Abstract
Genome-wide chemical mutagenesis screens in the zebrafish(Danio rerio) have led to the identification of novel genes affecting vertebrate erythropoiesis. In determining if this approach could also be used to clarify the molecular genetics of myelopoiesis, it was found that the developmental hierarchy of myeloid precursors in the zebrafish kidney is similar to that in human bone marrow. Zebrafish neutrophils resembled human neutrophils, possessing segmented nuclei and myeloperoxidase-positive cytoplasmic granules. The zebrafish homologue of the human myeloperoxidase (MPO) gene, which is specific to cells of the neutrophil lineage, was cloned and used to synthesize antisense RNA probes for in situ hybridization analyses of zebrafish embryos. Granulocytic cells expressing zebrafishmpo were first evident at 18 hours after fertilization (hpf) in the posterior intermediate cell mass (ICM) and on the anterior yolk sac by 20 hpf. By 24 hpf, mpo-expressing cells were observed along the ICM and within the developing vascular system. Thus, the mpo gene should provide a useful molecular probe for identifying zebrafish mutants with defects in granulopoiesis. The expression of zebrafish homologues was also examined in 2 other mammalian hematopoietic genes, Pu.1, which appears to initiate a commitment step in normal mammalian myeloid development, andL-Plastin, a gene expressed by human monocytes and macrophages. The results demonstrate a high level of conservation of the spatio-temporal expression patterns of these genes between zebrafish and mammals. The morphologic and molecular genetic evidence presented here supports the zebrafish as an informative model system for the study of normal and aberrant human myelopoiesis.
Introduction
During vertebrate hematopoietic development, common myeloid progenitors give rise to separate precursors for cells of the granulocyte/macrophage and erythrocyte/megakaryocyte lineages.1 Studies of the genetic control of normal myelopoiesis have focused on transcription factors and growth factors and their receptors, largely through targeted disruption of these genes in the mouse.2,3 Many of the genes known to regulate erythropoiesis in mammals have been identified in another vertebrate animal model, the zebrafish (Danio rerio).4Homologues of known mammalian hematopoietic transcription factors, such as gata-1, gata-2, c-myb, scl, fli-1, Imo2, andcbfb are all appropriately expressed during zebrafish embryogenesis.5-9 Chemical mutagenesis screens in this organism have resulted in the isolation of numerous zebrafish lines containing mutations that affect both embryonic and definitive erythropoiesis.5,10 Positional cloning of genes altered in these mutant lines has identified genes that are homologous to those linked to anemia,11,12 resulting in a new animal model for human congenital sideroblastic anemia.11Furthermore, the cloning of the reisling mutation, which causes a defect in zebrafish erythrocytes analogous to that found in human hereditary spherocytosis, led to the identification of zebrafishβ-spectrin.13 This conservation of the fundamental mechanisms controlling erythropoiesis between mammals and zebrafish4 raises the possibility that this powerful genetic system could be applied to uncover new classes of genes required in the normal development of granulocyte/macrophage cell lineages during vertebrate myelopoiesis.
The site of hematopoiesis in vertebrates changes during embryogenesis.14 In mammals, primitive hematopoiesis is largely erythropoietic and occurs outside the embryo in the blood islands of the yolk sac. In later stages of development, hematopoiesis moves to the aorta-gonad-mesonephros region and the fetal liver,15 whereas in adults definitive hematopoiesis occurs in the bone marrow where all blood cell lineages are produced.16,17 By contrast, primitive hematopoiesis in the zebrafish occurs within the embryo in a region between the notochord and endoderm of the trunk called the intermediate cell mass (ICM), as well as anteriorly in the paraxial mesoderm over the yolk cell and posteriorly in a small ventral cluster of cells in the developing tail referred to as the posterior blood island.7,18,19 The earliest site of zebrafish hematopoiesis is the ICM, a region analogous to the blood islands of the yolk sac in mammals. Hematopoietic progenitor cells from the posterior blood island enter the circulation slightly later than those in the ICM and may represent the end stage of primitive hematopoiesis.7 In the 48-hour zebrafish embryo, cells expressing c-myb are found scattered along the ventral wall of the dorsal aorta, an expression pattern analogous to the observed expression of this gene in the progenitors of definitive hematopoiesis in the aorta-gonad-mesonephros region of mammalian species.7 This observation suggests that these cells may represent the first progenitors of definitive hematopoiesis in the zebrafish.4,7 During later stages of zebrafish maturation and throughout adulthood the major site of definitive hematopoiesis is the kidney, although some hematopoietic activity may occur in the spleen.20
We propose that genome-wide mutagenesis screens in the zebrafish will detect genes whose dominant or recessive mutation leads to deficiencies or abnormal distributions of mature granulocytes and that a subset of these genes will have human counterparts in which loss-of-function contributes to myeloid cell neoplasias. The ultimate value of such screens will depend greatly on the reliability of markers of granulocyte development and the homology between zebrafish and mammalian myelopoiesis.
The number of myeloid cell lineages and their distinct morphologies vary among different fish species.21 For example, inSparus auratus, heterophils (neutrophils) and eosinophils are relatively numerous, whereas basophils are rare.22,23In other fish species, only 1 or 2 granulocyte lineages have been described,21,24,25 and the monocyte/macrophage lineage has been identified in only a few species.26 27 These considerations underscore the importance of defining normal myeloid cell development in the zebrafish before embarking on studies to dissect the genetic control of this process.
The morphologic, cytochemical, and genetic findings reported here indicate a high level of conservation of the cell lineages and genes involved in vertebrate granulopoiesis and support zebrafish mutagenesis as a feasible approach for defining the molecular mechanisms of myeloid cell development.
Materials and methods
Fish maintenance and embryonic staging
Zebrafish were maintained and developmentally staged according to Westerfield.28 Briefly, wild-type fish were mated, and the embryos were collected and grown in E3 media (5 mM NaCl, 0.17 mM KCl, 0.4 mM CaCl2, and 0.16 mM MgSO4) at 28.5°C. Between 18 and 24 hours after fertilization (hpf), embryos were transferred to E3 containing 0.003% 1-phenyl-2-thiourea (PTU) to inhibit pigment formation and to prolong their optical transparency. Embryos were briefly treated with pronase (1 mg/mL) in E3, removed from their outer chorions by gentle trituration with transfer pipettes, and fixed overnight at various stages of development in 4% paraformaldehyde at 4°C. Fixed embryos were washed in PBST (phosphate-buffered saline [PBS] with 0.1% Tween-20) and transferred into methanol for storage at −20°C.
For hematopoietic mutants, retsina and clochemutant embryos were generated from crosses between heterozygous males and females. Both lines were obtained from the laboratory of Leonard Zon (Howard Hughes Medical Institute, Children's Hospital, Boston, MA).
Blood collection and kidney harvesting
Normal adult zebrafish were euthanized with tricaine and ice water. Blood was obtained by cardiac puncture with a 10-μL heparinized glass microhematocrit tube, and smears were made onto glass slides. Kidneys were surgically removed through a lateral incision along the ventral side of adult fish, and touch preps and smears were prepared on glass slides. Cytospins were prepared by the centrifugation of resuspended kidney tissue in a solution of PBS and 1% bovine serum albumin at 700 rpm for 3 minutes onto glass slides with use of a Shandon 3 cytocentrifuge (Shandon, Pittsburgh, PA).
Cytochemical and histochemical stains
Blood and kidney smears, as well as kidney touch preparations and cytospins, were stained for the identification of specific myeloid cell lineages, using Wright-Giemsa, leukocyte AP, periodic acid-Schiff, myeloperoxidase, alpha-naphthyl butyrate esterase, chloroacetate esterase, Sudan black, and toluidine blue (all from Sigma, St Louis, MO). Staining procedures followed the manufacturer's instructions.
Electron microscopy
Adult zebrafish kidney tissue was isolated and fixed in 2.5% formaldehyde and 0.03% picric acid in 100 mM cacodylate buffer (pH 7.4). Samples were washed in buffer and postfixed with 1% osmium tetroxide/1.5% potassium ferrocyanide and embedded in Epon-araldite. Semithin (1 μm) sections were cut and evaluated by light microscopy. Thin sections (60 nm) were then cut and stained with saturated uranyl acetate and lead citrate. These sections were examined and photographed with a JEOL 1200EX electron microscope (JEOL, Tokyo, Japan).
Cloning of zebrafish myeloperoxidase
Degenerate polymerase chain reaction was performed to amplify a 450–base pair (bp) fragment of myeloperoxidase mpo gene from adult zebrafish kidney complementary DNA (cDNA). This fragment was used to screen an adult zebrafish kidney cDNA library, and more than 50 positive clones were identified by overlapping sequence analysis. Twenty of these positive clones were confirmed to encode the zebrafishmpo gene. The 3486-bp cDNA sequence we obtained encodes a predicted 762–amino acid protein (GenBank Accession No. AF349034). The human and zebrafish proteins share 76.5% similarity and 49.6% identity. In the MPO catalytic domain, the 2 sequences share 82.8% similarity and 56.8% identity (Figure1).
Alignment of the proteins encoded by the zebrafish and human MPO genes.
Sequence comparison was performed using the FASTA program in the Genetics Computer Group (GCG) software. Identical amino acids are denoted by bars and similar amino acids by double dots. The human and zebrafish proteins share 76.5% similarity and 49.6% identity at the amino acid level. In the MPO catalytic domain (underlined), the 2 sequences share 82.8% similarity and 56.8% identity.
Alignment of the proteins encoded by the zebrafish and human MPO genes.
Sequence comparison was performed using the FASTA program in the Genetics Computer Group (GCG) software. Identical amino acids are denoted by bars and similar amino acids by double dots. The human and zebrafish proteins share 76.5% similarity and 49.6% identity at the amino acid level. In the MPO catalytic domain (underlined), the 2 sequences share 82.8% similarity and 56.8% identity.
Whole-mount in situ hybridization
Digoxigenin- and fluorescein-labeled RNA probes were transcribed from linear cDNA constructs according to the manufacturer's instructions (Roche Molecular Biochemicals, Indianapolis, IN). Whole-mount in situ hybridization assays were performed according to the method of Schulte-Merker et al29 with minor modifications. Before in situ hybridization, fixed embryos (stored at −20°C in methanol) were rehydrated in PBST and incubated with proteinase K solution. All incubations were done at room temperature (RT); the proteinase K concentration and incubation times were determined according to the embryonic stage of development. Embryos younger than 24 hpf were not treated. Embryos collected at 24 to 30 hpf were incubated in proteinase K for 10 minutes (10 μg/μL), at 30 to 52 hpf for 20 minutes (20 μg/μL), and at 3 to 5 days past fertilization (dpf) for 20 minutes (100 μg/μL). Embryos were then fixed overnight in 4% paraformaldehyde at 4°C, washed in PBST, transferred to microfuge tubes containing hyb−solution (50% formamide, 5 × sodium chloride/sodium citrate [SSC], 0.1% Tween-20, preheated to 68°C) and incubated for 15 minutes. Hyb− solution was replaced with hyb+(hyb− with 5 mg/mL tortula yeast RNA type IV and 50 μg/mL heparin), and the embryos were incubated at 68°C for 1 to 4 hours with gentle agitation. Digoxigenin- or fluorescein-labeled antisense RNA probe was added, and the embryos were hybridized overnight at 68°C. After hybridization, they were washed at 68°C as follows: twice with 2 × SSCT SSC plus 0.1% Tween/50% formamide (30 minutes), once with 2 × SSCT (15 minutes), and twice with 0.2 × SSCT (30 minutes), after which they were transferred into 12-well plates, and washed 2 times at RT with MABT (100 mM maleic acid,150 mM NaCl, Tris pH 9.5 to bring the pH to 7.5, and 0.1% Tween-20). Embryos were then transferred to blocking solution (MABT with 10% heat-inactivated fetal calf serum and 2% blocking reagent, Roche Molecular Biochemicals, Indianapolis, IN) for 1 hour, followed by incubation with alkaline phosphatase (AP)-coupled antidigoxigenin or antifluorescein Fab fragment antibody and incubated overnight at 4°C. Embryos were washed once in blocking solution (30 minutes) and twice in MABT (30 minutes), followed by 3 washes (5 minutes) in staining buffer (50 mM MgCl2, 100 mM NaCl, 0.1% Tween-20, and 1 mM levamisol) containing 100 mM Tris at pH 9.5 for BCIP/NBT (5-bromo-4-chloro-3-indolyl-phosphate, 4-nitroblue tetrazolium chloride) staining or 100 mM Tris pH 8.2 for Fast Red staining (Roche Molecular Biochemicals). The staining reaction, with either BCIP/NBT or Fast Red, proceeded in the dark until satisfactory staining of embryos was obtained. Reactions were stopped by a brief rinse in PBST followed by storage in 4% paraformaldehyde at 4°C. cDNA clones for synthesis of RNA probes were provided by Christine and Bernard Thisse (L-plastin),30Graham Lieschke (pu.1, GenBank Accession No. AF321009; manuscript in preparation), and Leonard Zon (embryonic alpha globin [αE1]).11
For the hematopoietic mutants, at least 30 embryos from heterozygous incrosses were scored for each in situ hybridization assay, and the results were consistent with approximately 25% of the embryos exhibiting the homozygous recessive phenotype.
Double in situ hybridization
Double in situ hybridization assays were performed as described above employing simultaneous overnight incubations with digoxigenin- and fluorescein-labeled probes. Subsequent washes and blocking incubations followed the procedures outlined above, but antibody and staining reactions were done sequentially. Briefly, washed embryos were first incubated overnight with AP-coupled–antidigoxigenin Fab fragment, then washed, and reacted with BCIP/NBT. After detection, the embryos were washed twice (20 minutes) in MABT at RT, followed by an incubation (30 minutes) at 65°C in MABT with 10 mM EDTA to inactivate the antibody. The embryos were incubated in 100% methanol at RT to decrease background staining and were rehydrated through a series of 10-minute incubations (75%, 50%, and 25% methanol) back into MABT. The embryos were incubated in MABT for 1 hour, incubated with AP-coupled fluorescein overnight, and reacted with Fast Red, allowing detection of the expression of 2 different genes by the use of distinct chromagenic substrates.
Results
Histology
In the adult zebrafish, hematopoietic cells develop in the interstitium of the kidney (Figure 2A), which is positioned in the retroperitoneum along the dorsoposterior aspect of the body cavity. Hematopoietic tissue is situated between the renal tubules (Figure 2B), similar to the placement of developing mammalian blood cells among the fat and stromal elements of bone marrow. This compartment in the zebrafish contains a heterogeneous population of mature hematopoietic cells and their precursors (Figure2C). Two granulocyte lineages were observed: neutrophils and a unique type of granulocyte, which exhibits characteristics of both basophils and eosinophils (referred to here as a basophil/eosinophil; Figure 2D). Like their human counterparts, zebrafish neutrophils have segmented nuclei; however, their nuclei are divided into 2 or 3 lobes instead of the 5 lobes typically found in human neutrophils (Figure 2F). Two distinct populations of granules were observed in the cytoplasm of zebrafish neutrophils. One population consisted of small, azurophilic granules discernible by Wright-Giemsa staining, and the other population comprised larger granules that failed to stain distinctly with Wright-Giemsa. By contrast, the mature basophil/eosinophil has a relatively smaller, eccentrically placed, nonsegmented nucleus (Figure 2F). Like that of the neutrophil, the basophil/eosinophil cytoplasm was highly granular, but the granules were relatively large and spherical and did not stain with Wright-Giemsa (Figure 2D,F).
Zebrafish myelopoietic cytomorphology.
Myelopoietic cells detected by various histological and cytochemical methods. Low (A) and high (B) magnification micrographs of adult zebrafish kidney tissue sections stained with hematoxylin and eosin. Micrographs of adult zebrafish kidney smears (C,G-J) and cytospins (D-F), stained with Wright-Giemsa stain (C-F), MPO (G), demonstrating the neutrophil cytoplasm (brown precipitate, black arrowhead), and acid phosphatase showing reactivity with neutrophils (H; red stain, black arrowhead). PAS specifically stained basophils/eosinophils (I; bright pink stain, black arrowhead), and double staining with MPO and PAS (J) showed distinct neutrophils and basophils/eosinophils, respectively. T = renal tubule; Bp = basophil/eosinophil promyelocyte; E = erythrocyte; N = neutrophil; pm = promyelocyte; B = basophil/eosinophil; Me = metamyelocyte; Ba = band. (A) 50 ×; (B) 150 ×; (C,G-J) 250 ×; (D-F) 300 ×.
Zebrafish myelopoietic cytomorphology.
Myelopoietic cells detected by various histological and cytochemical methods. Low (A) and high (B) magnification micrographs of adult zebrafish kidney tissue sections stained with hematoxylin and eosin. Micrographs of adult zebrafish kidney smears (C,G-J) and cytospins (D-F), stained with Wright-Giemsa stain (C-F), MPO (G), demonstrating the neutrophil cytoplasm (brown precipitate, black arrowhead), and acid phosphatase showing reactivity with neutrophils (H; red stain, black arrowhead). PAS specifically stained basophils/eosinophils (I; bright pink stain, black arrowhead), and double staining with MPO and PAS (J) showed distinct neutrophils and basophils/eosinophils, respectively. T = renal tubule; Bp = basophil/eosinophil promyelocyte; E = erythrocyte; N = neutrophil; pm = promyelocyte; B = basophil/eosinophil; Me = metamyelocyte; Ba = band. (A) 50 ×; (B) 150 ×; (C,G-J) 250 ×; (D-F) 300 ×.
The maturation of zebrafish granulocytes roughly parallels that seen in mammalian myelopoiesis. Blast cells were not discernible in the adult zebrafish kidney, but occasional granulocytic promyelocytes were observed (Figure 2D). These large cells contained round, eccentrically placed nuclei and moderate-to-abundant cytoplasm. The zebrafish neutrophilic promyelocyte lacks the coarse, azurophilic primary granules characteristic of human promyelocytes. As neutrophil maturation progressed, the cells became smaller in size, the chromatin condensed and divided into lobes, and the cytoplasm acquired the characteristic granules of mature neutrophils (Figure 2F). Although early basophil/eosinophil precursors were uncommon, occasional immature forms were observed. These larger cells exhibited diffuse nuclear chromatin and fewer, less-distinct cytoplasmic granules (Figure 2C). Rare macrophages were also observed, characterized by a high cytoplasmic-to-nuclear ratio and diffuse nuclear chromatin (Figure 2E). The macrophage cytoplasm was agranular but was often filled with vacuoles containing phagocytic material, particularly pigment, most likely derived from the pigmented connective tissue surrounding the kidney.
Cytochemistry
Cytochemical stains are useful in distinguishing cells of different lineages in human bone marrow and are routinely used to evaluate cell differentiation in patients with acute leukemia. We therefore applied such stains to normal zebrafish myeloid cells to further define their enzymatic properties.
MPO is an abundant lysosomal enzyme present in the granules of mature human neutrophils and eosinophils. Monocytes are weakly positive for the enzyme, whereas basophils, erythrocytes, platelets, and lymphocytes are typically negative. In zebrafish, neutrophil granules were strongly MPO+, whereas the basophils/eosinophils were negative (Figure 2G).
Acid phosphatase activity is present in many human hematopoietic cells, including lymphocytes, monocytes, and granulocytes. The granules of zebrafish neutrophils reacted strongly with acid phosphatase, whereas the lymphocytes and basophils/eosinophils were negative (Figure 2H).
In human granulocytes, components of the cytoplasmic granules may react with periodic acid-Schiff (PAS). Human neutrophils show a fine, diffuse staining that is stronger in more mature cells, whereas human eosinophils and basophils are usually PAS− but can show weak diffuse staining. In the zebrafish, neutrophils were negative and the basophils/eosinophils strongly PAS+ (Figure 2I). Double-staining reactions with PAS and MPO clearly distinguished the morphologically distinct populations of myeloid cells: neutrophils showed MPO staining exclusively, whereas the basophils/eosinophils exhibited strong PAS reactivity (Figure 2J).
Other histochemical stains routinely used to identify the different cell lineages in human bone marrow, including Sudan Black, the monocytic esterases, chloroacetate esterase, toluidine blue, and AP, were also applied to the zebrafish kidney samples, but they failed to reveal distinctive staining patterns (results not shown).
Ultrastructural analysis
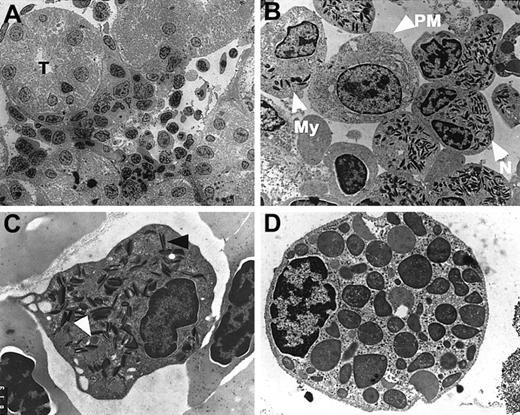
Electron microscopy was used to confirm the myeloid lineage assignments based on histochemical and cytochemical findings. At low magnification, hematopoietic cells were again observed among the renal tubules of the kidney (Figure 3A). The morphologic features of the hematopoietic cells were more clearly defined at higher magnification. The promyelocyte had a large, round nucleus with diffuse nuclear chromatin, and the cytoplasm contained a few small, round and dense granules (Figure 3B). As maturation progressed, neutrophil precursors at the myelocyte stage began to exhibit the 2 types of distinctive granules. The ultrastructure of one granule type was characterized by its round shape and homogeneous interior, whereas the second granule type showed needlelike paracrystalline inclusions (Figure 3C). Maturing neutrophils contained segmented nuclei and increasing numbers of both types of cytoplasmic granules (Figure 3B,C).
Ultrastructure of zebrafish hematopoietic tissue.
(A-D) Electron micrographs of myeloid cells in the zebrafish kidney at low (A) and high (B-D) magnifications. (C) Zebrafish neutrophil with round cytoplasmic granules (white arrowhead) and elongated granules with paracrystalline inclusions (black arrowhead). (D) Mature zebrafish basophil/eosinophil. T = renal tubule; PM = promyelocyte; My = myelocyte; N = neutrophil. (A) 1400 ×; (B) 7500 ×; (C) 15 750 ×; (D) 23 500 ×.
Ultrastructure of zebrafish hematopoietic tissue.
(A-D) Electron micrographs of myeloid cells in the zebrafish kidney at low (A) and high (B-D) magnifications. (C) Zebrafish neutrophil with round cytoplasmic granules (white arrowhead) and elongated granules with paracrystalline inclusions (black arrowhead). (D) Mature zebrafish basophil/eosinophil. T = renal tubule; PM = promyelocyte; My = myelocyte; N = neutrophil. (A) 1400 ×; (B) 7500 ×; (C) 15 750 ×; (D) 23 500 ×.
Although occasional immature cells were found, most of the basophils/eosinophils identified in the adult kidney were nearly fully mature, as in mammalian bone marrow (Figure 3D). The zebrafish basophil/eosinophil has a relatively small, eccentrically placed nucleus and abundant round cytoplasmic granules with homogeneous interiors that lack paracrystalline inclusions.
Molecular genetic analysis
pu.1 expression.
In mammals, the transcription factor Pu.1 is one of the earliest hematopoietic genes expressed and is essential for normal hematopoiesis, specifically in the B-lymphoid and myeloid cell lineages.31 In the zebrafish, pu.1 expression was observed by 16 hpf in the anterior yolk region of the developing embryo, as well as in the posterior ICM (Figure4A). The posterior expression was lost between 22 and 24 hpf (Figure 4B), whereas anterior expression persisted and was observed in cells spreading anteriorly over the yolk (Figure 4B,C). By 28 to 30 hpf, pu.1 expression was no longer observed (results not shown).
Zebrafish pu.1expression.
In situ hybridization using a digoxigenin-labeled RNA probe against zebrafish pu.1 at 16 hpf (A) and 22 hpf (B,C). Lateral views, dorsal upward, anterior to the left (A-B). Dorsal view, anterior to the left (C).
Zebrafish pu.1expression.
In situ hybridization using a digoxigenin-labeled RNA probe against zebrafish pu.1 at 16 hpf (A) and 22 hpf (B,C). Lateral views, dorsal upward, anterior to the left (A-B). Dorsal view, anterior to the left (C).
L-Plastin expression.
The actin-binding protein, L-Plastin, is present in human peripheral blood leukocytes, predominately in monocytes/macrophages, but also in B lymphocytes, T lymphocytes, granulocytes, and natural killer cells.32,33 The zebrafish homologue of theL-plastin gene has been cloned, and its expression was observed in macrophages migrating along the yolk of early embryos.30 In our studies, the expression ofL-plastin was first observed at 18 hpf and was limited to cells in the anterior yolk region of the embryo (Figure5A,B). By 28 hpf, L-plastinexpression was evident in cells in the posterior ICM and along the body of the embryo (Figure 5C). By 5 dpf, expression of the gene was drastically reduced, although a few cells continued to express it in the area of the developing gill arches and thymus (results not shown).
ZebrafishL-plastin expression.
In situ hybridization using a digoxigenin-labeled RNA probe to detect zebrafish L-plastin at 18 hpf (A,B) and 28 hpf (C). (C) Black arrowhead indicates L-plastin expression in the posterior tail region. Lateral views, dorsal upward, anterior to the left (A,C). Dorsal view, anterior to the left (B).
ZebrafishL-plastin expression.
In situ hybridization using a digoxigenin-labeled RNA probe to detect zebrafish L-plastin at 18 hpf (A,B) and 28 hpf (C). (C) Black arrowhead indicates L-plastin expression in the posterior tail region. Lateral views, dorsal upward, anterior to the left (A,C). Dorsal view, anterior to the left (B).
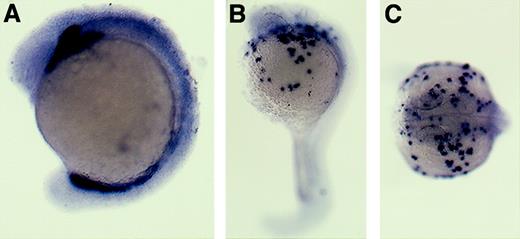
mpo expression.
In humans, MPO is present in the granules of neutrophils and eosinophils from the promyelocyte stage through maturity. We cloned the zebrafish homologue of mammalian Mpo (Figure 1) and used it to create an antisense RNA probe for whole-mount in situ hybridization to detect progenitors and differentiating cells of the granulocytic lineage (Figure 6). The earliest expression of zebrafish mpo was detected in cells at 18 hpf and, in contrast to L-plastin, was first observed in the posterior ICM. Within 1 to 2 hours, cells expressing mpowere also observed anteriorly, spreading along the yolk sac (Figure6A). At 3 dpf, the mpo-expressing granulocytic cells were evident over the anterior yolk, in the posterior ICM, and in the posterior blood island (Figure 6B); however, by 4 dpf they were distributed throughout the embryo (Figure 6C).
Zebrafish mpoexpression.
In situ hybridization using a digoxigenin-labeled RNA probe to detect zebrafish mpo in embryos at 22 hpf (A) and at 3 to 4 dpf (B-C) and in a paraffin-embedded adult kidney section (D). Sections counterstained with methyl green (Vector, Burlingame, CA). Black arrowheads indicate mpo expression in the posterior ICM (A-B). Black arrows indicate mpo expression in neutrophils, note the bi-lobed nuclei of positive cells. Lateral views, dorsal upward, anterior to the left (A-C); 150 × (D).
Zebrafish mpoexpression.
In situ hybridization using a digoxigenin-labeled RNA probe to detect zebrafish mpo in embryos at 22 hpf (A) and at 3 to 4 dpf (B-C) and in a paraffin-embedded adult kidney section (D). Sections counterstained with methyl green (Vector, Burlingame, CA). Black arrowheads indicate mpo expression in the posterior ICM (A-B). Black arrows indicate mpo expression in neutrophils, note the bi-lobed nuclei of positive cells. Lateral views, dorsal upward, anterior to the left (A-C); 150 × (D).
mpo expression in adult kidney sections.
The mpo RNA probe was also used for in situ hybridization assays on paraffin-embedded sections of normal adult zebrafish kidneys (Figure6D). Expression of the gene was observed in a subset of cells with the morphologic features of neutrophils. Distinct segmented nuclei were observed in the mpo+ cells (Figure 6D, inset), confirming the specificity of mpo expression for granulocytes. Other hematopoietic cells, as well as those of neighboring renal tubules, do not express the gene.
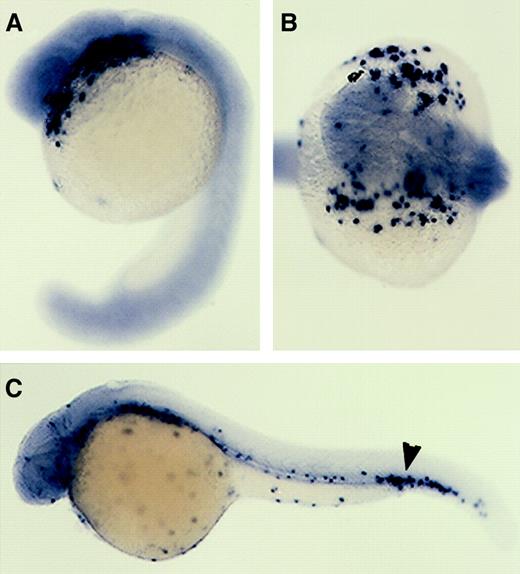
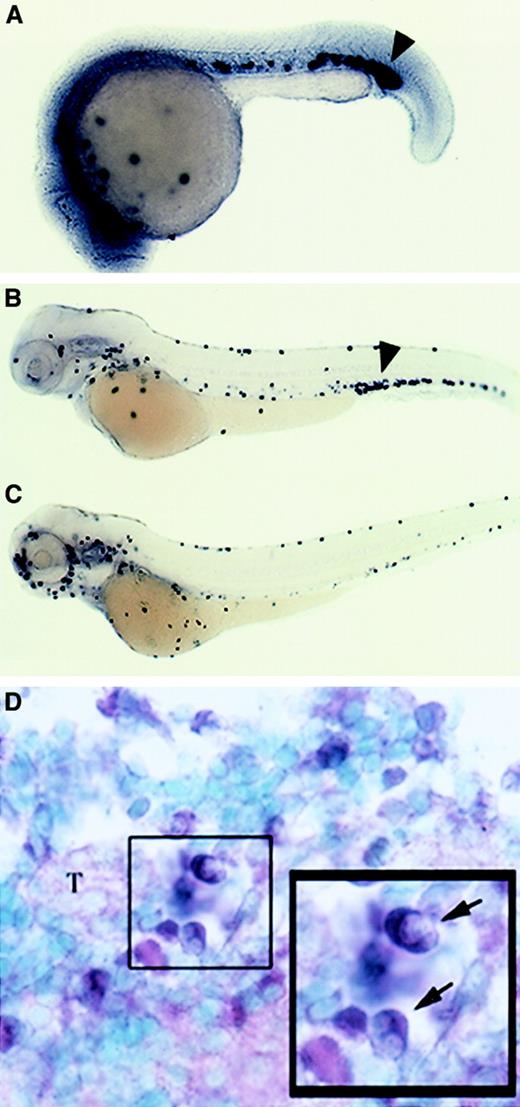
mpo expression in the hematopoietic mutants,cloche and retsina.
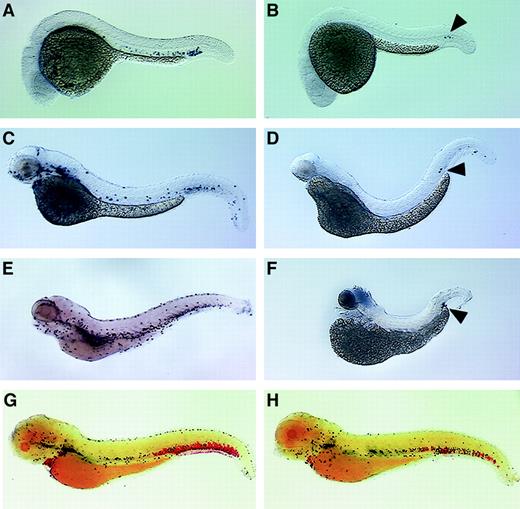
The zebrafish cloche mutation affects both endothelial and hematopoietic cell lineages, and these mutant embryos lack endocardium, head, and trunk vascular endothelium and nearly all blood cells.34 The ICM of mutant embryos is severely depleted of hematopoietic stem cells and transcription factors essential for early hematopoiesis, such as scl, gata-1, andgata-2, are not expressed except for a few cells in the posterior ICM.8 35 The expression of mpo incloche mutant embryos was examined at several different developmental stages and found to be absent throughout most of the embryo, except for a very small number of cells observed in the posterior ICM (Figure 7B,D,F). No cells positive for mpo were seen along the anterior yolk or in the trunk ICM as is seen in wild-type controls (Figure 7A,C,E).
Zebrafish mpo expression incloche and retsina mutant embryos.
In situ hybridization using a digoxigenin-labeled RNA probe against zebrafish mpo (purple) and αE1 (red). Expression of mpo in wild-type (A,C,E) and clochemutant embryos (B,D,F). Cloche mutants (bloodless) at 1 dpf (B), 2 dpf (D), and 4 dpf (F) lack mpo staining except for rare cells in the posterior blood island (B,D,F; black arrowhead). Wild-type siblings at 1 dpf (A), 2 dpf (C), and 4 dpf (E); note purple-stained cells expressing mpo. Expression ofαE1 and mpo in wild-type (G) andretsina mutant (H) embryos at 4 dpf. Retsinamutant at 4 dpf (H) with normal mpo expression (purple-stained cells) but low expression of αE1(red-stained cells) relative to a wild-type sibling (G). Lateral views, dorsal upward and anterior to the left.
Zebrafish mpo expression incloche and retsina mutant embryos.
In situ hybridization using a digoxigenin-labeled RNA probe against zebrafish mpo (purple) and αE1 (red). Expression of mpo in wild-type (A,C,E) and clochemutant embryos (B,D,F). Cloche mutants (bloodless) at 1 dpf (B), 2 dpf (D), and 4 dpf (F) lack mpo staining except for rare cells in the posterior blood island (B,D,F; black arrowhead). Wild-type siblings at 1 dpf (A), 2 dpf (C), and 4 dpf (E); note purple-stained cells expressing mpo. Expression ofαE1 and mpo in wild-type (G) andretsina mutant (H) embryos at 4 dpf. Retsinamutant at 4 dpf (H) with normal mpo expression (purple-stained cells) but low expression of αE1(red-stained cells) relative to a wild-type sibling (G). Lateral views, dorsal upward and anterior to the left.
The zebrafish retsina mutation (rettr265) causes a decrease in the red blood cell count after 3 days of development and is lethal during the first week of life.5 We performed double in situ hybridization experiments with retsina embryos using probes againstmpo and αE1, a member of the embryonic globin gene family expressed in red blood cells.11 At 4 dpf, the number of cells expressing αE1 is markedly decreased in the retsina mutant (Figure 7H) when compared with a wild-type sibling (Figure 7G). By contrast, the number and distribution of the mpo-expressing cells in the retsina mutant is indistinguishable from its wild-type sibling.
Double in situ hybridization.
Assays to detect the simultaneous expression of 2 genes within single cells demonstrated coexpression of mpo andpu.1 at 22 to 24 hpf in some cells of the anterior yolk sac and posterior ICM (Figure 8A,B,C). Coexpression was observed during the brief period of time whenpu.1 expression was decreasing overall and mpowas just beginning to be strongly expressed.
Zebrafish mpoand pu.1 expression.
Double in situ hybridization using RNA probes against mpo(red) and pu.1 (purple) at 22 hpf (A) and 24 hpf (B-C). Higher magnification images of the anterior head region (B) and the posterior tail region (C). mpo Expression alone (black arrow) and pu.1 expression alone (white arrow) in A and B. Coexpression of mpo and pu.1 is indicated by black arrowheads (B-C). Lateral views, dorsal upward, anterior to the left.
Zebrafish mpoand pu.1 expression.
Double in situ hybridization using RNA probes against mpo(red) and pu.1 (purple) at 22 hpf (A) and 24 hpf (B-C). Higher magnification images of the anterior head region (B) and the posterior tail region (C). mpo Expression alone (black arrow) and pu.1 expression alone (white arrow) in A and B. Coexpression of mpo and pu.1 is indicated by black arrowheads (B-C). Lateral views, dorsal upward, anterior to the left.
The double in situ hybridization studies with mpo andL-plastin confirmed our initial findings of independent expression of these 2 genes in distinct regions of the embryo,mpo in the posterior ICM and L-plastin along the anterior yolk sac of the embryo (Figure9A). By 28 hpf, a mixture of both cell types was evident along the entire length of the embryo (Figure 9B), and occasional coexpression was observed (Figure 9C). However, the majority of cells appeared to express only one or the other of these genes, particularly at later stages of development (4-5 dpf).
Zebrafish mpoand L-plastin expression.
Double in situ hybridization using mpo (red) andl-plastin (purple) at 22 hpf (A) and 24 hpf (B-C). Higher magnification images of the anterior head region (C), demonstrating coexpression of mpo and L-plastin in the same cells (black arrows). Black arrowheads indicate mpoexpression alone. White arrowheads indicate L-plastinexpression alone. Lateral views with dorsal upward and anterior to the left (A-B). Magnification in panel C is 50 ×.
Zebrafish mpoand L-plastin expression.
Double in situ hybridization using mpo (red) andl-plastin (purple) at 22 hpf (A) and 24 hpf (B-C). Higher magnification images of the anterior head region (C), demonstrating coexpression of mpo and L-plastin in the same cells (black arrows). Black arrowheads indicate mpoexpression alone. White arrowheads indicate L-plastinexpression alone. Lateral views with dorsal upward and anterior to the left (A-B). Magnification in panel C is 50 ×.
Discussion
Forward genetic screening in the zebrafish offers the opportunity to discover critical myelopoietic genes whose alteration leads to the differentiation arrest that characterizes congenital or acquired premalignant states or overt leukemia. One of the most promising strategies is to detect dominant or recessive mutations that cause a deficiency or abnormal distribution of mature granulocytes. The success of this approach will depend in large measure on the conservation of the genetic mechanisms regulating granulopoiesis in zebrafish versus mammals and the availability of reliable zebrafish markers of myeloid cell development.
The findings reported here demonstrate that the zebrafish neutrophil, like its human counterpart, has a segmented nucleus and granular cytoplasm. The cytoplasmic granules appear to be of 2 types, one round and homogeneous and the other elongated with paracrystalline inclusions. Although not characteristic of human neutrophilic granules, these paracrystalline inclusions are common among the neutrophilic granules found in other teleost species.21 The maturation of zebrafish neutrophil precursors shares many of the features of granulocyte development in humans. Although relatively abundant in the kidney of juvenile zebrafish,36 myeloblasts were rare and difficult to identify in the adult kidney. Promyelocytes, large immature-appearing cells containing nonsegmented nuclei with diffuse chromatin, were also present in the zebrafish kidney. The primary granules of zebrafish promyelocytes were not evident by light microscopy, but rare granules were identified by high-magnification electron microscopy. As these granulocytic cells became more differentiated, they acquired their distinctive granularity and nuclear segmentation. One of the features that clearly defines zebrafish granulocytes as neutrophils is their positive staining for MPO, an enzyme found in human neutrophilic granules.
A second granulocyte lineage identified in this study is more difficult to classify because these cells exhibit features of human basophils as well as eosinophils. Like human basophils and eosinophils, they possess highly granular cytoplasms, but, in contrast to the human cells, they lack segmented nuclei. The cytoplasmic granules are peroxidase negative, unlike those of human eosinophils, and the robust positive PAS reaction of the granules is uncharacteristic of either human cell type. The ultrastructural features of the granules most resemble those of human basophils and mast cells. In other teleost species, analogous cells have been identified as either basophils or eosinophils.21 Because of this ambiguity, we have tentatively classified these zebrafish cells as basophil/eosinophils. Conceivably, this unusual cell type may be the result of the divergence of mammals from teleosts and, therefore, may not be directly comparable to any single human myeloid cell type. Further functional analyses are needed for the definitive classification of this cell lineage.
The results of in situ hybridization experiments using zebrafish homologues of 3 early mammalian hematopoietic genes, Scl, Lmo2, and Gata-2, indicate analogous expression patterns during zebrafish hematopoiesis.6,7 At 12 hpf, these genes are expressed in lateral plate mesoderm as 2 stripes that converge medially by 24 hpf to form the ICM.6,7 In the zebrafish, both hematopoietic and vascular tissues arise from the ICM, which serves as the counterpart of the yolk blood islands in the mammalian embryo.6,7,19 Similar to findings in mammals, the developmental expression of zebrafish gata-1 follows that of scl, lmo2, and gata-2 and is observed by 18 hpf in the ICM.19 By 48 hpf,lmo2 and gata-2 are no longer expressed in blood, and gata-1 expression is severely reduced. Taken together, these results indicate a high level of conservation of the genes and their spatio-temporal expression patterns in early vertebrate hematopoiesis.
We cloned the zebrafish mpo gene and demonstrated its specificity for granulocytes by in situ hybridization of adult kidney sections in which the morphologic features of granulocytes could be clearly identified. Moreover, by whole-mount in situ hybridization we established that the expression of mpo persisted throughout early embryogenesis and was seen in regions of early myelopoiesis. These observations show that mpo expression is restricted to cells of the granulocyte lineage, as it is in humans. Furthermore, the profound depletion of mpo-expressing cells in the zebrafish bloodless mutant, cloche, confirms that nonhematopoietic cells do not express mpo. However, the mpoexpression in the anemic mutant, retsina, was normal, emphasizing its distinct expression in nonerythroid blood cells. The robust and specific expression of mpo in normal zebrafish granulocytes demonstrates the suitability of this gene as a marker of maturing cells within the granulocyte lineage.
We also analyzed the expression of the zebrafish homologues of 2 other known mammalian hematopoietic genes that are expressed in the pathway of myeloid differentiation, pu.1 and L-plastin. The expression of zebrafish pu.1, which is necessary for normal myeloid cell and B-lymphocyte development in the mouse,37 was observed in hematopoietic progenitors between 16 and 30 hpf but not in older embryos. Thus, cells expressingpu.1 appear later than those expressing scl,lmo2, and gata-2, consistent with findings in mammals showing that Pu.1 acts downstream of these transcription factors and may represent a commitment step in myeloid differentiation. In addition, the transient coexpression ofpu.1 and mpo at a time when pu.1expression was decreasing supports the idea that pu.1expression in early zebrafish myeloid progenitors may regulate their subsequent development into mature mpo-expressing neutrophils.
Zebrafish cells expressing mpo and L-plastinrepresent distinct hematopoietic cell lineages, which was particularly evident in the double in situ hybridization experiments demonstrating the general lack of coexpression of these 2 genes in zebrafish cells. These results correspond to findings in mammals, which show thatMpo expression is specific to granulocytes, whereasL-Plastin expression is specifically expressed in monocytes/macrophages and a subpopulation of lymphocytes.32 33
By injecting DNA constructs containing zebrafish promoter sequences fused to the green fluorescent protein (GFP) gene,38 it has been possible to generate transgenic zebrafish lines that exhibit tissue-specific expression ofGFP. The potential utility of this approach was further demonstrated by the creation of transgenic lines capable of erythroid-specific expression of GFP from thegata-1 promoter and of T-cell–specific expression from therag-1 promoter.38 39 We are presently developing a transgenic zebrafish line that expresses GFPunder control of the mpo promoter to visualize GFP-expressing granulocytes. The ability to analyze the dynamic spatial and temporal changes in developing granulocytes of living embryos will enable us to better define the origin, migration, and lineage specification of these cells.
Abnormalities of the genes controlling the myeloid differentiation program can lead to myelodysplastic syndrome and acute myeloid leukemia, a continuum of devastating diseases in children and adults in which both stem and progenitor cells of the myeloid lineage are affected. Heterozygous or homozygous inactivation of still-to-be-discovered genes has been inferred as a cause of acquired myelodysplastic syndrome in humans, based on the frequent finding of nonrandom chromosomal deletions in clonally developing bone marrow cells (eg, monosomy 7, 5q−, and 20q−).40 In addition, in 2 congenital neutropenic diseases, Kostmann and Shwachman syndromes, the inactivation of the genes causing these disorders predisposes patients to myeloid neoplasias, suggesting that the loss of gene function essential for normal granulocyte development contributes to the multistep pathway of genetic alterations leading to leukemic transformation.41-43 Kostmann syndrome (severe congenital neutropenia) is caused by a mutation in the gene, Ela2, which encodes neutrophil elastase.44 The defect responsible for the autosomal recessive Shwachman syndrome, a rare disorder characterized by impaired granulopoiesis and exocrine pancreatic insufficiency, has not been identified. The steps that lead to the leukemic transformation in both of these diseases have also not been elucidated, although activating mutations of the granulocyte colony-stimulating factor receptor have been documented in some patients with Kostmann syndrome who develop myeloid leukemia.45
Genome-wide chemical mutagenesis screens in the zebrafish with thempo probe described here should help to uncover genes in which heterozygous or homozygous inactivation leads to defective granulocyte development. Our morphologic, histochemical, and genetic analyses of myelopoiesis in the zebrafish support the use of this model to identify mutations that affect granulopoiesis. We postulate that the human homologues of many of the genes required for granulopoiesis in the zebrafish will prove to be the targets of inactivating mutations or deletions in congenital and acquired myelodysplastic syndromes and that forward genetic analysis in the zebrafish will prove to be a useful approach to gene discovery and the generation of reliable animal models for these diseases.
We thank John Gilbert for editorial review, Massimo Loda and his staff at the Dana-Farber In Situ Hybridization Core Facility for their assistance with tissue sectioning and processing, Bernard and Christine Thisse and Graham Lieschke for cDNA clones used for RNA probes, the HMS Electron Microscopy Core Facility, and members of the Look and Zon laboratory for their helpful comments and scientific discussion.
Supported in part by grant DK 02593 from the National Institutes of Health (B.H.P.). B.H.P. is supported in part by a Postdoctoral Fellowship for Physicians from the Howard Hughes Medical Institute.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
A. Thomas Look, Department of Pediatric Oncology, Mayer-630, Dana-Farber Cancer Institute, 44 Binney St, Boston, MA 02115; e-mail: thomas_look@dfci.harvard.edu.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal