Serotonin (5-hydroxytryptamine, or 5-HT), released from activated platelets, not only accelerates aggregation of platelets but also is known to promote mitosis, migration, and contraction of vascular smooth muscle cells (VSMCs). These effects are considered to contribute to thrombus formation and atherosclerosis. The aim of this study was to investigate the effects of 5-HT on the expressions of coagulative and fibrinolytic factors in rat aortic endothelial cells. Endothelial cells were stimulated with various concentrations of 5-HT (0.1∼10 μM), and the expressions of tissue factor (TF), tissue factor pathway inhibitor (TFPI), plasminogen activator inhibitor-1 (PAI-1), and tissue-type plasminogen activator (TPA) messenger RNAs (mRNAs) were evaluated by Northern blot analysis. The activities of TF and PAI-1 were also measured. TF and PAI-1 mRNA were increased significantly in a concentration- and time-dependent manner. However, TFPI and TPA mRNA expression did not change. The inductions of TF and PAI-1 mRNAs were inhibited by a 5-HT1/5-HT2 receptor antagonist (methiothepin) and a selective 5-HT2A receptor antagonist (MCI-9042). These results indicate that 5-HT increases procoagulant activity and reduces fibrinolytic activities of endothelial cells through the 5-HT2A receptor. It was concluded that the modulation of procoagulant and hypofibrinolytic activities of endothelial cells by 5-HT synergistically promotes thrombus formation at the site of vessel injury with the platelet aggregation, VSMC contraction, and VSMC proliferation.
Introduction
Serotonin (5-hydroxytryptamine, or 5-HT) is synthesized and secreted into the blood stream by enterochromaffin cells in the gastrointestinal tract and is rapidly taken up and stored in small dense granules in platelets.1 When platelets adhere and aggregate at a site of vessel injury, 5-HT is secreted and directly accelerates platelet aggregation and potentiates the response of platelets to other agonists such as adenosine diphosphate, collagen, and thromboxane A2.2 5-HT also promotes the proliferation,3-5 migration,6 and contraction7-9 of vascular smooth muscle cells (VSMCs). In addition to physiologic hemostasis, these vascular responses play a pivotal role in the development and progression of thrombotic diseases.10 11 Although the effects of 5-HT on platelets and smooth muscle cells (SMCs) are well documented, little information on its effect in vascular endothelial cells (ECs) is available, especially concerning the regulation of coagulation and fibrinolysis.
Vascular ECs produce many modulatory proteins that regulate coagulation and fibrinolysis, such as tissue factor (TF) and plasminogen activator inhibitor-1 (PAI-1), which accelerate the development of thrombotic states.12,13 TF is a 47-kd membrane-bound glycoprotein that plays an important role in the extrinsic pathway of blood coagulation.14 TF present on cell membrane surfaces forms complexes with coagulation factor VII/VIIa. The TF-VIIa complex activates circulating factors X and IX by limited proteolysis, leading to thrombin formation and deposition of insoluble fibrin. The inhibitory regulator of this pathway is tissue factor pathway inhibitor (TFPI), a 42-kd plasma glycoprotein previously referred to as lipoprotein-associated coagulation inhibitor, or extrinsic pathway inhibitor.15 PAI-1 is a 50-kd glycoprotein and the primary physiologic inhibitor of plasminogen activation. PAI-1 plays a critical role in the regulation of fibrinolysis, serving as the primary inhibitor of tissue-type plasminogen activator (TPA).16These 4 proteins are involved in the regulation of both coagulation and fibrinolysis by ECs.17 18
In this study we examined the effects of 5-HT on the expression of TF, TFPI, PAI-1, and TPA in cultured rat aortic endothelial cells (RAECs).
Materials and methods
Cell cultures
RAECs were isolated from male Sprague-Dawley rats (200-250 g) by the primary explant technique as previously described.19Cells were cultured in Dulbecco modified Eagle medium (DMEM) supplemented with 15% fetal bovine serum (FBS), EC growth supplement (150 μg/mL), and antibiotics (50 μg/mL ampicillin, 15 μg/mL gentamicin, 1 μg/mL minomycin, and 1 μg/mL amphotericin B). Biocoat cell ware (rat tail collagen, type 1), 35 mm/100-mm diameter tissue culture dishes from Becton Dickinson Labware (Bedford, MA) were used for the RAEC culture. Experiments were performed on cells after 4 to 6 passages from the primary culture. After reaching confluence, RAECs were placed in conditioned medium (DMEM containing 1.0% bovine serum albumin [BSA] and the antibiotics) for 48 hours before experiments were performed to induce quiescence.
Measurements of TF, TFPI, PAI-1, and TPA messenger RNA expression
Total cellular RNA from RAECs was prepared, using RNeasy kits (Qiagen GmbH, Hilden, Germany). RNA pellets were dissolved in water, and their concentrations were determined by the absorbance at 260 nm. The relative amounts of specific messenger RNAs (mRNAs) were quantified by Northern blot analysis, using specific complementary DNA (cDNA) probes. The level of each specific expression was normalized to the expression of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA. cDNA probes were generated by reverse transcription-polymerase chain reaction (RT-PCR), using synthetic oligonucleotide primers (Sawady Technology Inc, Tokyo, Japan) for rat TF, TFPI, PAI-1, TPA, and GAPDH cDNA sequence.
Total RNA samples (10 μg) were size fractionated on 1% agarose/formaldehyde gels and capillary transferred onto nitrocellulose membranes, Hybond-N (Amersham International plc, Buckinghamshire, England). After baking at 80°C for 1 hour, the membranes were prehybridized at 42°C for 2 hours in a mixture of 50% formamide, 4 × sodium chloride and sodium citrate (SSC), 5 × Denhardt solution, 0.2% SDS, and 100 μg/mL of heat-denatured sonicated salmon sperm DNA. Membranes were hybridized overnight with specific cDNA probes at 42°C in a shaker, washed with 2 × SSC-0.1% sodium dodecyl sulfate (SDS) at room temperature for 10 minutes, air dried, and exposed to X-OMAT AR film (Eastman Kodak Co, Rochester, NY) at −80°C.
Measurements of TF and PAI-1 activities
TF activity of RAEC surfaces was measured by using a chromogenic assay because TF is a membrane-associated protein.20 After stimulation, RAECs were washed 3 times in Tris-buffered saline (50 mM Tris HCl,120 mM NaCl, 2.7 mM KCl, 3 mg/mL BSA, pH 7.4) and incubated with 1.0 mL prothrombin complex (PPSB-HT: factor II, VII, IX, and X concentrates; Nippon Pharmaceutical Co, Tokyo, Japan) diluted 2-fold with Tris-buffered saline containing 10 mM CaCl2. Samples were incubated for 30 minutes at 37°C, and 200 μL supernatant was added to 100 μL of 2 mM S-2765 (Chromogenix AB, Möndal, Sweden), a chromogenic substrate of factor Xa. After a 15-minute incubation at 25°C, the reaction was stopped by the addition of 200 μL of 50% (vol/vol) acetic acid. The amidolytic activity of generated factor Xa was determined by the absorbance at 405 nm. TF activity is expressed as arbitrary units (AU), using reference curves generated with a standard human placenta-derived TF, Thromborel S (Behringwerke AG, Marburg, Germany) containing 106 AU/mL. The curve was linear from 50 to 1000 AU/mL.
For the measurement of PAI-1 concentration in the medium, conditioned medium was immediately collected and stored at −80°C. Functionally active PAI-1 present in the culture medium was measured, using an enzyme-linked immunosorbent assay kit (RPAIKT, Molecular Innovations Inc, Royal Oak, MI). Briefly, functionally active rat PAI-1 present in the culture medium was reacted with human TPA coated on microtiter plates, and a rabbit antirat PAI-1 primary antibody was bound to the PAI-1. Then, the bound primary antibody was complexed with a secondary antibody conjugated to biotin and subsequently detected with avidin conjugated to alkaline phosphatase. Para-nitrophenyl phosphate substrate was used for color development, which was quantified by the absorbance at 405 nm.
Reagents
EC growth supplement was obtained from Becton Dickinson Labware (Bedford, MA). DMEM and heat-inactivated FBS were from Gibco BRL Life Technologies Inc (Grand Island, NY). BSA and antibiotics were purchased from Sigma Chemical (St Louis, MO). 5-HT was obtained from Sigma Chemical Co. MCI-9042, 5-HT2A receptor antagonist, was kindly provided by Tokyo Tanabe Inc (Tokyo, Japan). 5-carboxamidotryptamine maleate (a 5-HT1 receptor agonist), 2-methyl-5-hydroxytryptamine hydrochloride (a 5-HT3 receptor agonist), methiothepin maleate (a 5-HT1/5-HT2 receptor antagonist), and MDL-72222 (a 5-HT3 receptor antagonist) were purchased from Tocris Cookson Inc (Ballwin, MO).
Data analysis
The intensity of bands of interest from Northern blots was quantified by reflectance densitometry. The TF, TFPI, PAI-1, and TPA mRNA expression was normalized GAPDH mRNA expression. The relative TF, TFPI, PAI-1, and TPA mRNA expression levels are expressed as the fold increase in normalized TF, TFPI, PAI-1, and TPA mRNA expression relative to the expression in vehicle-treated or quiescent RAECs. All values are expressed as the mean ± SEM, for n = 3. Statistical analysis was performed by analysis of variance, and Fisher protected least significant difference was used for individual comparisons. Values of P < .05 were considered significant.
Results
Effects of 5-HT on TF, TFPI, PAI-1, and TPA mRNA expression in RAECs
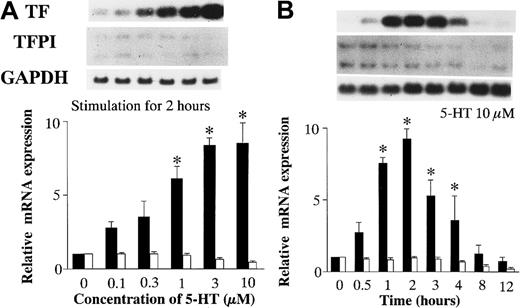
After quiescent RAECs were incubated with vehicle or various concentrations of 5-HT for 2 hours, total RNA was isolated from the RAECs to examine TF and TFPI mRNA expression by Northern blot analysis (Figure 1A). In the RAECs cultured with 5-HT at concentrations ≥ 1 μM, the expression of TF mRNA increased significantly in a concentration-dependent manner, whereas TFPI mRNA expression did not change relative to vehicle-treated RAECs.
Effects of 5-HT on TF and TFPI mRNA expression.
(A) Dose-dependent effects of 5-HT. (B) Time-dependent effects of 5-HT. TF and TFPI mRNA expression was normalized to GAPDH mRNA expression. Relative TF mRNA expression, ▪; TFPI, ■. Each bar represents the mean ± SEM of 3 independent experiments (*P < .05 versus vehicle-treated controls). Relative TF and TFPI mRNA expressions represent the fold increase in normalized TF and TFPI mRNA expression compared to vehicle-treated controls, respectively.
Effects of 5-HT on TF and TFPI mRNA expression.
(A) Dose-dependent effects of 5-HT. (B) Time-dependent effects of 5-HT. TF and TFPI mRNA expression was normalized to GAPDH mRNA expression. Relative TF mRNA expression, ▪; TFPI, ■. Each bar represents the mean ± SEM of 3 independent experiments (*P < .05 versus vehicle-treated controls). Relative TF and TFPI mRNA expressions represent the fold increase in normalized TF and TFPI mRNA expression compared to vehicle-treated controls, respectively.
To investigate the time-dependent effects of 5-HT on TF and TFPI mRNA expression, total RNA was isolated from RAECs stimulated with 10 μM 5-HT for various periods of time (Figure 1B). Northern blot analysis of the isolated RNA shows that TF mRNA induction by 5-HT stimulation began within 1 hour and reached a peak expression after 2 hours. The peak TF mRNA expression was 9.23 ± 0.71-fold higher than the basal level.
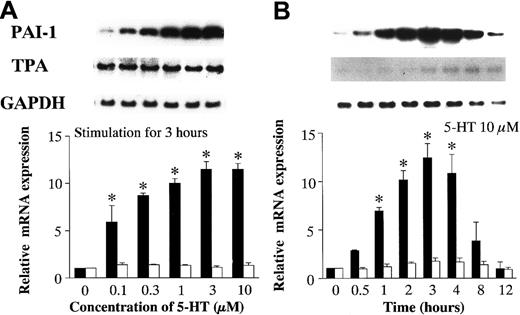
To characterize PAI-1 and TPA mRNA expression, the dose-dependent and time-dependent effects of 5-HT were studied as described above for TF and TFPI mRNA. PAI-1 mRNA expression increased significantly in a concentration-dependent manner, at concentrations of 5-HT ≥ 0.1 μM. In contrast, TPA mRNA expression did not change (Figure2A). PAI-1 mRNA induction by 5-HT stimulation began within 1 hour and reached a peak after 3 hours. The peak level of PAI-1 mRNA expression was 12.42 ± 1.46-fold higher than the basal level. There was no evidence of time-dependent induction of TPA mRNA (Figure 2B).
Effects of 5-HT on PAI-1 and TPA mRNA expression.
(A) Dose-dependent effects of 5-HT. (B) Time-dependent effects of 5-HT. Graph showing relative PAI-1 (▪) and TPA (■) mRNA expression represents the fold increase in corrected PAI-1 and TPA mRNA expression compared to vehicle-treated controls, respectively. Each bar represents the mean ± SEM of 3 independent experiments (*P < .05 versus vehicle-treated controls).
Effects of 5-HT on PAI-1 and TPA mRNA expression.
(A) Dose-dependent effects of 5-HT. (B) Time-dependent effects of 5-HT. Graph showing relative PAI-1 (▪) and TPA (■) mRNA expression represents the fold increase in corrected PAI-1 and TPA mRNA expression compared to vehicle-treated controls, respectively. Each bar represents the mean ± SEM of 3 independent experiments (*P < .05 versus vehicle-treated controls).
Effects of 5-HT on the activities of TF on the surface of RAECs and PAI-1 in the culture medium
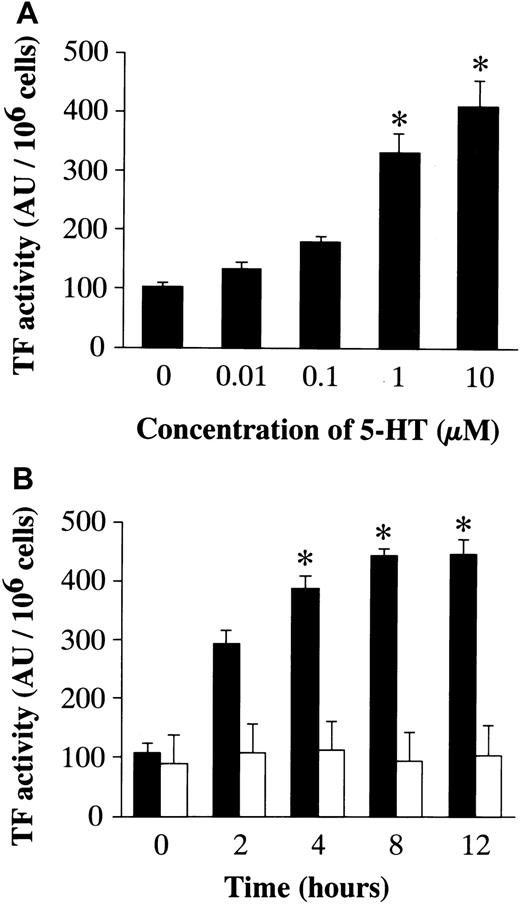
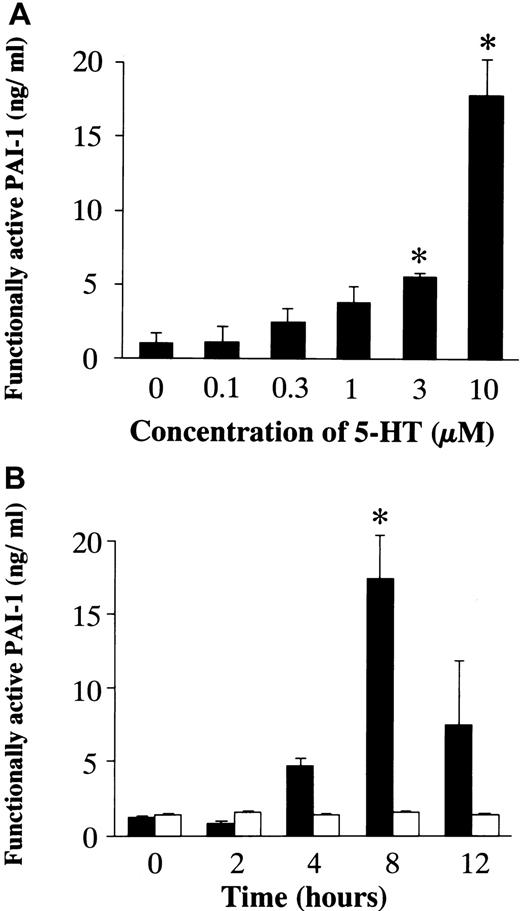
To confirm a relationship between mRNA expression and the activity/amount of protein, the activity of TF and the concentration of PAI-1 were examined (Figure 3). After a 48-hour incubation in conditioned medium, RAECs were stimulated with 5-HT (0.01, 0.1, 1, and 10 μM) for 12 hours. 5-HT stimulation resulted in a significant increase in TF activity on the surface of RAECs in a dose-dependent manner (Figure 3A). The time course for changes in TF activity induced by 10 μM 5-HT was examined. A significant increase in TF activity, compared with vehicle-treated controls, was seen at 4 hours of stimulation with 5-HT, and the maximum TF activity on the cell surface was noted 12 hours after the addition of 5-HT (Figure 3B). To quantify functionally active PAI-1 produced by RAECs, they were stimulated with 5-HT (0.1, 0.3, 1, 3, and 10 μM) for 8 hours after a 48-hour incubation in conditioned medium. A significant increase in the PAI-1 concentration in the culture medium was attained at a 5-HT concentration ≥ 3 μM (Figure4A). In the time course study, a significant increase in the PAI-1 concentration was observed 8 hours after stimulation (Figure 4B).
Effects of 5-HT on the TF activity on the RAEC surface.
(A) Dose-dependent effects of 5-HT. Each bar represents the mean ± SEM of 3 independent experiments (*P < .05 versus vehicle-treated controls). (B) Time-dependent effect of 5-HT. ▪, 5-HT, 10 μM; ■, vehicle.
Effects of 5-HT on the TF activity on the RAEC surface.
(A) Dose-dependent effects of 5-HT. Each bar represents the mean ± SEM of 3 independent experiments (*P < .05 versus vehicle-treated controls). (B) Time-dependent effect of 5-HT. ▪, 5-HT, 10 μM; ■, vehicle.
Effects of 5-HT on the concentration of functionally active PAI-1 in the medium.
(A) Dose-dependent effects of 5-HT. Each bar represents the mean ± SEM of 3 independent experiments (*P < .05 versus vehicle-treated controls). (B) Time-dependent effects of 5-HT. ▪, 5-HT, 10 μM; ■, vehicle.
Effects of 5-HT on the concentration of functionally active PAI-1 in the medium.
(A) Dose-dependent effects of 5-HT. Each bar represents the mean ± SEM of 3 independent experiments (*P < .05 versus vehicle-treated controls). (B) Time-dependent effects of 5-HT. ▪, 5-HT, 10 μM; ■, vehicle.
Effects of 5-HT receptor antagonists on 5-HT–induced TF and PAI-1 mRNA expression
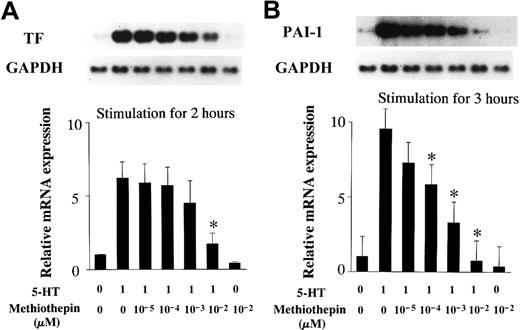
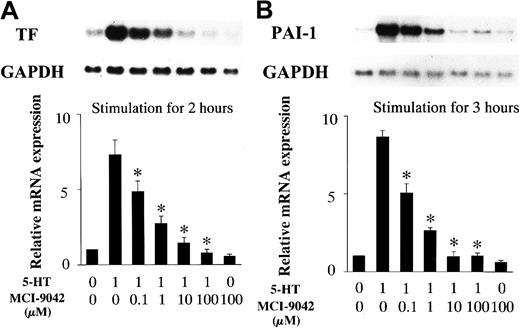
RAECs were incubated with 5-HT receptor antagonists for 30 minutes before stimulation with 1 μM 5-HT. Total RNA was extracted from the RAECs after 2- or 3-hour incubations with 5-HT. Northern blot analysis revealed that preincubation with methiothepin, a 5-HT1/5-HT2 receptor antagonist, blocked the increase in TF and PAI-1 mRNA expression, at a concentration of 10−2 μM and 10−4 ∼ 10−2μM, respectively (Figure 5). MDL-72222, a 5-HT3 receptor antagonist, had no effect on 5-HT–stimulated TF and PAI-1 mRNA expression in RAECs (data not shown). Pretreatment with MCI-9042, a 5-HT2A receptor antagonist, decreased TF and PAI-1 mRNA induction by 5-HT, at concentrations ≥ 1 μM and completely blocked induction of both at a concentration of 10 μM (Figure 6).
Inhibitory effects of methiothepin (a 5-HT1/5-HT2 receptor antagonist) on 5-HT–induced mRNA expression.
(A) TF mRNA expression. (B) PAI-1 mRNA expression. Quiescent RAECs were preincubated with the indicated concentrations of methiothepin for 30 minutes and then cultured with serotonin 1 μM for 2 or 3 hours. Relative TF and PAI-1 mRNA expression represent the fold increase in normalized TF and PAI-1 mRNA expression compared to vehicle-treated controls, respectively. Each bar represents the mean ± SEM of 3 independent experiments (*P < .05 versus RAECs treated with 5-HT only).
Inhibitory effects of methiothepin (a 5-HT1/5-HT2 receptor antagonist) on 5-HT–induced mRNA expression.
(A) TF mRNA expression. (B) PAI-1 mRNA expression. Quiescent RAECs were preincubated with the indicated concentrations of methiothepin for 30 minutes and then cultured with serotonin 1 μM for 2 or 3 hours. Relative TF and PAI-1 mRNA expression represent the fold increase in normalized TF and PAI-1 mRNA expression compared to vehicle-treated controls, respectively. Each bar represents the mean ± SEM of 3 independent experiments (*P < .05 versus RAECs treated with 5-HT only).
Inhibitory effects of MCI-9042 (a 5-HT2Areceptor antagonist) on 5-HT–induced mRNA expression.
(A) TF mRNA expression. (B) PAI-1 mRNA expression. Quiescent RAECs were preincubated with the indicated concentrations of MCI-9042 for 30 minutes and then cultured with serotonin 1 μM for 2 or 3 hours. Relative TF and PAI-1 mRNA expression represent the fold increase in normalized TF and PAI-1 mRNA expression compared to expression in vehicle-treated controls. Each bar represents the mean ± SEM of 3 independent experiments (*P < .05 versus RAECs treated with 5-HT only).
Inhibitory effects of MCI-9042 (a 5-HT2Areceptor antagonist) on 5-HT–induced mRNA expression.
(A) TF mRNA expression. (B) PAI-1 mRNA expression. Quiescent RAECs were preincubated with the indicated concentrations of MCI-9042 for 30 minutes and then cultured with serotonin 1 μM for 2 or 3 hours. Relative TF and PAI-1 mRNA expression represent the fold increase in normalized TF and PAI-1 mRNA expression compared to expression in vehicle-treated controls. Each bar represents the mean ± SEM of 3 independent experiments (*P < .05 versus RAECs treated with 5-HT only).
Discussion
The 3 major factors responsible for thrombus formation are “abnormality of the vascular wall,” “decrease in blood flow,” and “imbalance of coagulative and fibrinolytic factors,” which are known as the Virchow triad.21,22 Injury of the endothelium begins in the first place, which would cause platelets to aggregate and release 5-HT. 5-HT directly accelerates platelet aggregation and also potentiates the response of platelets to other agonists such as adenosine diphosphate, collagen, and thromboxane A2.2 5-HT is known to promote the contraction of VSMCs.7-9 Vikenes et al23 recently reported that elevated concentrations of 5-HT in platelet- rich plasma promote platelet aggregation and VSMC contraction and is associated with the development of coronary artery disease and cardiac events. Vasoconstriction, together with platelet aggregation, would reduce arterial blood flow and would increase the risk of thrombus formation.24
5-HT also promotes the proliferation3-5 and migration of VSMCs6 and may contribute to the progression of atherosclerosis, which is considered to be the major risk factor of arterial thrombotic disease. 5-HT not only interacts synergistically with platelet-derived growth factor4 but also has a direct mitogenic effect on cultured aortic VSMCs.3-5,25,26 It has also been reported that 5-HT stimulates the expression of thrombin receptors in VSMCs probably by 5-HT2 receptors, which would further potentiate the contraction and proliferation of VSMCs by thrombin.27 These effects could cause narrowing of the vessel lumen and contribute to both the development of atherosclerosis and the restenosis observed after angioplasty.11,28 29
In respect to the imbalance of coagulative and fibrinolytic factors, vascular ECs play an important role by producing modulatory proteins such as TF, TFPI, PAI-1, and TPA. Many factors, including vasoactive substances affect this regulation.30-32 Expression and balance of these proteins is known to control the development of thrombosis. Following platelet aggregation, the activation of coagulation cascade would result in thrombin activation and fibrin formation, which is known as secondary hemostasis. When platelets are activated, the expressions of phosphatidyl-serine (PS), one of the major negatively charged phospholipids on the surface of platelets, are enhanced and bind activated factors V and X, which is supposed to augment the activation of following coagulation reactions.33 However, little information is available about the effects of 5-HT released from activated platelets on this regulation. In the present study, 5-HT was shown to increase the TF and PAI-1 mRNA and protein expression in a concentration- and time-dependent manner in RAECs. In contrast, 5-HT had no significant effect on the expressions of TFPI and TPA. On the basis of these results, 5-HT causes ECs to be procoagulative and hypofibrinolytic by increasing TF relative to TFPI and increasing PAI-1 relative to TPA. These findings suggest that 5-HT is involved in physiologic hemostasis by modifying the properties of vascular ECs, in addition to causing platelet aggregation and SMC contraction.
In applying these findings to in vivo status, however, several limitations must be considered. Recent studies demonstrate that the function of cultured ECs is different, depending on the source of endothelial cells. For example, the differences of adhesion molecule expressions or permeability to various vasoactive substances between cultured human arterial and venous ECs were reported.34 35RAECs were used in this study, because platelets participate more potently in the arterial thrombus formation than in the venous thrombus formation. Whether the same reactions are seen in venous ECs or not remain uncertain.
Second, the concentration of 5-HT used in this investigation must be compared with those in vivo. In healthy humans and animals, the normal concentration of 5-HT in plasma is reported to be low, because 5-HT is rapidly taken up by platelets or metabolized by monoamine oxidase in ECs and SMCs. Rat plasma concentration of 5-HT is reported to be approximately 0.1 μM,36 which is equivalent to the minimum concentration of 5-HT used in our experiments. This concentration is regarded as the basal concentration that ECs are usually exposed in vivo. With platelet aggregation, the local 5-HT concentration is considered to increase from basal level, which can trigger the local actions involving ECs. In the 5-HT concentration-dependent study, the significant induction of TF and PAI-1 mRNA was obtained at the concentrations of 5-HT 1 μM and higher. These results from concentration-dependent study seem to be relevant. 5-HT is known to be rapidly taken by platelets; therefore, the local concentration of an injured vessel might be rapidly decreased at local sites. As Ashton et al37 demonstrated, however, the persistent mobilization of platelets from circulating blood and the adherence of the activated platelets to the initial layer contribute the increased 5-HT concentration at the site. This means that physiologic primary hemostasis is maintained by activated platelets, which can exist until secondary hemostasis is completed. In a rabbit model of angioplasty involving the femoral artery, Pakala et al38 found that there was platelet deposition at the site of vascular injury with progressive accumulation of 5-HT. The concentration of 5-HT measured at the local vascular tissue in their model exceeded the concentrations required in vitro to stimulate ECs. Moreover, under these circumstances, the dysfunction of the ECs, which accompanies a decrease in monoamine oxidase activity, may result in the local accumulation of 5-HT by the decrease in its breakdown.39
In the present study, the stimulation of RAECs to produce TF and PAI-1 required high concentration and prolonged incubation of 5-HT; however, these are in agreement with the earlier reports in other cell types and in other responses related to 5-HT.7,27,40 41 On the basis of these observations, it is considered to be relevant that high 5-HT concentration levels persist at local sites until TF and PAI-1 proteins express.
The 5-HT receptors, 5HT1-7 receptors, have been characterized pharmacologically.1 The 5-HT2receptor is responsible for the platelet aggregation induced by 5-HT, because the effect of 5-HT is blocked by antagonists such as ketanserin.2 The 5-HT2 receptor is also involved in the proliferation of VSMCs induced by 5-HT.4,42 Moreover, Pakala et al43 suggested that the mitogenic effect of 5-HT to canine aortic ECs is mediated by the 5-HT2 receptor. Because MCI-9042, a selective 5-HT2A receptor antagonist,44 inhibited the induction of TF and PAI-1 mRNA by 5-HT in the present study, it was suggested that the responsible receptor for these reactions is the 5-HT2A receptor. The effect of 5-carboxamidotryptamine maleate, a 5-HT1 receptor agonist, and 2-methyl-5-hydroxytryptamine, a 5-HT3 receptor agonist, were also investigated. Both agonists induced TF and PAI-1 mRNA expression in a dose-dependent manner, respectively, which was contrary to the results we had expected. However, because MCI-9042 completely inhibited these effects, we consider that these results were due to cross-reaction of these agonists with the 5-HT2A receptor (data not shown). Taken together, we concluded that the effects of 5-HT were mediated by the activation of 5-HT2A receptors on the endothelial surface. The existence of endothelial 5-HT2Areceptors was confirmed by RT-PCR (data not shown).
In conclusion, 5-HT causes ECs to become thrombogenic via the 5-HT2A receptor, which is a novel and interesting property of 5-HT, in light of the interactions between platelets and ECs. These results demonstrate the contribution of 5-HT to the development of thrombotic diseases in combination with other properties, such as platelet aggregation, VSMC contraction, and VSMC proliferation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Hidehiko Kawano, Second Department of Medicine, Kyoto Prefectural University of Medicine, Kawaramachi-Hirokoji, Kamigyo-ku, Kyoto, Japan; e-mail: kawano@gemini.kpu-m.ac.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal