Abstract
Protein tyrosine phosphatase (PTP) ε (PTPε) exists as 2 forms generated by alternative promoter usage. It has recently been reported that a cytosolic isoform of PTPε (PTPεC) when over-expressed in murine M1 myeloid cells inhibits interleukin-6 (IL-6)– and leukemia inhibitory factor–induced activation of Janus kinsases (JAKs), thereby suppressing STAT3 tyrosine phosphorylation and STAT3 signaling. This study characterizes an inhibitory action of PTPεC on IL-6 signaling and also reveals that PTPεC inhibitory activity is independent of other potential negative regulators, such as SHP-2 and SOCS family proteins. Furthermore, it analyzes the selectivity of PTPεC action toward several cytokines. On IL-6 stimulation, expression of PTPεC-DA, a catalytically inactive mutant of PTPεC, results in an earlier onset of STAT3 tyrosine phosphorylation, suggesting different modes of action between PTPεC and other negative regulators. In addition, the study shows PTPεC-DA enhances activation of STAT1 by IL-6 as well. In terms of specificity to cytokines, over-expressed PTPεC also inhibits IL-10–induced tyrosine phosphorylation of STAT3 in M1 cells, whereas PTPεC does not affect either interferon-β– and interferon-γ–induced tyrosine phosphorylation of STATs or expression of STAT transcriptional targets. Among cytokines tested, the inhibitory effect of PTPεC is selective to IL-6– and IL-10–induced JAK-STAT signaling.
Introduction
Numerous cellular functions, such as growth, differentiation, and cell death, are regulated by cytokines. It is well established that the Janus kinase (JAK)–STAT signaling pathway plays a pivotal role in signal transduction via cytokine receptors.1-4 To date 7 members of the STAT family of proteins have been identified in mammals, and each STAT protein has been implicated in intracellular signaling elicited by distinct cytokines.5 It has been shown that phosphotyrosine-based motifs residing in receptor subunits determine which particular STAT(s) is activated by a specific cytokine.6 Although the mechanisms of signal transmission through the JAK-STAT pathway have been extensively studied, negative regulation of such signaling has thus far not been well characterized. Studies have identified a suppressors of cytokine signaling (SOCS)/STAT-induced STAT inhibitor (SSI)/cytokine-inducible SH2-containing protein (CIS) family of proteins that inhibits cytokine-induced JAK-STAT signaling(s).7-9 Because SOCS/SSI/CIS expression is cytokine inducible, this family of proteins is supposed to form a negative feedback loop in cytokine signaling.
Interleukin-10 (IL-10) is an 18-kD polypeptide that is mainly secreted from helper T cells, B cells, thymocytes, keratinocytes, activated monocytes, and macrophages.10 IL-10 acts on natural killer cells, B cells, T-helper lymphocyte 1 cells, monocytes, and macrophages to down-regulate and/or to limit their inflammatory and immune responses.10,11 On binding of IL-10 to the IL-10 receptor (IL-10-R), which belongs to the class II cytokine receptor family,12,13 receptor-associated JAK tyrosine kinases are activated and stimulate downstream signaling.14-18 JAK1, tyrosine kinase 2 (TYK2), and STAT3 are all involved in this signaling cascade.17 19 Activated STAT3 protein, for example, translocates to the nucleus and regulates specific gene expression.
Protein tyrosine phosphatase (PTP) ε (PTPε) exists as 2 forms generated by alternative promoter usage from a single gene: a transmembrane (PTPεM) and a cytosolic (PTPεC) form (PTPεC is also referred to as cyt-PTPε.).20-22 Although PTPεM is expressed in brain, testis, and lung, expression of PTPεC is mainly restricted to hematopoietic tissues such as spleen, thymus, and peritoneal macrophages.21,22 PTPεC expression is up-regulated during differentiation and/or activation of macrophages.21,22 Peretz et al23 have reported that PTPεC/cyt-PTPε is also expressed in Schwann cells. Targeted disruption of the PTPε gene in mice (both isoforms are disrupted) revealed its essential role in regulation of myelination of Schwann cells.23 The role of PTPεC/cyt-PTPε in other cell types remains obscure.
Recently, we reported that forced expression of PTPεC suppresses IL-6– and leukemia inhibitory factor (LIF)–induced JAK activation of murine M1 leukemia cells.24 In this study, to further define the molecular mechanism of this suppression, we characterized PTPεC-mediated suppression of IL-6 signaling and examined whether PTPεC is a general inhibitor of cytokine signaling. We found that PTPεC-mediated suppression is selective for certain JAK-STAT signaling pathways through corresponding cytokine receptors such as gp130 and IL-10-R. These findings shed light on the regulatory mechanisms of cytokine signaling.
Materials and methods
Materials
Murine interferon γ (IFN-γ) and IFN-β were generous gifts of Dr T. Abo (Niigata University) and Dr T. Minagawa (Hokkaido University), respectively. Human IL-6 was provided by Ajinomoto (Yokohama, Japan). Mouse IL-10 was purchased from Peprotech (London, United Kingdom). Specific antibodies to phosphotyrosine 701 of STAT1 and STAT1 were obtained from New England Biolabs (Beverly, MA).
IL-10 binding assay
The levels of IL-10-Rs on the cells were assessed by an IL-10-R detection kit (Genzyme, Minneapolis, MN), following the instructions of the manufacturer. The signal due to binding of biotinylated IL-10 was almost completely abolished by addition of 5-fold excess of nonbiotinylated mouse IL-10 (data not shown).
Stable transfectants
Establishment of M1 clones stably expressing PTPεC or PTPεC-DA has been described elsewhere.24 In every experiment, 2 to 3 independent clones of passage 5 to 20 were analyzed. Results using representative clones, M1-εC-8 and M1-εC-DA-15, are shown throughout unless otherwise described. Cells were cultured in RPMI 1640 medium (Gibco-BRL, Rockville, MD) supplemented with 10% fetal calf serum (FCS; Intergen, Purchase, NY), 2 mM glutamate, 250 μg/mL Geneticin (Gibco-BRL), streptomycin, and penicillin.
Northern blot analysis
Total RNAs prepared from cell lines treated with cytokines were analyzed by Northern blotting as described previously.25 Probes used were mouse interferon regulatory factor 1 (IRF-1) complementary DNA (cDNA),24 mouse FcγRI cDNA (nucleotides 680-1221), mouse SOCS-1 cDNA (nucleotides 275-650), and mouse SOCS-3 cDNA (nucleotides 440-872). NIH3T3 cells were cultured in Dulbecco modified Eagle medium (Sigma, St Louis, MO) supplemented with 10% FCS.
Western blot analysis
Cells were treated with 10 ng/mL IL-6 for 10 minutes, 25 ng/mL IFN-γ for 30 minutes, or 20 ng/mL IL-10 for 20 minutes. Immunoprecipitation and Western blot analysis were done as described previously.24
Results
IL-6–induced activation of STATs in M1 cells expressing wild-type and a dominant-negative form of PTPεC
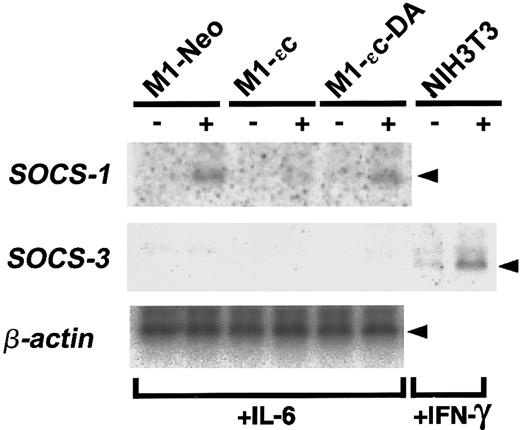
We previously reported that PTPεC negatively regulates IL-6– and LIF-induced JAK-STAT signaling when overexpressed in murine M1 cells.24 Specifically, activation of STAT3 is impaired in M1 cells expressing PTPεC (M1-εC). By contrast, in cells expressing a catalytically inactive form of PTPεC, PTPεC-DA (M1-εC-DA), IL-6 activation of STAT3 was enhanced compared with parent M1 cells and mock-transfected (M1-Neo) cells.24 These results further suggest that PTPεC-DA may act as a dominant negative and that endogenous PTPεC negatively regulates IL-6–induced JAK-STAT signaling. Studies have identified a SOCS/SSI/CIS family of proteins that inhibits cytokine-induced JAK-STAT signaling(s).7-9In the SOCS/SSI/CIS family, SOCS-1 and SOCS-3 have been shown to inhibit JAK-STAT signaling induced by IL-6.7,9,26-29PTPεC may function in the up-regulation of protein(s) of the SOCS family. If so, SOCS expression might be observed in M1-εC cells even under unstimulated conditions. To address this question, the steady state levels of SOCS-1 and SOCS-3 messenger RNA (mRNA) in M1-stable clones were determined (Figure 1). Expression of SOCS-1 mRNA was not detected in all clones before IL-6 stimulation. IL-6 treatment of M1-Neo cells was accompanied by up-regulation of SOCS-1 mRNA. In M1-εC cells, IL-6–mediated up-regulation of SOCS-1 mRNA was reduced. These results are consistent with the previous report that IL-6–induced expression of SOCS-1 mRNA is under the control of STAT3, and IL-6–induced activation of STAT3 is suppressed in M1-εC cells.9,24 Additionally, expression of SOCS-3 mRNA was very low in M1 stable clones before and after IL-6 treatment, whereas they were highly expressed in IFN-γ–treated NIH3T3 cells as reported30 (Figure 1). Taken together, these results suggest that negative regulation of cytokine signaling by PTPεC is independent of SOCS family proteins.
Expression of SOCS mRNAs in M1-PTPε clones.
Total RNA was prepared from M1-stable clones cultured for 4 hours in the absence or presence of 25 ng/mL IL-6 and analyzed for expression of SOCS-1, SOCS-3, and β-actin mRNA by Northern blotting. RNA from NIH3T3 cells treated with 25 ng/mL IFN-γ was also analyzed for SOCS-3 expression as a positive control.
Expression of SOCS mRNAs in M1-PTPε clones.
Total RNA was prepared from M1-stable clones cultured for 4 hours in the absence or presence of 25 ng/mL IL-6 and analyzed for expression of SOCS-1, SOCS-3, and β-actin mRNA by Northern blotting. RNA from NIH3T3 cells treated with 25 ng/mL IFN-γ was also analyzed for SOCS-3 expression as a positive control.
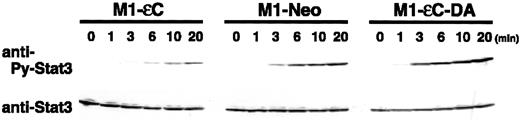
To characterize PTPεC-mediated regulation of STAT3, a time course of STAT3 phosphorylation/activation induced by IL-6 in M1-εC and M1-εC-DA cells was undertaken (Figure2). PTPεC and PTPεC-DA produced delayed and earlier onset of STAT3 tyrosine phosphorylation following IL-6 stimulation, respectively, suggesting that PTPεC may work as a negative regulator at the onset of STAT3 tyrosine phosphorylation. Another PTP SHP-2 has been reported to be involved in negative regulation of gp130 signaling.31-33 Introduction of a point mutation into gp130 that abrogates recruitment of SHP-2 apparently resulted in loss of this negative regulation and in prolonged activation of STAT3 without affecting the onset.31,33 These results suggest different modes of action for the 2 PTPs, PTPεC and SHP-2.31 33
Effect of PTPεC on the onset of IL-6–induced STAT3 tyrosine phosphorylation.
M1 stable clones were treated with IL-6 at 10 ng/mL for the times indicated and lysed. Phosphorylated STAT3 was detected by Western blotting with an antibody specific to tyrosine-phosphorylated STAT3 (upper panels). Blots were stripped and reprobed with the anti-STAT3 antibody (lower panels).
Effect of PTPεC on the onset of IL-6–induced STAT3 tyrosine phosphorylation.
M1 stable clones were treated with IL-6 at 10 ng/mL for the times indicated and lysed. Phosphorylated STAT3 was detected by Western blotting with an antibody specific to tyrosine-phosphorylated STAT3 (upper panels). Blots were stripped and reprobed with the anti-STAT3 antibody (lower panels).
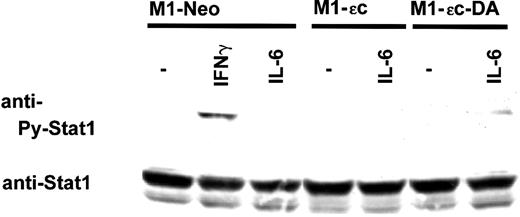
Although STAT1 was activated by IL-6 to a lesser extent than was STAT3, the activation was gp130-tyrosine phosphorylation–dependent.34 35 Therefore, the effect of PTPεC on STAT1 activation was analyzed. STAT1 phosphorylation in M1-Neo cells was undetectable under our experimental conditions. However, in M1-εC-DA cells, IL-6 induced tyrosine phosphorylation of STAT1 (Figure 3). This experiment showed that PTPεC negatively regulates the activation of STAT1 as well as that of STAT3.
PTPεC inhibits IL-6–induced activation of STAT1.
M1 stable clones were treated with IL-6 at 10 ng/mL or with IFN-γ at 25 ng/mL for 20 minutes and lysed. Phosphorylated STAT1 was detected by Western blotting with an antibody specific to tyrosine-phosphorylated STAT1 (upper panels). Blots were stripped and reprobed with the anti-STAT1 antibody (lower panels).
PTPεC inhibits IL-6–induced activation of STAT1.
M1 stable clones were treated with IL-6 at 10 ng/mL or with IFN-γ at 25 ng/mL for 20 minutes and lysed. Phosphorylated STAT1 was detected by Western blotting with an antibody specific to tyrosine-phosphorylated STAT1 (upper panels). Blots were stripped and reprobed with the anti-STAT1 antibody (lower panels).
Negative regulation of IL-10 signaling by PTPεC
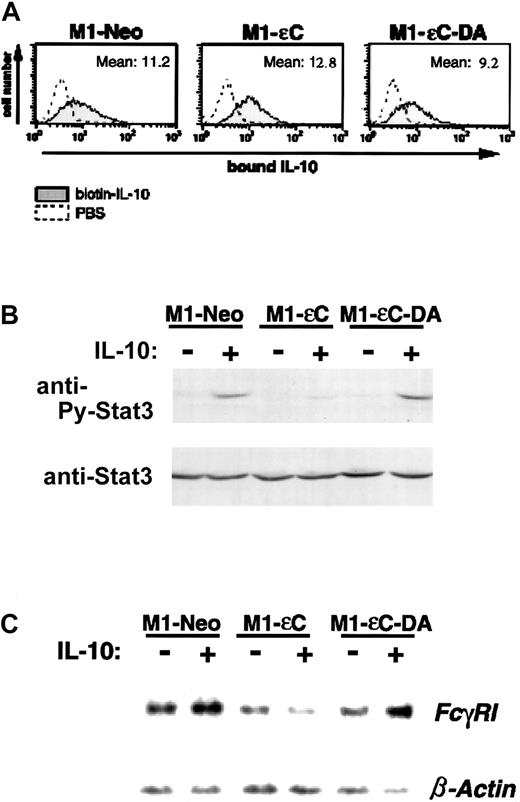
Because the JAK-STAT signaling pathway is used by many cytokines, it was important to analyze whether the inhibitory effect of PTPεC was specific to signaling induced by IL-6. We therefore investigated the effect of PTPεC on IL-10–induced signaling because JAK1, TYK2, and STAT3 are involved in IL-10 signaling as in IL-6–signaling cascade.17 19 First, we determined whether IL-10-Rs were expressed on M1-εC and M1-εC-DA cells by an IL-10 binding assay. As shown in Figure 4A, these cells bound IL-10 to a similar extent as mock-transfected M1-Neo cells, suggesting similar levels of receptor expression on the surface of all the stable clones. Stable clones were treated with IL-10 for 20 minutes and analyzed for STAT tyrosine phosphorylation. As illustrated in Figure4B, IL-10 treatment preferentially induced tyrosine phosphorylation of STAT3 in M1-Neo cells. At the same time, STAT1 tyrosine phosphorylation was barely detected (data not shown). In M1-εC cells, the level of tyrosine phosphorylation of STAT3 by IL-10 was lower than that seen in M1-Neo cells.
PTPεC inhibits IL-10–induced activation of STAT3.
(A) Binding of biotinylated IL-10 to M1 stable clones was analyzed by flow cytometry as described in “Materials and methods.” (B) M1 stable clones were treated with IL-10 at 20 ng/mL for 20 minutes and lysed. STAT3 tyrosine phosphorylation was analyzed as in Figure 2. (C) Total RNA was prepared from M1 stable clones cultured for 20 hours in the presence of 20 ng/mL IL-10 and analyzed for expression of FcγRI and β-actin mRNA by Northern blotting (upper panel). Data represent 1 of 3 independent experiments.
PTPεC inhibits IL-10–induced activation of STAT3.
(A) Binding of biotinylated IL-10 to M1 stable clones was analyzed by flow cytometry as described in “Materials and methods.” (B) M1 stable clones were treated with IL-10 at 20 ng/mL for 20 minutes and lysed. STAT3 tyrosine phosphorylation was analyzed as in Figure 2. (C) Total RNA was prepared from M1 stable clones cultured for 20 hours in the presence of 20 ng/mL IL-10 and analyzed for expression of FcγRI and β-actin mRNA by Northern blotting (upper panel). Data represent 1 of 3 independent experiments.
It has been shown that IL-10 up-regulates expression of theFcγRI gene through STAT3 activation.16,36 37To determine whether a downstream target of STAT3 induced by IL-10 was also suppressed in M1-εC cells, cells were treated with IL-10 and subjected to Northern blot analysis to determine whether theFcγRI gene is up-regulated (Figure 4C). A treatment of M1-Neo cells with IL-10 resulted in a slight increase in FcγRI mRNA compared with untreated controls (Figure 4C). Significantly, in M1-εC cells, IL-10 treatment failed to up-regulate FcγRIexpression. These results indicate that forced expression of PTPεC can inhibit not only IL-6– and LIF-induced signaling but also IL-10–induced JAK-STAT signaling. We also investigated the levels of tyrosine phosphorylation of JAK1 and TYK2 as determined by immunoprecipitation–Western blot analysis (data not shown). However, tyrosine phosphorylation of JAK1 or TYK2 was not detected following IL-10 stimulation of our cell lines. This finding is likely due to the low levels of JAK proteins that are activated in these cells.
Effects of PTPεC expression on IFN-induced JAK-STAT signaling
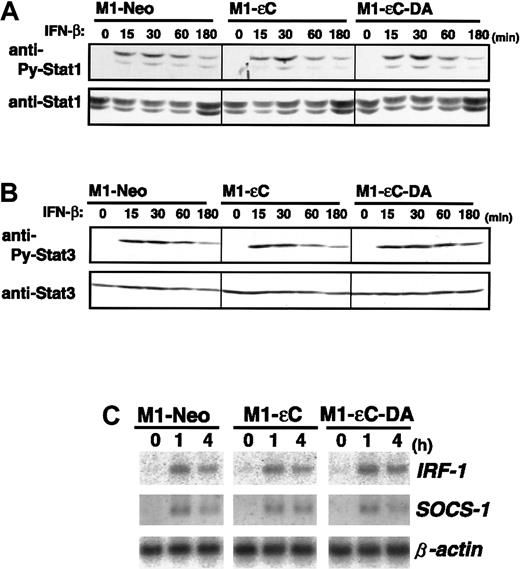
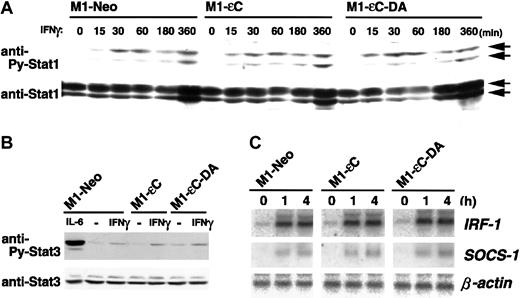
Subunits of the IL-10-R, IL-10-RI, and CRF2-4 are structurally subgrouped as belonging to the class II cytokine receptor family with IFN-α/β-R and IFN-γ-R.12,13,38 Given that PTPεC negatively regulates IL-10-R–mediated activation of STAT3, we investigated whether PTPεC is a common negative regulator of class II receptor-mediated JAK-STAT signaling. To address this issue, M1 stable clones were treated with IFN-β and IFN-γ and analyzed for STAT1 tyrosine phosphorylation. In sharp contrast to IL-10 treatment, PTPεC did not block IFN-β– and IFN-γ–induced tyrosine phosphorylation of STAT1. Figures 5A and 6A show the time courses of STAT1 tyrosine phosphorylation in M1 stable clones treated with IFN-β and IFN-γ, respectively. In each case, expression of PTPεC or PTPεC-DA did not affect STAT1 tyrosine phosphorylation, at least up to 3 or 6 hours. In addition to STAT1, tyrosine phosphorylation of JAK1 and STAT3 was not affected by PTPεC (Figures 5B and 6B, and data not shown). IRF-1, a downstream effector of IFNs, is induced by IFNs through STAT1-STAT2 heterodimers and STAT1 homodimers.39As shown in Figures 5C and 6C, expression of IRF-1 mRNA was induced in M1-εC cells following IFN-β and IFN-γ treatment, similar to what was observed in M1-Neo cells. SOCS-1 is also reported to be IFN inducible.7 40 Expression of SOCS-1 mRNA by IFNs was similarly induced in all stable clones (Figures 5C and 6C). Taken together, PTPεC does not appear to affect IFN-γ–induced JAK-STAT signaling.
Effect of PTPεC on IFN-β-induced STAT activation.
(A) M1 stable clones were treated with IFN-β at 1000 U/mL for the times indicated and lysed. STAT1 activation was analyzed as in Figure3, and (B) STAT3 activation was analyzed as in Figure 2. Cells were treated with IFN-β for the times indicated. Total RNA was analyzed for IRF-1 and SOCS-1 expression by Northern blotting. (C) Expression levels of β-actin were also estimated.
Effect of PTPεC on IFN-β-induced STAT activation.
(A) M1 stable clones were treated with IFN-β at 1000 U/mL for the times indicated and lysed. STAT1 activation was analyzed as in Figure3, and (B) STAT3 activation was analyzed as in Figure 2. Cells were treated with IFN-β for the times indicated. Total RNA was analyzed for IRF-1 and SOCS-1 expression by Northern blotting. (C) Expression levels of β-actin were also estimated.
Effect of PTPεC on IFN-γ–induced STAT activation.
(A) M1 stable clones were treated with IFN-γ at 25 ng/mL for the times indicated and lysed. STAT1 activation was analyzed as in Figure3, and (B) STAT3 activation was analyzed as in Figure 2B. Cells were treated with IFN-γ for the times indicated. Total RNA was analyzed for IRF-1 and SOCS-1 mRNA by Northern blotting. (C) The expression levels of β-actin were also estimated.
Effect of PTPεC on IFN-γ–induced STAT activation.
(A) M1 stable clones were treated with IFN-γ at 25 ng/mL for the times indicated and lysed. STAT1 activation was analyzed as in Figure3, and (B) STAT3 activation was analyzed as in Figure 2B. Cells were treated with IFN-γ for the times indicated. Total RNA was analyzed for IRF-1 and SOCS-1 mRNA by Northern blotting. (C) The expression levels of β-actin were also estimated.
Discussion
We and others have reported that expression of PTPεC is up-regulated during macrophage differentiation and/or activation. As noted previously, PTPεC inhibited IL-10–induced STAT3 activation but not IFN-γ–induced STAT1 activation. It is well established that IFN-γ and IL-10 have pleiotrophic effects on regulation of macrophage activation and de-activation, respectively.10,41 42 In this context, PTPεC might play important roles in facilitating activation of macrophages and/or in maintaining activated states by selective down-regulation of the de-activating IL-10 signal. Because PTPεC is expressed not only in myeloid/monocytic lineages but also in lymphocytes (K.N. and N.T., unpublished results, March 2001), it would be of great interest and importance to determine whether PTPεC plays negative regulatory roles in cytokine signaling in lymphocytes.
In summary, results in this study reveal an inhibitory effect of PTPεC on STAT activation induced by IL-10 as well as by IL-6. Negative regulation by PTPεC does not involve SOCS family proteins (at least not SOCS-1 and -3) and may have a different mode of action from SHP-2. In contrast, PTPεC appears not to interfere with IFN-β– and IFN-γ–induced JAK-STAT signaling. Our results indicate that PTPεC inhibition of JAK-STAT activation is not specified by particular JAKs or STATs. Instead, PTPεC-mediated suppression is selective for certain JAK-STAT signaling pathways through corresponding cytokine receptors such as gp130 and IL-10-R. Identification of target(s) of PTPεC-mediated suppression will enable us to fully understand the molecular basis for regulation of cytokine signaling.
We thank Dr T. Abo for murine IFN-γ and Dr T. Minagawa for murine IFN-β. Additionally, we thank Dr N. Urushibara for help with some of the experiments, and Y. Saito and E. Yoshida for secretarial assistance.
Supported in part by Grant-in-Aids for Scientific Research provided by the Japan Society for the Promotion of Science and a Grant-in-Aid for Scientific Research on Priority Areas provided by the Ministry of Education, Culture, Sports, Science and Technology of Japan.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Kunimi Kikuchi, Division of Biochemical Oncology and Immunology, Institute for Genetic Medicine, Hokkaido University, Kita-15, Nishi-7, Kita-Ku, Sapporo 060-0815, Japan; e-mail:kikuchi@imm.hokudai.ac.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal