Abstract
Various therapeutic options are available for patients with chronic myelogenous leukemia. Allogeneic stem cell transplantation, though often curative, is associated with high nonrelapse mortality and long-term morbidity, particularly when cells from unrelated donors are used. Many physicians and patients opt for a trial of interferon-α (IFN)–based therapy first, reserving transplantation for patients with inadequate response or intolerance to IFN. Data were analyzed on 740 patients receiving unrelated donor transplants for chronic myelogenous leukemia in first chronic phase provided by the International Bone Marrow Transplant Registry and the National Marrow Donor Program to see whether IFN pretreatment compromised transplantation outcome. A total of 489 (66%) had received IFN prior to transplantation; 251 (34%) had not. Disease characteristics in the 2 groups were similar at diagnosis but at the time of transplantation, hematologic parameters and weight were lower in IFN patients and the interval between diagnosis and transplantation was longer. After adjustment for baseline covariates, no effect of IFN exposure was found on overall survival, leukemia-free survival, nonrelapse mortality, engraftment, relapse, or acute or chronic graft-versus-host disease. Evaluation of effects based on duration of therapy and time off IFN prior to transplantation was limited by missing data and confounding with IFN intolerance and disease responsiveness. In conclusion, no evidence was found for an independent adverse effect of IFN pretreatment on the outcome of subsequent unrelated donor transplantation.
Introduction
Chronic myelogenous leukemia (CML) is curable by allogeneic hematopoietic stem cell transplantation (SCT), but most patients lack an HLA-matched sibling donor. Recent studies report long-term disease-free survival rates of 60% to 70% in younger patients receiving transplants early from HLA-matched unrelated donors.1 2 However, a high incidence of early nonrelapse mortality and late complications make transplantation an undesirable first-line therapy for many patients.
Several nontransplant therapies have proven efficacy in CML. The best established treatment is interferon-α (IFN), alone or combined with other drugs, which induces complete cytogenetic remissions in 6% to 25% of treated patients. IFN-related mortality is essentially nonexistent, although morbidity is considerable and up to 20% of patients stop therapy due to intolerable side effects.3-13Biologically, IFN exerts many immunomodulatory effects, such as up-regulation of class I and class II major histocompatibility antigen expression, and may contribute to a fibrotic appearance of marrow in treated patients.14 These effects could have either beneficial or deleterious effects on the subsequent success of allogeneic SCT.
The difference in early mortality between treatment approaches has prompted many investigators to recommend IFN first for patients with CML, holding allogeneic transplantation in reserve for those who fail to achieve a complete cytogenetic remission or who cannot tolerate IFN. This allows identification of patients likely to do well with nontransplant therapy. However, because an adequate IFN trial requires several months of therapy, this approach delays allogeneic transplantation in all patients and results in prolonged pretransplant IFN exposure for those who eventually proceed to transplantation.
Knowledge of the effect of IFN on transplantation outcome is critical to assessing the risk-benefit ratio of this strategy. Additionally, an understanding of the effects of IFN would help guide management of patients already treated with IFN who are preparing to undergo transplantation. Studies analyzing the impact of prior IFN therapy on allogeneic transplantation outcome report conflicting results.15-25 Many of these studies are limited to single-institution experiences with relatively few patients and different donor types. Five studies found no effect of IFN on transplantation outcome.15,17,20,21,25 In studies showing an adverse effect, both the duration of IFN therapy16,19,24and interval between discontinuation and transplantation18,23 have been implicated. In the only study limited to recipients of unrelated donor marrow, IFN was found to have an adverse effect on survival if given for longer than 6 months before transplantation.19 Excess deaths occurred between 3 months and 1 year after transplantation and were due to refractory chronic graft-versus-host disease (GVHD) in patients with prior severe acute GVHD.
The International Bone Marrow Transplant Registry (IBMTR) and National Marrow Donor Program (NMDP) maintain observational databases of sequential transplantation procedures from a network of centers. This retrospective study sought to determine whether IFN had an adverse effect on the subsequent success of unrelated donor transplantation for CML reported to the IBMTR and NMDP.
Patients and methods
Patients
Eligibility criteria included: (1) Philadelphia (Ph)–positive CML in first chronic phase; (2) age 16 years or older; (3) bone marrow transplantation between 1991 and 1997 from a serologically or molecularly HLA-matched unrelated donor; (4) cyclosporine or tacrolimus plus methotrexate for GVHD prophylaxis; and (5) transplantation between 3 and 48 months after diagnosis. Patients were excluded if the bone marrow was depleted of T cells.
Data collection instrument and methods
The IBMTR and NMDP collect detailed pretransplant and posttransplant clinical information on prospectively identified transplant recipients. Patients reported to both registries were identified and included only once in the analysis. Eligible patients were identified by the IBMTR and the NMDP and a 2-page supplementary form was sent to the transplant centers. This form collected additional data on IFN administration, response to IFN, and toxicity. All patients meeting the eligibility criteria were included whether or not the transplant center provided supplementary IFN data. Cytogenetic response was classified as complete (0% Ph-positive metaphases), partial (1%-34% Ph positive), minor (35%-94% Ph positive) or none (> 95% Ph positive). Major responses included complete and partial responses.
Although information on cytogenetic, extramedullary, and hematologic relapses is requested by the registries, only hematologic relapse was included in the analysis because centers are known to differ in the frequency, type, and interpretation of monitoring studies after transplantation. This restriction likely underestimates the true relapse rate but was felt to be necessary given the current controversies in assessing disease status after transplant.
Biostatistical analysis
Patient-, disease- and transplant-related factors were compared between the 2 groups using the χ2 or Mantel-Haenszel χ2 test for categorical variables and the Wilcoxon 2-sample test for continuous variables. Year of transplant was dichotomized as 1994 or earlier versus after 1994 based on the median of the sample. According to prior conventions, interval from diagnosis to transplant was divided into less than 1 year versus greater than or equal to 1 year2 and conditioning regimens into those incorporating total body irradiation (TBI) versus no TBI. Because of prior published observations, duration of IFN treatment (< 6 months versus ≥ 6 months) and interval off IFN prior to transplant (≤ 90 days versus > 90 days) were also evaluated. Other cutoffs for these variables were not examined.
Patients who were exposed to IFN prior to transplant (IFN) were compared to those who were not (no IFN). Overall survival, leukemia-free survival, nonrelapse mortality, engraftment, relapse, and acute and chronic GVHD were analyzed using the Kaplan-Meier estimator and log-rank test. Analyses of acute and chronic GVHD were limited to patients who successfully engrafted. Due to concerns about accurate grading of GVHD, modeling was performed based on the presence or absence of GVHD regardless of severity, although conclusions were confirmed in analyses including only grades II to IV acute or extensive chronic GVHD. Candidate variables included: (1) patient age, race, sex, donor-patient sex match, cytomegalovirus (CMV) status, weight, and Karnofsky performance status (KPS) at transplant; (2) disease variables including hematologic parameters at diagnosis and transplant, presence of additional cytogenetic abnormalities beyond the Philadelphia chromosome; and (3) transplant specifics including donor age, IBMTR versus NMDP, year of transplant, whether or not transplant was performed within 1 year of diagnosis, and use of TBI. Variables were tested for proportional hazards assumptions, and time-dependent terms used in cases of nonproportional hazards, prior to initial analysis. All models included the variable for pretransplant treatment (IFN or no IFN). Interactions between pretransplant treatment and each variable were tested in univariate analyses. A P less than .01 was required to declare significance for all main effects and interactions given the multiple tests performed. The effects of duration of treatment (< 6 months versus ≥ 6 months) and interval off IFN prior to transplantation (≤ 90 days versus > 90 days), which were only available for a subset of patients, were tested separately in the final models, including all other significant covariates from the initial analyses.
Results
Patient characteristics
The characteristics of the study population are shown in Table1. The group exposed to IFN underwent transplantation more recently, had a higher rate of CMV seropositivity or CMV-positive donors, and had a higher proportion of non-Caucasian patients. Patients treated with IFN were otherwise similar to the no IFN group in source of data (IBMTR versus NMDP), age, donor age, sex, patient-donor sex match, and conditioning regimen. IFN-treated patients had a lower leukocyte count at diagnosis, but were otherwise clinically similar based on available information. The Sokal Score26,27 and New Prognostic Score28could not be calculated because spleen size was not quantified. Myelofibrosis could not be analyzed because of missing data.
Patient characteristics
| Variable . | No IFN . | IFN . | P . |
|---|---|---|---|
| N (%) | 251 (34) | 489 (66) | |
| Registry, N | .26 | ||
| IBMTR | 94 | 204 | |
| NMDP | 157 | 285 | |
| Age, y (median, range) | 37 (16-58) | 37 (16-62) | .85 |
| Donor age, y (median, range)* | 36 (21-56) | 37 (19-58) | .81 |
| Male, N (%) | 155 (62) | 281 (57) | .26 |
| Donor-sex match (%)* | .38 | ||
| M into M | 110 (44) | 181 (37) | |
| M into F | 49 (20) | 106 (22) | |
| F into M | 44 (18) | 99 (20) | |
| F into F | 47 (19) | 98 (20) | |
| Caucasian, N (%) | 243 (97) | 441 (90) | .002 |
| CMV+ patient or donor, N (%)* | 141 (57) | 322 (67) | .01 |
| Conditioning regimen, N (%) | .82 | ||
| Cytoxan + TBI +/− other | 203 (81) | 386 (79) | |
| Busulfan + cytoxan +/− other | 45 (18) | 97 (20) | |
| Other | 3 (1) | 6 (1) | |
| Year of transplant, N | < .0001 | ||
| 1991 | 17 | 10 | |
| 1992 | 21 | 26 | |
| 1993 | 44 | 33 | |
| 1994 | 44 | 73 | |
| 1995 | 40 | 119 | |
| 1996 | 38 | 123 | |
| 1997 | 47 | 105 | |
| N survivors at last follow-up | 123 | 254 | † |
| Follow-up survivors, y (median, range) | 4.1 (0.7-8.3) | 3.1 (0.3-8.7) | < .0001 |
| Cause of death, N (%) | .20 | ||
| Primary disease | 8 (6) | 6 (3) | |
| Acute GVHD | 30 (23) | 38 (16) | |
| Chronic GVHD | 17 (13) | 33 (14) | |
| Infection | 29 (23) | 56 (24) | |
| Other | 34 (27) | 83 (35) | |
| Missing | 10 (8) | 19 (8) |
| Variable . | No IFN . | IFN . | P . |
|---|---|---|---|
| N (%) | 251 (34) | 489 (66) | |
| Registry, N | .26 | ||
| IBMTR | 94 | 204 | |
| NMDP | 157 | 285 | |
| Age, y (median, range) | 37 (16-58) | 37 (16-62) | .85 |
| Donor age, y (median, range)* | 36 (21-56) | 37 (19-58) | .81 |
| Male, N (%) | 155 (62) | 281 (57) | .26 |
| Donor-sex match (%)* | .38 | ||
| M into M | 110 (44) | 181 (37) | |
| M into F | 49 (20) | 106 (22) | |
| F into M | 44 (18) | 99 (20) | |
| F into F | 47 (19) | 98 (20) | |
| Caucasian, N (%) | 243 (97) | 441 (90) | .002 |
| CMV+ patient or donor, N (%)* | 141 (57) | 322 (67) | .01 |
| Conditioning regimen, N (%) | .82 | ||
| Cytoxan + TBI +/− other | 203 (81) | 386 (79) | |
| Busulfan + cytoxan +/− other | 45 (18) | 97 (20) | |
| Other | 3 (1) | 6 (1) | |
| Year of transplant, N | < .0001 | ||
| 1991 | 17 | 10 | |
| 1992 | 21 | 26 | |
| 1993 | 44 | 33 | |
| 1994 | 44 | 73 | |
| 1995 | 40 | 119 | |
| 1996 | 38 | 123 | |
| 1997 | 47 | 105 | |
| N survivors at last follow-up | 123 | 254 | † |
| Follow-up survivors, y (median, range) | 4.1 (0.7-8.3) | 3.1 (0.3-8.7) | < .0001 |
| Cause of death, N (%) | .20 | ||
| Primary disease | 8 (6) | 6 (3) | |
| Acute GVHD | 30 (23) | 38 (16) | |
| Chronic GVHD | 17 (13) | 33 (14) | |
| Infection | 29 (23) | 56 (24) | |
| Other | 34 (27) | 83 (35) | |
| Missing | 10 (8) | 19 (8) |
Missing some data.
Significance not tested.
At the time of transplant, IFN patients had significantly lower hematologic parameters including white blood count, hemoglobin, platelet count, percentage of blasts in blood and bone marrow, and weight (Table 2). The interval between diagnosis and transplant was longer for patients receiving IFN (17 months versus 10 months, P < .0001) so that fewer were transplanted within the first year of diagnosis (32% versus 59%,P < .0001). The IFN group had higher European Group for Blood and Marrow Transplantation (EBMT) risk scores (3.2 versus 2.9,P < .0001) primarily because of the longer time from diagnosis to transplant; the 2 groups did not differ in the other variables that make up the score.29 Information on interval between transplant evaluation and transplantation was collected on 322 IFN-treated patients and 61 no IFN patients and did not differ (216 days versus 209 days, P = .23). Duration of follow-up for the 254 survivors in the IFN arm is shorter at 3.1 years (versus 4.1 years for the 123 in the no IFN group,P < .0001), reflecting the more recent years of transplantation.
Patient characteristics at diagnosis and time of transplantation
| Variable . | No IFN . | IFN . | P . | ||
|---|---|---|---|---|---|
| N . | Median (range) . | N . | Median (range) . | ||
| Total N | 251 | 489 | |||
| At diagnosis | |||||
| White blood count, × 1000/μL | 237 | 159 (4-668) | 448 | 119 (3-835) | .01 |
| Hemoglobin, g/dL | 197 | 11.8 (5.3-17) | 354 | 11.7 (3.7-16) | .44 |
| Platelets, × 1000/μL | 229 | 358 (44-1788) | 391 | 401 (22-3275) | .12 |
| Blasts in blood, % | 156 | 1 (0-46) | 298 | 1 (0-25) | .63 |
| Blasts in bone marrow, % | 55 | 2 (0-12) | 90 | 3 (0-10) | .05 |
| Additional cytogenetic abnormality* | 219 | 14 (6) | 405 | 31 (8) | .56 |
| At transplantation | |||||
| White blood count, × 1000/μL | 251 | 10.2 (1-211) | 488 | 9 (2-286) | .001 |
| Hemoglobin, g/dL | 242 | 13.3 (7-17.4) | 469 | 12.5 (7.2-17.6) | < .0001 |
| Platelets, × 1000/μL | 248 | 292 (69-1570) | 485 | 251 (17-1234) | .0002 |
| Blasts in blood, % | 186 | 0 (0-8) | 412 | 0 (0-6) | .005 |
| Blasts in bone marrow, % | 160 | 2 (0-19) | 327 | 1 (0-13) | .001 |
| KPS | 251 | 100 (60-100) | 488 | 90 (60-100) | .06 |
| Weight, kg | 250 | 81 (44-177) | 489 | 75 (44-149) | < .0001 |
| Interval diagnosis to transplantation, mo | 251 | 10 (4-45) | 489 | 17 (3-48) | < .0001 |
| Interval transplant evaluation to transplantation, median | 61 | 209 (7-1359) | 322 | 216 (11-1341) | .23 |
| Transplanted within 1 y of diagnosis* | 251 | 147 (59) | 489 | 155 (32) | < .0001 |
| Variable . | No IFN . | IFN . | P . | ||
|---|---|---|---|---|---|
| N . | Median (range) . | N . | Median (range) . | ||
| Total N | 251 | 489 | |||
| At diagnosis | |||||
| White blood count, × 1000/μL | 237 | 159 (4-668) | 448 | 119 (3-835) | .01 |
| Hemoglobin, g/dL | 197 | 11.8 (5.3-17) | 354 | 11.7 (3.7-16) | .44 |
| Platelets, × 1000/μL | 229 | 358 (44-1788) | 391 | 401 (22-3275) | .12 |
| Blasts in blood, % | 156 | 1 (0-46) | 298 | 1 (0-25) | .63 |
| Blasts in bone marrow, % | 55 | 2 (0-12) | 90 | 3 (0-10) | .05 |
| Additional cytogenetic abnormality* | 219 | 14 (6) | 405 | 31 (8) | .56 |
| At transplantation | |||||
| White blood count, × 1000/μL | 251 | 10.2 (1-211) | 488 | 9 (2-286) | .001 |
| Hemoglobin, g/dL | 242 | 13.3 (7-17.4) | 469 | 12.5 (7.2-17.6) | < .0001 |
| Platelets, × 1000/μL | 248 | 292 (69-1570) | 485 | 251 (17-1234) | .0002 |
| Blasts in blood, % | 186 | 0 (0-8) | 412 | 0 (0-6) | .005 |
| Blasts in bone marrow, % | 160 | 2 (0-19) | 327 | 1 (0-13) | .001 |
| KPS | 251 | 100 (60-100) | 488 | 90 (60-100) | .06 |
| Weight, kg | 250 | 81 (44-177) | 489 | 75 (44-149) | < .0001 |
| Interval diagnosis to transplantation, mo | 251 | 10 (4-45) | 489 | 17 (3-48) | < .0001 |
| Interval transplant evaluation to transplantation, median | 61 | 209 (7-1359) | 322 | 216 (11-1341) | .23 |
| Transplanted within 1 y of diagnosis* | 251 | 147 (59) | 489 | 155 (32) | < .0001 |
indicates N (%).
IFN versus no IFN
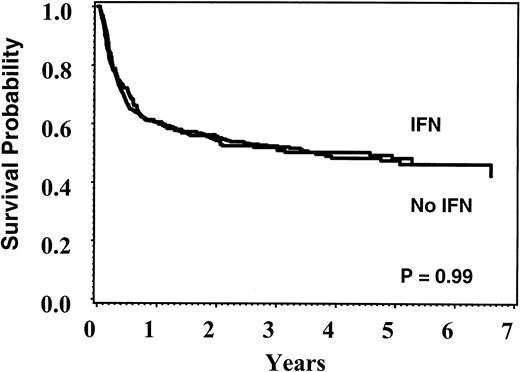
Table 3 shows the reported number of events used for modeling. At 5 years, overall survival was 48% (95% CI, 41%-54%) for the no IFN group and 48% (95% CI, 43%-54%) for the IFN group (P = .99) (Figure 1); disease-free survival was 45% (95% CI, 9%-52%) and 44% (95% CI, 39%-49%), respectively (P = .71). Failure to engraft and relapse occurred in fewer than 10% of patients in both groups. Acute and chronic GVHD occurred equally in both groups and had a similar severity spectrum. Table 4 summarizes the results of the multivariate Cox models. Lower weight at time of transplantation, lack of additional cytogenetic abnormalities, CMV seronegativity in patient and donor, Caucasian race, and more recent year of transplant were associated with better survival and were included in the final models. None of the immediate pretransplant hematologic variables or KPS was associated with outcome once pretreatment group was considered, so these variables were not included in the final analysis. Because of concern that weight at time of transplantation and more recent year of transplantation correlated and thus acted as surrogates for IFN exposure, models were rerun excluding these variables; conclusions were identical. Separate analyses of overall and leukemia-free survival limited to patients transplanted within the first year of diagnosis or after 1995 yielded similar conclusions.
Reported number of events for modeling
| Variable . | No IFN . | IFN . |
|---|---|---|
| Deaths, N (%) | 128 (51) | 235 (48) |
| Nonrelapse mortality, N (%) | 111 (45) | 216 (44) |
| Failure to engraft, N (%) | 14 (6) | 32 (7) |
| Hematologic relapses, N (%) | 18 (7) | 37 (8) |
| Acute GVHD, N (%) | 194 (78) | 386 (79) |
| Maximum grade acute GVHD, % | ||
| I | 25 | 29 |
| II | 30 | 36 |
| III | 26 | 20 |
| IV | 20 | 16 |
| Chronic GVHD, N (%) | 121 (49) | 226 (47) |
| Maximum grade chronic GVHD, % | ||
| Limited | 31 | 35 |
| Extensive | 69 | 65 |
| Variable . | No IFN . | IFN . |
|---|---|---|
| Deaths, N (%) | 128 (51) | 235 (48) |
| Nonrelapse mortality, N (%) | 111 (45) | 216 (44) |
| Failure to engraft, N (%) | 14 (6) | 32 (7) |
| Hematologic relapses, N (%) | 18 (7) | 37 (8) |
| Acute GVHD, N (%) | 194 (78) | 386 (79) |
| Maximum grade acute GVHD, % | ||
| I | 25 | 29 |
| II | 30 | 36 |
| III | 26 | 20 |
| IV | 20 | 16 |
| Chronic GVHD, N (%) | 121 (49) | 226 (47) |
| Maximum grade chronic GVHD, % | ||
| Limited | 31 | 35 |
| Extensive | 69 | 65 |
Unadjusted Kaplan-Meier plots of overall survival according to pretransplant treatment.
Unadjusted Kaplan-Meier plots of overall survival according to pretransplant treatment.
Multivariate modeling
| Outcome . | RR with IFN4-150 . | 95% CI . | P . |
|---|---|---|---|
| Overall survival | 0.99 | 0.80-1.24 | .95 |
| Leukemia-free survival | 1.14 | 0.92-1.42 | .24 |
| Nonrelapse mortality | 1.03 | 0.82-1.30 | .80 |
| Engraftment | 0.92 | 0.78-1.08 | .30 |
| Hematologic relapse | 1.16 | 0.66-2.03 | .62 |
| Acute GVHD | 1.10 | 0.93-1.31 | .27 |
| Chronic GVHD | 0.94 | 0.76-1.18 | .62 |
| Outcome . | RR with IFN4-150 . | 95% CI . | P . |
|---|---|---|---|
| Overall survival | 0.99 | 0.80-1.24 | .95 |
| Leukemia-free survival | 1.14 | 0.92-1.42 | .24 |
| Nonrelapse mortality | 1.03 | 0.82-1.30 | .80 |
| Engraftment | 0.92 | 0.78-1.08 | .30 |
| Hematologic relapse | 1.16 | 0.66-2.03 | .62 |
| Acute GVHD | 1.10 | 0.93-1.31 | .27 |
| Chronic GVHD | 0.94 | 0.76-1.18 | .62 |
Lower weight at time of transplantation, lack of additional cytogenetic abnormalities, CMV seronegativity in patient and donor, Caucasian race, and more recent year of transplantation were associated with better survival and were included in the final models. All forms of acute and chronic GVHD were included. Conclusions were identical when the analysis was limited to grades II-IV or III-IV acute GVHD or extensive chronic GVHD.
In summary, no effect of IFN pretreatment was detected on overall survival, leukemia-free survival, nonrelapse mortality, engraftment, relapse, or occurrence of acute and chronic GVHD.
IFN treatment characteristics
To test hypotheses advanced in the literature, additional data were requested from transplant centers to classify patients into groups according to the duration of IFN treatment or whether IFN was given within 90 days of transplantation. A total of 324 of 489 (66%) requests for additional information were returned. Among patients with adequate data for analysis, 164 of 293 (56%) received IFN within 90 days of transplant and 172 of 246 (70%) received more than 6 months of IFN. Patients for whom details of IFN administration were available were less likely to be transplanted within the first year of diagnosis (27% versus 40%, P = .005) and had lower KPS at transplant (median 90% versus 100%, P < .0001). Posttransplant outcomes including overall survival, leukemia-free survival, nonrelapse mortality, engraftment, relapse, and chronic GVHD were similar in the groups who did and did not provide IFN information. However, acute GVHD was more frequent in the group with IFN information (RR 1.41, 95% CI 1.14-1.77, P = .0015) although there was no difference in the spectrum of severity. All other variables presented in Tables 1 and 2 did not differ between these groups. Table5 shows the characteristics of patients for whom supplementary IFN information was available.
Interferon variables
| Variable . | N . | Value . |
|---|---|---|
| Number receiving IFN | 489 | |
| Number returning supplementary IFN form | 324 (66%) | |
| Interval from diagnosis to start of IFN, median | 291 | 1.8 mo |
| Duration of IFN therapy, median | 305 | 9.6 mo |
| Starting dose, median | 247 | 3 MU/d |
| Maximum dose, median | 242 | 8 MU/d |
| Time to best response, median | 145 | 6.7 mo |
| Best response to IFN | 303 | |
| Complete response | 2% | |
| Partial response | 4% | |
| Minor response | 13% | |
| Hematologic response, unknown cytogenetics | 19% | |
| Hematologic response, no cytogenetics | 42% | |
| None | 20% | |
| Interval between IFN discontinuation and transplant, median | 293 | 74 d |
| Toxicity | 278 | |
| None | 41% | |
| Minor | 47% | |
| Major | 12% | |
| Reason for stopping IFN | 320 | |
| Transplant | 62% | |
| Did not tolerate | 14% | |
| No/inadequate response | 17% | |
| Progression after response | 3% | |
| Other | 4% | |
| Received IFN more than 6 mo | 246 | 70% |
| Received IFN within 90 d of transplant | 293 | 56% |
| Additional medications | ||
| Hydroxyurea | 318 | 71% |
| Busulfan | 308 | 1% |
| Cytarabine | 308 | 5% |
| Variable . | N . | Value . |
|---|---|---|
| Number receiving IFN | 489 | |
| Number returning supplementary IFN form | 324 (66%) | |
| Interval from diagnosis to start of IFN, median | 291 | 1.8 mo |
| Duration of IFN therapy, median | 305 | 9.6 mo |
| Starting dose, median | 247 | 3 MU/d |
| Maximum dose, median | 242 | 8 MU/d |
| Time to best response, median | 145 | 6.7 mo |
| Best response to IFN | 303 | |
| Complete response | 2% | |
| Partial response | 4% | |
| Minor response | 13% | |
| Hematologic response, unknown cytogenetics | 19% | |
| Hematologic response, no cytogenetics | 42% | |
| None | 20% | |
| Interval between IFN discontinuation and transplant, median | 293 | 74 d |
| Toxicity | 278 | |
| None | 41% | |
| Minor | 47% | |
| Major | 12% | |
| Reason for stopping IFN | 320 | |
| Transplant | 62% | |
| Did not tolerate | 14% | |
| No/inadequate response | 17% | |
| Progression after response | 3% | |
| Other | 4% | |
| Received IFN more than 6 mo | 246 | 70% |
| Received IFN within 90 d of transplant | 293 | 56% |
| Additional medications | ||
| Hydroxyurea | 318 | 71% |
| Busulfan | 308 | 1% |
| Cytarabine | 308 | 5% |
Duration of IFN treatment and interval off IFN before transplantation
Of the patients who could be classified with IFN data, 56% received IFN within 90 days of transplant (recent IFN) versus 44% who discontinued IFN more than 90 days prior to transplantation (distant IFN). Patients had similar disease characteristics at diagnosis. At the time of transplant, recent IFN patients had lower hemoglobin (12.2 g/dL versus 13 g/dL, P = .005), shorter interval between diagnosis and transplant (15.2 months versus 20 months,P = .004), and longer duration of exposure to IFN (11.2 months versus 7 months, P < .0001) than distant IFN patients. Among the recent IFN patients, 88% stopped IFN because of transplant, whereas this reason was listed for only 31% of distant IFN patients. Instead, drug intolerance (28% versus 3%) and inadequate or no response (31% versus 6%) were more frequently identified as reasons for discontinuation in distant IFN patients.
No differences in overall survival, leukemia-free survival, nonrelapse mortality, engraftment, and acute or chronic GVHD were seen when recent and distant IFN patients were compared with each other and with the no IFN group. However, recent and distant IFN patients did have different relapse risks (Figure2). At 4 years, the cumulative incidence of relapse was 20% for distant IFN patients, 4% for recent IFN patients, and 8% for no IFN patients (3-way comparison, P = .001). Findings were similar when the analysis was limited to patients stopping IFN for transplantation, when poor response versus all other reasons for stopping IFN was added as a potential explanatory variable, and when a variable representing interval between diagnosis and transplantation was included in the regression. Hazards between the different groups were not proportional over time. Although the risk of relapse within the first year was similar between the distant IFN patients and the no IFN group (P = .49), the relapse incidence for the distant IFN patients was greater in the second and subsequent year after transplantation (RR 7.5, 95% CI 2.4-23.6, P = .0006). The risk of relapse within 1 year of transplant was less in the IFN patients who received IFN within 90 days of SCT (RR = 0.2, 95% CI, 0.1-0.7, P = .007) and equivalent thereafter (P = .73) to patients not exposed to IFN. Comparison of distant and recent IFN patients showed a proportional RR = 5.6 (95% CI 2.1-15.0, P = .0006). Thus, within the first year the relapse rate was statistically lower for the recent IFN patients compared to the other groups, whereas after the first year, the relapse rate was higher for the distant IFN patients compared to the other groups.
Cumulative incidence of relapse.
(A) Distant IFN (discontinue > 90 days before SCT). (B) Recent IFN (continue within 90 days of SCT). (C) No IFN. Relapse incidence at 4 years: distant IFN, 20%; recent IFN, 4%; no IFN, 8%;P = .001.
Cumulative incidence of relapse.
(A) Distant IFN (discontinue > 90 days before SCT). (B) Recent IFN (continue within 90 days of SCT). (C) No IFN. Relapse incidence at 4 years: distant IFN, 20%; recent IFN, 4%; no IFN, 8%;P = .001.
Seventy percent received IFN for at least 6 months (long IFN) versus 30% who received the drug for less than 6 months (short IFN). Comparison of long IFN to short IFN patients showed they had a longer interval between diagnosis and transplant (20.3 months versus 10.8 months, P < .0001), a shorter interval off IFN prior to transplantation (median 57 days versus 112 days,P = .002), and were more likely to stop IFN due to transplant (69% versus 47%). Short IFN patients were more likely intolerant of IFN (27% versus 8%). Duration of IFN exposure (< 6 months versus > 6 months) was not associated with any of the outcomes evaluated. Conclusions were similar if duration of treatment was dichotomized as greater than or less than 12 months.
Discussion
We investigated the effect of IFN exposure prior to unrelated donor marrow transplantation for CML in first chronic phase. No differences in overall survival, leukemia-free survival, nonrelapse mortality, engraftment, relapse, and acute or chronic GVHD were observed between patients who did and did not receive IFN before transplantation.
Our ability to evaluate the independent effects of duration of therapy and time off IFN before transplantation was compromised because we were unable to classify 34% of IFN patients. Nevertheless, restricting the analysis to available data, we were not able to confirm other reports in the literature implicating prolonged or recent exposure to IFN as risk factors for poor outcomes.16,18,19,23,24 We suspect that differences in the study populations and the relatively small number of patients in other studies may explain these conflicting findings. Whereas our analysis was restricted to HLA-matched unrelated donors, most other studies also included HLA-mismatched or related donors, various methods of GVHD prophylaxis, and a significant percentage of patients exposed to busulfan. Beelen and colleagues studied 135 heterogeneous patients and reported that IFN treatment for more than 12 months before transplantation was associated with worse outcomes, mostly attributable to graft failure and infections.16 Morton and colleagues studied 184 patients with chronic phase CML undergoing unrelated donor transplantation and found worse overall survival, 43% versus 60%, for patients receiving 6 months or more of IFN therapy (n = 48) compared to those receiving no IFN or less than 6 months of IFN (n = 136). The excess mortality was due to refractory chronic GVHD in patients with prior severe acute GVHD, and occurred 3 to 12 months after transplantation.19Hehlmann and colleagues studied 152 patients in chronic phase undergoing either related or unrelated donor transplantation following randomization to IFN or no IFN as part of the German CML studies. They found a worse overall survival, 46% versus 78%, in patients who received IFN within 90 days of transplantation (n = 50) compared to those who discontinued IFN more than 90 days in advance (n = 36).23 These results should be compared to an overall survival of 61% for their patients who were randomized to hydroxyurea prior to transplantation. A predominant cause of excess deaths could not be identified.
Each team of investigators tested the hypotheses published by others. When Morton and colleagues compared their patients exposed to recent IFN (IFN within 90 days of transplantation) versus distant IFN (> 90 days off IFN prior to transplantation), they found a trend towardbetter overall survival in the former group (unpublished observations, C.A., personal communication, February 2001). Hehlmann and coworkers23 reported that duration of IFN exposure (when dichotomized at 5 months, 1 year, or 2 years) was not associated with overall survival in their patient group. Beelen and colleagues24 updated their original 1995 observation with a letter published in Blood in which they confirmed the observations of both Morton and Hehlmann, but maintain that treatment duration more than 12 months was most predictive of poor survival.
Two surprising findings in our analysis were the difference in relapse rates between recent and distant IFN subgroups and the lack of an association between transplantation within the first year of diagnosis and better disease-free survival. Other than statistical artifact, the most likely explanation for our observation about relapse is that interval off IFN prior to transplantation is acting as a surrogate for other unmeasured clinical characteristics. Indeed, we know that distant IFN patients had a longer interval between diagnosis and transplant and were more likely to stop IFN due to intolerance or lack of response. However, findings were similar after controlling for these variables. Alternatively IFN could actually be affecting relapse incidence or detection, although it is difficult to explain the differential effects between recent and distant IFN exposure. If IFN is exerting a biologic effect on relapse, then it is probably multifactorial and not easily attributable to a single mechanism. The lack of an association between early transplantation (within 1 year of diagnosis) and improved survival following transplantation may be due to the fact we limited our analysis to patients undergoing transplantation within 4 years of diagnosis. In contrast, recent analyses that include all patients despite interval from diagnosis to transplant continue to document a favorable outcome for patients having transplants early.2
A number of limitations must be kept in mind when interpreting these data. Most importantly, this is a retrospective observational study as were the previous published studies. Biases may be introduced by selection of pretransplant therapy, referral patterns, eligibility criteria for transplantation, patient decision to proceed with transplantation, and other unknown factors. Indeed, we found differences in race, CMV exposure, and year of transplant between the IFN and no IFN groups. However, no effect of IFN was detected even after adjustment for these variables in multivariate modeling. Second, all donor-recipient pairs were reportedly HLA-matched, although because patients underwent transplantation during 1991 to 1997, this matching was determined by a combination of serologic and molecular typing. IFN patients were transplanted more recently and probably benefited from improved typing methods more than the no IFN group. However, we adjusted for year of transplantation and still found no adverse effects of IFN. Third, the analysis is limited by lack of complete IFN data. Supplementary data on 34% of patients known to have received IFN were not available, interfering with our ability to examine hypotheses about subsets of IFN patients as proposed in the literature. Fourth, many centers now use combined IFN and cytarabine therapy, which is more effective but possibly more toxic.11,30 Because only 5% of the patients in this dataset received combination therapy, we were not able to evaluate the impact of this combination. Finally, nontransplant therapy of CML is evolving and the effect of each new agent on transplant success will have to be evaluated. STI571 (Gleevec) has shown great promise in treating CML and is already becoming an essential component of nontransplant therapy.31 32 We do not know whether our conclusions extend to patients who receive both STI571 and IFN prior to transplantation.
Nevertheless, in this large, retrospective, registry-based study we did not find evidence for an adverse effect of IFN on the outcome of unrelated donor transplantation. Patients who prefer a trial of IFN-based therapy and assessment of cytogenetic response before to proceeding to unrelated donor transplantation may be reassured that we did not find an independent adverse effect of this exposure if they later undergo SCT. For patients already on IFN who intend to undergo SCT, our data also suggest that there is no benefit to delaying transplantation for 90 days simply to achieve a 3-month window off IFN prior to undergoing the procedure.
The authors especially wish to acknowledge the transplant centers that submitted supplementary data for this analysis.
Supported in part by National Institutes of Health grant CA75267-03 and the Amy Strelzer-Manasevit Scholars Program. Schering-Plough Oncology/Biotech and Roche Laboratories provided an unrestricted educational grant. Additional support came from Public Health Service grant U24-CA76518 from the National Cancer Institute, the National Institute of Allergy and Infectious Diseases, and the National Heart, Lung and Blood Institute. Grants were also from Abgenix, AmCell Corporation, American Cancer Society, American Society of Clinical Oncology, Amgen, Anonymous, Aventis Pharmaceuticals, Berlex Laboratories, Blue Cross and Blue Shield Association, Lynde and Harry Bradley Foundation, Bristol-Myers Squibb Oncology, Center for Advanced Studies in Leukemia, Cerus Corporation, Chimeric Therapies, Chiron Therapeutics, Eleanor Naylor Dana Charitable Trust, Deborah J. Dearholt Memorial Fund, Empire Blue Cross Blue Shield, Fujisawa Healthcare, Gambro BCT, Genentech, GlaxoSmithKline, Human Genome Sciences, ICN Pharmaceuticals, IDEC Pharmaceuticals, Immunex, IntraBiotics Pharmaceuticals, Kettering Family Foundation, Kirin Brewery Company, Robert J. Kleberg Jr and Helen C. Kleberg Foundation, LifeTrac/Allianz, The Liposome Company, Nada and Herbert P. Mahler Charities, Market Certitude LLC, Mayer Ventures, MedImmune, Merck & Co, Milliman & Robertson, Milstein Family Foundation, The Greater Milwaukee Foundation/Elsa Schoeneich Research Fund, NeoRx, Nexell Therapeutics, Novartis Pharmaceuticals, Orphan Medical, Ortho Biotech, John Oster Family Foundation, Pfizer U.S. Pharmaceuticals, Pharmacia Corporation, Principal Life Insurance Company, Response Oncology, RGK Foundation, SangStat, Schering AG, Stackner Family Foundation, The Starr Foundation, SuperGen, TheraTechnologies, Unicare Life & Health Insurance, and Wyeth/ Genetics Institute.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Mary M. Horowitz, Statistical Center, International Bone Marrow Transplant Registry, Medical College of Wisconsin, 8701 Watertown Plank Rd, PO Box 26509, Milwaukee, WI 53226; e-mail: marymh@mcw.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal