Abstract
Cyclosporine A (CsA) inhibits P-glycoprotein (Pgp)–mediated cellular export of anthracyclines at clinically achievable concentrations. This randomized controlled trial was performed to test the benefit of CsA addition to treatment with cytarabine and daunorubicin (DNR) in patients with poor-risk acute myeloid leukemia (AML). A total of 226 patients were randomly assigned to sequential treatment with cytarabine and infusional DNR with or without intravenous CsA. Remitting patients received one course of consolidation chemotherapy that included DNR with or without CsA as assigned during induction. Addition of CsA significantly reduced the frequency of resistance to induction chemotherapy (31% versus 47%,P = .0077). Whereas the rate of complete remission was not significantly improved (39% versus 33%, P = .14), relapse-free survival (34% versus 9% at 2 years,P = .031) and overall survival (22% versus 12%,P = .046) were significantly increased with CsA. The effect of CsA on survival was greatest in patients with moderate or bright Pgp expression (median 12 months with CsA versus 4 months for controls) compared to patients with absent or low Pgp expression (median 6 months in both arms). The frequency of induction deaths was 15% with CsA and 18% in controls. Steady-state serum concentrations of DNR (P = .0089) and daunorubicinol (P < .0001) were significantly higher in CsA-treated patients. Survival (P = .0003) and induction response (P = .028) improved with increasing DNR concentration in CsA-treated patients but not in controls, suggesting a targeted interaction by CsA to enhance anthracycline cytotoxicity. These results indicate that addition of CsA to an induction and consolidation regimen containing infusional DNR significantly reduces resistance to DNR, prolongs the duration of remission, and improves overall survival in patients with poor-risk AML.
Introduction
P-glycoprotein (Pgp) is a highly conserved 170-kd plasma membrane protein that displays structural homology to bacterial transport proteins.1,2 In human leukemias and other malignancies, Pgp functions as an adenosine triphosphate (ATP)–dependent multidrug exporter with broad specificity for natural product-derived antineoplastics.3 Overexpression of Pgp is associated with reduced cellular accumulation and relative in vitro resistance to anthracyclines that can be overcome by concurrent exposure to Pgp antagonists such as cyclosporine A (CsA).4-6 In acute myeloid leukemia (AML), Pgp overexpression is associated with a lower rate of complete remission (CR) and inferior disease-free and overall survival (OS) in patients receiving conventional anthracycline-containing induction and consolidation regimens.7-12
Cyclosporine A is a potent competitive inhibitor of Pgp transport function at concentrations ranging from 1000 to 2000 ng/mL. In a pilot study performed in patients with poor-risk AML, coadministration of CsA and infusional daunorubicin (DNR) yielded a high rate of CR and eliminated MDR1-overexpressing clones in relapse specimens.13 Although dose-limiting toxicity was not encountered, treatment with CsA delayed the hepatic elimination of bilirubin and DNR, resulting in reversible conjugated hyperbilirubinemia and increased systemic anthracycline exposure.13 14
To test whether CsA enhances the antileukemia effect of anthracyclines, the Southwest Oncology Group (SWOG) conducted a randomized clinical trial comparing treatment outcomes of adult patients with high-risk AML or myelodysplastic syndrome (MDS) treated with a DNR- and cytarabine-containing induction and consolidation chemotherapy regimen with or without the addition of CsA.
Patients, materials, and methods
Eligibility
Eligible patients were 18 to 70 years of age, with histologically confirmed diagnoses of AML refractory to induction chemotherapy or in relapse following a chemotherapy-induced remission or stem cell transplantation; or previously untreated (1) AML secondary to MDS, chemotherapy, or radiotherapy, or (2) refractory anemia with excess blasts in transformation (RAEB-t).15 All patients had SWOG performance status of 2 or less, serum creatinine and total bilirubin levels of 1.5 times or less the institutional upper limits of normal (IULN); and normal left ventricular function with ejection fraction 50% or higher. Patients with active fungal infection requiring treatment with amphotericin-B or prior cytarabine-related neurotoxicity were ineligible. All patients gave written informed consent in accordance with federal and institutional requirements.
Study design
Patients were stratified by age (≤ 55 years versus > 55 years) and disease type and randomly assigned to treatment with cytarabine (AraC) 3 g/m2 daily administered on induction days 1 to 5 by 3-hour intravenous infusion, followed by DNR 45 mg/m2 per day administered by continuous infusion on days 6 to 8, or the same regimen with CsA.16 CsA was administered by 72-hour continuous intravenous infusion at a dosage of 16 mg/kg per day concurrently with DNR, following a 2-hour intravenous loading dose (6 mg/kg) and 6-hour infusion of CsA (4 mg/kg) on day 6 to ensure achievement of steady state prior to DNR administration.13Bone marrow response was evaluated on day 14 of the induction regimen. Patients with persistent leukemia who experienced a more than 50% reduction in blast percentage received a second course of induction therapy on or before day 21. Patients with 50% or less reduction in blast percentage at day 14 and patients who did not achieve CR after a second course of induction chemotherapy were considered treatment failures and received no further protocol-directed therapy. Patients who achieved CR were eligible to receive one course of consolidation chemotherapy consisting of the assigned induction regimen with the AraC schedule abbreviated to days 1 to 3, and DNR with or without CsA administered on days 4 to 6.
There was no adjustment of chemotherapy dosage for hematologic toxicity. Treatment with DNR with or without CsA on day 6 was delayed up to 48 hours if serum bilirubin level exceeded 3 times the IULN. Protocol-directed therapy was discontinued if bilirubin level persisted above 3 times the IULN after 48 hours. Neither DNR nor CsA dosage was adjusted or interrupted for elevations in serum bilirubin level resulting from CsA administration. AraC was immediately discontinued for patients developing grade 2 or higher central nervous system toxicity, and treatment with DNR with or without CsA initiated on the following treatment day. Toxicity was graded using standard SWOG toxicity criteria.17
Criteria for response
Response was evaluated according to SWOG criteria as modified from National Cancer Institute guidelines.15 OS was measured from the date of randomization until death from any cause. Relapse-free survival (RFS) was measured from the date of CR until relapse or death from any cause, with observation censored at the date of last contact for patients last known to be alive without report of relapse. Patients who failed to achieve CR after induction therapy were classified according to type of failure: resistant disease (RD), death during aplasia, or indeterminate.15
Flow cytometric detection of Pgp
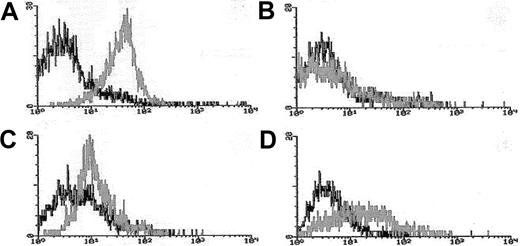
Blasts from pretreatment bone marrow or peripheral blood were enriched by density gradient separation. Expression of Pgp was measured using the MDR1 specific antibody, MRK16 (Kamiya, Thousand Oaks, CA) in 3-color flow cytometry assays in which blasts were costained with MRK16, the hematopoietic stem/progenitor cell antigen CD34, and the panmyeloid antigen CD33, as previously described.10,18Analyses were performed using an isotype control on a FACScan flow cytometer using Lysis II software (Becton Dickinson). Expression of Pgp on gated leukemic blasts compared to control cells was measured using a modification of the Kolmogorov-Smirnov statistic, denoted D, which measures the difference between 2 distribution functions and generates a value ranging from 0 to 1.0.19The method was modified by ascribing a negative value to cases where the median fluorescence intensity of the control cells was brighter than that of antibody-stained cells.10 18 MRK16 staining intensity was categorized for descriptive purposes as follows: moderate or bright (D > 0.20 [Pgp+]), dim and negative (D < 0.20 [Pgp−]); however, analyses of clinical outcomes in relation to Pgp expression were performed using the D value as a continuous variable. Figure 1 provides representative flow histograms illustrating the range of Pgp expression detected in patient leukemia specimens and corresponding control cell lines.
Flow cytometric histograms of Pgp expression in representative AML cases.
Leukemia blasts enriched over density gradients were assessed for Pgp expression using the fluorescence-conjugated Pgp-specific monoclonal antibody MRK16. MRK16 fluorescence, and that of appropriately matched isotype controls, were detected using highly sensitive flow cytometric assays. MRK16 expression was quantitated with the Kolmogorov-Smirnov statistic D. Representative histograms for AML cases in the present study are shown for (A) case considered “Pgp+ high” (defined as D > 0.3) with a D value of 0.45 relative to the isotype control; (B) case considered “Pgp−”(defined as D < 0.2) with a D value of 0.10; (C) “PgP+ low” AML (defined as 0.2 < D < 0.3) with a D value of 0.25 relative to the isotype control; and (D) the Pgp+ DOX 6 cell line used as a positive control for MRK16 staining, with a D value of 0.42 relative to the isotype control.
Flow cytometric histograms of Pgp expression in representative AML cases.
Leukemia blasts enriched over density gradients were assessed for Pgp expression using the fluorescence-conjugated Pgp-specific monoclonal antibody MRK16. MRK16 fluorescence, and that of appropriately matched isotype controls, were detected using highly sensitive flow cytometric assays. MRK16 expression was quantitated with the Kolmogorov-Smirnov statistic D. Representative histograms for AML cases in the present study are shown for (A) case considered “Pgp+ high” (defined as D > 0.3) with a D value of 0.45 relative to the isotype control; (B) case considered “Pgp−”(defined as D < 0.2) with a D value of 0.10; (C) “PgP+ low” AML (defined as 0.2 < D < 0.3) with a D value of 0.25 relative to the isotype control; and (D) the Pgp+ DOX 6 cell line used as a positive control for MRK16 staining, with a D value of 0.42 relative to the isotype control.
Daunorubicinol pharmacokinetics
Serum samples were obtained from a peripheral venous source just prior to the completion of the DNR infusion on induction day 9 (ie, estimate of DNR steady-state serum concentration), and 24 hours later (day 10) on all study patients.13 A reference (pre-DNR) sample was obtained just before initiation of chemotherapy on day 1. Serum samples were cryopreserved and stored at −80°C before being thawed and DNR and daunorubicinol (DNR-ol) concentration analyzed by reverse-phase high-pressure liquid chromatography (HPLC) using a method modified after Peng and coworkers20 as previously described.13 Measurements below limits of detection (10 and 5 ng/dL for DNR and DNR-ol, respectively) were included in analyses as censored observations.
Whole-blood CsA concentration
EDTA-anticoagulated blood obtained from a peripheral venous source was obtained from patients assigned to treatment with CsA on day 6 of induction therapy immediately before administration of the 24-hour infusions of CsA and DNR. Specimens were cryopreserved and stored at −80°C before being thawed and centrally analyzed by radioimmunoassay as previously described.13
Serum sensitization assay
The ability of day 6 patient serum to enhance DNR cytotoxicity in the Pgp+ myeloma cell line, 8226/Dox6, was evaluated using a microculture dye reduction assay.21 Cryopreserved patient serum obtained immediately prior to DNR administration was thawed, and aliquots of patient or volunteer (control) serum were incubated with 8226/Dox6 cells for 10 minutes prior to the addition of DNR (0.1-50 μg/mL) and incubated for 1 hour at 37°C. Cells were then washed twice and plated in triplicate in 96-well microtiter plates. Cell viability was determined by assessment of dye fluorescence using 3-[4,5-dimethylthiazol-2-yl]-2,5diphenyltetrazolium bromide in the methyl-thiazol-diphenyl-tetrazolium (MTT) assay after a 4-day incubation.21 The magnitude of enhancement of DNR cytotoxicity in Dox6 cells (ie, sensitization factor) was determined by dividing the DNR concentration, which yielded a 50% reduction in cell viability (IC50) in 8226/Dox6 cells incubated with control serum (−CsA) by the IC50 obtained from cells incubated with patient serum (+CsA).
Cytogenetic analysis
Cytogenetic studies were performed using standard G-banding with trypsin-Giemsa or trypsin-Wright staining in SWOG-approved cytogenetics laboratories. Karyotypes were interpreted using International System for Cytogenetic Nomenclature (ISCN) criteria.22 Karyotypes were considered normal diploid if no clonal abnormalities were detected in a minimum of 20 metaphases examined and if 2 growth/harvesting methods were used. Each karyotype was independently reviewed by at least 2 members of the SWOG Cytogenetics Committee. Karyotypic abnormalities were grouped into prognostic categories based on published literature for correlation with clinical outcome.10 23-26 Karyotypes that could not be assigned to risk categories were regarded as not classified.
Statistical analysis
The study design called for randomization of 220 eligible patients, providing statistical power of 82% to detect a 50% increase in the CR rate from 35% to 53% (one-tailed test at 5% critical level). This would also provide 90% power to detect a mortality hazard ratio (CsA to control) of 0.67, assuming patient accrual over 66 months, 6 additional months' follow-up, and median OS of 6 months for controls. This study was monitored throughout its accrual and follow-up phases by a Data and Safety Monitoring Committee of which the investigators were not members. Two interim analyses were performed but did not require modification or early termination of the study.
Treatment comparisons were based on assigned treatments, that is, CsA-assigned patients who did not receive CsA were retained in the CsA arm. CR and RD rates were analyzed using the Fisher exact test and logistic regression analysis.27 Distributions of OS and RFS were estimated by the method of Kaplan and Meier, and analyzed according to treatment assignment and patient and disease characteristics using proportional hazards regression models.28 29 Wilcoxon rank sum test and its generalization for censored data were used for comparisons of quantitative variables such as Pgp expression level or DNR and DNR-ol concentrations. Because the principal objective of the study was to assess whether CsA improves treatment outcomes, the statistical significance of treatment differences in outcomes was measured by one-sided P values for superior outcome in the CsA arm. All other test results are reported using 2-sided P values. Results are based on data available November 19, 1999.
Results
Patients and randomization
Among 231 patients registered for study treatment between March 1993 and March 1998, 5 were ineligible due to excluded diagnoses (4 patients) or abnormal liver function (1 patient). Of 226 eligible patients, 107 patients were randomized to treatment with AraC-DNR and 119 to treatment with AraC-DNR+CsA. The distributions of age, gender, and diagnosis were similar in the 2 treatment groups (Table1). Approximately 75% of patients in each treatment arm had relapsed or refractory AML; the remaining had untreated secondary AML or RAEB-t. The proportion with unfavorable karyotypes was somewhat lower in the CsA arm (36 of 85, 42%) compared to the controls (42 of 78, 54%). Among 197 patients with adequate specimens for Pgp analysis, approximately one third in each arm had moderate or bright Pgp expression. The proportion of Pgp+patients was somewhat higher in the previously untreated group (48%) compared to the relapsed/refractory group (31%), although the difference in Pgp expression levels was not statistically significant (P = .12 by Wilcoxon rank sum test).
Characteristics of 226 patients with high-risk AML or RAEB-t by assigned treatment
| . | . | AraC-DNR (N = 107) . | AraC-DNR + CsA (N = 119) . | ||
|---|---|---|---|---|---|
| No. . | Percent . | No. . | Percent . | ||
| Age | Median | 53 y | 54 y | ||
| Disease status | First relapse | 61 | 57 | 71 | 60 |
| Second or later relapse | 7 | 7 | 6 | 5 | |
| Refractory | 17 | 16 | 15 | 13 | |
| Secondary | 22 | 21 | 27 | 23 | |
| AML/RAEB-t | |||||
| Sex | Female | 52 | 49 | 53 | 45 |
| Male | 55 | 51 | 66 | 55 | |
| Performance status | 0-1 | 93 | 87 | 108 | 91 |
| 2 | 14 | 13 | 11 | 9 | |
| WBC (1000/μL) | Median | 4.3 | 5.4 | ||
| Range | 0.3-178 | 0.5-211 | |||
| FAB (local) | M1 | 20 | 19 | 21 | 18 |
| M2 | 26 | 24 | 36 | 30 | |
| M3 | 5 | 5 | 5 | 4 | |
| M4 | 28 | 26 | 26 | 22 | |
| M5 | 5 | 5 | 11 | 9 | |
| M6 | 3 | 3 | 4 | 3 | |
| M7 | 0 | 0 | 1 | 1 | |
| M0 | 7 | 7 | 3 | 3 | |
| AML, NOS | 9 | 8 | 5 | 4 | |
| RAEB-t | 4 | 4 | 7 | 6 | |
| Cytogenetic pattern | Favorable | 8 | 9 | 11 | 11 |
| Intermediate | 28 | 33 | 38 | 39 | |
| Unfavorable | 42 | 49 | 36 | 37 | |
| Not classified | 8 | 9 | 13 | 13 | |
| Not evaluable | 21 | — | 21 | — | |
| Pgp expression (MRK16) | Negative (D < 0.20) | 59 | 66 | 70 | 65 |
| Positive (D ≥ 0.20) | 31 | 34 | 37 | 35 | |
| Unknown | 17 | — | 12 | — | |
| . | . | AraC-DNR (N = 107) . | AraC-DNR + CsA (N = 119) . | ||
|---|---|---|---|---|---|
| No. . | Percent . | No. . | Percent . | ||
| Age | Median | 53 y | 54 y | ||
| Disease status | First relapse | 61 | 57 | 71 | 60 |
| Second or later relapse | 7 | 7 | 6 | 5 | |
| Refractory | 17 | 16 | 15 | 13 | |
| Secondary | 22 | 21 | 27 | 23 | |
| AML/RAEB-t | |||||
| Sex | Female | 52 | 49 | 53 | 45 |
| Male | 55 | 51 | 66 | 55 | |
| Performance status | 0-1 | 93 | 87 | 108 | 91 |
| 2 | 14 | 13 | 11 | 9 | |
| WBC (1000/μL) | Median | 4.3 | 5.4 | ||
| Range | 0.3-178 | 0.5-211 | |||
| FAB (local) | M1 | 20 | 19 | 21 | 18 |
| M2 | 26 | 24 | 36 | 30 | |
| M3 | 5 | 5 | 5 | 4 | |
| M4 | 28 | 26 | 26 | 22 | |
| M5 | 5 | 5 | 11 | 9 | |
| M6 | 3 | 3 | 4 | 3 | |
| M7 | 0 | 0 | 1 | 1 | |
| M0 | 7 | 7 | 3 | 3 | |
| AML, NOS | 9 | 8 | 5 | 4 | |
| RAEB-t | 4 | 4 | 7 | 6 | |
| Cytogenetic pattern | Favorable | 8 | 9 | 11 | 11 |
| Intermediate | 28 | 33 | 38 | 39 | |
| Unfavorable | 42 | 49 | 36 | 37 | |
| Not classified | 8 | 9 | 13 | 13 | |
| Not evaluable | 21 | — | 21 | — | |
| Pgp expression (MRK16) | Negative (D < 0.20) | 59 | 66 | 70 | 65 |
| Positive (D ≥ 0.20) | 31 | 34 | 37 | 35 | |
| Unknown | 17 | — | 12 | — | |
WBC indicates white blood cell; NOS, not otherwise specified.
Induction outcome and survival
Among the eligible patients randomized, 118 (99%) patients assigned treatment with CsA and 105 (98%) controls received their protocol-directed therapy. Forty-seven of the 119 eligible CsA patients achieved CR (39%, 95% confidence interval [CI], 31%-48%), compared to 35 of the 107 controls (33%, CI, 24%-42%;P = .14; Table 2). A significantly greater proportion of patients treated with CsA achieved CR after one course of induction therapy (38%, 45 of 119; CI, 29%-47%) compared to the controls (26%, 28 of 107; CI, 18%-34%;P = .032). Only 2 (4%) of the 47 CsA patients required 2 courses of induction therapy compared to 7 (20%) of the 35 controls (P = .024). Median time from the start of treatment to achievement of CR was 48 days (range, 24-98 days) with CsA and 52 days (range, 24-419 days) for controls. Among the 51 patients with indeterminate or inadequately assessed response according to the NCI guidelines,15 10 (7 CsA, 3 control) were considered probable complete responders because they survived at least 90 days and achieved either normal marrow or peripheral blood examinations. The proportion of documented or probable CRs was 45% (CI, 36%-54%) with CsA and 36% (CI, 26%-45%) for controls (P = .065). Treatment with CsA was associated with a significant reduction in the frequency of RD: 37 of 119 (31%, CI, 23%-39%) with CsA versus 50 of 107 (47%, CI, 37%-56%) among controls (P = .0077).
Response to induction therapy by assigned treatment
| . | AraC-DNR (N = 107) . | AraC-DNR + CsA (N = 119) . | ||
|---|---|---|---|---|
| No. of patients . | Percent of patients (CI) . | No. of patients . | Percent of patients (CI) . | |
| CR | 35 | 33 (24-42) | 47 | 39 (31-48) |
| RD | 50 | 47 (37-56) | 37 | 31 (23-39) |
| Died during aplasia | 3 | 3 | 3 | 3 |
| Indeterminate* | 16 | 15 | 22 | 18 |
| Not adequately assessed | 3 | 3 | 10 | 8 |
| . | AraC-DNR (N = 107) . | AraC-DNR + CsA (N = 119) . | ||
|---|---|---|---|---|
| No. of patients . | Percent of patients (CI) . | No. of patients . | Percent of patients (CI) . | |
| CR | 35 | 33 (24-42) | 47 | 39 (31-48) |
| RD | 50 | 47 (37-56) | 37 | 31 (23-39) |
| Died during aplasia | 3 | 3 | 3 | 3 |
| Indeterminate* | 16 | 15 | 22 | 18 |
| Not adequately assessed | 3 | 3 | 10 | 8 |
Died within 7 days after completing induction therapy; or died later with (1) no posttreatment marrow examination and (2) no posttreatment peripheral blood smear demonstrating leukemia.
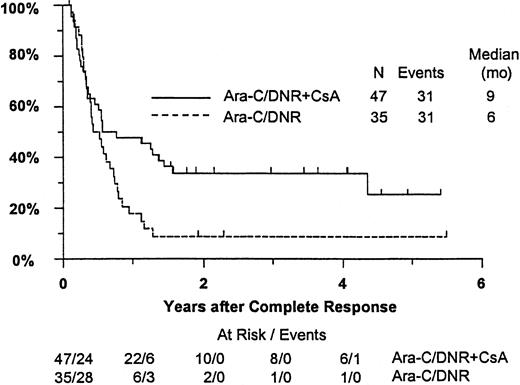
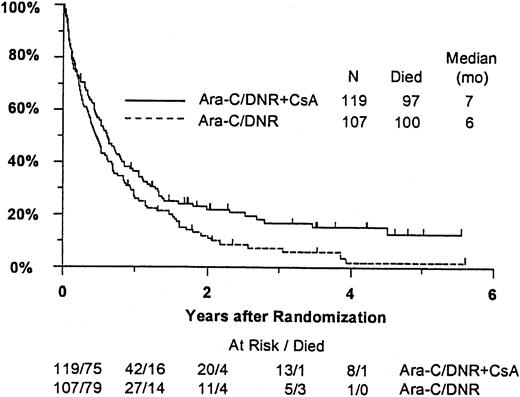
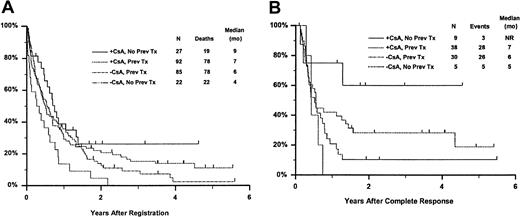
Fifty-seven remitting patients (30 CsA, 27 controls) received protocol-directed consolidation chemotherapy. RFS (Figure2) among all 82 remitting patients was significantly higher in the CsA arm (P = .031). The estimated probability of RFS at 2 years was 34% (CI, 20%-48%) with CsA compared to 9% (CI, 0%-18%) for controls (relative risk [RR], 0.59; CI, 0.34-1.03). OS was significantly improved in the CsA arm (RR, 0.78; CI, 0.58-1.04; P = .046), with estimated 2-year survival of 22% (CI, 14%-30%) compared to 12% (CI, 6%-18%) for controls (Figure 3). There was no significant difference in the benefit of CsA treatment between patients who had received prior chemotherapy (ie, relapsed and refractory) and those previously untreated (ie, secondary AML and RAEB-t): CR,P = .66; RD, P = 0.76 (Tables3 and4); OS, P = .057 and RFS, P = .26 (Figure4). The marginally significant interaction between the effects of prior treatment and CsA on OS is largely attributable to the particularly poor survival among the 22 control patients with untreated secondary AML or RAEB-t; all 22 of whom succumbed to disease within 2 years (median OS, 4 months; CI, 1-7 months), compared to a median OS of 9 months (CI, 6-13 months) among the 27 previously untreated CsA patients.
Kaplan-Meier estimate of RFS for patients achieving CR, according to assigned treatment.
Tick marks indicate surviving patients in continuous CR. RFS was significantly higher in the CsA arm (P = .031).
Kaplan-Meier estimate of RFS for patients achieving CR, according to assigned treatment.
Tick marks indicate surviving patients in continuous CR. RFS was significantly higher in the CsA arm (P = .031).
Kaplan-Meier estimate of OS for all patients according to assigned treatment.
Tick marks indicate surviving patients. OS was significantly higher in the CsA arm (P = .046).
Kaplan-Meier estimate of OS for all patients according to assigned treatment.
Tick marks indicate surviving patients. OS was significantly higher in the CsA arm (P = .046).
OS by prior treatment status and treatment arm
| . | Relapsed/refractory patients (N = 177) . | Previously untreated patients (N = 49) . | ||||||
|---|---|---|---|---|---|---|---|---|
| OS at 2 y (%) . | 95% CI . | RR . | 95% CI . | OS at 2 y (%) . | 95% CI . | RR . | 95% CI . | |
| Control | 14 | 6 -21 | 1.00 | — | 5 | 0 -23 | 1.00 | — |
| CsA | 21 | 12 -29 | 0.84 | 0.61 -1.16 | 26 | 9 -43 | 0.41 | 0.22 -0.76 |
| . | Relapsed/refractory patients (N = 177) . | Previously untreated patients (N = 49) . | ||||||
|---|---|---|---|---|---|---|---|---|
| OS at 2 y (%) . | 95% CI . | RR . | 95% CI . | OS at 2 y (%) . | 95% CI . | RR . | 95% CI . | |
| Control | 14 | 6 -21 | 1.00 | — | 5 | 0 -23 | 1.00 | — |
| CsA | 21 | 12 -29 | 0.84 | 0.61 -1.16 | 26 | 9 -43 | 0.41 | 0.22 -0.76 |
RFS by prior treatment status and treatment arm
| . | Relapsed/refractory patients (N = 68) . | Previously untreated patients (N = 14) . | ||||||
|---|---|---|---|---|---|---|---|---|
| RFS at 2 y (%) . | 95% CI . | RR . | 95% CI . | RFS at 2 y (%) . | 95% CI . | RR . | 95% CI . | |
| Control | 10 | 0 -21 | 1.00 | — | 5 | 0 -52 | 1.00 | — |
| CsA | 28 | 14 -43 | 0.67 | 0.39 -1.15 | 60 | 24 -96 | 0.20 | 0.05 -0.84 |
| . | Relapsed/refractory patients (N = 68) . | Previously untreated patients (N = 14) . | ||||||
|---|---|---|---|---|---|---|---|---|
| RFS at 2 y (%) . | 95% CI . | RR . | 95% CI . | RFS at 2 y (%) . | 95% CI . | RR . | 95% CI . | |
| Control | 10 | 0 -21 | 1.00 | — | 5 | 0 -52 | 1.00 | — |
| CsA | 28 | 14 -43 | 0.67 | 0.39 -1.15 | 60 | 24 -96 | 0.20 | 0.05 -0.84 |
Kaplan-Meier estimate of OS and RFS according to treatment arm and prior treatment.
Panel A is OS and panel B is RFS. Prev indicates previous; NR, not reached.
Kaplan-Meier estimate of OS and RFS according to treatment arm and prior treatment.
Panel A is OS and panel B is RFS. Prev indicates previous; NR, not reached.
Among remitting patients under age 60 at study entry, 9 of 25 controls (36%) and 17 of 39 CsA patients (43%) received stem cell transplants in remission. In an analyses of RFS limited to this age group, the estimated RR comparing patients after receiving their transplants to patients not receiving transplants was 0.70 (CI, 0.34-1.46). This indicates that the risk of relapse or death was somewhat lower following transplantation, though not statistically significant. Therefore, we investigated whether the apparent benefit of CsA might be attributable to the slightly higher proportion of patients receiving transplants in the CsA arm. In a proportional hazards regression model that allowed the prognosis for RFS to improve following transplantation, the resulting adjusted estimate of the treatment RR was 0.42 (CI, 0.22-0.81). This is nearly identical to the estimate of 0.44 (CI, 0.23-0.84) obtained without adjusting for the effect of transplantation, suggesting that the CsA effect was not attributable to transplantation in remission.
Effect of Pgp and cytogenetic pattern
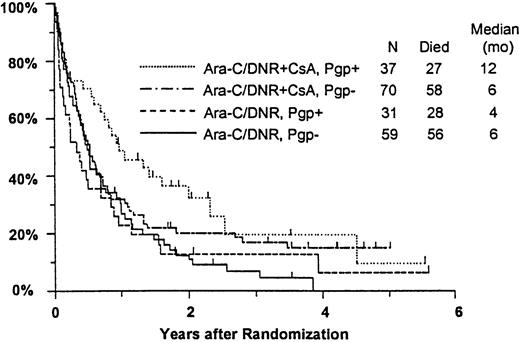
Among all study patients, treatment response and OS did not vary markedly in relation to myeloblast expression of Pgp (Pgp+versus Pgp−: CR, 37% versus 36%; RD, 37% versus 42%; median OS, 8 versus 6 months), although RFS was rather better in the Pgp+ group (median, 15 versus 6 months,P = .050). Response to induction chemotherapy according to Pgp expression and treatment arm is summarized in Table5. Although these data suggest that the benefit of CsA was somewhat greater in Pgp+ patients, the treatment effect did not vary significantly over the range of Pgp expression analyzed (test for interaction: CR, P = 0.61; RD, P = .83). OS was also analyzed in relation to blast expression of Pgp and treatment arm (Figure5). Median survival in Pgp+patients was 12 months (CI, 7-19 months) in CsA-treated patients, but only 4 months in controls (CI, 2-6 months). Among Pgp−patients, in contrast, median OS was 6 months (CI, 4-8 months) in both treatment arms; however, the treatment difference did not vary significantly over the range of Pgp expression levels (interactionP = .63). A similar pattern was seen for RFS, with medians of 17 and 7 months (CIs 7-52 and 4-15 months) for CsA-treated Pgp+ and Pgp− patients, respectively, and 7 and 5 months (CIs 5-9 and 3-9 months) for Pgp+ and Pgp− controls (interaction P = .21).
Induction outcome by Pgp expression and treatment arm
| . | AraC-DNR (N = 59) . | AraC-DNR (N = 31) . | AraC-DNR + CsA (N = 70) . | AraC-DNR + CsA (N = 37) . | ||||
|---|---|---|---|---|---|---|---|---|
| No. of patients . | Percent of patients (CI) . | No. of patients . | Percent of patients (CI) . | No. of patients . | Percent of patients (CI) . | No. of patients . | Percent of patients (CI) . | |
| Pgp− (D < 0.20) patients only | ||||||||
| CR | 20 | 34 (22-47) | 27 | 39 (27-51) | ||||
| RD | 29 | 49 (36-63) | 25 | 36 (25-48) | ||||
| Died during aplasia | 2 | 3 | 0 | 0 | ||||
| Indeterminate5-150 | 6 | 10 | 12 | 17 | ||||
| Not adequately assessed | 2 | 6 | 6 | 9 | ||||
| Pgp+ (D ≥ 0.20) patients only | ||||||||
| CR | 8 | 26 (12-45) | 17 | 46 (29-63) | ||||
| RD | 14 | 45 (27-64) | 11 | 30 (16-47) | ||||
| Died during aplasia | 1 | 3 | 2 | 5 | ||||
| Indeterminate5-150 | 8 | 26 | 4 | 11 | ||||
| Not adequately assessed | 0 | 0 | 3 | 8 | ||||
| . | AraC-DNR (N = 59) . | AraC-DNR (N = 31) . | AraC-DNR + CsA (N = 70) . | AraC-DNR + CsA (N = 37) . | ||||
|---|---|---|---|---|---|---|---|---|
| No. of patients . | Percent of patients (CI) . | No. of patients . | Percent of patients (CI) . | No. of patients . | Percent of patients (CI) . | No. of patients . | Percent of patients (CI) . | |
| Pgp− (D < 0.20) patients only | ||||||||
| CR | 20 | 34 (22-47) | 27 | 39 (27-51) | ||||
| RD | 29 | 49 (36-63) | 25 | 36 (25-48) | ||||
| Died during aplasia | 2 | 3 | 0 | 0 | ||||
| Indeterminate5-150 | 6 | 10 | 12 | 17 | ||||
| Not adequately assessed | 2 | 6 | 6 | 9 | ||||
| Pgp+ (D ≥ 0.20) patients only | ||||||||
| CR | 8 | 26 (12-45) | 17 | 46 (29-63) | ||||
| RD | 14 | 45 (27-64) | 11 | 30 (16-47) | ||||
| Died during aplasia | 1 | 3 | 2 | 5 | ||||
| Indeterminate5-150 | 8 | 26 | 4 | 11 | ||||
| Not adequately assessed | 0 | 0 | 3 | 8 | ||||
Died within 7 days after completing induction therapy; or died later with (1) no posttreatment marrow examination and (2) no posttreatment peripheral blood smear demonstrating leukemia.
Kaplan-Meier estimate of OS by assigned treatment and Pgp expression.
Survival estimates include only patients evaluable for Pgp expression (n = 197). Pgp expression is measured by Kolmogorov-Smirnov D value, with Pgp+ and Pgp− defined as D ≥ 0.20 and D < 0.20, respectively. Tick marks indicate surviving patients.
Kaplan-Meier estimate of OS by assigned treatment and Pgp expression.
Survival estimates include only patients evaluable for Pgp expression (n = 197). Pgp expression is measured by Kolmogorov-Smirnov D value, with Pgp+ and Pgp− defined as D ≥ 0.20 and D < 0.20, respectively. Tick marks indicate surviving patients.
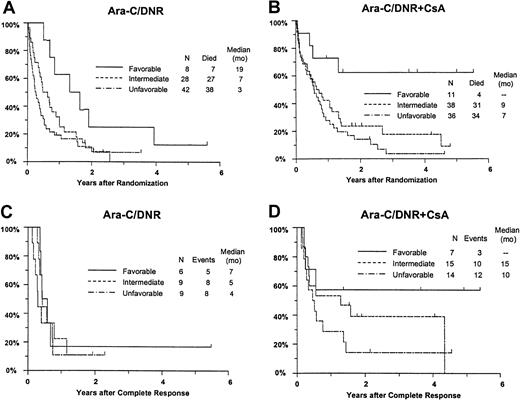
Response and OS varied significantly between the favorable, intermediate, and unfavorable cytogenetic groups (CR,P = .0080; RD, P = .016; OS,P = .0001; RFS, P = .18). For example, estimated survival at 2 years was 44% (CI, 21%-68%), 18% (95% CI, 9%-27%), and 12% (95% CI, 5%-19%) for patients with a favorable, intermediate, and unfavorable karyotype, respectively. Additional analyses were performed to determine whether imbalance in distribution of cytogenetic patterns between the treatment arms might explain the apparent benefit of CsA. After adjusting for differences between the cytogenetic groups (Table 5), CsA continued to be associated with a nonsignificant increase in CR rate (P = .086), a significant decrease in RD (P = .019), and significantly improved OS (P = .013) with an estimated RR of 0.68 (CI, 0.49-0.96) (Figure 6A,B). CsA treatment was associated with a similar improvement in RFS after adjustment for cytogenetic pattern (RR, 0.61; CI, 0.34-1.10), although this effect was of borderline significance (P = .051) (Figure 6C,D).
Kaplan-Meier estimate of OS and RFS by assigned treatment and cytogenetic pattern.
Panels A and B are OS; panels C and D are RFS. Survival estimates include only patients evaluable for cytogenetic pattern (n = 163) defined as follows: Favorable, inv16/t(16;16), t(15;17) with or without other abnormalities, t(8;21) lacking del(9q) or complex karyotype; intermediate, normal karyotype, +8, +6, del(12p); unfavorable, −5/del(5q), −7/del(7q), inv(3q), abn 11q, abn 20q, t(9;22), abn 17p, and complex karyotype (> 3 abnormalities). Tick marks indicate censored observations.
Kaplan-Meier estimate of OS and RFS by assigned treatment and cytogenetic pattern.
Panels A and B are OS; panels C and D are RFS. Survival estimates include only patients evaluable for cytogenetic pattern (n = 163) defined as follows: Favorable, inv16/t(16;16), t(15;17) with or without other abnormalities, t(8;21) lacking del(9q) or complex karyotype; intermediate, normal karyotype, +8, +6, del(12p); unfavorable, −5/del(5q), −7/del(7q), inv(3q), abn 11q, abn 20q, t(9;22), abn 17p, and complex karyotype (> 3 abnormalities). Tick marks indicate censored observations.
Pharmacologic measurements and treatment outcome
Median day 6 whole blood concentration of CsA, measured in 58 CsA patients, was 1774 ng/mL (range, 559-14 230, with 9 censored values reported as 1500+). Serum from 49 CsA patients significantly augmented ex vivo DNR cytotoxicity in Pgp-overexpressing 8226/Dox6 cells with a median sensitization factor of 5.7, compared to 2.2 in 70 controls (P = .0001). Adequate pharmacokinetic sampling was available from 145 patients for analysis of DNR or DNR-ol (or both) serum concentration. Although there was considerable individual variance in steady-state serum concentration of the anthracyclines, the median estimates of day 9 DNR (P = .0028) and DNR-ol (P < .0001) concentrations were significantly higher in CsA patients (Table 6). Elimination of the anthracycline species was rapid after termination of the CsA infusion. Nonetheless, day 10 DNR (P = .0005) and DNR-ol (P < .0001) serum concentrations remained significantly elevated in CsA patients.
Serum DNR and DNR-ol concentrations (ng/mL) by assigned treatment and day of measurement
| Variable . | AraC-DNR . | AraC-DNR + CsA . | P6-150 . | ||||
|---|---|---|---|---|---|---|---|
| Median . | Range . | N . | Median . | Range . | N . | ||
| DNR d 9 | 11.8 | < 10 -348.6 | 62 | 23.1 | < 10 -609.2 | 60 | .0026 |
| DNR-ol d 9 | 51.1 | < 5 -647.5 | 62 | 201.4 | 23.7 -760.3 | 64 | < .0001 |
| DNR d 10 | < 10 | < 10 -521.7 | 58 | < 10 | < 10 -196.0 | 63 | .0005 |
| DNR-ol d 10 | 17.1 | 5.0 -542.4 | 60 | 57.4 | < 10 -987.5 | 65 | < .0001 |
| Variable . | AraC-DNR . | AraC-DNR + CsA . | P6-150 . | ||||
|---|---|---|---|---|---|---|---|
| Median . | Range . | N . | Median . | Range . | N . | ||
| DNR d 9 | 11.8 | < 10 -348.6 | 62 | 23.1 | < 10 -609.2 | 60 | .0026 |
| DNR-ol d 9 | 51.1 | < 5 -647.5 | 62 | 201.4 | 23.7 -760.3 | 64 | < .0001 |
| DNR d 10 | < 10 | < 10 -521.7 | 58 | < 10 | < 10 -196.0 | 63 | .0005 |
| DNR-ol d 10 | 17.1 | 5.0 -542.4 | 60 | 57.4 | < 10 -987.5 | 65 | < .0001 |
Two-tailed P based on Wilcoxon test with drug concentration as a continuous variable censored at lower limits of detection (10 and 5 ng/mL for DNR and DNR-ol, respectively).
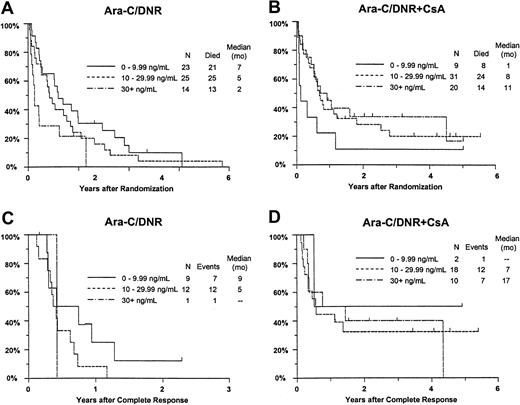
Additional analyses were performed to investigate whether greater systemic exposure to DNR-ol alone or an interaction with CsA might explain the effects of CsA on outcomes (Table7). CR rates improved with increasing day 9 DNR concentration in the CsA arm, but decreased slightly with increasing DNR concentration among controls (interactionP = .033). The analogous interaction of study treatment and day 9 DNR-ol concentration on remission rate was not statistically significant (P = .35). Similarly, the frequency of resistance to induction therapy decreased with increasing DNR serum concentration in CsA-treated patients, but not in controls (P = .078). A similar nonsignificant relationship was detected between DNR-ol concentration and induction resistance (P = .091).
Response to induction therapy by day 9 serum DNR and DNR-ol concentration and assigned treatment
| DNR or DNR-ol (ng/mL) . | AraC-DNR . | AraC-DNR + CsA . | ||||
|---|---|---|---|---|---|---|
| Patients . | Percent CR . | Percent RD . | Patients . | Percent CR . | Percent RD . | |
| DNR | ||||||
| 0-9.99 | 23 | 39 | 48 | 9 | 22 | 56 |
| 10-29.99 | 25 | 48 | 32 | 31 | 58 | 23 |
| ≥ 30 | 14 | 7 | 50 | 20 | 50 | 25 |
| DNR-ol | ||||||
| 0-49.99 | 30 | 47 | 37 | 5 | 20 | 60 |
| 50-199.99 | 27 | 22 | 48 | 27 | 41 | 41 |
| ≥ 200 | 5 | 40 | 40 | 32 | 63 | 13 |
| DNR or DNR-ol (ng/mL) . | AraC-DNR . | AraC-DNR + CsA . | ||||
|---|---|---|---|---|---|---|
| Patients . | Percent CR . | Percent RD . | Patients . | Percent CR . | Percent RD . | |
| DNR | ||||||
| 0-9.99 | 23 | 39 | 48 | 9 | 22 | 56 |
| 10-29.99 | 25 | 48 | 32 | 31 | 58 | 23 |
| ≥ 30 | 14 | 7 | 50 | 20 | 50 | 25 |
| DNR-ol | ||||||
| 0-49.99 | 30 | 47 | 37 | 5 | 20 | 60 |
| 50-199.99 | 27 | 22 | 48 | 27 | 41 | 41 |
| ≥ 200 | 5 | 40 | 40 | 32 | 63 | 13 |
Although there was no improvement in survival in relation to DNR concentration among all study patients (P = .58), OS improved with increasing day 9 serum DNR concentration in CsA-treated patients, but decreased with increasing DNR concentration in controls (interaction P = .0003; Figure7A,B). Similar interactions with treatment were detected for day 9 serum DNR-ol concentration in the analysis of OS (P = .0068), and for day 9 serum DNR concentration in the analysis of RFS (Figure 7C,D;P = .062).
Kaplan-Meier estimate of OS and RFS by assigned treatment and day 9 serum DNR concentration.
Panels A and B show OS; panels C and D show RFS. Estimates include only patients evaluable for day 9 DNR serum concentration (n = 122). Tick marks indicate censored observations.
Kaplan-Meier estimate of OS and RFS by assigned treatment and day 9 serum DNR concentration.
Panels A and B show OS; panels C and D show RFS. Estimates include only patients evaluable for day 9 DNR serum concentration (n = 122). Tick marks indicate censored observations.
Treatment-related toxicity and mortality
Three patients were excluded from the analysis of induction toxicity: one control died before initiating treatment, another erroneously received CsA, and one CsA patient did not receive protocol-directed therapy. Induction deaths occurred in 15% (CI, 9%-22%) of CsA patients and 18% (CI, 11%-25%) of controls. One CsA patient died of pulmonary hemorrhage, whereas infection was the cause of all other induction deaths. Grade 4 hyperbilirubinemia occurred with significantly greater frequency in the CsA-treated cohort (31% versus 4%, P < .0001). Except for hyperbilirubinemia, the frequency of grade 3+ nonhematologic toxicities was similar in the 2 treatment groups. Grade 3 nausea was more common in CsA patients (11%) than controls (3%; P = .016). There was no significant difference in the frequency or severity of stomatitis (CsA, 4%; controls, 8 ≥ grade 3), or renal toxicity as measured by creatinine elevation (CsA, 2%; controls, 3%, grade 3).
Discussion
In this randomized clinical trial, the coadministration of high-dose CsA with infusional DNR during induction and consolidation therapy significantly reduced the frequency of resistance to induction chemotherapy and the probability of leukemia relapse in remitting patients with poor-risk AML or RAEB-t. The estimated 2-year RFS of remitting CsA patients was 34%, compared to 9% for controls. Moreover, survival was significantly improved with CsA treatment, with estimated 2-year survival probability of 22% with CsA compared to 12% in the control group.
Inhibition of Pgp transport function in preclinical models requires CsA concentrations ranging from 1000 to 2000 ng/mL.4-6 The median whole-blood CsA concentration of 1774 ng/mL achieved in this trial was within the targeted therapeutic range and was pharmacologically active as confirmed in the ex vivo cytotoxicity assay. Indeed, treatment with CsA extended median survival in patients with Pgp-overexpressing AML 3-fold (12 months versus 4 months), whereas no difference in median survival was evident in patients with absent or minimal Pgp expression. Similarly, the probability of survival was improved with CsA treatment when adjusted for cytogenetic pattern, with the benefit of study treatment extending to both previously treated and untreated patients.
In addition to its inhibitory effect on the multidrug transporter Pgp, CsA like other cyclosporines, alters the hepatobiliary elimination of bilirubin and anthracyclines.13,30-32 Transient and sometimes extreme conjugated hyperbilirubinemia occurred with higher frequency in CsA-treated patients (31% versus 4%); however, sustained hepatic organ toxicity was uncommon. Although treatment with CsA approximately doubled the steady-state serum concentration of DNR, there was no associated increase in treatment-related toxicity as measured by the occurrence of congestive heart failure, mucositis, or induction deaths. The apparent equivalence in toxicities may relate in part to the extensive individual variation in DNR elimination and the prolonged schedule of DNR administration. A 24-hour infusion of DNR was used in this trial to optimize potentiation of anthracycline antileukemia activity in the presence of CsA. Preclinical investigations indicate that sensitization of multidrug-resistant tumor cells to anthracyclines or other natural products by Pgp antagonists is both concentration- and time-dependent, and is thereby optimized by prolonged exposure to the targeted antineoplastic.33-36Extending the duration of DNR infusion attenuates peak serum concentration (Cmax), which in this trial remained considerably lower than the micromolar concentrations expected with bolus administration of conventional doses. These differences may account for the necessity for significant reduction in antineoplastic dosage in other trials testing cyclosporine modulators to obviate excessive toxicity when the anticancer drug is administered by bolus or rapid intravenous infusion.
The notion that CsA improved treatment outcome by enhancing leukemia sensitivity to DNR rather than by simply increasing DNR exposure per se is supported by the relationship between steady-state serum DNR concentration and efficacy end points. The rate of CR increased and the frequency of resistance to induction therapy decreased with increasing DNR and DNR-ol serum concentration in CsA-treated patients, whereas a similar relationship was not observed in the control group. Analogous effects on RFS and OS were evident in CsA-treated patients; however, in contrast, survival inversely correlated with serum DNR concentration in the control group. If confirmed, these observations have important therapeutic implications for anthracycline therapy in AML. Steady-state serum DNR concentration may be a critical determinant of treatment outcome when the anthracycline is administered in the presence of a cell defense inhibitor such as CsA, whereas in its absence, greater systemic DNR exposure may contribute to an inferior outcome by increasing nonhematologic toxicity.
This study represents the first randomized trial to demonstrate a significant clinical benefit of Pgp modulation in adults with AML, and more importantly, it is the first randomized cooperative group study using modern chemotherapy and supportive care to yield a significant improvement in survival in patients with poor-risk AML. A French cooperative group study compared remission induction therapy with mitoxantrone and cytarabine with or without the addition of the Pgp modulator, quinine sulfate.37 Although the addition of quinine conferred a nonsignificant reduction in the frequency of induction resistance, this study was limited by the heterogeneity in leukemia types and greater mucosal and cardiac toxicity in the quinine-treated group. The Medical Research Council (MRC) recently completed a randomized trial in older AML patients that compared 2 DNR- and etoposide-containing induction regimens with secondary randomization to the addition of CsA.38 This trial showed greater induction toxicity with CsA, but used a CsA dosage (5 mg/kg per day) insufficient for systemic blockade of Pgp transport function. In addition, the MRC trial included etoposide, an agent with low affinity for Pgp,39 40 and in both trials the targeted antineoplastic was administered by rapid intravenous infusion. These differences from the current study design suggest that both the schedule of antineoplastic administration and the selection of an antileukemia agent whose activity is limited by high affinity for Pgp are potentially important determinants of the success of modulator strategies in AML.
Four randomized trials evaluating the more potent and selective, second-generation Pgp antagonist, PSC 833, were recently completed, but yielded disappointing results.39-42 Studies performed by the Eastern Cooperative Oncology Group (ECOG) and Cancer and Leukemia Group-B (CALGB) were prematurely suspended due to lack of benefit or excessive toxicity in the PSC 833-treatment cohorts, respectively.41,42 In each of these trials, the targeted antineoplastic(s) were administered by rapid infusion, necessitating dose reduction to accommodate average changes in drug elimination, thereby raising concern that anthracene underdosing undermined the potential for benefit in up to one third of patients in whom a pharmacokinetic interaction is not anticipated.43Nevertheless, these trials raise the notion that CsA represents a potentially more effective modulator of chemotherapy resistance by virtue of its actions on alternate mechanisms of cell defense, in addition to Pgp. Indeed, CsA moderately enhances DNR retention and cytotoxicity in multidrug-resistant tumor cells that overexpress the ATP-binding cassette (ABC) transporters, multidrug-resistance protein-1 (MRP-1),44-46 or the recently described breast cancer- or mitoxantrone-resistance protein (BCRP, MXR, ABCp).47,48 In addition, through its interaction with cyclophilin, CsA suppresses angiogenic response to vascular endothelial growth factor,49 a cytokine implicated in myeloblast self-renewal and which carries adverse prognostic relevance in AML.50-52 It is plausible that the capacity for CsA to inhibit the above or other biologic targets that promote leukemia survival53-56 served to magnify the benefit of Pgp modulation in this study. Nevertheless, these data provide convincing evidence for the potential benefit of such Pgp-modulation strategies in patients with poor-risk AML.
We are indebted to many people who contributed to the success of this study, in particular Dr William Dalton for advice regarding study design, Dr Sheila Dobin for cytogenetic review, the many SWOG clinical investigators for their commitment to quality patient care, Ms Laura Kingsbury for her efforts in data coordination and evaluation, and to the late Dr Sydney Salmon, whose mentorship and intellectual advice were invaluable.
Supported in part by the following Public Health Service Cooperative Agreement grant numbers awarded by the National Cancer Institute, Department of Health and Human Services: CA38926, CA32102, CA13612, CA12213, CA46368, CA14028, CA35431, CA58416, CA20319, CA04919, CA46282, CA76448, CA58882, CA63845, CA76462, CA35261, CA46441, CA35176, CA12644, CA58686, CA46113, CA52654, CA35090, CA46136, CA35281, CA04920, CA58861, CA35192, CA45450, CA76447, CA42777, CA35178, CA67663, CA16385, CA45560, CA63850, CA35128, CA76132, CA96429, CA22433, CA58415.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Alan F. List, Southwest Oncology Group (SWOG-9126), Operations Office, 14980 Omicron Dr, San Antonio, TX 78245; e-mail: alist@azcc.arizona.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal