Plasminogen activators urokinase-type plasminogen activator (uPA) and tissue-type plasminogen activator (tPA) are extracellular proteases involved in various tissue remodeling processes. A requirement for uPA activity in skeletal myogenesis was recently demonstrated in vitro. The role of plasminogen activators in skeletal muscle regeneration in vivo in wild-type, uPA-deficient, and tPA-deficient mice is investigated here. Wild-type and tPA−/− mice completely repaired experimentally damaged skeletal muscle. In contrast, uPA−/− mice had a severe regeneration defect, with decreased recruitment of blood-derived monocytes to the site of injury and with persistent myotube degeneration. In addition, uPA-deficient mice accumulated fibrin in the degenerating muscle fibers; however, the defibrinogenation of uPA-deficient mice resulted in a correction of the muscle regeneration defect. A similar severe regeneration deficit with persistent fibrin deposition was also reproducible in plasminogen-deficient mice after injury, suggesting that fibrinolysis by uPA-mediated plasminogen activation plays a fundamental role in skeletal muscle regeneration. In conclusion, the uPA-plasmin system is identified as a critical component of the mammalian skeletal muscle regeneration process, possibly because it prevents intramuscular fibrin accumulation and contributes to the adequate inflammatory response after injury. These studies demonstrate the requirement of an extracellular proteolytic cascade during muscle regeneration in vivo.
Introduction
Activation of the zymogen plasminogen (Plg) into the active serine proteinase, plasmin, is a highly regulated and widely used mechanism for the generation of extracellular proteolytic activity. Activation of Plg is exerted by 2 distinct Plg activators, tissue-type plasminogen activator (tPA) and urokinase-type plasminogen activator (uPA). The plasminogen system is a key factor in the dissolution of fibrin matrices and is critical in the maintenance of hemostatic balance. By its action in concert with other proteolytic systems, it is also thought to play a role in the degradation of extracellular matrices in physiologic and pathologic tissue remodeling and cell migration events, such as ovulation, trophoblast invasion, post-lactational mammary involution, wound healing, angiogenesis, and tumor cell invasion (reviewed in1).
Extracellular proteolysis takes place during skeletal muscle formation and pathologic muscle regeneration, in which muscle precursor satellite cells play a major role. In response to muscle injury, damaged tissue is infiltrated by fibroblasts, inflammatory cells, and macrophages.2 Necrotic tissue is removed, revascularization starts, and proliferation of satellite cells is initiated. Numerous proteolytic enzymes have been proposed to play a role during muscle regeneration, either in the inflammatory response or in the migration of myoblasts across the basal lamina and in their further fusion to form the terminal muscle fiber.3,4Metalloproteinases (MMPs) such as MMP-2 and MMP-9, meltrin-α, and cathepsin B seem to be required for myotube formation in vitro.4-7 Moreover, the expression of MMP-2 and MMP-9 has also been reported in the degeneration-regeneration process of myofibers in vivo.8 The mechanism of MMP activation in most cell types has been found to involve a proteolytic activation cascade initiated by uPA. Most MMPs then can be directly activated by plasmin cleavage to a protein of lower molecular weight.9 Expression of uPA and tPA has been reported in cell cultures from chicken, mouse, rat, and human muscle.10-12 We have recently shown that the inhibition of uPA proteolytic activity with an anti-uPA antibody abrogated migration, fusion, and differentiation of C2C12 myoblasts in vitro.13Most of the above reports, however, refer to in vivo and in vitro correlations between myogenesis and the expression of different proteases, making an accurate assessment of the physiologic role of these proteases difficult. Here we analyze uPA- and tPA-deficient mice (uPA−/− and tPA−/−, respectively)14 and show that the regeneration capacity of muscle tissue in uPA-deficient mice is reduced. On the basis of striking dystrophic changes in regenerating muscle of uPA-deficient mice and the concomitant accumulation of intramuscular fibrin, we propose that uPA, but not tPA, is required for efficient muscle regeneration in vivo. Moreover, using mice genetically deficient in Plg (Plg−/−),15 we have begun an analysis of the contribution of uPA-mediated Plg activation to this deficit.
Materials and methods
Animals
The mice used were 9- to 12-week-old animals from the C57Bl16 control strain and the corresponding uPA-, tPA-, and plasminogen-deficient mice.14 15 All were maintained as a breeding colony and kept at room temperature with a natural night-day cycle. The uPA- and tPA- deficient mice were kindly provided by Dr P. Carmeliet (Centre for Transgene Technology and Gene Therapy, Leuven, Belgium). Before manipulation, mice were anesthetized by an intraperitoneal injection of dormicum-droperidol.
Induction of muscle regeneration
Regeneration of skeletal muscle was induced by either of 2 standard procedures—a single freeze-crush injury of the musculus tibialis anterior (TA), as described by McGeachie and Grounds,16 or an intramuscular injection of 50 μL 50% glycerol (vol/vol) in the gastrocnemius muscle group of the mice, as described by Kawai et al.17 The experiments were performed in right hindlimb muscles, and contralateral intact muscles were used as control. Morphologic and biochemical examinations were performed at 2, 5, 7, 9, and 20 days after injury. Four animals were used for each time point.
Histologic and immunohistochemical analysis
At selected times, muscles of control and knockout mice were removed after cervical dislocation. They were carefully dissected, frozen in isopentane-chilled liquid nitrogen, and stored at −80°C before sectioning. Transverse cryostat sections (10 μm thick) were stained with hematoxylin-eosin (H&E). Immunohistochemistry was performed using the Vectastain Elite kit (Vector Laboratories, Burlingame, CA) and diaminobenzidine for single-labeling experiments. The following antibodies were used: mouse monoclonal antibody against myosin developmental-type heavy chain (MHCd) (Novocastra, Newcastle, United Kingdom) 1:50, rat monoclonal anti-CD31 antibody (Pharmingen, San Diego, CA) 1:25, goat polyclonal M-cadherin (sc-6470; Santa Cruz Biotechnology, Santa Cruz, CA) 1:25, and rabbit antimouse fibrinogen (kindly provided by Dr Keld Dano, Finsenlab, Denmark) 1:1000. Sections stained with antimouse fibrinogen were co-stained with hematoxylin to visualize cellular structures. For single-immunofluorescence staining, sections were incubated with rat monoclonal antibodies anti–Mac-1 (1:30) or anti–Gr-1 (1:30; Pharmingen) conjugated with fluorescein and mounted with Vectashield (Vector Laboratories). For double-immunofluorescence staining, sections were incubated overnight simultaneously with Mac-1/uPA, Gr-1/uPA, and M-cadherin–uPA primary antibodies, washed, and overlaid with the appropriate secondary antibodies conjugated with rhodamine (for M-cadherin), Texas Red (for Mac-1 and Gr-1), or fluorescein (for uPA); slides were then washed and mounted with Vectashield (Vector Laboratories). Sections were photographed using an Olympus BX60 microscope (Tokyo, Japan). Control experiments without primary antibody demonstrated that the immunofluorescence signals observed were specific (data not shown).
RNA analysis
Total RNA was extracted and purified from freshly isolated muscle tissue according to the procedure of Chomczynski and Sacchi.18 Three micrograms total RNA was subjected to Northern blot analysis, and blots were sequentially hybridized with radiolabeled complementary DNA (cDNA) probes corresponding to murine uPA, tPA, MyoD, and myogenin as previously described.13 19To normalize signal intensity, blots were later rehybridized with a radiolabeled 18S oligonucleotide probe. For reverse transcription–polymerase chain reaction (RT-PCR) analysis, 2 μg total RNA was reverse transcribed using the first-strand cDNA synthesis kit (Pharmacia, Uppsala, Sweden) in a 35-μL reaction. Amplification parameters were denaturation at 95°C for 45 seconds; annealing for 2 minutes at 55°C (uPA), 60°C (MyoD), 60°C (myogenin), 55°C (glyceraldehyde phosphate dehydrogenase [GAPDH]); and extension at 72°C for 2 minutes. Aliquots of 10% of the reaction were removed from the assay after different cycles, as indicated, and analyzed on 2% agarose gels. Primers for the detection of reverse transcriptase products were derived from different exons to distinguish RT-PCR products from genomic DNA contaminations. Primer sequences were: uPA, 5′-GGCAGTGTACTTGGAGCTCCT-3′ and 5′-TAGAGCCTTCTGGCCACACTG-3′; MyoD, 5′-AGGCTCTGCTGCGCGACC-3′ and 5′-TGCAGTCGATCTCTCAAAGCACC-3′; myogenin, 5′-GAGCGCGATCTCCGCTACAGAGG-3′ and 5′-CTGGCTTGTGGCAGGCCCAGG-3′; GAPDH, 5′-ACTCCCACTCTTCCACCTTC-3′ and 5′-TCTTGCTCAGTGTCCTTGC-3′. Expected product sizes were: uPA, 450 base pairs (bp); MyoD, 471 bp; myogenin, 379 bp; GAPDH, 185 bp.
Preparation of muscle extracts
Gastrocnemius muscles were dissected, sectioned, weighed, and pounded in an ice-cold Potter tube with 0.1 mM Tris-HCl buffer, pH 7.6, containing 2 mM EDTA and 0.4% Triton X-100. The resultant extracts were centrifuged for 20 minutes at 4°C at 12 000g, and the supernatants were stored as aliquots at −80°C until use. Protein concentration was determined in the supernatants using the Bio-Rad protein assay (Bio-Rad, Hercules, CA).
Plasmin kinetics
Plasmin content in muscles was measured using the S-2251–based assay, which was performed in triplicate in microtiter plates at 37°C and calibrated with purified plasmin. The chromogenic substrate selective for plasmin, S-2251 (Chromogenix, Milan, Italy), was used to follow the initial rate of plasminogen activation by measuringp-nitroaniline generation. Forty micrograms muscle extract from wild-type mice (obtained on different days after injury; see Figure 7) or standard samples was mixed with a buffer containing 0.1 M Tris-HCl and 2 mM EDTA, pH 7.6, and 1.6 mM S-2251 as substrate. The generation of plasmin was detected by measuringp-nitroaniline release from the substrate, as indicated above.
Zymography
Three hundred micrograms muscle extracts was size-fractionated on a 10% nonreducing sodium dodecyl sulfate (SDS) acrylamide gel, which was washed for 30 minutes in 2.5% Triton X-100–phosphate-buffered saline and for 30 minutes in distilled water. The gel was subsequently placed in contact with a casein gel containing 2% (wt/vol) nonfat dry milk, 0.25 mM Tris-HCl, pH 7.6, 1% (wt/vol) agarose, 0.25 × phosphate-buffered saline, and 15 μg/mL plasminogen (Chromogenix), and incubated in a humid chamber at 37°C until caseinolytic bands were visualized and photographed.
Systemic defibrinogenation
Then uPA-deficient mice were anesthetized, and 14-day mini-osmotic pumps (model 1002; Alza, Palo Alto, CA) filled with a buffered solution of 500 U/mL ancrod (Sigma Chemical, St Louis, MO) were implanted subcutaneously into their backs (one mini-pump per animal). The insertion sites were sutured closed. The pumps deliver 0.25 μL/h, so the mice received 3 U ancrod/d. In control animals, saline-filled mini-pumps were implanted. On day 3 of ancrod or saline infusion, injury was induced by intramuscular injection of 50% glycerol. Nine days after injury, mice were killed and gastrocnemius muscles were dissected, frozen, and analyzed by H&E staining. Fibrinogen levels in citrated blood from ancrod- or saline-treated mice were analyzed by SDS–polyacrylamide gel electrophoresis (PAGE; 6% polyacrylamide gel) followed by Western blotting using an antimouse fibrinogen antibody (1:1000).
Results
Expression of uPA messenger RNA and proteolytic activity are induced after skeletal muscle injury
We have previously demonstrated a role for uPA in myogenesis in vitro because the inhibition of uPA activity abrogated both differentiation and fusion of C2C12 myoblasts in culture.13 Our aim in the current study was to decipher the role of uPA during skeletal muscle regeneration in vivo. Muscle regeneration was induced in mice either by freeze-crush16or by intramuscular injection of 50% glycerol,17 and the expression of uPA was investigated in regenerating muscle after injury and compared with that of the contralateral control muscle.
RNA was isolated from muscles on the third day after glycerol-induced injury, and uPA, MyoD, and myogenin expression was analyzed by semiquantitative RT-PCR. As shown in Figure1A, significantly fewer amplification cycles were needed with RNA from regenerating muscle to yield comparable signals for uPA than with RNA from control muscle, whereas no differences were observed when primers specific for GAPDH were used (Figure 1A). As a control for muscle regeneration, the expression of myogenic regulators MyoD and myogenin (up-regulated by satellite cells during muscle regeneration in vivo)20 21 was also examined. Detectable PCR signal for MyoD and myogenin was obtained after fewer amplification cycles with RNA from glycerol-treated muscle than with RNA from control muscle. Similarly, when muscle regeneration was induced by traumatic freeze-crush, the expression of uPA, MyoD, and myogenin mRNA was dramatically induced in regenerating muscle of wild-type mice as assessed by Northern blot analysis (Figure 1B top; see WT panel, control, and wound). We observed that, as with uPA, tPA expression is induced in regenerating skeletal muscle, though to a lesser extent, suggesting that uPA rather than tPA might may be involved in skeletal muscle regeneration in vivo (Figure 1B bottom; see WT panel, control, and wound). Thus, injured muscle up-regulates uPA and tPA mRNA.
uPA-deficient mice show a regeneration defect following muscle injury.
(A) uPA expression is increased in regenerating muscle tissue of wild-type (WT) mice after glycerol injury. RNA was isolated from the gastrocnemius muscle group of WT mice (control) or 3 days after glycerol injury (wound). RT-PCR was performed to detect the expression of uPA, GAPDH, MyoD, and myogenin. One tenth of the reaction volume was removed after 10, 15, 20, 22, 24, 26, and 30 amplification cycles. The number of amplification cycles is indicated at the top of each lane. Purified murine cDNAs corresponding to uPA, MyoD, and myogenin were used as positive controls for the PCR reaction (+). Expression of uPA, MyoD, and myogenin increased several times in regenerating muscle compared with control muscle. (B) Impaired up-regulation of MyoD and myogenin mRNAs in skeletal muscle of uPA-deficient mice after freeze-crush injury. RNA was isolated from the musculus tibialis anterior of WT, uPA-deficient, and tPA-deficient mice (control) or 3 days after freeze-crush injury (wound). Northern blot analysis was performed to detect the expression of uPA, MyoD, myogenin and 18S (top) and of tPA and 18S (bottom). Transcript levels of uPA, tPA, MyoD, and myogenin were induced in regenerating muscle of WT mice. Expression of MyoD and myogenin was lower in regenerating muscle of uPA-deficient mice than in regenerating muscle of WT or tPA-deficient mice. As expected, no uPA or tPA mRNA was detected in uPA- or tPA-deficient mice, respectively, whereas levels of 18S were similar in all lanes. (C) Proteolytic activity of uPA is increased in regenerating muscle tissue of WT mice after glycerol injury. Gel zymographies. Forty micrograms protein from muscle extracts of noninjured (0 days after injury) and injured skeletal muscle (2, 5, 7, and 9 days after injury) was analyzed, after SDS-PAGE, by PA zymography (top). Two nanograms purified human uPA (55 kd) was used as a standard (+). Forty micrograms protein from muscle extracts of noninjured (lane 1) and 5-day injured skeletal muscle (lane 2) of uPA-deficient mice and from 2-day injured muscle of WT mice (lane 3) was analyzed by PA zymography (bottom). The approximately 45-kd molecular mass species, indicated by an arrow, corresponds to murine uPA and was calculated according to standard molecular mass markers electrophoresed in an adjacent lane and stained with Coomassie blue. Photographs were taken after overnight incubation at 4°C and 4 hours at 37°C.
uPA-deficient mice show a regeneration defect following muscle injury.
(A) uPA expression is increased in regenerating muscle tissue of wild-type (WT) mice after glycerol injury. RNA was isolated from the gastrocnemius muscle group of WT mice (control) or 3 days after glycerol injury (wound). RT-PCR was performed to detect the expression of uPA, GAPDH, MyoD, and myogenin. One tenth of the reaction volume was removed after 10, 15, 20, 22, 24, 26, and 30 amplification cycles. The number of amplification cycles is indicated at the top of each lane. Purified murine cDNAs corresponding to uPA, MyoD, and myogenin were used as positive controls for the PCR reaction (+). Expression of uPA, MyoD, and myogenin increased several times in regenerating muscle compared with control muscle. (B) Impaired up-regulation of MyoD and myogenin mRNAs in skeletal muscle of uPA-deficient mice after freeze-crush injury. RNA was isolated from the musculus tibialis anterior of WT, uPA-deficient, and tPA-deficient mice (control) or 3 days after freeze-crush injury (wound). Northern blot analysis was performed to detect the expression of uPA, MyoD, myogenin and 18S (top) and of tPA and 18S (bottom). Transcript levels of uPA, tPA, MyoD, and myogenin were induced in regenerating muscle of WT mice. Expression of MyoD and myogenin was lower in regenerating muscle of uPA-deficient mice than in regenerating muscle of WT or tPA-deficient mice. As expected, no uPA or tPA mRNA was detected in uPA- or tPA-deficient mice, respectively, whereas levels of 18S were similar in all lanes. (C) Proteolytic activity of uPA is increased in regenerating muscle tissue of WT mice after glycerol injury. Gel zymographies. Forty micrograms protein from muscle extracts of noninjured (0 days after injury) and injured skeletal muscle (2, 5, 7, and 9 days after injury) was analyzed, after SDS-PAGE, by PA zymography (top). Two nanograms purified human uPA (55 kd) was used as a standard (+). Forty micrograms protein from muscle extracts of noninjured (lane 1) and 5-day injured skeletal muscle (lane 2) of uPA-deficient mice and from 2-day injured muscle of WT mice (lane 3) was analyzed by PA zymography (bottom). The approximately 45-kd molecular mass species, indicated by an arrow, corresponds to murine uPA and was calculated according to standard molecular mass markers electrophoresed in an adjacent lane and stained with Coomassie blue. Photographs were taken after overnight incubation at 4°C and 4 hours at 37°C.
Next, we analyzed whether the induction of uPA and tPA messenger RNAs (mRNAs) caused by muscle injury was followed by an induction of their corresponding enzymatic activities. Tissue extracts were prepared from noninjured and injured muscles on days 2, 5, 7, and 9 after injury and were analyzed by zymography (Figure 1C top). This assay allows clear distinction between uPA and tPA, which migrate at 45 and 72 kd, respectively. In tissue extracts from noninjured muscle, uPA activity was not detectable. However, it was induced in the regenerating muscle samples, as demonstrated by the appearance of a casein degradation band of 45 kd, corresponding to murine uPA active enzyme. Under the same conditions, tPA activity was undetectable in all the tissue extracts analyzed. As expected, no uPA activity was detected in tissue extracts from muscle of uPA-deficient mice, demonstrating the specificity of the assay (Figure 1C bottom; see lanes 1-2). These results suggested that uPA rather than tPA might be involved in skeletal muscle regeneration in vivo.
Expression of MyoD and myogenin mRNA is reduced in regenerating muscle tissue of uPA-deficient mice
To evaluate the importance of uPA and tPA for the skeletal muscle regeneration process, we performed freeze-crush injury-induced regeneration experiments in uPA- and tPA-deficient mice.14RNA levels for MyoD and myogenin from wild-type and uPA- or tPA-deficient mice were examined 3 days after injury by Northern blotting (Figure 1B). MyoD expression was below detectable levels in quiescent muscle from wild-type mice and was dramatically induced in injured muscle (Figure 1B; compare control and wound in WT). MyoD transcript levels were induced to a lesser extent in uPA-deficient mice than in wild-type or tPA-deficient mice after injury (Figure 1B; compare wound in WT, uPA−/−, and tPA−/−). Similarly, myogenin mRNA expression was reduced in the regenerating muscle of uPA-deficient mice compared to that of tPA-deficient and wild-type mice, whereas the 18S RNA level was comparable in all lanes. As expected, uPA and tPA transcripts were absent in muscle from uPA- and tPA-deficient mice, respectively (Figure 1B). These results indicate that uPA, but not tPA, is required for efficient skeletal muscle regeneration in vivo.
uPA deficiency exacerbates histologic features of skeletal muscle degeneration
To determine the functional significance of the increased uPA expression in skeletal muscle after damage and to evaluate further the importance of uPA for skeletal muscle regeneration, we analyzed comparatively the histopathologic changes induced by intramuscular injection of glycerol in the muscle fibers of wild-type and uPA- and tPA-deficient mice. As shown in Figure2A, skeletal muscle of uPA-deficient mice displayed a prominent regeneration defect after glycerol-induced injury, whereas regeneration in tPA-deficient and wild-type mice proceeded normally. This regeneration defect in uPA−/− mice was apparent by 5 days after injury, but it was most striking 9 to 20 days after injury. Analysis of H&E-stained cross-sections of wild-type, uPA-deficient, and tPA-deficient mice 2 days after injury showed that the muscles of all mice were edematous and had fibrotic infiltrates within the enlarged intercellular space separating the necrotic myofibers. Analysis of cross-sections of muscle 5 days after injury revealed well-advanced regeneration in wild-type and tPA-deficient mice, with many new myofibers characterized by its small size and single nuclei and with a reduction in fibrotic infiltrates (Figure 2A). In contrast, in uPA-deficient mice at 5 days after injury, the muscle appeared edematous, and no new uninucleated, small myofibers were detected yet. Seven days after injury, most injured fibers regenerated into groups of centrally nucleated myotubes, a clear sign of advanced regeneration, and few necrotic fibers could occasionally be observed in wild-type and tPA-deficient mice; in addition, the size of newly formed myofibers had augmented in the wild-type and tPA−/− animals. In uPA-deficient mice, however, the muscle had a necrotic appearance, showing still extensive fibrosis 7 days after injury. Nine days after injury, virtually no sign of previous damage was detectable in wild-type and tPA−/− mice, and centrally located nuclei were observed inside the regenerated fibers, which also exhibited increases in the cross-sectional areas, indicating complete regeneration (Figure 2A). In uPA-deficient mice, however, a high number of degenerated myotubes was still visible. Twenty days after injury, the lesion was no longer noticeable in wild-type and tPA−/− mice, except for the central myonuclei. In uPA−/− mice, however, the muscle showed extensive fibrosis with high numbers of degenerated myotubes. Furthermore, staining of wild-type and uPA-deficient muscle tissue at 5 and 9 days after injury with a monoclonal antibody against developmental myosin heavy chain (MHCd, an MHC isoform expressed only in embryonic and early neonatal life, whose expression is re-induced on muscle regeneration in adult life) showed regenerating myotubes expressing MHCd at 5 days after injury in both wild-type and uPA−/− mice (Figure 2B). In contrast, at 9 days after injury, MHCd expression was detected in uPA−/− mice but absent in wild-type mice. These results reveal the persistence of ongoing muscle degeneration-regeneration in uPA-deficient mice at stages at which muscle regeneration is more advanced in wild-type mice.
(A) Glycerol-induced injuries result in impaired skeletal muscle regeneration of uPA- but not tPA-deficient mice. Frozen sections of muscles from wild-type (WT), uPA-deficient, and tPA-deficient mice were stained with H&E 2, 5, 7, 9, and 20 days after injury, as indicated. Contralateral control muscles were also stained with HE (0 days after injury). In WT and tPA-deficient mice, well-advanced regeneration is visible after 5 days, and regeneration is complete after 9 days. In uPA-deficient mice, a regeneration defect is already visible 5 days after injury but is most striking at 9 and 20 days. In WT and tPA-deficient mice, virtually no sign of the previous injury was detectable after 20 days, except for the centrally located myonuclei. Original magnification, 400 ×. (B) Persistence of developmental MHC-positive myofibers 9 days after glycerol injury in uPA-deficient mice. Cryostat frozen sections from WT and uPA-deficient mice were reacted with a monoclonal antibody against developmental MHC (MHCd) at 5 and 9 days after injury, as indicated in the figure (5 and 9 days after injury). Large numbers of MHCd-expressing myofibers are visible in regenerating muscle of uPA-deficient mice 5 and 9 days after intramuscular glycerol injection. In WT mice, fibers positive for MHCd are observed 5 days after injury, whereas no MHCd expression is detected 9 days after injury.
(A) Glycerol-induced injuries result in impaired skeletal muscle regeneration of uPA- but not tPA-deficient mice. Frozen sections of muscles from wild-type (WT), uPA-deficient, and tPA-deficient mice were stained with H&E 2, 5, 7, 9, and 20 days after injury, as indicated. Contralateral control muscles were also stained with HE (0 days after injury). In WT and tPA-deficient mice, well-advanced regeneration is visible after 5 days, and regeneration is complete after 9 days. In uPA-deficient mice, a regeneration defect is already visible 5 days after injury but is most striking at 9 and 20 days. In WT and tPA-deficient mice, virtually no sign of the previous injury was detectable after 20 days, except for the centrally located myonuclei. Original magnification, 400 ×. (B) Persistence of developmental MHC-positive myofibers 9 days after glycerol injury in uPA-deficient mice. Cryostat frozen sections from WT and uPA-deficient mice were reacted with a monoclonal antibody against developmental MHC (MHCd) at 5 and 9 days after injury, as indicated in the figure (5 and 9 days after injury). Large numbers of MHCd-expressing myofibers are visible in regenerating muscle of uPA-deficient mice 5 and 9 days after intramuscular glycerol injection. In WT mice, fibers positive for MHCd are observed 5 days after injury, whereas no MHCd expression is detected 9 days after injury.
Impact of uPA deficiency on the inflammatory response after muscle injury
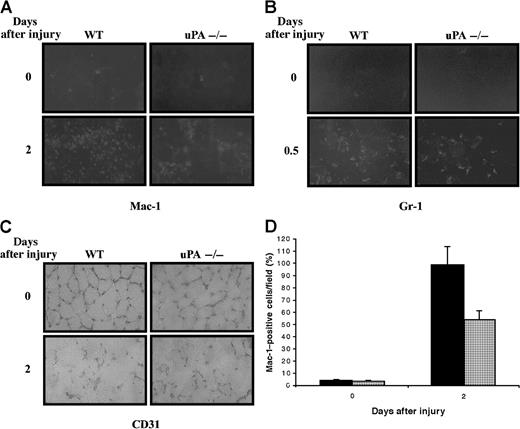
Blood-borne monocytes-macrophages are recruited after injury to skeletal muscle during the inflammatory phase. These cells play a major role in the phagocytosis of tissue debris after muscle injury.2 To analyze the distribution of macrophages in normal and regenerating muscle of wild-type and uPA-deficient mice, we performed immunohistochemical studies using an antibody against a well-characterized macrophage marker, Mac-1. A few resident Mac-1–positive cells were detected in the noninjured muscle sections of wild-type and uPA−/− mice (Figure3A; 0 days after injury). There was an increase of Mac-1–positive cells at the injury site 2 days after injury; however, the extent of Mac-1 staining in uPA-deficient mice was reduced to almost 50% compared with that in wild-type mice (Figure 3A [2 days after injury] and 3D), suggesting that macrophage recruitment to the injured muscle was reduced in the absence of uPA. Although macrophages have been identified as the predominant cell type in the inflammatory infiltrate in experimentally induced muscle injury, neutrophils have also been detected during the very early hours of the muscle injury response.2 To investigate the presence of neutrophil infiltrates in wild-type and uPA−/− mice, we performed immunohistochemical studies using an antibody against a neutrophil marker, Gr-1. No Gr-1–positive cells were detected in noninjured muscle sections of wild-type or uPA-deficient mice (Figure 3B; 0 days after injury). Twelve hours after injury, there was an increase in Gr-1–expressing cells at the injury site of both types of animals, though the neutrophil response was more marked in wild-type than in uPA−/− mice at the site of injury (Figure 3B; 0.5 days after injury). It is known that uPA has important functions in neovascularization and capillary angiogenesis, such as after cardiac infarct.22To assess whether angiogenesis in regenerating muscle of uPA−/− mice was impaired, immunohistochemistry with a vascular endothelial cell marker, CD31/PECAM-1, was carried out.23 We found no significant differences in the number of CD31-stained microvessels between uPA−/− and wild-type mice (Figure 3C). However, we cannot completely rule out that small differences in the amount or diameter of microvessels exist between uPA−/− and wild-type mice. Taken together, these data suggest that the reduced muscle regeneration of uPA-deficient mice may be caused by a decreased inflammatory response shortly after injury.
Immunohistochemical demonstration of the presence of inflammatory cells in muscles of wild-type and uPA-deficient mice after glycerol-induced injury.
Detection of macrophages (Mac-1+) (A) and neutrophils (Gr-1+) (B) in transverse sections of control and injured skeletal muscles of wild-type (WT) and uPA-deficient mice at the indicated times after injury. Mac-1–expressing cells increased 2 days after injury in muscle of WT and uPA-deficient mice; however, the number of Mac-1–positive cells was lower in uPA−/− mice muscle. Gr-1–expressing cells were maximal 12 hours after injury in both WT and uPA-deficient mice, though there were fewer Gr-1–positive cells in the uPA-deficient mice. (C) Detection of endothelial (CD31+) cells in skeletal muscle of WT and uPA-deficient mice. No significant differences in the number of CD31-stained microvessels were found between uPA-deficient and WT mice. Original magnification, 400×. (D) Quantitative analysis of Mac-1–positive cells in regenerating muscles of WT ▪ and uPA-deficient ░ mice. Muscles were analyzed for Mac-1 immunofluorescence at 0 and 2 days after injury. Mac-1–positive cells were counted in 10 random fields per section, and data from 12 sections per muscle were pooled. Mean values for both limbs were to provide a mean value for each animal (3 animals were studied at each time point). Histograms show the percentage of reduction (46%) of Mac-1–positive cells in uPA−/− mice with respect to WT mice.
Immunohistochemical demonstration of the presence of inflammatory cells in muscles of wild-type and uPA-deficient mice after glycerol-induced injury.
Detection of macrophages (Mac-1+) (A) and neutrophils (Gr-1+) (B) in transverse sections of control and injured skeletal muscles of wild-type (WT) and uPA-deficient mice at the indicated times after injury. Mac-1–expressing cells increased 2 days after injury in muscle of WT and uPA-deficient mice; however, the number of Mac-1–positive cells was lower in uPA−/− mice muscle. Gr-1–expressing cells were maximal 12 hours after injury in both WT and uPA-deficient mice, though there were fewer Gr-1–positive cells in the uPA-deficient mice. (C) Detection of endothelial (CD31+) cells in skeletal muscle of WT and uPA-deficient mice. No significant differences in the number of CD31-stained microvessels were found between uPA-deficient and WT mice. Original magnification, 400×. (D) Quantitative analysis of Mac-1–positive cells in regenerating muscles of WT ▪ and uPA-deficient ░ mice. Muscles were analyzed for Mac-1 immunofluorescence at 0 and 2 days after injury. Mac-1–positive cells were counted in 10 random fields per section, and data from 12 sections per muscle were pooled. Mean values for both limbs were to provide a mean value for each animal (3 animals were studied at each time point). Histograms show the percentage of reduction (46%) of Mac-1–positive cells in uPA−/− mice with respect to WT mice.
Macrophage and satellite cells contribute to the expression of uPA during skeletal muscle regeneration
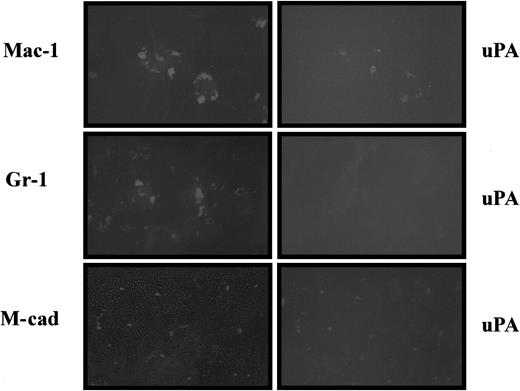
We have demonstrated that both uPA mRNA and proteolytic activity are induced after muscle injury in wild-type mice (Figure 1). To determine precisely where uPA is expressed during the regeneration process, we performed double-immunohistochemical staining on sections of regenerating muscle using an antibody against uPA together with antibodies against Mac-1, Gr-1, and M-cadherin, specific markers for macrophages, neutrophils, and satellite cells, respectively. As shown in Figure 4, uPA was detected in Mac-1–positive cells, but not in Gr-1–expressing cells, suggesting that uPA is expressed by macrophages but not by neutrophils during the inflammatory response to skeletal muscle injury. In addition, M-cadherin was found to be co-expressed with uPA, demonstrating that uPA was synthesized by skeletal muscle stem cells in vivo (Figure 4).
uPA is expressed by macrophages and satellite cells after muscle injury.
Double-immunohistochemical staining of cross-sections from regenerating muscle of wild-type mice visualizing uPA, together with Mac-1, Gr-1, and M-cadherin, respectively. uPA is expressed by macrophages, but not by neutrophils, because abundant uPA– and Mac-1–double-positive cells were found 2 days after injury, whereas no uPA– or Gr-1–double-positive cells were found 12 hours after injury. uPA is expressed in satellite cells because it was detected in M-cadherin–positive cells 5 days after injury.
uPA is expressed by macrophages and satellite cells after muscle injury.
Double-immunohistochemical staining of cross-sections from regenerating muscle of wild-type mice visualizing uPA, together with Mac-1, Gr-1, and M-cadherin, respectively. uPA is expressed by macrophages, but not by neutrophils, because abundant uPA– and Mac-1–double-positive cells were found 2 days after injury, whereas no uPA– or Gr-1–double-positive cells were found 12 hours after injury. uPA is expressed in satellite cells because it was detected in M-cadherin–positive cells 5 days after injury.
Increased fibrin deposition in damaged muscle of uPA-deficient mice
Given that the established role of plasminogen activation is fibrinolysis (reviewed in24), we analyzed whether the loss of uPA resulted in increased fibrin deposition after muscle injury. Fibrin content in regenerating muscle from wild-type and uPA-deficient mice was analyzed by fibrin immunohistochemistry, using an antimouse fibrin(ogen) antibody (Figure5). In 9-day–injured muscle of wild-type mice, minimal fibrin immunoreactivity was found (Figure 5; WT). In contrast, abundant deposits of fibrin were detected in muscles of uPA-deficient mice at the same time after injury (Figure 5; uPA−/−). These results correlate with the persistence of muscle degeneration features observed in uPA−/− mice 9 days after injury (Figure 2; uPA−/−, 9 days after injury).
Immunologic detection of fibrin in regenerating muscle of uPA-deficient mice.
Frozen cross-sections from regenerating muscle (9 days after injury) of wild-type (WT) mice and uPA-deficient mice (uPA−/−) were stained with a rabbit antimurine fibrin(ogen) antibody. Brown indicates positivity. Immunoperoxidase staining showed that fibrin deposits were abundant in uPA-deficient mice, but not in WT mice, at 9 days after injury. Original magnification, 400 ×.
Immunologic detection of fibrin in regenerating muscle of uPA-deficient mice.
Frozen cross-sections from regenerating muscle (9 days after injury) of wild-type (WT) mice and uPA-deficient mice (uPA−/−) were stained with a rabbit antimurine fibrin(ogen) antibody. Brown indicates positivity. Immunoperoxidase staining showed that fibrin deposits were abundant in uPA-deficient mice, but not in WT mice, at 9 days after injury. Original magnification, 400 ×.
Systemic fibrinogen depletion restores muscle regeneration in uPA-deficient mice
Administration of ancrod (a viper venom) has been shown to lead to the consumption of systemic fibrinogen.25 Ancrod- or saline-delivering osmotic pumps (3 U/d) were implanted in uPA−/− mice for 3 days before muscle injury and then throughout the experimental period (up to 9 days after injury). As shown in Figure6A, ancrod administration resulted in a significant reduction of plasma fibrinogen, as assessed by Western blotting, without any effect on survival (compare ancrod versus saline). Moreover, signs of improved muscle regeneration were detectable in ancrod-treated uPA-deficient mice 9 days after injury, with centrally located nuclei inside the regenerated fibers. In contrast, histologic features of muscle degeneration persisted in saline-treated uPA-deficient mice at the same stage after injury, including a high number of degenerated myotubes and fibrosis in the intercellular space throughout the damaged muscle (Figure 6B). The extent of muscle regeneration of ancrod-treated uPA-deficient mice 9 days after injury was comparable to the regeneration status of wild-type mice at the same time after injury. This observation supports the idea of a potential pathogenic role of fibrin(ogen) accumulation during muscle regeneration in uPA-deficient mice.
Effect of systemic fibrinogen depletion on muscle regeneration of uPA-deficient mice.
uPA-deficient mice were implanted 3 days before glycerol-induced muscle degeneration with ancrod-filled mini-osmotic pumps (3 U/d over 12 days) (Ancrod) or with buffered-filled pumps (Saline). (A) Ancrod treatment reduces fibrinogen in blood of uPA-deficient mice. Forty micrograms sodium citrate–treated blood obtained from saline- or ancrod-infused mice was analyzed in a 6% polyacrylamide gel by Western blotting using a polyclonal antimouse fibrinogen antibody (1:1000). The 340-kd approximate molecular mass species corresponds to murine fibrinogen and was calculated according to standard molecular mass markers electrophoresed in an adjacent lane. (B) Fibrinogen depletion restores muscle regeneration in uPA-deficient mice. Cryosections from saline- and ancrod-treated uPA-deficient mice were stained with H&E 9 days after injury. Although well-advanced regeneration is visible in ancrod-treated uPA−/− mice, a regeneration defect is still observed in saline-treated mice 9 days after injury.
Effect of systemic fibrinogen depletion on muscle regeneration of uPA-deficient mice.
uPA-deficient mice were implanted 3 days before glycerol-induced muscle degeneration with ancrod-filled mini-osmotic pumps (3 U/d over 12 days) (Ancrod) or with buffered-filled pumps (Saline). (A) Ancrod treatment reduces fibrinogen in blood of uPA-deficient mice. Forty micrograms sodium citrate–treated blood obtained from saline- or ancrod-infused mice was analyzed in a 6% polyacrylamide gel by Western blotting using a polyclonal antimouse fibrinogen antibody (1:1000). The 340-kd approximate molecular mass species corresponds to murine fibrinogen and was calculated according to standard molecular mass markers electrophoresed in an adjacent lane. (B) Fibrinogen depletion restores muscle regeneration in uPA-deficient mice. Cryosections from saline- and ancrod-treated uPA-deficient mice were stained with H&E 9 days after injury. Although well-advanced regeneration is visible in ancrod-treated uPA−/− mice, a regeneration defect is still observed in saline-treated mice 9 days after injury.
Plasminogen-deficient mice show a severe regeneration defect with enhanced fibrosis and myotube degeneration
The results shown above provide evidence of a role for uPA in skeletal muscle regeneration in vivo. To investigate whether the effect of uPA is plasminogen dependent (ie, whether the lack of uPA prevents muscle regeneration because of a failure of plasminogen processing), we measured plasmin activity in regenerating muscle tissues at different times after injury. Using a chromogenic substrate for the activity of plasmin, we observed that plasmin generation was increased during skeletal muscle regeneration in wild-type mice, with a peak of activity occurring 2 to 5 days after injury and decreasing by day 9 (Figure7). To test the role of plasmin in skeletal muscle regeneration, we performed glycerol-induced muscle injury in wild-type mice and in mice deficient in plasminogen (Plg−/−) and comparatively analyzed skeletal muscle regeneration. By day 9 after injury, virtually no sign of previous damage was detectable in wild-type mice except for the presence of centrally located nuclei inside the regenerating fiber, indicating complete regeneration. In Plg-deficient mice, however, muscle presented histologic features of degeneration (Figure 8A). The presence of fibrin in cross-sections of regenerating muscle of wild-type and Plg−/− mice was also analyzed in both types of mice. Although no fibrin immunoreactivity was detected in regenerating muscle of wild-type mice, extensive fibrin deposition was observed in regenerating muscle of Plg-deficient mice 9 days after injury (Figure8B). These results indicated that, like uPA-deficient mice, Plg-deficient mice developed significant fibrin deposition on muscle injury, leading to a defective muscle regeneration process.
Plasmin generation is increased during skeletal muscle regeneration.
Histograms show plasmin-specific activity (SA) (expressed as mOD/min per milligram protein) in muscle of wild-type mice 0, 2, 5, 7, and 9 days after injury. The assays were performed in 0.1 M Tris-HCl and 2 mM EDTA, pH 7.6, containing the chromogenic substrate S-2251. No plasmin activity is detected in extracts from noninjured muscle tissue, but it is induced on muscle injury.
Plasmin generation is increased during skeletal muscle regeneration.
Histograms show plasmin-specific activity (SA) (expressed as mOD/min per milligram protein) in muscle of wild-type mice 0, 2, 5, 7, and 9 days after injury. The assays were performed in 0.1 M Tris-HCl and 2 mM EDTA, pH 7.6, containing the chromogenic substrate S-2251. No plasmin activity is detected in extracts from noninjured muscle tissue, but it is induced on muscle injury.
Glycerol-induced injury results in impaired skeletal muscle regeneration of plasminogen-deficient mice.
(A) Frozen muscle sections from wild-type (WT) and Plg-deficient mice were stained with H&E 9 days after injury. In WT mice, regeneration is almost complete 9 days after injury, whereas it is impaired in Plg-deficient mice. (B) Immunohistochemical detection of fibrin in regenerating muscle of Plg-deficient mice. Sections from 9-day–injured muscles of WT and Plg-deficient mice were stained with a rabbit antimurine fibrin(ogen) antibody and counterstained with hematoxylin. Fibrin deposits were observed in regenerating muscle of Plg-deficient mice but not of WT mice. Original magnification, 400 ×.
Glycerol-induced injury results in impaired skeletal muscle regeneration of plasminogen-deficient mice.
(A) Frozen muscle sections from wild-type (WT) and Plg-deficient mice were stained with H&E 9 days after injury. In WT mice, regeneration is almost complete 9 days after injury, whereas it is impaired in Plg-deficient mice. (B) Immunohistochemical detection of fibrin in regenerating muscle of Plg-deficient mice. Sections from 9-day–injured muscles of WT and Plg-deficient mice were stained with a rabbit antimurine fibrin(ogen) antibody and counterstained with hematoxylin. Fibrin deposits were observed in regenerating muscle of Plg-deficient mice but not of WT mice. Original magnification, 400 ×.
Discussion
In the current study, we describe the consequences of the inactivation of plasminogen activation system genes during skeletal muscle regeneration. Mice lacking uPA, but not tPA, show a pronounced regeneration defect after experimentally induced injury of muscle, suggesting a protective role for uPA in the muscle regeneration process. The persistence of muscle degeneration in uPA-deficient mice was reproduced in Plg-deficient mice. This indicates that between the 2 pathways of Plg activation (uPA- or tPA-mediated), uPA-mediated Plg activation is the major one in the muscle regeneration process. This conclusion is supported by zymographic analysis of regenerating muscles of wild-type mice, which only showed uPA activity. Interestingly, in C2C12 murine myoblasts, uPA activity was also predominant.13 Finally, the similar phenotypes of uPA- and Plg-deficient mice indicates, in this animal model, the predominance of uPA-dependent plasmin effects.
We have demonstrated a significant accumulation of extravascular fibrin in regenerating muscle of uPA−/− and Plg−/− mice. Extravascular fibrin deposition is a key feature in abnormalities characterized by inflammation and tissue repair, including impaired skin wound healing26 and glomerulonephritis.27 In these 2 situations, failure to remove fibrin was attributable to reduced uPA- or tPA-mediated fibrinolysis, respectively. In experimentally induced muscle degeneration, we also found that uPA deficiency led to increased levels of muscular fibrin, indicating the importance of uPA in extravascular fibrin clearance. Fibrin accumulation in the extracellular basal membrane may have deleterious effects, such as the impediment of normal nutrition to the muscle tissue. The link between increased fibrin levels and prolonged muscle degeneration was further explored by defibrinogenation of uPA-deficient mice. Ancrod administration reduced plasma fibrinogen levels in uPA-deficient mice, resulting in a substantial restoration of the normal muscle regeneration process. Altogether, these results demonstrate that excessive and persistent fibrin(ogen) accumulation has a pathogenic role in sustaining muscle degeneration.
Our finding that loss of Plg activation impedes muscle regeneration raises the question of how the uPA-Plg system is involved in tissue repair. Inflammation is a process frequently associated with tissue repair because degenerating tissues are invaded by inflammatory cells. Our study showed that in response to glycerol-induced muscle injury, macrophages accumulated near the injury site 48 hours after injury. Similar results in macrophage recruitment were reported in a study of regeneration after bupivacaine-induced muscle injury in the rat28 and after crush injury in the mouse.2We have observed that mice with a specific deficit in uPA show a reduced staining for Mac-1–positive cells 48 hours after injury, indicating that the number of macrophages reaching the injury site is reduced in the absence of uPA. This suggests that uPA activity may have a profound effect on inflammation and inflammation-related muscle disease. Recent work29 with uPA-deficient mice has demonstrated that uPA is required for the pulmonary inflammatory response to Cryptococcus neoformans because a lack of uPA resulted in inadequate macrophage recruitment, uncontrolled infection, and death. In addition, endogenously produced uPA could amplify tumor necrosis factor-α neosynthesis by mononuclear phagocytes, representing a novel mechanism by which a phagocyte-derived protease contributes to the generation of proinflammatory signals.30 It is, therefore, tempting to speculate that uPA might play a similar role in the inflammation caused by muscle injury. Thus, the reduced presence of macrophages in the injured muscles of uPA−/− mice might be due either to a decrease in the migration capacity of inflammatory cells devoid of uPA or to a decrease in the potential of these cells to traverse fibrin-rich matrices. In addition, in vitro studies31 have shown that factors produced by damaged skeletal muscle (eg, fibroblast growth factor [FGF] and platelet-derived growth factor [PDGF]) are highly chemoattractant for neutrophils and macrophages, and this activity was apparent within muscle tissue 6 hours after injury and was increased by 24 to 48 hours. This correlates with commencement of the influx of macrophages in vivo shown in the current study. Furthermore, it has been shown that activated macrophages (equivalent to those that accumulate at the site of muscle damage), but not neutrophils, produce soluble factors (FGF and PDGF) that are highly chemoattractant and are mitogenic for muscle precursor cells.31 Thus, activated macrophages that accumulate in response to muscle damage may not only phagocytose necrotic tissue, they may facilitate the repair of damaged myofibers. These processes will be altered if macrophages do not accumulate at the injury site, providing a potential explanation for the persistent muscle degeneration observed in uPA-deficient mice.
The hypothesis that, in general, Plg activation facilitates cellular penetration of fibrin-containing matrices is in accordance with a putative scenario occurring in normal skeletal muscle regeneration, with local conversion of Plg to plasmin and subsequent fibrin degradation. Plasmin is probably one member of a team of carefully regulated and specialized matrix-degrading enzymes, including serine, metallo, and other classes of proteases, that together serve in matrix remodeling and cellular reorganization of wound fields. Our data suggest that plasmin is particularly useful in fibrin solubilization; without it, cellular reorganization of fibrin-rich matrices is severely impeded. We conclude that the uPA-Plg system is required for the normal regeneration and resolution of muscle damage in mice. The reduced presence of macrophages at the injury site suggests that a key factor slowing muscle repair in uPA-deficient animals may be the diminished ability of responding macrophages to proteolytically dissect their way through the extracellular matrix until they reach the injury site. In this model, one major matrix component within damaged areas that may represent a particular impediment to inflammatory cell migration in the absence of uPA (or Plg) is fibrin. However, based on the existing data, we cannot exclude that the uPA-Plg deficiency may impede cell migration because of the lost contribution of plasmin to degrade other matrix components or the lack of other key matrix proteinases or of growth factors. Apart from its effects on extracellular matrix proteins, uPA-plasmin can cleave and activate latent forms of growth-angiogenic factors such as transforming growth factor-β and hepatocyte growth factor–scatter factor.32,33 Both factors are expressed within injured muscle and are believed to have important muscle regeneration effects by promoting the activation of quiescent satellite cells after injury in vivo.34 Therefore, modulation of uPA-mediated proteolytic activity may indirectly influence cell recruitment and the growth and differentiation of cellular constituents in regenerating muscle, though this role remains to be demonstrated both in vitro and in vivo. Recently, a defect in the differentiation of satellite cells of MyoD-deficient mice has been described,35 and, as a consequence, the skeletal muscle regeneration capacity of these mice is compromised. Interestingly, the phenotypes of uPA-deficient mice (this study) and MyoD-deficient mice appear to overlap partially.
In conclusion, our results demonstrate that uPA fulfills a beneficial role in skeletal muscle regeneration, mainly through uPA-mediated fibrinolytic activity. Compounds aimed at decreasing fibrin levels in muscle may be clinically useful in therapy for muscular dystrophy. Future experiments will be directed to further definition of the benefit of defibrinogenation, anticoagulant, or fibrinolytic agents in dystrophinopathy. The prevention of plasminogen activation has revealed the requirement of uPA-plasmin and the dispensability of tPA in the regeneration of skeletal muscle. This report constitutes the first demonstration for a role of a proteolytic system in skeletal muscle regeneration in vivo. Rescue experiments by injection of recombinant proteases or retroviruses will show whether exogenously supplied uPA can compensate for the mutation. If this is the case, uPA might prove to be useful for the treatment of muscle injuries or muscle dystrophies, particularly if uPA evokes the response preferentially in muscle.
We thank Dr Keld Dano for the antifibrinogen and Dr Lourdes Gombau, A. Martı́n, and D. Fernández for assistance.
Supported by DGES grant PM97-008 and by a grant from Fundació La Marató-TV3 (P.M.C., M.R.), by FISS94-0968, by the Medical Research Council and the British Council (S.M.H.), and by the European Union (P.M.C., S.M.H., P.C.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Pura Muñoz-Cánoves, Centre d'Oncologia Molecular, Institut de Recerca Oncològica, Aut Castelldefels, km 2.7, 08907 L'Hospitalet de LL, Barcelona, Spain; e-mail: pmunoz@iro.es.







This feature is available to Subscribers Only
Sign In or Create an Account Close Modal