Abstract
Splenectomy increases the number of B cells in the blood of humans and animals. It is unknown whether this is due to changes in migration, proliferation, or both. The numbers of naı̈ve (IgD+IgM+), memory (IgD−IgMhigh), newly formed (IgMhighCD90high), early recirculating follicular (IgMlowCD90high), recirculating follicular (IgMlowCD90−), and marginal zone (IgMhighCD90−) phenotype B cells were determined in control and splenectomized rats by flow cytometry. All subsets increased significantly in the blood after splenectomy. Because surface molecules are involved in the regulation of migration and proliferation, their expression (lymphocyte function-associated antigen 1 [LFA-1], intercellular adhesion molecule 1 (ICAM-1), L-selectin, α4-integrins, CD44, major histocompatability complex class II, interleukin 2 receptor-α chain) was determined on B- and T-cell subsets of both groups. B cells, but not T cells, showed a significantly reduced LFA-1 and ICAM-1 expression in blood and lymph nodes, whereas the expression of the other surface molecules analyzed remained unchanged. The down-regulation of these molecules did not influence the adherence of B cells to high endothelial venules in vitro. In vivo, however, ICAM-1low–expressing B cells migrated significantly faster through lymph nodes (ICAM-1low 41 ± 5 hours versus ICAM-1high58 ± 3 hours), whereas proliferation of B cells in bone marrow, lymph node, and blood remained unchanged. Thus, the presence of one organ is necessary for appropriate expression of LFA-1 and ICAM-1 on B cells in other, distant organs. The more rapid transit of ICAM-1low B cells through lymph nodes may be responsible for the increased B-cell number in the blood after splenectomy.
Introduction
Antigens circulating in the blood are retained in the spleen and presented to a huge number of B and T cells migrating through its different compartments.1,2 On removal of the spleen the incidence of severe infections increases both in children and adults,3,4 and there are changes in many immunologic parameters.5 One lasting change is a high increase in the number of B lymphocytes in the blood, an effect that is constantly observed both in humans6 and in experimental animals,7,8 and that comparably affects the 2 compartments of the blood, the peripheral and the marginal pools.9
Because it is not known whether all or only certain blood B-cell subsets increase in number after the loss of the spleen, the numbers of naı̈ve (IgD+IgM+), memory (IgD−IgMhigh), newly formed (NF; IgMhighCD90high), early recirculating follicular (ERF; IgMlowCD90high), recirculating follicular (RF; IgMlowCD90−), and marginal zone (MZ; IgMhighCD90−) phenotype B cells10 were determined in blood, lymph nodes, and bone marrow of control and splenectomized animals. In principle, the splenectomy-induced increase in blood B-cell numbers could be due to an altered migration pattern or to increased proliferation of B cells caused, for example, by a latent infection.
Because migration and proliferation of B cells are regulated by surface molecules, our hypothesis was that their expression might be altered by splenectomy. Therefore, the expression of molecules involved in initial adhesion of lymphocytes to the endothelium, in transmigration through the endothelium and the tissue, and in B-cell activation within the tissue was analyzed in all B-cell subsets in blood, lymph nodes, and bone marrow (α4-integrins, CD44, L-selectin, lymphocyte function-associated antigen1 [LFA-1], intercellular adhesion molecule 1 [ICAM-1], major histocompatability complex [MHC] class II, interleukin [IL]-2 receptor-α [IL2-Rα] chain11-14). The functional consequences of possible changes in surface molecule expression were tested in vitro by adhesion assays and in vivo by following the traffic of labeled B cells from blood to thoracic duct lymph. In addition, the number of proliferating B cells was determined in the bone marrow, in the B-cell area of lymph nodes, and in the blood.
Our data show that loss of the spleen leads to a decrease in LFA-1 and ICAM-1 expression of B cells but not T cells in blood and lymph nodes. This accelerates the migration of B cells through lymph nodes and results in higher numbers of B cells in the thoracic duct lymph and, subsequently, in the blood.
Materials and methods
Animals
Normal rats (LEW/Ztm), rats with high numbers of B cells (BH.1L/Won15), and rats lacking T cells (LEW/Ztm-rnu16) were obtained from the Central Animal Facility at the Medical School of Hannover and were barrier-maintained in a room with controlled environment (22 ± 2°C, 55% ± 5% relative humidity, 12-hour light/dark cycle).
Splenectomy
Splenectomy was performed as described previously.3,7 The animals were kept under barrier-maintained conditions for 3 months before analysis to allow recovery from the operation and adjustment to the asplenic situation. Sham splenectomy was performed as described above with the exception that after cutting the splenic ligaments the spleen was put back into the abdominal cavity. Routine microbiologic monitoring according to Federation of European Laboratory Animal Science Associations (FELASA) recommendations17 was conducted on animals from both groups. They did not reveal any evidence of infection with common rat pathogens except for Pasteurella pneumotropica, which was found in both control and splenectomized rats. To further rule out infections due to the absence of the spleen, Northern blot analysis of α2-macroglobulin was performed according to standard procedures using liver tissue. Total RNA was isolated by the guanidium isothiocyanate method.18 Total RNA (15 μg) was analyzed through a 1% 3-(N-morpholino)propane sulfonic acid (Sigma, Munich, Germany) agarose gel, followed by transfer to Gene Screen Plus (NEN, Boston, MA). The α2-macroglobulin and β-actin complementary DNA (cDNA) probe was labeled with αCTP32 according to Random priming (Boehringer Mannheim, Mannheim, Germany). The hybridization procedure was performed as described before.18 The ratio of α2-macroglobulin to β-actin expression was set to 1. Changes in the expression level of α2-macroglobulin in splenectomized rats were calculated as fold activation compared to control animals. If inflammation would have been present due to loss of the spleen, the α2-macroglobulin expression level would have been over a 100 times higher in splenectomized rats than in control animals.18 However, the level ranged between 0.4 and 2.53 for both groups.
Collection of lymphocytes from blood, lymph nodes, and bone marrow
Blood was collected from the abdominal aorta under ether anesthesia. Mesenteric lymph nodes and both femora were removed and cell suspensions were prepared as described.19 The volume and the total number of leukocytes (Coulter Counter, Luton, United Kingdom) were determined for the different cell suspensions. The B-cell subsets were analyzed by flow cytometry (FACScan, Becton Dickinson, Mountain View, CA) as described below.
Identification of B-cell subsets
Blood, lymph node, bone marrow, and thoracic duct lymph cell suspensions were washed and adjusted to 1 × 106cells/well. The different B-cell subsets were identified using a biotinylated antibody against IgM,20 which was revealed with streptavidin-conjugated Red 670 (FL3; Gibco, Gaithersburg, MD) in combination with fluorescein isothiocyanate (FITC)–conjugated monoclonal antibody Ox7 against CD9021 (FL1) and a monoclonal antibody against IgD,20 which was revealed using a phycoerythrin (PE)–conjugated secondary antibody (FL2; Becton Dickinson). By staining first for FL2 and then for FL1 and FL3, no cross-reaction occurred between the PE-conjugated secondary antibody (antimouse) and the subsequently applied anti-CD90 antibody (both generated in the mouse). Six B-cell populations were identified: naı̈ve (IgD+IgM+), memory (IgD−IgMhigh), NF (IgMhighCD90high), ERF (IgMlowCD90high), RF (IgMlowCD90−), and MZ phenotype B cells (IgMhighCD90−).10 The incubation period was 30 minutes at 4°C for each antibody. Using a FACScan and PC-LYSYS software, about 1 × 105 viable cells were analyzed and the percentage of the respective population among B cells was determined.
Surface molecule expression on B- and T-cell subsets
Cell suspensions were treated as described above. In the following experiments 2 colors (FL1 and FL3) were used to define either B-cell subpopulations (NF, ERF, RF, and MZ phenotype) or T-cell subsets (CD4, CD8), and the third color was used to determine the surface molecule expression. In brief, cells were incubated with antibodies against ICAM-1 (1A29), LFA-1 (WT.1), CD44 (Ox49), α4-integrin (HP2/1), L-selectin (HRL-3 and Ox85), IL-2 receptor (Ox39), and MHC class II (Ox6).22 The antibodies were revealed with a PE-conjugated antimouse Ig secondary antibody (FL2). Because one of the anti–L-selectin antibodies (HRL-3) is produced in hamster it was revealed using a PE-conjugated antihamster antibody (Caltag, Burlingame, CA). Next, the cells were incubated with an FITC-conjugated monoclonal antibody against CD90 (Ox7; FL1) and a biotinylated antibody against IgM (FL3) to identify the B-cell subsets, or the cells were incubated with an FITC-conjugated monoclonal antibody against the T-cell receptor (R73; FL1) and a biotinylated antibody against CD4 (FL3) to identify the T-cell subsets. Finally, the biotinylated antibody was revealed with streptavidin-Red 670 (FL3). All incubations were performed for 30 minutes at 4°C. An irrelevant mouse antibody was used as a control. To compare the surface molecule expression, blood, lymph node, and bone marrow cells of control and splenectomized animals were always analyzed on the same day, and the different suspensions were treated identically with respect to the applied suspension medium, erythrocyte lysis, and setting of FACS parameters. Using a FACScan and the PC-LYSYS software the surface molecule expression was analyzed by gating on the respective population. Forward and side scatter analysis revealed that the size of the different B-cell subsets did not change after splenectomy. This allowed direct comparison of the fluorescence intensities of the various surface molecules between the subsets of both groups.
In vitro binding of B cells to high endothelial venules
In the in vitro adhesion assay the binding of blood B cells of control and splenectomized rats to high endothelial venules (HEVs) of various tissues was compared to that of a standard population. The assay was performed as described previously.23 24 Blood lymphocytes were obtained from normal and splenectomized animals. Standard lymphocytes from mesenteric lymph nodes of normal rats were labeled with FITC. Lymph node cryostat sections were overlaid with 100 μL of a suspension containing 1.5 × 106 lymphocytes (mixture of blood lymphocytes and standard cells). Lymph node sections from control animals were overlaid with blood cells from control rats and lymph node sections from splenectomized animals were overlaid with blood cells from splenectomized rats. The slides were agitated during incubation. For immunohistochemical staining the sections were fixed in glutaraldehyde 0.2% for 10 minutes, 1% paraformaldehyde or 30 minutes, and acetone for 2 minutes.
Blood B cells were identified by alkaline phosphatase labeling of surface antigens, and the FITC-labeled standard cells were revealed by an anti-FITC antibody and peroxidase staining described above. The incubation with antibodies was performed at room temperature in a humid chamber, each step lasting 30 minutes. The first incubation step was performed using a monoclonal mouse antibody for B lymphocytes (His14).25 For the second incubation step a cocktail was used, which consisted of goat antimouse IgG (Dianova, Hamburg, Germany) and rabbit anti-FITC. Finally a cocktail of alkaline phosphatase–antialkaline phosphatase (APAAP) mouse monoclonal and swine antirabbit was added to the sections. The peroxidase reaction was developed first (standard lymphocytes) and then the B cells were revealed using Fast Blue BB salt.23 24 The sections were counterstained with hemalum, washed in triethanolamine-buffered saline (TBS) and mounted in mounting medium (Dako, Hamburg, Germany). The ratio of blood B cells and standard cells bound to the high endothelial venules was determined.
Determination of the mean transit time of ICAM-1high and ICAM-1low B cells from blood to lymph
The thoracic duct was cannulated and lymph was collected as described.22,26 After the operation the rats were allowed to adjust to their surroundings for 24 hours. Thoracic duct lymphocytes were labeled with 10 μg/mL FITC as described previously.27 Then, 50 × 106 cells were either taken into culture to generate the ICAM-1high–expressing B cells (for 24 hours at 37°C, 5% CO2, in RPMI 1640 medium containing 10% fetal calf serum [FCS], 1 mmol l-glutamine, 50 IU/mL penicillin, 50 μg/mL streptomycin), or taken directly as ICAM-1low–expressing B cells. Before injection, surface molecule expression on B cells was determined as described above. Then FITC-labeled B cells (either ICAM-1high or ICAM-1low) were injected, and the lymph was collected in portions every 6 hours for 144 hours. The mean transit time from blood to lymph, that is, the time it takes for 50% of the injected cells to be recovered in the thoracic duct lymph, was determined and compared between both populations. The recovery was always greater than 90% of the injected cells within 144 hours of collection. There was no difference in the recovery of B cells expressing high or low levels of ICAM-1. This suggests that differences in the mean transit time are due to the different level of ICAM-1 expression rather than to the different times the 2 populations were cultured in vitro.
Identification of proliferating B cells in bone marrow, blood, and B-cell area of lymph nodes
Control and splenectomized animals were given 5 mg/100 g body weight 5-bromo-2-deoxyuridine (BrdU; Sigma) intravenously 12 weeks after surgery to determine the number of proliferating B cells immunohistologically.28 One hour and 24 hours after BrdU injection the animals were anesthetized and exsanguinated via the abdominal aorta. Blood, both femora, and the mesenteric lymph nodes were removed for further processing. The femora were flushed with medium and cytospots were prepared for blood and bone marrow as described.28 Both on cytospots and on cryostat sections 2 antigens were simultaneously revealed to identify proliferating B cells as described.29 B cells were identified using the appropriate antibodies (His14,25 IgM, and IgD20) and visualized in blue.30 After denaturation the BrdU+ cells were identified and visualized in red as described.29
Statistics
The statistical package SPSS for Windows 10.0 (SPSS, Chicago, IL) was used to calculate the means and SDs and to determine significant differences (P < .05 in the Mann-WhitneyU test or Wilcoxon matched-pairs signed-rank test).
Results
B-cell numbers of all subtypes analyzed increase in the blood after splenectomy
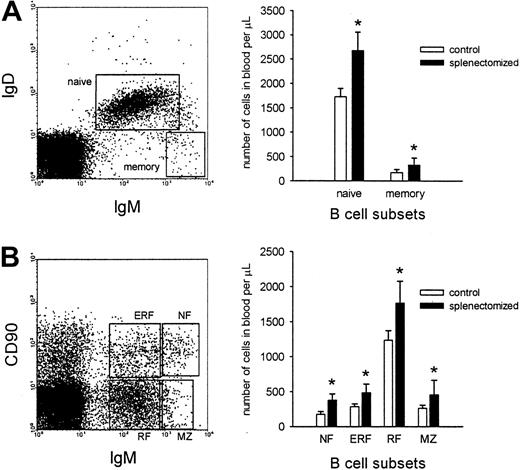
Three months after splenectomy the number of B cells in the blood was significantly increased, whereas the number of T cells, natural killer (NK) cells, monocytes, and neutrophils was unchanged (Table1). This increase in blood B-cell numbers was found both among naı̈ve and memory B cells (Figure1A). In addition, when the B cells were further subdivided into NF, ERF, RF, and MZ phenotypes, a significant increase was observed for all subsets (Figure 1B).
Only the number of B cells is increased in the blood after splenectomy
| Cell type . | Control . | Splenectomized . |
|---|---|---|
| B cells | 1976 ± 243 | 3088 ± 466* |
| T cells | 6295 ± 861 | 6151 ± 623 |
| NK cells | 209 ± 18 | 154 ± 29 |
| Monocytes | 540 ± 263 | 831 ± 306 |
| Neutrophils | 1750 ± 583 | 1736 ± 428 |
| Cell type . | Control . | Splenectomized . |
|---|---|---|
| B cells | 1976 ± 243 | 3088 ± 466* |
| T cells | 6295 ± 861 | 6151 ± 623 |
| NK cells | 209 ± 18 | 154 ± 29 |
| Monocytes | 540 ± 263 | 831 ± 306 |
| Neutrophils | 1750 ± 583 | 1736 ± 428 |
The number of cells per μL (mean ± SD) of peripheral blood in control and splenectomized rats are presented. The asterisk indicates a significant difference between control and splenectomized rats (Mann-Whitney U test; P < .001; n = 4-5 for each group).
In the blood B cell numbers of all subsets increase after splenectomy.
(A) Representative dot plot of naı̈ve (IgD+IgM+) and memory (IgD−IgMhigh) B-cell populations in the blood is shown (left). The number of cells of the framed populations was determined 3 months after either sham operation or splenectomy, and expressed as the number of cells per microliter (right). (B) Representative dot plot of NF (IgMhighCD90high), ERF (IgMlowCD90high), RF (IgMlowCD90−), and MZ (IgMhighCD90−) B-cell subsets is shown (each subset is framed). The graph demonstrates the number of B cells of the different subsets in blood of rats either sham operated or splenectomized. The values for both graphs are given as the mean ± SD (n = 8 for each group). The asterisk represents significant differences between the B-cell number of sham-operated and splenectomized animals (Mann-Whitney U test;P < .05).
In the blood B cell numbers of all subsets increase after splenectomy.
(A) Representative dot plot of naı̈ve (IgD+IgM+) and memory (IgD−IgMhigh) B-cell populations in the blood is shown (left). The number of cells of the framed populations was determined 3 months after either sham operation or splenectomy, and expressed as the number of cells per microliter (right). (B) Representative dot plot of NF (IgMhighCD90high), ERF (IgMlowCD90high), RF (IgMlowCD90−), and MZ (IgMhighCD90−) B-cell subsets is shown (each subset is framed). The graph demonstrates the number of B cells of the different subsets in blood of rats either sham operated or splenectomized. The values for both graphs are given as the mean ± SD (n = 8 for each group). The asterisk represents significant differences between the B-cell number of sham-operated and splenectomized animals (Mann-Whitney U test;P < .05).
Down-regulation of LFA-1 and ICAM-1 expression on B-cell subsets after splenectomy
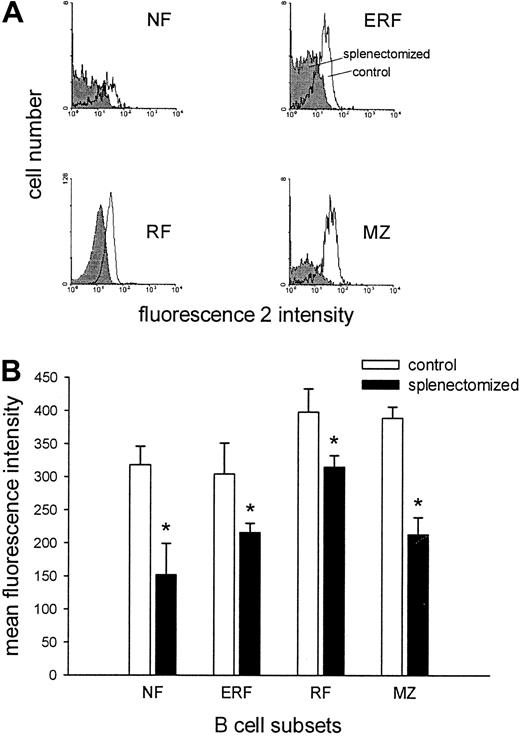
Surface molecules are involved in the regulation of migration and proliferation, 2 possible causes of the observed increase in B-cell numbers. Therefore, the expression of LFA-1, ICAM-1, L-selectin, α4-integrins, IL-2–Rα chain, CD44, and MHC class II was determined by flow cytometry on the different B-cell subsets in the blood. Three months after splenectomy no difference in the expression of the adhesion molecules analyzed was observed between the 2 groups with the exception of LFA-1. LFA-1 expression was significantly reduced on all B-cell subsets in the blood of splenectomized animals compared with control animals (Figure 2).
LFA-1 expression is lower on various blood B-cell subsets in splenectomized animals.
(A) Representative histograms of the LFA-1 expression of NF (IgMhighCD90high), ERF (IgMlowCD90high), RF (IgMlowCD90−), and MZ (IgMhighCD90−) blood B cells in control (white) and splenectomized (gray) animals are demonstrated. (B) The LFA-1 expression of various B-cell subsets in the blood is given as the mean of the mean fluorescence intensity ± SD (n = 5-6 for each group). The asterisk represents a significant difference between the LFA-1 expression of control versus splenectomized animals (Mann-WhitneyU test; P < .05).
LFA-1 expression is lower on various blood B-cell subsets in splenectomized animals.
(A) Representative histograms of the LFA-1 expression of NF (IgMhighCD90high), ERF (IgMlowCD90high), RF (IgMlowCD90−), and MZ (IgMhighCD90−) blood B cells in control (white) and splenectomized (gray) animals are demonstrated. (B) The LFA-1 expression of various B-cell subsets in the blood is given as the mean of the mean fluorescence intensity ± SD (n = 5-6 for each group). The asterisk represents a significant difference between the LFA-1 expression of control versus splenectomized animals (Mann-WhitneyU test; P < .05).
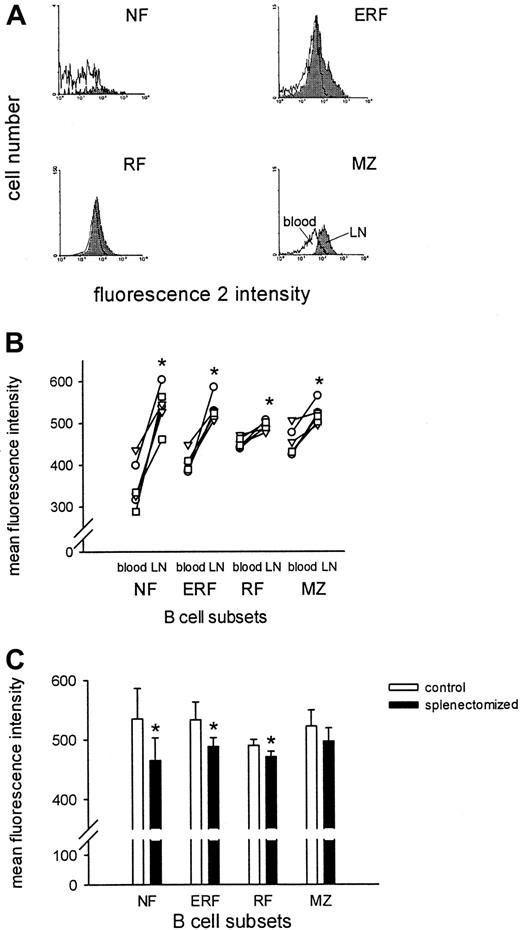
Compared to blood B cells, lymph node B cells in control animals showed significantly higher expression levels of ICAM-1 (Figure3A,B) and CD44 and MHC class II (data not shown). Expression of L-selectin was reduced (data not shown). Splenectomized animals also demonstrated increased levels of ICAM-1, CD44, and MHC class II and a reduced level of L-selectin in lymph node compared to blood (data not shown). However, compared to control animals the difference in ICAM-1 expression between B cells in blood and lymph nodes was much smaller in splenectomized animals. This resulted in a significantly lower ICAM-1 expression on B cells from splenectomized than from control animals (Figure 3C). These results demonstrate that the spleen is necessary for the expression of LFA-1 and ICAM-1 on B cells in the blood and lymph nodes. In contrast, the expression of LFA-1 and ICAM-1 on CD4+ and CD8+T cells did not change after splenectomy (data not shown).
ICAM-1 expression is lower on various lymph node B-cell subsets in splenectomized animals.
(A) Representative histograms of the ICAM-1 expression for NF (IgMhighCD90high), ERF (IgMlowCD90high), RF (IgMlowCD90−), and MZ (IgMhighCD90−) B cells are demonstrated in blood (white) versus lymph node (gray) of normal animals. (B) The ICAM-1 expression of the B-cell subsets in blood and lymph node is depicted for each normal animal. The values for the blood and lymph node of each rat are connected by a line. (C) The ICAM-1 expression of B-cell subsets in lymph node of control and splenectomized animals is shown. The values are given as the mean of the mean fluorescence intensity ± SD (n = 5-6 for each group). The asterisk represents a significant difference (Wilcoxon matched-pairs signed rank test or Mann-Whitney U test; P < .05).
ICAM-1 expression is lower on various lymph node B-cell subsets in splenectomized animals.
(A) Representative histograms of the ICAM-1 expression for NF (IgMhighCD90high), ERF (IgMlowCD90high), RF (IgMlowCD90−), and MZ (IgMhighCD90−) B cells are demonstrated in blood (white) versus lymph node (gray) of normal animals. (B) The ICAM-1 expression of the B-cell subsets in blood and lymph node is depicted for each normal animal. The values for the blood and lymph node of each rat are connected by a line. (C) The ICAM-1 expression of B-cell subsets in lymph node of control and splenectomized animals is shown. The values are given as the mean of the mean fluorescence intensity ± SD (n = 5-6 for each group). The asterisk represents a significant difference (Wilcoxon matched-pairs signed rank test or Mann-Whitney U test; P < .05).
After splenectomy, nude animals (genetically lacking T cells) also developed B lymphocytosis in the blood (sham-operated, 2300 ± 354; splenectomized, 4786 ± 1031; n = 6 for each group) accompanied by a significantly decreased LFA-1 expression, as well as a significantly reduced ICAM-1 expression on lymph node B cells (data not shown). This demonstrates that also in nude animals the spleen controls the regulation of LFA-1 and ICAM-1 expression of B cells in blood and lymph nodes, suggesting that T cells are not involved in this process.
ICAM-1 down-regulation on B cells accelerates their migration through lymph nodes
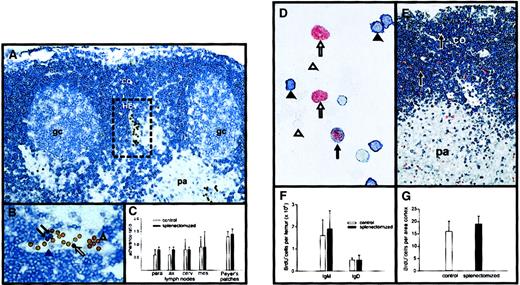
Next, it was investigated whether decreased LFA-1 expression on blood B cells would impair their entry into lymph nodes. The binding of blood B cells from control and splenectomized animals to HEVs of control and splenectomized animals, respectively, was studied in an in vitro adhesion assay. As shown in Figure4, panels A and B, blood B cells and standard cells could be clearly identified. The standard lymphocytes were derived from mesenteric lymph nodes of control rats and the binding of blood B cells from either normal or splenectomized animals was related to them. This permitted a comparison of the binding of the 2 populations. The B cells of control and splenectomized rats showed comparable binding to HEVs of different lymph nodes and Peyer patches (Figure 4C). This suggests that the entry of B cells into these organs was not impaired by the splenectomy-induced decrease of LFA-1 expression.
Splenectomy does not influence adherence of B cells to HEVs in vitro or B-cell proliferation in vivo.
(A) After incubation of blood and standard cells on a mesenteric lymph node cryostat section the adherence on HEVs was demonstrated and B cells (blue) and standard lymphocytes (brown) were identified immunohistochemically. Co indicates cortex; pa, paracortex; gc, germinal center; HEV, high endothelial venule, framed. Original magnification × 250. (B) Depicted is the framed HEV that is shown in panel A. The standard cells are visible in brown. The black arrows demonstrate blood B cells, the black arrowhead a blood non-B cell, the white arrow a standard B cell, and the open arrowhead a standard non-B cell. Original magnification × 500. (C) The adherence on parathymic (para), axillary (ax), cervical (cerv), and mesenteric (mes) lymph nodes and Peyer patches is shown for control and splenectomized animals. On tissue from control animals blood B cells from control animals were used, and on tissue from splenectomized rats blood B cells from splenectomized rats were used. The values are given as the mean of the adherence ratio ± SD (n = 6 for each group). (D) Cytospot of bone marrow cells is depicted. Bone marrow cells were isolated 1 hour after BrdU injection. B cells that were IgM positive (black arrowhead), proliferating B cells that had incorporated BrdU (black arrow), proliferating non-B cells (white arrow), and nonproliferating non-B cells (white arrowhead) are shown. (E) Mesenteric lymph nodes were removed 1 hour after BrdU injection and tissue sections were stained for B cells and BrdU incorporation. The black arrows show proliferating B cells (original magnification D: × 600 and E: × 150; co indicates cortex; pa, paracortex). (F) The graph demonstrates the number of proliferating IgD+ and IgM+ B cells in the bone marrow of control and splenectomized rats. (G) Indicated is the number of B cells per area (mm2 × 1000) proliferating in the B-cell area in control versus splenectomized rats. For panels F and G the mean ± SD are given (n = 4-5 for each group).
Splenectomy does not influence adherence of B cells to HEVs in vitro or B-cell proliferation in vivo.
(A) After incubation of blood and standard cells on a mesenteric lymph node cryostat section the adherence on HEVs was demonstrated and B cells (blue) and standard lymphocytes (brown) were identified immunohistochemically. Co indicates cortex; pa, paracortex; gc, germinal center; HEV, high endothelial venule, framed. Original magnification × 250. (B) Depicted is the framed HEV that is shown in panel A. The standard cells are visible in brown. The black arrows demonstrate blood B cells, the black arrowhead a blood non-B cell, the white arrow a standard B cell, and the open arrowhead a standard non-B cell. Original magnification × 500. (C) The adherence on parathymic (para), axillary (ax), cervical (cerv), and mesenteric (mes) lymph nodes and Peyer patches is shown for control and splenectomized animals. On tissue from control animals blood B cells from control animals were used, and on tissue from splenectomized rats blood B cells from splenectomized rats were used. The values are given as the mean of the adherence ratio ± SD (n = 6 for each group). (D) Cytospot of bone marrow cells is depicted. Bone marrow cells were isolated 1 hour after BrdU injection. B cells that were IgM positive (black arrowhead), proliferating B cells that had incorporated BrdU (black arrow), proliferating non-B cells (white arrow), and nonproliferating non-B cells (white arrowhead) are shown. (E) Mesenteric lymph nodes were removed 1 hour after BrdU injection and tissue sections were stained for B cells and BrdU incorporation. The black arrows show proliferating B cells (original magnification D: × 600 and E: × 150; co indicates cortex; pa, paracortex). (F) The graph demonstrates the number of proliferating IgD+ and IgM+ B cells in the bone marrow of control and splenectomized rats. (G) Indicated is the number of B cells per area (mm2 × 1000) proliferating in the B-cell area in control versus splenectomized rats. For panels F and G the mean ± SD are given (n = 4-5 for each group).
To test whether a decrease of ICAM-1 expression affects B-cell migration in vivo, thoracic duct B cells that differ in their ICAM-1 expression were labeled. These cells did not differ in their LFA-1 or L-selectin expression (data not shown). Either ICAM-1high(mean fluorescence intensity 581 ± 42) or ICAM-1low(mean fluorescence intensity 524 ± 23) B cells were injected. ICAM-1low B cells had a significantly faster mean transit time (time point when 50% of the injected cells were recovered) from blood via lymph nodes to thoracic duct lymph than ICAM-1high B cells (41 ± 5 hours versus 58 ± 3 hours; each n = 4). This shows that the decreased ICAM-1 expression leads to faster migration of B cells through the lymph nodes.
Splenectomy does not change proliferation of B cells
To analyze whether the down-regulation of LFA-1 and ICAM-1 on B cells after splenectomy influences their proliferation rate, control and splenectomized rats intravenously received BrdU, which labels proliferating cells in the S phase of the cell cycle.29One hour later, the number of BrdU+ B cells was determined in bone marrow and lymph nodes. Proliferating bone marrow B cells (Figure 4D) and lymph node B cells (Figure 4E) could be clearly identified. The bone marrow of control and splenectomized animals contained comparable numbers of proliferating B cells (Figure 4F), and the percentage of NF B cells within the bone marrow remained constant after splenectomy (control, 6% ± 2% versus splenectomized 7% ± 3%; each n = 4). In addition, both the number of BrdU+ cells in lymph nodes (Figure 4G) and the percentage of BrdU+ cells among the blood B cells was comparable in control and splenectomized animals (control, 1.0% ± 0.4%, n = 5; splenectomized, 1.3% ± 0.4%, n = 5) 24 hours after BrdU injection.
Together, these results indicate that changes in migration properties rather than changes in proliferation cause the B lymphocytosis in the blood after splenectomy. This conclusion is further supported by the observation that B cells from BH.1L rats, which have much higher numbers of B cells than Lewis rats, also showed a marked increase in blood B-cell number after splenectomy (Table2).
Increased B-cell number in the blood of BH.1L rats 3 months after splenectomy
| Operation . | Animal . | Percent B cells before surgery (number/μL) . | Percent B cells after surgery (number/μL) . |
|---|---|---|---|
| Sham | 1 | 36.2 (2209) | 35.0 (1834) |
| 2 | 42.0 (2933) | 47.0 (3339) | |
| Splenectomized | 3 | 39.1 (1240) | 55.4 (5886) |
| 4 | 42.0 (3150) | 63.8 (6200) |
| Operation . | Animal . | Percent B cells before surgery (number/μL) . | Percent B cells after surgery (number/μL) . |
|---|---|---|---|
| Sham | 1 | 36.2 (2209) | 35.0 (1834) |
| 2 | 42.0 (2933) | 47.0 (3339) | |
| Splenectomized | 3 | 39.1 (1240) | 55.4 (5886) |
| 4 | 42.0 (3150) | 63.8 (6200) |
The percentage of B cells (Ox12+)7 in the blood of the same rat before and after either sham operation or splenectomy is presented. Normal Lewis rats demonstrate 22.5% ± 2.5%, whereas splenectomized Lewis rats show 31.1% ± 2.9% B cells in the blood (n = 6).
Discussion
Splenectomy leads to an increase in the number of blood B cells in humans, sheep, and rats.6-8 The present study extends these observations by showing that all B-cell subsets increase in the blood after splenectomy.
In addition, our study further shows that expression of LFA-1 and ICAM-1 is down-regulated on B cells after removal of the spleen, suggesting that these molecules are involved in the generation of the B lymphocytosis. This conclusion is supported by the observation that after splenectomy T cells do not show down-regulation of LFA-1 and ICAM-1, and, as already mentioned, T lymphocytosis is not observed. Thus, the questions arise (1) how the spleen influences the expression of LFA-1 and ICAM-1 on B cells in blood and lymph nodes and (2) how the reduced LFA-1 and ICAM-1 expression is involved in the generation of the B lymphocytosis in blood.
The MZ of the spleen influences LFA-1 and ICAM-1 expression on B cells in blood and lymph nodes
The spleen contains 4 compartments (periarteriolar lymphatic sheath [PALS], red pulp, follicles, and MZ) which, together or individually, could be the sites involved in the regulation of LFA-1 and ICAM-1 expression on B cells. Our results show that splenectomy of nude animals has the same consequences as splenectomy of normal animals (B lymphocytosis, LFA-1 and ICAM-1 down-regulation). Because nude rats have no or only very few T cells,16 this indicates that the T cells of the splenic T-cell area (PALS) are not involved in LFA-1 and ICAM-1 regulation.
Splenic transplantation experiments point toward the MZ as the most likely site of regulation. After splenectomy, splenic tissue can be implanted into the greater omentum. Following a phase of necrosis the implanted splenic tissue regenerates.3,31 Although red pulp and follicles of such splenic transplants are well developed31 and functional,2,32 a marked B lymphocytosis is still observed in these animals,2,7suggesting that red pulp and follicles are not involved in LFA-1 and ICAM-1 regulation on B cells. In contrast, the MZ is poorly developed3,31,33 and not functional as shown by the increased susceptibility to infections.33 This suggests a role of the MZ in the maintenance of LFA-1 and ICAM-1 expression on B cells in blood and lymph nodes. Two observations further support this conclusion. First, the MZ is unique to the spleen,34 and after splenectomy no substitute is available.35 Second, the MZ is poorly developed in young children up to 2 years of age. Here, the main finding is a low expression of CD21 on MZ B cells.35 Interestingly, in children of this age a B lymphocytosis is observed that is comparable to that seen after splenectomy.36 37
It remains to be shown why only LFA-1 and ICAM-1 are affected by splenectomy, why its expression on B cells but not on T cells is modulated, and which cell types within the MZ are responsible for the maintenance of LFA-1 and ICAM-1. In addition, it is unclear whether B cells are required to migrate through the MZ or whether MZ cells produce soluble factors that influence LFA-1 and ICAM-1 expression of B cells while they are in the blood and lymph nodes. Furthermore, it should be analyzed whether the continuous presence of the MZ is necessary or whether its presence at some time of B-cell development is sufficient.38
Splenectomy changes B-cell migration but not B-cell proliferation
At first glance the cause for lymphocytosis in the blood does not seem difficult to understand. An organ is removed, which is constantly entered by many B cells,1,2 and if it is missing B cells accumulate in the blood. However, T cells also migrate in high numbers into the spleen,2 and their numbers in the blood are not increased after splenectomy (Table 1) making the “lack of space” argument unlikely.
The comparable binding of B cells from control and splenectomized animals to the HEVs of various lymph nodes and Peyer patches suggests that the entry into these organs is not affected. This is not surprising because B cells of splenectomized animals still express LFA-1 (albeit at a reduced level) and even lymphocytes completely deficient in LFA-1 are able to enter lymph nodes and Peyer patches via HEVs in numbers ranging between 20% and 70% of control cells.39 In addition, a higher number of injected lymphocytes leaves the blood of splenectomized sheep compared to control animals.8 Together these studies indicate that the entry of B cells is not impaired. However, when lymphocytes were infused into the blood of pigs 5 days after splenectomy a slower disappearance rate was noted.40 This shows that the effects of splenectomy on lymphocyte entry change over time, which is in agreement with a recent study in rats showing that B lymphocytosis develops from 4 weeks after splenectomy onward and lasts for at least 18 months.7
Once the B cells are within the tissue, the decreased expression of LFA-1 and ICAM-1 on B cells might weaken their interactions with other cells within the lymph nodes. In turn, this might lead to a faster migration of B cells through the tissue and might result in higher numbers of B cells in the efferent lymph, consequently causing an increased re-entry of B cells into the blood. This scenario is in accordance with the observation that labeled B cells with a reduced ICAM-1 expression migrate significantly faster from blood via the lymph nodes and efferent lymphatics back into the blood (present study), and the number of B cells in the thoracic duct is doubled after splenectomy.41 Because lymph nodes contain about 40% of all B cells and the blood about 2%,42,43 small changes in the number of B cells leaving the lymph nodes are sufficient to cause large changes in the number of B cells in the blood. It remains to be determined which cell types are involved in the interactions occurring during transmigration of the lymph nodes,44 and whether and to what extent the migration through nonlymphoid organs such as lung, liver, and gut is affected by the changes in LFA-1 and ICAM-1 expression after splenectomy.
Another explanation for the B lymphocytosis could be that splenectomy increases B-cell production in the bone marrow and at other sites, such as secondary lymphoid organs. However, the number of proliferating B cells in the bone marrow, in the B-cell area of lymph nodes, and in the blood of rats (present study) and humans45 is not affected by splenectomy, arguing against an effect of splenectomy on B-cell proliferation.
In conclusion, after splenectomy LFA-1 and ICAM-1 expression on B cells in blood and lymph nodes is reduced. This shows that the expression of adhesion molecules in one organ can depend on the presence of another distant organ and demonstrates an additional level in the regulation of surface molecule expression. It is probably the MZ that regulates the expression level of LFA-1 and ICAM-1 on B cells. The down-regulation of LFA-1 and ICAM-1 probably accelerates the migration of B cells through lymph nodes and provides an explanation for the well-known B lymphocytosis after splenectomy. Thus, the overwhelming postsplenectomy infections (reviewed in Pabst et al3) may not exclusively be due to the absence of the splenic MZ per se.35,46,47They could be the result of the accelerated migration of B cells through the lymph nodes leaving less time for the cellular interactions necessary for initiation and regulation of an effective immune response.48 49
The authors would like to thank R. Pabst and R. Schwinzer (Hannover, Germany) for their critical comments and K. Bankes, I. Dressendörfer, S. Lopez-Kostka, and F. Weidner for the technical assistance.
Supported by the Deutsche Forschungsgemeinschaft (We 1175/4-3). N.M.M. is a Fellow of the Alexander von Humboldt-Foundation, Bonn, Germany.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Jürgen Westermann, Institute of Anatomy, Medical University of Lübeck, Ratzeburger Allee 160, 23538 Lübeck, Germany; e-mail: westermann@anat.mu-luebeck.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal