM EGTA; 1 mM PMSF; 10 μg/ml leupeptin; and 10 μg/ml pepstatin). After centrifugation at 800g for 10 minutes at 4°C, supernatants were collected and 5 mM EDTA was added. Subsequently, the extracts were centrifuged at 280 000g for 60 minutes. DTT (2 mM) was added to the supernatants, and protein concentration was determined by using Bradford protein assay.
Immunoprecipitation
For immunoprecipitation, 5 × 106 adherent BaF3 cells were lysed in ice-cold modified RIPA buffer. The lysates were cleared by centrifugation for 15 minutes at 12 000g. Tyrosin-phosphorylated proteins were immunoprecipitated from lysates by addition of 10 μg agarose-conjugated antiphosphotyrosine antibody (Sigma) and incubated for 17 hours at 4°C. Immunocomplexes were then washed 4 times with ice-cold RIPA buffer, resuspended in SDS sample buffer, and separated on 7.5% SDS polyacrylamide gels.
Western blots
Proteins (50 μg each lane) were run on SDS-polyacrylamide gels with the Tris glycine buffer system. Proteins were transferred to polyvinylidene fluoride membranes (Boehringer Mannheim, Germany), using a semi-dry transfer system as recommended by the manufacturer (Bio-Rad, Munich, Germany). Loading of proteins was controlled by ponceau staining prior to antigen detection. The membranes were blocked by incubating in 1% blocking solution (Boehringer Mannheim) for 1 hour. The blots were then incubated with either anti-Abl (1 μg/mL; Pharmingen), anti–Bcl-2 (4 μL/mL; Santa-Cruz, Heidelberg, Germany), anti–Bcl-XL (1 μg/mL; Transduction, Lexington), anti–cytochrome c (1 μg/mL; Pharmingen), anti-AKT (1:1000 dilution; New England Biolabs, Beverly, MA), or antiphospho-AKT (Ser473) antibody (1:1000 dilution; New England Biolabs) in Tris-buffered saline (TBS) with 0.5% blocking solution overnight. Blots were washed twice in TBS with Tween-20 (0.1%) (TBST) for 10 minutes and afterward blocked 2 times with 0.5% blocking solution in TBS for 10 minutes. Then blots were incubated with goat antimouse or goat antirabbit secondary antibody conjugated to horseradish peroxidase (diluted 1:10 000; Jackson Immunotech, West Grove, USA) for 60 minutes, and proteins were detected by chemoluminescence (Boehringer Mannheim).
TUNEL assay
Cells were plated into ECM-coated 6-well plates at 2 × 105/well in 2.7 mL of their respective media. After a growing period of 24 hours at 37°C, nonadherent cells were removed. Adherent cells were washed twice with phosphate-buffered saline (PBS), supplemented with fresh medium, and irradiated with 0, 8, or 50 Gy, respectively. Cells were incubated for 17 hours at 37°C and then harvested by trypsinization. After washing with PBS, cells were fixed in 2% paraformaldehyde in PBS for 30 minutes at room temperature. They were then washed twice with PBS and permeabilized by incubating with 100 μL 0.1% Triton X-100, 0.1% sodium citrate for 2 minutes on ice. After 2 wash steps, the TUNEL reaction was carried out by incubating cells with 0.3 nmol fluorescein isothiocyanate (FITC)-12-dUTP (Boehringer Mannheim), 3 nmol dATP, 2 μL 25 mM CoCl2, 25 U TdT (Boehringer, Mannheim), and TdT buffer (30 mM Tris pH 7.2, 140 mM sodium cacodylate) in a reaction volume of 50 μL at 37°C for 45 minutes. After washing twice, samples were analyzed using a FACScan (Becton Dickinson, San Jose, CA).
Annexin-V staining
Cells were plated into ECM-coated 6-well plates at 2 × 105/well in 2.7 mL of their respective media. After an incubation period of 17 to 24 hours at 37°C as indicated, cells were left untreated or were γ-irradiated with 8 and/or 50 Gy. Following an additional incubation at 37°C for the indicated time period, cells were harvested by trypsinization. FITC-, or phycoerythrin (PE)-conjugated Annexin V was purchased from Pharmingen and used according to the manufacturer's instructions. Harvested cells were washed once in ice-cold PBS and then washed once in cold binding buffer (10 mM HEPES, 140 mM NaCl, 25 mM CaCl2, pH 7.4). The cell pellet was resuspended in 100 μL binding buffer containing 5μL Annexin V-FITC and 10 μL propidium iodide (50-μg/mL stock solution in PBS), or 5 μL Annexin V-PE. The suspension was gently vortexed and incubated at room temperature for 15 minutes. An additional 400 μL binding buffer was added to the cells before analysis using a FACScan and CELLQuest software (Becton Dickinson, San Jose, CA).
Cell cycle analysis
Cell cycle analyses were performed by determination of DNA content with propidium iodide. Cells were washed in PBS and fixed in 70% ethanol for at least 1 hour at 4°C. Shortly before flow cytometry analysis cells were rehydrated in cold PBS, treated with 50 μg/mL RNase A (Boehringer Mannheim) for at least 15 minutes at room temperature, and stained with 50 μg/mL propidium iodide. The stained cells were analyzed by FACScan (Becton Dickinson). The fraction of cells with a DNA content of less than 2N, 2N, 2N to 4N, and 4N was determined by means of the Modfit 2.0 software (Becton Dickinson).
Immunofluorescence and flow cytometry
Cells were washed in EPICS buffer (0.2% BSA (wt/vol); 0.1% sodium azide in PBS) and incubated with either normal rat immunoglobulin G control (Santa-Cruz) or integrin subunit antibodies (1:250 dilution in EPICS buffer) for 30 minutes at 4°C followed by a EPICS buffer wash step and a 30-minute incubation with a goat anti–rat FITC-conjugated antibody (1:250 dilution; Santa-Cruz). Cells were washed twice in EPICS buffer and analyzed by flow cytometry (Becton Dickinson).
Immunofluorescence and confocal laser scan microscopy
Cells in the respective media were allowed to attach for 17 hours to glass cover slips coated with fibronectin or polylysin. Nonadherent cells were gently removed by washing with PBS. After fixation with 4% paraformaldehyde in PBS for 15 minutes, cells were permeabilized with 0.25% Triton X-100 in PBS for 5 minutes, washed with 0.1% BSA in PBS, and stained with rhodamine phalloidin (25 μL/mL of a 200-U methanolic stock solution in PBS supplemented with 1% [wt/vol] BSA; Molecular Probes, Eugene, OR) for 30 minutes at room temperature. Immunofluorescence was measured by using a Leica TCS confocal microscope with a Leica ×63 objective (Leica Microsystems, Heidelberg, Germany).
Statistical analysis
Results of experimental points obtained from multiple experiments are reported as mean ± SEM. Results obtained from triplicates are reported as mean ± SD.
Results
STI571 restores growth factor dependency in Bcr-Abl–transfected BaF3 cells
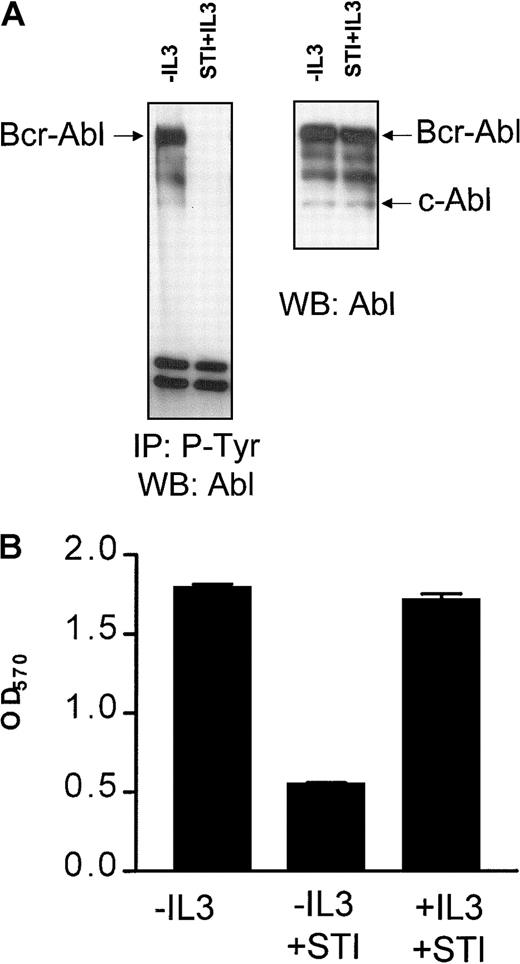
It has been shown previously that Bcr-Abl expression abrogates the requirement of BaF3 cells for exogenous IL-3.2 STI571 is a selective inhibitor of the Abl tyrosine kinase and has been shown to inhibit the Bcr-Abl–mediated growth signals by blocking its tyrosine kinase activity.22 23 Consistent with this, BaF3p185 cells grown neither with IL-3 nor with STI571 Bcr-Abl or proteins bound to Bcr-Abl were highly phosphorylated at tyrosine residues (Figure1A, left panel). This phosphorylation completely disappeared following treatment with 1 μM STI571 for 17 hours even in the presence of the exogenous growth factor IL-3 (Figure1A, left panel). STI571 did not affect Bcr-Abl or c-Abl protein expression, as shown in the Western blot analysis probed with anti-Abl antibody (Figure 1A, right panel).
STI571 completely inhibits Bcr-Abl autophosphorylation and leads to a growth factor-dependent proliferation of Bcr-Abl+ cells.
(A) Immunoprecipitation of tyrosine phosphorylated proteins in Bcr-Abl–expressing BaF3p185 cells with (first lanes) or without STI571+IL-3 (second lanes). Precipitates (left panel) or complete lysates (right panel) were separated in a 7.5% SDS-polyacrylamide gel electrophoresis. Immunoblot was performed by using a monoclonal Abl antibody. (B) Cells were plated into 96-well plates at 1 × 104/well. The viable cells in each well were assayed to their ability to transform MTT into a purple formazan. The absorbance of the samples was measured in an ELISA immunosorbent assay reader at 570 nm. Data shown are representative for 3 independent examinations. Values are means ± SD of triplicates.
STI571 completely inhibits Bcr-Abl autophosphorylation and leads to a growth factor-dependent proliferation of Bcr-Abl+ cells.
(A) Immunoprecipitation of tyrosine phosphorylated proteins in Bcr-Abl–expressing BaF3p185 cells with (first lanes) or without STI571+IL-3 (second lanes). Precipitates (left panel) or complete lysates (right panel) were separated in a 7.5% SDS-polyacrylamide gel electrophoresis. Immunoblot was performed by using a monoclonal Abl antibody. (B) Cells were plated into 96-well plates at 1 × 104/well. The viable cells in each well were assayed to their ability to transform MTT into a purple formazan. The absorbance of the samples was measured in an ELISA immunosorbent assay reader at 570 nm. Data shown are representative for 3 independent examinations. Values are means ± SD of triplicates.
A 48-hour incubation with 1 μM STI571 did not affect the viability of parental BaF3 cells maintained in the presence of growth factor IL-3 (data not shown). Inhibition of the Bcr-Abl kinase activity by STI571 specifically blocked proliferation of IL-3–independent Bcr-Abl+ BaF3p185 cells (Figure 1B). Recent studies from our laboratory demonstrated that BaF3p185 cells rapidly undergo apoptotic cell death after exposure to STI571.12Readdition of murine IL-3 entirely rescued BaF3p185 cells from apoptotic death induced by STI571 (Figure 1B). Similar results were also obtained with bcr-abl–transfected 32D cells expressing p210Bcr-Abl (not shown). Therefore, this system enabled us to compare cells expressing kinase-active Bcr-Abl or kinase-inactive Bcr-Abl with cells lacking Bcr-Abl expression within the same cell type.
Adhesion to immobilized fibronectin selectively protects cells expressing the active Bcr-Abl kinase
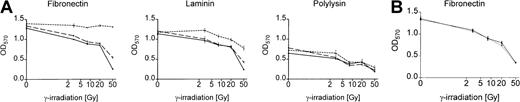
We analyzed survival of Bcr-Abl+ and Bcr-Abl− BaF3 cells adhering to the matrix proteins fibronectin and laminin or binding to polylysin after γ-irradiation by using an MTT-based survival assay. Bcr-Abl+ BaF3 cells adherent to immobilized fibronectin survived DNA damage induced by γ-irradiation significantly better than those grown on laminin or polylysin (Figure 2A). This effect was dependent on the Bcr-Abl kinase activity and could not be detected in cells treated with STI571/IL-3 or in Bcr-Abl− parental BaF3 cells (Figure 2A). Even with high doses of irradiation, cell proliferation was not completely abrogated in BaF3 cells. Twenty hours after irradiation, cells were pulse labeled with BrdU and counterstained with propidium iodide. More than 10% of cells irradiated with 50 Gy remained in S-phase and incorporated BrdU (data not shown).
Bcr-Abl kinase-active BaF3p185 cells adherent to fibronectin exhibit a significant survival advantage following γ-irradiation.
(A) Bcr-Abl+ cells with (dashed line) or without (dotted line) STI571+IL-3 and Bcr-Abl− BaF3 cells (solid line) adherent to fibronectin, laminin, or polylysin were γ-irradiated with indicated dosages. After a growing period of 17 hours, nonadherent cells were removed and survival of adherent cells was measured by using MTT survival assay. The absorbance of the samples was analyzed in an ELISA reader at 570 nm. Data shown are representative for 3 independent experiments. Values are means ± SD of triplicates. (B) Viability of Bcr-Abl− BaF3 cells adherent to fibronectin 17 hours after irradiation with 3, 8, 20, or 50 Gy. Data shown are representative for 3 independent examinations. Values are means ± SD of triplicates.
Bcr-Abl kinase-active BaF3p185 cells adherent to fibronectin exhibit a significant survival advantage following γ-irradiation.
(A) Bcr-Abl+ cells with (dashed line) or without (dotted line) STI571+IL-3 and Bcr-Abl− BaF3 cells (solid line) adherent to fibronectin, laminin, or polylysin were γ-irradiated with indicated dosages. After a growing period of 17 hours, nonadherent cells were removed and survival of adherent cells was measured by using MTT survival assay. The absorbance of the samples was analyzed in an ELISA reader at 570 nm. Data shown are representative for 3 independent experiments. Values are means ± SD of triplicates. (B) Viability of Bcr-Abl− BaF3 cells adherent to fibronectin 17 hours after irradiation with 3, 8, 20, or 50 Gy. Data shown are representative for 3 independent examinations. Values are means ± SD of triplicates.
To rule out the possibility that the effect of STI571 in Bcr-Abl+ cells depends on the inhibition of endogenous c-Abl kinase or other related kinases, we treated Bcr-Abl−, c-Abl+ parental BaF3 cells (wtBaF3) with the combination STI571/IL-3. As shown in Figure 2B, STI571 had no effect on the survival following γ-irradiation in Bcr-Abl− wtBaF3 cells adhered to fibronectin.
Induction of apoptosis following γ-irradiation is reduced selectively in Bcr-Abl+ BaF3 cells adherent to immobilized fibronectin
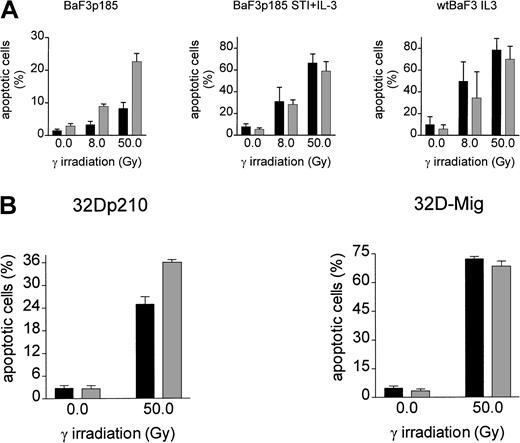
To analyze the effect of adhesion to fibronectin on the induction of apoptosis following γ-irradiation, adherent Bcr-Abl−(grown with IL-3), Bcr-Abl kinase-active (grown in the absence of exogenous growth factor), and Bcr-Abl kinase-inactive cells (with STI571 and IL-3) were irradiated with 0, 8, or 50 Gy. Following a 17-hour incubation at 37°C, induction of apoptosis was measured by using TUNEL reaction. BaF3p185 expressing an active Bcr-Abl kinase showed a lower percentage of apoptotic cells compared with Bcr-Abl kinase-inactive BaF3p185 or Bcr-Abl− wtBaF3 cells (Figure3A). This finding is in accordance with studies by other investigators showing a Bcr-Abl–dependent antiapoptotic effect.55 56 Adhesion to immobilized fibronectin conferred significant further protection from apoptosis exclusively in Bcr-Abl kinase-active cells. In contrast, fibronectin had no protective effect in Bcr-Abl expressing BaF3p185 treated with STI571/IL-3 or parental BaF3 subsequent to irradiation when compared with cells grown on polylysin. Similar results were obtained with 32D cells expressing p210Bcr-Abl (Figure 3B). Apoptotic cells were detected by means of Annexin V-PE. Seventeen hours subsequent to γ-irradiation (50 Gy) we observed an apoptotic fraction of 24.9% ± 1.9% when adhered to fibronectin compared with 36.0% ± 0.7% when bound to polylysin (Figure 3B). Again, in Bcr-Abl− 32D cells treated with IL-3 no difference was seen between polylysin- or fibronectin-bound cells.
Induction of apoptosis by γ-irradiation is selectively reduced in Bcr-Abl+ BaF3 cells following adhesion to fibronectin.
(A) Bcr-Abl+ BaF3 cells with or without STI571/IL-3 and Bcr-Abl− wtBaF3 cells were cultured for 24 hours on immobilized fibronectin (▪) or polylysin (░). Selectively adherent cells were then γ-irradiated with indicated dosages. After a growing period of 17 hours, the percentage of apoptotic cells was determined by TUNEL-assay. Values reflect the mean ± SEM of 3 independent examinations. (B) Percentage of apoptotic cells following irradiation with 0 Gy and 50 Gy of bcr-abl–transfected 32D (left panel) and Mig-transfected 32D cells (right panel). Apoptosis was detected with Annexin V-PE. Values reflect the mean ± SEM of 3 independent examinations. ▪ indicates fibronectin; ░, polylysin.
Induction of apoptosis by γ-irradiation is selectively reduced in Bcr-Abl+ BaF3 cells following adhesion to fibronectin.
(A) Bcr-Abl+ BaF3 cells with or without STI571/IL-3 and Bcr-Abl− wtBaF3 cells were cultured for 24 hours on immobilized fibronectin (▪) or polylysin (░). Selectively adherent cells were then γ-irradiated with indicated dosages. After a growing period of 17 hours, the percentage of apoptotic cells was determined by TUNEL-assay. Values reflect the mean ± SEM of 3 independent examinations. (B) Percentage of apoptotic cells following irradiation with 0 Gy and 50 Gy of bcr-abl–transfected 32D (left panel) and Mig-transfected 32D cells (right panel). Apoptosis was detected with Annexin V-PE. Values reflect the mean ± SEM of 3 independent examinations. ▪ indicates fibronectin; ░, polylysin.
To determine whether adhesion to immobilized fibronectin is essential for the observed antiapoptotic effect in Bcr-Abl active cells, BaF3p185 cells were cultured either in suspension on polyHEMA-coated dishes with soluble fibronectin (10 μg/mL) or on immobilized fibronectin. Seventeen hours after irradiation apoptotic cells were determined by means of FITC-conjugated Annexin V. In contrast to the antiapoptotic mechanism in Bcr-Abl kinase-active BaF3p185 cells mediated by immobilized fibronectin, soluble fibronectin had no protective effect on BaF3p185 cells in suspension (Figure4, upper panel). Neither immobilized nor soluble fibronectin protects BaF3p185 cells treated with the combination STI571 and IL-3 (Figure 4, lower panel).
Soluble fibronectin does not provide any benefit to Bcr-Abl+ cells.
Bcr-Abl+ BaF3 cells were cultured either in suspension on polyHEMA-coated dishes in the presence (░) or absence (■) of soluble fibronectin or on immobilized fibronectin (▪). Selectively adherent cells (on immobilized fibronectin) or all cells (in suspension with soluble fibronectin) were irradiated with 0, 8, or 50 Gy. After a growing period of 17 hours, the percentage of apoptotic cells was determined by using Annexin V-FITC assay. Values reflect the mean ± SEM of 3 independent examinations.
Soluble fibronectin does not provide any benefit to Bcr-Abl+ cells.
Bcr-Abl+ BaF3 cells were cultured either in suspension on polyHEMA-coated dishes in the presence (░) or absence (■) of soluble fibronectin or on immobilized fibronectin (▪). Selectively adherent cells (on immobilized fibronectin) or all cells (in suspension with soluble fibronectin) were irradiated with 0, 8, or 50 Gy. After a growing period of 17 hours, the percentage of apoptotic cells was determined by using Annexin V-FITC assay. Values reflect the mean ± SEM of 3 independent examinations.
Antibodies to the fibronectin receptors VLA4 (integrins α4/β1) and VLA5 (integrins α5/β1) block the adhesion-mediated antiapoptotic effect
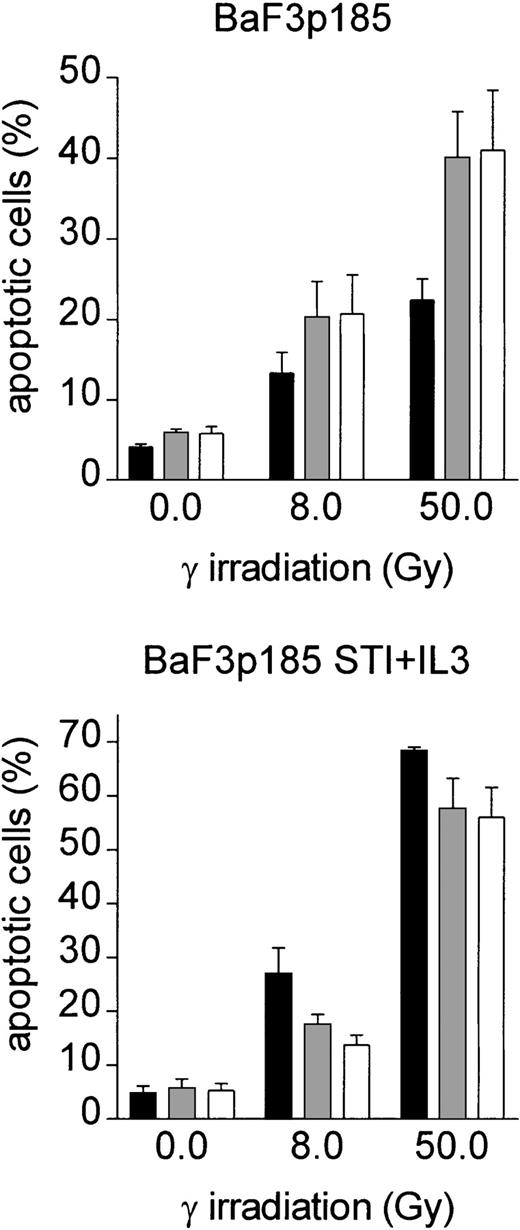
To assess the role of specific integrins, we performed adhesion assays on fibronectin in the presence of antibodies against β1, α4, and α5 integrins. Neither the anti-β1 antibody, nor the anti-α4 antibody, or the anti-α5 antibody alone blocked the fibronectin-induced effect on induction of apoptosis (not shown). However, blocking both integrin chains by combining β1/α4 antibodies (VLA4) or β1/α5 antibodies (VLA5) at least partially inhibited the fibronectin-induced antiapoptotic effect of BaF3p185 (Figure 5, left panel). In contrast, anti-VLA4 or anti-VLA5 antibodies had no effect on induction of apoptosis in BaF3p185 cells expressing the active Bcr-Abl kinase when grown on polylysin (Figure 5, middle panel). In addition, induction of apoptosis is independent of fibronectin receptor blockade when Bcr-Abl kinase activity is inhibited by STI571 (Figure 5, right panel). Thus, this protective mechanism is dependent on the specific adhesion of Bcr-Abl+ cells to fibronectin through binding to VLA4 and/or VLA5 receptors.
Treatment of Bcr-Abl+ BaF3p185 cells with antibodies to the fibronectin receptor chains leads to a significant reduction of the fibronectin-mediated antiapoptotic effect.
Bcr-Abl+ BaF3 cells with or without STI571/IL-3 were cultured in the absence (■) or presence of anti-α5/β1 (░) or anti-α4/β1 (■) integrin antibodies. Cells were then plated on immobilized fibronectin or polylysin and cultured overnight at 37°C. All cells (nonadherent and adherent cells) were then irradiated with 0 Gy or 50 Gy and grown for an additional 8 hours. The percentage of apoptotic cells was determined by using Annexin V-FITC staining. Values reflect the mean ± SEM of 3 independent examinations.
Treatment of Bcr-Abl+ BaF3p185 cells with antibodies to the fibronectin receptor chains leads to a significant reduction of the fibronectin-mediated antiapoptotic effect.
Bcr-Abl+ BaF3 cells with or without STI571/IL-3 were cultured in the absence (■) or presence of anti-α5/β1 (░) or anti-α4/β1 (■) integrin antibodies. Cells were then plated on immobilized fibronectin or polylysin and cultured overnight at 37°C. All cells (nonadherent and adherent cells) were then irradiated with 0 Gy or 50 Gy and grown for an additional 8 hours. The percentage of apoptotic cells was determined by using Annexin V-FITC staining. Values reflect the mean ± SEM of 3 independent examinations.
Expression pattern of integrin chains β1, α4, and α5 on Bcr-Abl+ and Bcr-Abl− BaF3 cells and adhesion features of the cell lines
To investigate the mechanism of the fibronectin-induced antiapoptotic effect in Bcr-Abl+ cells, we first examined the expression levels of the integrin chains β1, α4, and α5 on Bcr-Abl+ BaF3p185 and parental BaF3 cells (wtBaF3) by FACS analysis. As shown in Figure 6A, β1, α4, and α5 chains were expressed at comparable levels at the surface of both parental (wtBaF3) and p185bcr-abl–transformed BaF3 (BaF3p185) cells.
Effects of fibronectin binding in BaF3 cells expressing kinase active Bcr-Abl.
(A) Expression of β1, α4, and α5 integrin chains in wt andbcr-abl–transfected BaF3 cells were analyzed by flow cytometry. Bcr-Abl+ and Bcr-Abl− BaF3 cells were stained with an isotype control antibody (filled curves) or with monoclonal antibodies directed against β1, α4, and α5 integrin subunits (solid lines). (B) Adhesion of Bcr-Abl+ and Bcr-Abl− cells to fibronectin. Wild-type BaF3 cells supplemented with IL-3 and Bcr-Abl+ BaF3p185 cells in the absence or presence of STI571/IL-3 were cultured for 17 hours on immobilized fibronectin. Numbers of cells in suspension and adherent cells were determined by using a hemocytometer. Values reflect the mean ± SEM of 3 independent examinations. (C) Percentage of S-phase in adherent cells grown on immobilized fibronectin. Cell cycle analysis of adherent Bcr-Abl+ cells with or without STI571+IL-3 and wtBaF3 cells grown on fibronectin was determined by propidium iodide staining and FACScan. Values reflect the mean ± SEM of 3 independent examinations. (D) Differences in actin staining of Bcr-Abl–transformed BaF3 cells with or without STI571/IL-3 grown on fibronectin or polylysin. Adherent cells were fixed and stained for F-actin by using rhodamine-labeled phalloidin.
Effects of fibronectin binding in BaF3 cells expressing kinase active Bcr-Abl.
(A) Expression of β1, α4, and α5 integrin chains in wt andbcr-abl–transfected BaF3 cells were analyzed by flow cytometry. Bcr-Abl+ and Bcr-Abl− BaF3 cells were stained with an isotype control antibody (filled curves) or with monoclonal antibodies directed against β1, α4, and α5 integrin subunits (solid lines). (B) Adhesion of Bcr-Abl+ and Bcr-Abl− cells to fibronectin. Wild-type BaF3 cells supplemented with IL-3 and Bcr-Abl+ BaF3p185 cells in the absence or presence of STI571/IL-3 were cultured for 17 hours on immobilized fibronectin. Numbers of cells in suspension and adherent cells were determined by using a hemocytometer. Values reflect the mean ± SEM of 3 independent examinations. (C) Percentage of S-phase in adherent cells grown on immobilized fibronectin. Cell cycle analysis of adherent Bcr-Abl+ cells with or without STI571+IL-3 and wtBaF3 cells grown on fibronectin was determined by propidium iodide staining and FACScan. Values reflect the mean ± SEM of 3 independent examinations. (D) Differences in actin staining of Bcr-Abl–transformed BaF3 cells with or without STI571/IL-3 grown on fibronectin or polylysin. Adherent cells were fixed and stained for F-actin by using rhodamine-labeled phalloidin.
As noted in “Materials and methods,” we used a BaF3 cell clone highly adherent to fibronectin. By performing an adhesion assay for 17 hours, we observed that BaF3p185 cells expressing the active Bcr-Abl kinase showed a slightly decreased fraction of adherent cells (74.85% ± 5.77%) compared with 88.75% ± 5.77% in Bcr-Abl− wtBaF3 or 85.67% ± 2.98% in Bcr-Abl–expressing cells treated with STI571/IL-3 (Figure 6B). In addition, Bcr-Abl kinase-active BaF3p185 cells had no advantage in proliferation on fibronectin as shown in Figure 6C. Accordingly, the S-phase fraction of BaF3p185 cells adhered to fibronectin was slightly decreased when compared with Bcr-Abl− or Bcr-Abl kinase-inactive BaF3 cells (Figure 6C).
However, the morphology of Bcr-Abl+ cells differed significantly from cells without Bcr-Abl or cells exposed to STI571. In cells expressing an active Bcr-Abl kinase, binding to fibronectin displayed more extensive actin polymerization, cell spreading, and more actin-containing protrusions such as pseudopodia and filopodia when compared with the same cells grown on polylysin (Figure 6D, compare left and right panels). This cellular response was significantly reduced by treatment with STI571 (Figure 6D, compare left and middle panels). Therefore, we conclude that in BaF3 cells both Bcr-Abl and fibronectin in concert induce cell shape changes and the protection to radiation-induced apoptosis. These effects are not associated with changes on cell cycle or adhesion.
Adhesion to fibronectin inhibits the release of cytochrome c from mitochondria following γ-irradiation in Bcr-Abl–expressing cells
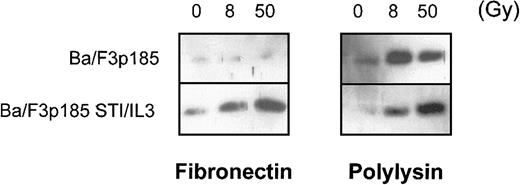
The major mechanism of processing procaspases in mammalian cells is mediated by Apaf-1. Apaf-1 is activated by binding to cytochrome c that then is released from the mitochondria during early stages of apoptosis.57 We examined whether the antiapoptotic effect in BaF3p185 cells adherent to fibronectin is mediated by blocking the release of cytochrome c from mitochondria. Following γ-irradiation of 8 and 50 Gy, we observed cytochrome c in the cytoplasmic fraction in BaFp185 cells treated with Bcr-Abl inhibitor STI571 and IL-3 both when adherent to fibronectin and to polylysin (Figure 7, right and left lower panels). Irradiation of BaF3p185 cells grown on polylysin also caused significant cytosolic accumulation of cytochrome c (Figure 7, upper-right panel). However, cytochrome c release was completely blocked in Bcr-Abl kinase-active cells adherent to immobilized fibronectin (Figure 7, upper-left panel). Thus, the inhibition of cytochrome c release into the cytoplasm caused by the adhesion to fibronectin seems to be an important mechanism mediating the protection of Bcr-Abl–active cells from γ-irradiation induced apoptotic cell death.
Bcr-Abl+ cells adherent to fibronectin do not accumulate cytochrome c in the cytoplasm following γ-irradiation.
Bcr-Abl+ BaF3p185 were cultured in the presence or absence of STI571/IL-3 overnight in serum-free medium on fibronectin or polylysin. Nonadherent cells were removed, selectively adherent cells were γ-irradiated with 0, 8, or 50 Gy as indicated. After a growing period of 17 hours, Western blot analysis of cytochrome c in the cytoplasmic fraction (5 μg/lane) was performed.
Bcr-Abl+ cells adherent to fibronectin do not accumulate cytochrome c in the cytoplasm following γ-irradiation.
Bcr-Abl+ BaF3p185 were cultured in the presence or absence of STI571/IL-3 overnight in serum-free medium on fibronectin or polylysin. Nonadherent cells were removed, selectively adherent cells were γ-irradiated with 0, 8, or 50 Gy as indicated. After a growing period of 17 hours, Western blot analysis of cytochrome c in the cytoplasmic fraction (5 μg/lane) was performed.
Expression of Bcl-XL and Bcl-2
It has been shown previously that overexpression of Bcl-XL and Bcl-2 can block the mitochondrial release of cytochrome c.58 To assess whether the integrin/Bcr-Abl–mediated inhibition of cytochrome c release is caused by regulation of Bcl-XL or Bcl-2, we analyzed the protein levels of these antiapoptotic proteins under the appropriate conditions. For Bcl-XL, we observed a Bcr-Abl–dependent up-regulation (Figure 8A, compare upper- and lower-left panels). This is in agreement with reports from different groups.12,56,59 Adhesion to fibronectin, however, had no further effect on Bcl-XL expression in Bcr-Abl+ BaF3p185 cells (Figure 8A, compare lower-left and right panels). In contrast, Bcr-Abl expression led to a remarkable down-regulation of Bcl-2 (Figure 8B, compare upper- and lower-left panels). The latter has also been reported for Bcr-Abl–expressing HL60 and K562 cells by Amarante-Mendes et al.60 Nevertheless, as observed for Bcl-XL, we found no difference in Bcl-2 protein expression dependent on the matrix used for cell adhesion. Moreover, no change of Bcl-XL and Bcl-2 expression was seen subsequent to γ-irradiation (Figure 8A,B). Therefore, we conclude that the inhibition of cytochrome c release in Bcr-Abl–expressing BaF3p185 cells adherent to fibronectin is not caused by an up-regulation of the Bcl-XL or Bcl-2 protein.
Adhesion to fibronectin has no effect on Bcl-XL or Bcl-2 protein expression.
Bcr-Abl+ and Bcr-Abl− BaF3 cells adherent to fibronectin or polylysin were γ-irradiated with 0 Gy or 8 Gy. After a 17-hour incubation, cells were washed and lysed in lysis buffer, and 50 μg/lane was subjected to immunblot analysis by using anti–Bcl-XL antibody (A) or anti–Bcl-2 antibody (B).
Adhesion to fibronectin has no effect on Bcl-XL or Bcl-2 protein expression.
Bcr-Abl+ and Bcr-Abl− BaF3 cells adherent to fibronectin or polylysin were γ-irradiated with 0 Gy or 8 Gy. After a 17-hour incubation, cells were washed and lysed in lysis buffer, and 50 μg/lane was subjected to immunblot analysis by using anti–Bcl-XL antibody (A) or anti–Bcl-2 antibody (B).
Adhesion-induced inhibition of apoptosis in Bcr-Abl+cells is dependent on PI-3K activation but not on mitogen-activated protein kinase
Adhesion of different cell types to fibronectin activates different promitogenic and antiapoptotic pathways, namely the Ras/Raf-1/mitogen-activated protein kinase (MAPK) and the PI-3K pathway through integrin-mediated signal transduction (for review, see Giancotti and Ruoslahti61). We examined whether the observed fibronectin-induced antiapoptotic mechanism in Bcr-Abl kinase-active cells depends on the activation of the Ras/Raf/Mek/Erk or PI-3K pathway by using pathway-specific inhibitors. As shown in Figure9, inhibition of MAPK kinase 1 and 2 (MEK-1/2) by PD98059 had no effect on γ-irradiation–induced apoptosis of BaF3p185 cells grown on fibronectin. In contrast, inhibition of PI-3K by LY294002 completely reversed the fibronectin-induced protection from DNA damage. As shown in Figure 9, the percentage of apoptotic cells following γ-irradiation in BaF3p185 cells adherent to fibronectin and pretreated with LY294002 was 44.90% ± 2.80% versus 19.80% ± 3.26% when grown on fibronectin without inhibitor or with PD980059.
The PI-3K inhibitor LY294002 completely antagonizes the fibronectin-induced antiapoptotic effect in Bcr-Abl+ cells.
Bcr-Abl+ BaF3p185 cells cultured on fibronectin were treated without (■) or with PD98059 (░) or LY294002 (■). After a growing period of 24 hours, all cells (adherent and nonadherent cells) were irradiated with 0 Gy or 50 Gy and incubated with or without inhibitor for an additional 8 hours. The percentage of apoptotic cells was then determined by using Annexin V-FITC staining. Values reflect the mean ± SEM of 3 independent examinations.
The PI-3K inhibitor LY294002 completely antagonizes the fibronectin-induced antiapoptotic effect in Bcr-Abl+ cells.
Bcr-Abl+ BaF3p185 cells cultured on fibronectin were treated without (■) or with PD98059 (░) or LY294002 (■). After a growing period of 24 hours, all cells (adherent and nonadherent cells) were irradiated with 0 Gy or 50 Gy and incubated with or without inhibitor for an additional 8 hours. The percentage of apoptotic cells was then determined by using Annexin V-FITC staining. Values reflect the mean ± SEM of 3 independent examinations.
Hyperactivation of AKT (protein kinase B) in Bcr-Abl kinase-active cells adherent to fibronectin as a consequence of Bcr-Abl and integrin signaling
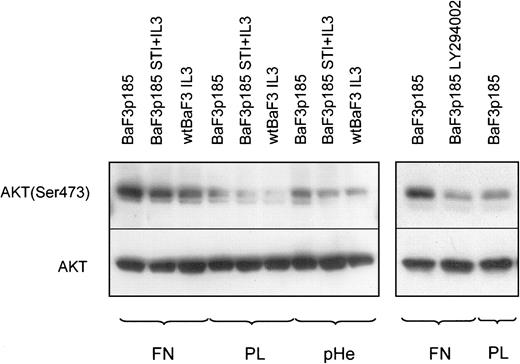
AKT/protein kinase B (PKB) has been identified as a direct target of PI-3K.62 Phosphorylation of Thr-308 and Ser-473 is required for activation of AKT. Activated AKT induces cell survival and suppresses apoptotic cell death in a number of cell types. In this context it has been reported recently that AKT inhibits apoptosis by preventing the release of cytochrome c.63 We therefore examined whether AKT phosphorylation is a mediator of the observed fibronectin-mediated inhibition of cytochrome c release in Bcr-Abl kinase-active cells. To address this situation, cells were harvested after a growing period of 45 minutes on fibronectin, polylysin, or polyHEMA and analyzed for AKT phosphorylation at Ser-473. Whereas AKT protein expression is not dependent on Bcr-Abl kinase activity or on fibronectin adhesion, Ser-473 phosphorylation of AKT was induced in Bcr-Abl+ and Bcr-Abl− cells when they adhered to fibronectin (Figure 10, left panel). This finding was not unexpected because integrin signaling leads to AKT activation in a variety of cells (for review, see Kumar39). Consistent with other reports64 65we also detected a higher Ser-473 phosphorylation of AKT in Bcr-Abl kinase-active cells (Figure 10, left panel). Both Bcr-Abl signaling and fibronectin-induced integrin signaling led to a hyperphosphorylation of AKT. Thus, the crosstalk between Bcr-Abl and integrin signals led to a more than additive phosphorylation on Ser-473 of AKT (Figure 10, left panel, lane 1). In Bcr-Abl kinase-inactive cells or in Bcr-Abl− cells treated with IL-3, we did not observe this effect (Figure 10, left panel, lanes 2 and 3). AKT phosphorylation in Bcr-Abl+ cells adherent to fibronectin completely disappeared following treatment with LY294002 (Figure 10, right panel).
AKT is hyperphosphorylated in BaF3p185 cells adherent to fibronectin.
Bcr-Abl− BaF3 cells with IL-3 and Bcr-Abl+BaF3p185 cells with or without STI571/IL-3 were cultured in serum-free RPMI medium for 4 hours in the presence or absence of 5 μM LY294002. Cells were then plated onto fibronectin (FN)-, polylysin (PL)-, or polyHEMA (pHe)-coated dishes with or without inhibitor and incubated for 45 minutes in serum-free RPMI at 37°C. Lysates were prepared from adherent cells and analyzed by Western blotting by using antiphospho-AKT or anti-AKT.
AKT is hyperphosphorylated in BaF3p185 cells adherent to fibronectin.
Bcr-Abl− BaF3 cells with IL-3 and Bcr-Abl+BaF3p185 cells with or without STI571/IL-3 were cultured in serum-free RPMI medium for 4 hours in the presence or absence of 5 μM LY294002. Cells were then plated onto fibronectin (FN)-, polylysin (PL)-, or polyHEMA (pHe)-coated dishes with or without inhibitor and incubated for 45 minutes in serum-free RPMI at 37°C. Lysates were prepared from adherent cells and analyzed by Western blotting by using antiphospho-AKT or anti-AKT.
Discussion
The extracellular environment is known to control growth, survival, and differentiation of cells via signals mediated by the interaction of adhesion receptors with ECM molecules (for review, see Boudreau and Jones66). An altered interaction between matrix proteins and transformed cells has been described in various models for malignant diseases, including CML.67-71
In the present study we analyzed the consequences of adhesion ofbcr-abl–transformed cells to different matrix proteins on survival and apoptosis. Our results strongly suggest a crosstalk between the transforming activity of Bcr-Abl and integrin signaling, leading to an enhanced protective effect against apoptosis following DNA damage by γ-irradiation. In contrast, no such crosstalk could be observed between the growth factor IL-3 and integrins.
A number of previous studies suggested that Bcr-Abl could influence adhesion-dependent biological processes.20,72,73Philadelphia chromosome-positive progenitor cells of CML patients show a decreased adhesion to fibronectin and stromal cells when compared with nonleukemic progenitors. The cause for this seems to be an altered function of β1 integrin.49 These observations can be interpreted in 2 different ways. Integrins present on CML progenitors may be in a low-affinity state, which results in diminished ligand binding. Defective function of β1 integrins may be caused by the abnormal reorganization of integrins after ligand binding in Bcr-Abl+ cells.74 Nevertheless, direct studies on the affinity of integrin-ligand interaction have not yet been published. An alternative explanation may be the existence of an intracellular crosstalk between the signaling cascades induced by integrin binding and Bcr-Abl kinase activity. Previous published data and our own observations favor the latter hypothesis: Bcr-Abl–transformed cells have an increased spontaneous motility on fibronectin-coated surfaces when compared with Bcr-Abl−cells.20 In addition, morphologic changes suggest a specific alteration of cytoskeletal function induced by the combined Bcr-Abl–fibronectin signal.20 These results were obtained both in Bcr-Abl+ cell lines and in primary CML cells. Thus, despite the decreased overall adhesion of primary CML cells to fibronectin as described, fibronectin binding of Bcr-Abl+cells can induce specific functional alterations. Our experiments show a direct biochemical consequence of such a crosstalk between Bcr-Abl and integrin signaling: Activation of both pathways is required for maximum AKT phosphorylation in bcr-abl–transformed BaF3 cells. In summary, these data support the view that an intracellular crosstalk between Bcr-Abl and integrin signaling is partially responsible for the functional alterations observed in Bcr-Abl+ cells.
Previously, it has been shown that Bcr-Abl+ progenitors that adhere to stroma (20%-40% of the cells) are not subject to normal integrin-mediated negative cell cycle regulation by matrix proteins.50 One mechanism underlying this loss of integrin-mediated growth inhibition mediated by Bcr-Abl is the relocation of p27 to the cytoplasm that was observed in fibronectin-adherent CML CD34+ cells.75Similar to Bcr-Abl in CML progenitors, physiologic factors such as IL-3 and stem cell factor in normal progenitors also override β1 integrin-mediated inhibition of cell proliferation.76 Our observation that Bcr-Abl–transformed cells do not show any significant changes in proliferation or adhesive properties on fibronectin when compared with IL-3–treated control cells is therefore not unexpected.
The abnormal response to integrin-mediated signals in Bcr-Abl–transformed cells may have important consequences for the progression of the disease. First, it may exert an influence on cell cycle regulation by preventing p27 activity by a yet unknown mechanism that may contribute to abnormal proliferation. Second, it may contribute to the down-regulation of apoptotic pathways leading to an enhanced survival following DNA damage. Together there might be a synergy resulting in the survival and accumulation of genetically altered cells contributing to the progression of the disease.
The active Bcr-Abl kinase itself exhibits a variety of antiapoptotic properties in murine and human cell lines as well as in primary CML cells. The molecular details of Bcr-Abl–induced protection from apoptosis after DNA damage and growth factor withdrawal are not fully understood. In bcr-abl–transfected cell lines an elevated expression of Bcl-255 or Bcl-XL12,56,59 has been described. In addition, Bcr-Abl inhibits apoptosis by Bad phosphorylation.65 The biologic and molecular characteristics of the antiapoptotic pathway elucidated by our experiments are different from those previously described. Neither Bcl-2 nor Bcl-XL appears to be regulated by the interaction with fibronectin. In addition, exogenous exposure to IL-3 is not capable of replacing Bcr-Abl in delivering the survival signal in concert with fibronectin binding. These observations contrast studies regarding the regulation of Bcl-XL by Bcr-Abl in which IL-3 was sufficient to restore the Bcr-Abl signal.12 In the present study inhibition of the Bcr-Abl kinase by STI571, even in the presence of IL-3, and specific blocking of the fibronectin receptors by integrin antibodies antagonized the antiapoptotic effect. These findings support the notion that maximum protection against apoptosis in Bcr-Abl+ cells requires signals from both Bcr-Abl and β1-integrins.
A critical step in the apoptotic cascade is the loss of integrity of the outer mitochondrial membrane accompanied by the release of cytochrome c into the cytoplasm. In the present study, we demonstrate that the crosstalk between the transforming Bcr-Abl–mediated signal and the physiologic integrin-induced pathway led to inhibition of cytochrome c release from the mitochondria after γ-irradiation. In addition, we show that the adhesion-dependent protection from γ-irradiation–induced apoptosis in BaF3p185 cells was abolished in the presence of LY294002, a pharmacologic inhibitor of PI-3K. This lipid kinase has been demonstrated to play an important role in integrin signal transduction.77,78 PI-3K regulates cell migration directly or by stimulation of Rac or Cdc42.79Another target of PI-3K is the serine threonine kinase AKT.80 AKT is a general mediator of growth factor-induced survival and has been shown to inhibit apoptosis by various stimuli.80 PI-3K is also essential for Bcr-Abl–induced transformation both in vivo and in vitro.64 Thus, integrin-mediated prevention of apoptosis following γ-irradiation in Bcr-Abl–transformed cells may require activation of the PI-3K pathway. However, the block of apoptosis and mitochondrial events does not involve Bcl-2 or BclXL.
The findings of the present study provide new insights into the biology of Bcr-Abl–induced malignancies and their resistance to therapeutic intervention. The signals described so far for Bcr-Abl are largely overlapping with those of physiologic growth factors such as IL-3. However, our data support the hypothesis that there are significant biological and molecular differences between Bcr-Abl and IL-3. The protection from apoptosis by integrins requires an active Bcr-Abl kinase also in the presence of IL-3. This enhanced protection from apoptosis compared with that mediated by physiologic growth factors might lead to inappropriate survival of damaged cells. Furthermore, the integrins involved in Bcr-Abl–induced antiapoptotic effect are also involved in homing and survival of hematopoietic stem cells (for review, see Papayannopoulou and Craddock81). A selective protection of Bcr-Abl+ cells by adhesion to fibronectin possibly alters the response of early hematopoietic cells to DNA damage specifically. These mechanisms may contribute to the continuing genetic damage of immature Bcr-Abl+ cells and thereby lead to the clonal evolution of the disease.
Bcr-Abl mediates its survival signal at least partly by altering intracellular signaling cascades of other exogenous survival signals. The cooperative signals investigated in this study are active predominantly in the bone marrow environment. This fact may be of great importance for potential treatment options of the disease. Treatment of CML with chemotherapy leads to a transient reduction of the number of leukemic cells.82 However, the leukemic clone usually recovers quicker than normal hematopoiesis. Therefore, these therapies do not mediate durable remissions in CML patients. In addition, high-dose chemotherapy treatment combined with stem cell transplantation does not eradicate the disease in most patients as indicated by the poor efficacy of syngeneic twin transplantation in CML.83 These data show that Bcr-Abl+hematopoietic stem cells are resistant to apoptosis induced by chemotherapeutic agents. Our present study suggests that part of the resistance of CML to treatment with DNA-damaging agents is mediated by reversible interactions with the ECM. A further exploration of this mechanism may therefore allow the development of strategies interfering with the cell-matrix interaction, leading toward a more effective treatment of CML. Use of integrin antibodies or disintegrins, for example, may reverse the fibronectin-induced chemotherapy resistance of CML.
We thank H. Michels for technical assistance and Dr E. Buchdunger (Novartis, Basel, Switzerland) for the generous gift of STI571.
Supported by the Robert Bosch Foundation project no. O8-99 and by grants (J.D.) from the German Mildred-Scheel-Stiftung and the BMBF, SFB456. C.M. is supported by a fellowship from the Deutsche José-Carreras Stiftung.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Walter E. Aulitzky, Robert-Bosch-Krankenhaus, Auerbachstr 110, 70376 Stuttgart, Germany; e-mail:walter.aulitzky@rbk.de.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal