Abstract
A reduced-intensity preparative regimen consisting of melphalan and a purine analog was evaluated for allogeneic transplantation in 86 patients who had a variety of hematologic malignancies and were considered poor candidates for conventional myeloablative therapies because of age or comorbidity. Seventy-eight patients received fludarabine 25 mg/m2 daily for 5 days in combination with melphalan 180 mg/m2 (n = 66) or 140 mg/m2 (n = 12). Eight patients received cladribine 12 mg/m2 continuous infusion for 5 days with melphalan 180 mg/m2. The median age was 52 years (range, 22-70 years). Disease status at transplantation was either first remission or first chronic phase in 7 patients, untreated first relapse or subsequent remission in 16 patients, and refractory leukemia or transformed chronic myelogenous leukemia in 63 patients. Nonrelapse mortality rates on day 100 were 37.4% for the fludarabine/melphalan combination and 87.5% for the cladribine/melphalan combination. The median percentage of donor cells at 1 month in 75 patients was 100% (range, 0%-100%). The probability of grade 2-4 and 3-4 acute graft-versus-host disease was 0.49 (95% CI, 0.38-0.60) and 0.29 (95% CI, 0.18-0.41), respectively. Disease-free survival at 1 year was 57% for patients in first remission or chronic phase and 49% for patients with untreated first relapse or in a second or later remission. On multivariate analysis the strongest predictor for disease-free survival was a good or intermediate risk category. In summary, fludarabine/melphalan combinations are feasible in older patients with associated comorbidities, and long-term disease control can be achieved with reduced-intensity conditioning in this population.
Introduction
High-dose chemoradiotherapy with allogeneic bone marrow transplantation (BMT) has been extensively used in young patients with advanced hematologic malignancies. Approximately 30% of patients can achieve long-term disease control, with transplantation-related deaths accounting for 30% of treatment failures and relapse rates of 50% to 60% commonly reported.1-3 Allogeneic bone marrow transplantation has been limited to young patients because of the increased risk for regimen-related toxicities and graft-versus-host disease (GVHD) that occurs with increasing age.4,5 Improvements in supportive care, GVHD prophylaxis, and cytomegalovirus (CMV) prophylaxis has now enabled many centers to increase the age limit for BMT, but few are performing allogenic stem cell transplantation on patients older than 55 years. Older patients, however, constitute a large group of patients with extremely poor prognosis if conventional induction therapy is used. Consequently, novel therapeutic options must be explored.6 7
The use of nonablative regimens to obtain donor cell engraftment followed by donor lymphocytes to exploit a graft-versus-leukemia effect has demonstrated that it is possible to achieve mixed and full chimerism after nonmyeloablative therapy. However, in patients with refractory disease remissions are usually short-lived, and there is little time to exploit a potentially beneficial graft-versus-leukemia effect.8 9
High-dose melphalan has been successful in effecting durable remissions in patients with acute leukemia and other hematologic malignancies with little extramedullary toxicity.10,11 Similarly, purine analogs have been shown to inhibit the mechanisms of DNA repair after alkylator-induced damage.12 Therefore, reduced-intensity conditioning with melphalan/purine analog combinations could potentially achieve disease remission and engraftment of allogeneic cells with acceptable toxicity. Herein we report the results of our initial trials using purine analog/melphalan reduced-intensity combinations in patients with a variety of hematologic malignancies who underwent allogeneic progenitor cell transplantation at MD Anderson Cancer Center and were considered poor candidates for conventional myeloablative therapy because of age or concurrent medical conditions.
Patients, materials, and methods
Patient eligibility
Patients with hematologic malignancies were eligible for this program if they were between 50 and 75 years. They were also eligible if they were younger than 50 but were deemed poor candidates for conventional myeloablative therapy because of concurrent medical conditions (cardiac ejection fraction less than 50%; forced expiratory volume in first second, forced vital capacity, or diffusion capacity of carbon monoxide less than 50% predicted; abnormal findings on liver function tests; poor performance status; or extensive prior therapy). Patients required either a full human leukocyte antigen (HLA)–matched unrelated donor (serologic match for class 1 antigens and high resolution molecular matching for class II antigens) or a fully matched or one-antigen–mismatched related donor willing and able to donate peripheral blood or bone marrow progenitor cells using conventional techniques. This study received the approval of the Institutional Review Board of the University of Texas MD Anderson Cancer Center, and patients and donors were required to provide written, informed consent. Unrelated donors were procured and gave consent through the National Marrow Donor Program according to current accepted standards and procedures.
Treatment plan
This report summarizes the results of 3 subsequent trials of reduced-intensity conditioning with melphalan and purine analogs at the University of Texas MD Anderson Cancer Center. From March 1996 to April 1997, patients received fludarabine 25 mg/m2 intravenously daily for 5 days in combination with melphalan 90 mg/m2daily for 2 days or melphalan 140 mg/m2 as a single intravenous dose as part of a pilot trial. Patients with prior exposure to fludarabine received cladribine (2 chloro-deoxyadenosine; 2CDA) instead of fludarabine at a dose of 12 mg/m2 continuous infusion for 5 days. From April 1997 to December 1998, patients participated in a randomized phase 2 protocol comparing fludarabine 25 mg/m2 intravenously daily for 5 days and melphalan 90 mg/m2 daily for 2 days versus cladribine 12 mg/m2 by continuous infusion daily for 5 days with the same melphalan dose and schedule as the fludarabine group. After June 1997 no additional patients received cladribine/melphalan because of excessive toxicity in these patients. From November 1997 to December 1998, 11 patients received melphalan 70 mg/m2 daily for 2 days instead of 90 mg/m2 because of a history of extensive prior therapy or renal dysfunction. Drugs were dosed according to adjusted body weight in patients whose actual weight was 20% more than the ideal body weight. Recipients of unrelated donor progenitor cells or one-antigen–mismatched related donors received non–T-cell–depleted donor bone marrow obtained using standard techniques. Recipients of progenitor cells from HLA-matched related donors received filgrastim-stimulated peripheral blood using standard apheresis procedures and filgrastim doses of 10 to 12 μg/kg daily for 4 days, with collection on the fifth day, for a target dose of 4 × 106 CD34+ cells/kg recipient body weight.
Supportive care
GVHD prophylaxis consisted of tacrolimus (FK506; Prograf, Fujisawa, Japan) dosed to maintain blood levels between 5 and 10 ng/dL and methotrexate 5 mg/m2 on days 1, 3, 6, and 11 in 81 patients. During the pilot trial 3 patients received cyclosporine/steroids as GVHD prophylaxis because of the unavailability of assays to monitor tacrolimus levels, and 2 patients received tacrolimus/steroids. GVHD was continued for a minimum of 3 months for patients with high-risk disease and a minimum of 6 months for patients with low-risk disease or for recipients of unrelated or mismatched donor bone marrow. Donor lymphocyte infusions were not routinely administered to patients unless cytogenetic or morphologic relapse was documented.
Antibacterial, antifungal, and antiviral prophylaxes were performed according to institutional protocols. These included trimethrophim/sulfamethoxazole for Pneumocystis cariniiprophylaxis, acyclovir for herpes simplex virus prophylaxis, fluconazole for fungal prophylaxis, and penicillin and norfloxacillin or ciprofloxacillin for bacterial prophylaxis. CMV prophylaxis consisted of biweekly surveillance blood and urine cultures for CMV using shell-vial and rapid-antigen techniques. Recipients of unrelated or mismatched related donor transplants or patients on high-dose steroid therapy received prophylactic ganciclovir 5 mg/kg intravenously 5 times weekly until day 100. Patients who became antigenemic or had positive shell-vial cultures were treated with ganciclovir 5 mg/kg intravenously twice daily for 14 days then daily for 8 weeks.
All patients received filgrastim 5 μg/kg subcutaneously daily from day 7 until an absolute neutrophil count of more than 1.5 × 109/L was achieved for 3 days in a row according to our current institutional guidelines. Packed red blood cells were transfused to maintain hemoglobin levels of 8 gm/dL or more, and platelets were transfused to keep the platelet count at 20 × 109/L or greater. All blood products were filtered and irradiated to 1500 cGy.
Patients with neutropenic fever were treated with broad-spectrum antibiotics according to institutional protocols. Patients with grade 2 or greater acute GVHD received at least 0.5 mg/kg methylprednisolone every 6 hours and could be enrolled on any existing protocol for the treatment of GVHD. Bone marrow aspiration was performed routinely 3 to 4 weeks after infusion and then 3 and 12 months later; aspirates were sent for morphologic evaluation, fluorescence in situ hybridization, cytogenetics, and chimerism evaluation.
Clinical endpoints
Major endpoints included survival and disease-free survival as of June 1, 1999 using Kaplan-Meier estimates.13 Neutrophil recovery was defined as an absolute neutrophil count of more than 0.5 × 109/L for 3 consecutive days. Platelet recovery was defined as a platelet count of more than 20.0 × 109/L independent of platelet transfusions for at least 7 days. Complete remission was determined by standard disease-specific criteria as defined by the International Bone Marrow Transplant Registry (IBMTR). Acute and chronic GVHD were scored according to published guidelines.14,15 Chimerism was determined on bone marrow samples by cytogenetics or restriction fragment-length polymorphisms using established techniques.16,17 Toxicity was graded according to Bearman criteria.18 For chronic myelogenous leukemia (CML), acute lymphoblastic leukemia (ALL), myelodysplastic syndromes (MDS), and acute myelogenous leukemia (AML), relapse was defined by standard morphologic criteria, conventional cytogenetic analysis, or both. Cytogenetic relapse was documented by the presence and persistence in more than 2 consecutive tests of the cytogenetic abnormality that defined the disease (Philadelphia chromosome for CML and patient-specific cytogenetic abnormalities for AML, MDS, and ALL). Patients with cytogenetic relapse were scored as treatment failures for the purpose of disease-free survival (DFS) analysis, regardless of their subsequent response to salvage therapy. The presence of minimal residual disease using polymerase chain reaction–based technology was not used to guide therapy or to define relapse after transplantation.
Statistical methods
Associations between pairs of covariates were assessed graphically for pairs of numerical variables by examining scatterplots, by Wilcoxon-Mann-Whitney and Kruskal-Wallis19 tests for categorical and continuous variables, and by the Fisher exact test20 and its generalizations21 for pairs of categorical variables. Unadjusted probabilities of survival, DFS, regimen-related death (nonrelapse mortality), and times to grade 2 or greater or grade 4 or greater GVHD were estimated using the Kaplan-Meier method.13 Unadjusted between-group comparisons of survival and DFS were made using the log rank test.22 The Cox proportional hazards model23and its generalizations24 were used to assess the ability of treatments and patient characteristics to predict survival and DFS, with goodness-of-fit assessed by the Grambsch-Therneau test,25 Schoenfeld residual plots, martingale residual plots, and likelihood ratio statistics. All scatterplots were smoothed using the lowess method of Cleveland,26with variables transformed as appropriate based on these plots. Confidence intervals for probabilities were computed using the hrinkage method of Ghosh.27 Each multivariate Cox model was obtained by first performing a backward elimination withP cutoff of .05, then allowing either treatment-covariate interactions or any variable previously deleted to enter the final model if P < .05. For these model fits, covariates exhibiting high collinearity were segregated so that no 2 collinear variables were allowed in the same model. All computations were carried out on a DEC Alpha 2100 5/250 system computer (Digital Electronics Corporation, Nashua, NH) in Splus28 and StatXact (Cytel Software, Cambridge, MA) using both the standard Splus functions and the Splus survival analysis package of Therneau.28-30
Results
Patient and disease characteristics
From February 1996 to December 1998, 86 patients received preparative regimens with melphalan and purine analogs. Patient and disease characteristics are summarized in Table1. In brief, the median age was 52 years (range, 22-70 years); 52 patients were men and 34 were women. Thirty-four patients had AML, 9 had MDS, and 27 had CML, whereas 13 patients had lymphoma, and 3 had ALL. Median time to transplantation was 547 days (range, 27-6626 days). The median number of prior therapies was 3 (range, 0-8), with 24 patients having undergone earlier transplantations with a different source of stem cells (except one patient). Seven patients were treated in either first remission or first chronic phase (good-risk category), and 16 patients were in an untreated first relapse or a subsequent remission (intermediate risk). Forty-two patients had either refractory disease or were beyond first salvage, and 21 patients had transformed CML according to the criteria used by the IBMTR (bad risk).
Patient and disease characteristics
| N | 86 |
| Median age, y (range) | 52 (22-70) |
| Sex | 52 M, 34 F |
| Diagnosis and status at transplantation | |
| AML/MDS | 43 |
| CR1 | 1 |
| Untreated 1st relapse | 10 |
| Remission ≥ CR2 | 5 |
| Refractory | 27 |
| CML | 27 |
| CR1 | 6 |
| Transformed | 21 |
| ALL/lymphoma | 16 |
| Remission ≥ CR2 | 1 |
| Refractory | 15 |
| Median no. prior therapies (range) | 3 (0-8) |
| Median time to transplantation, d (range) | 553 (27-6627) |
| Prior transplantation | 24 |
| Autologous | 16 |
| Allogeneic different donor | 5 |
| Allogeneic same donor | 3 |
| Donor type | |
| 6 of 6 Matched related | 39 |
| 5 of 6 Mismatched related | 7 |
| 6 of 6 Matched unrelated | 40 |
| Stem cell source | |
| Bone marrow | 52 |
| Peripheral blood | 34 |
| CMV Status | |
| Neg/neg | 6 |
| Donor/recipient sex | |
| Donor F/recipient M | 23 |
| Donor F/recipient F | 19 |
| Other | 44 |
| Preparative regimen | |
| Fludarabine/melphalan 180 | 66 |
| Fludarabine/melphalan 140 | 12 |
| Cladribine/melphalan 180 | 8 |
| GVHD prophylaxis | |
| Tacrolimus/methotrexate | 81 |
| Other | 5 |
| N | 86 |
| Median age, y (range) | 52 (22-70) |
| Sex | 52 M, 34 F |
| Diagnosis and status at transplantation | |
| AML/MDS | 43 |
| CR1 | 1 |
| Untreated 1st relapse | 10 |
| Remission ≥ CR2 | 5 |
| Refractory | 27 |
| CML | 27 |
| CR1 | 6 |
| Transformed | 21 |
| ALL/lymphoma | 16 |
| Remission ≥ CR2 | 1 |
| Refractory | 15 |
| Median no. prior therapies (range) | 3 (0-8) |
| Median time to transplantation, d (range) | 553 (27-6627) |
| Prior transplantation | 24 |
| Autologous | 16 |
| Allogeneic different donor | 5 |
| Allogeneic same donor | 3 |
| Donor type | |
| 6 of 6 Matched related | 39 |
| 5 of 6 Mismatched related | 7 |
| 6 of 6 Matched unrelated | 40 |
| Stem cell source | |
| Bone marrow | 52 |
| Peripheral blood | 34 |
| CMV Status | |
| Neg/neg | 6 |
| Donor/recipient sex | |
| Donor F/recipient M | 23 |
| Donor F/recipient F | 19 |
| Other | 44 |
| Preparative regimen | |
| Fludarabine/melphalan 180 | 66 |
| Fludarabine/melphalan 140 | 12 |
| Cladribine/melphalan 180 | 8 |
| GVHD prophylaxis | |
| Tacrolimus/methotrexate | 81 |
| Other | 5 |
y indicates years; M, male; F, female; AML, acute myelogenous leukemia; MDS, myelodysplastic syndromes; CR, chronic phase; CML, chronic myelogenous leukemia; ALL, acute lymphoblastic leukemia; d, days; CMV, cytomegalovirus; Neg, negative; GVHD, graft-versus-host disease.
Patients were considered poor candidates for conventional myeloablative therapy either because of age (56% of the patients were older than 50) or concurrent medical condition, as shown in Table2. Nine patients had no obvious comorbid condition but were considered poor candidates for conventional myeloablative therapy because of previous medical complications such as congestive heart failure, fungal infection, pericarditis with chronic steroid therapy, or history of serious organ dysfunction and chemotherapy.
Comorbid and concurrent medical conditions
| Comorbid conditions . | N . |
|---|---|
| Age > 50 | 48 |
| Ejection fraction < 50% | 5 |
| Pulmonary function < 50% | 4 |
| GPT > 120 | 11 |
| ≥ 3 Prior therapies | 28 |
| Prior BMT | 24 |
| PS = 2 | 14 |
| Infection | 10 |
| ≥ 2 Comorbid conditions | 46 |
| Comorbid conditions . | N . |
|---|---|
| Age > 50 | 48 |
| Ejection fraction < 50% | 5 |
| Pulmonary function < 50% | 4 |
| GPT > 120 | 11 |
| ≥ 3 Prior therapies | 28 |
| Prior BMT | 24 |
| PS = 2 | 14 |
| Infection | 10 |
| ≥ 2 Comorbid conditions | 46 |
GPT indicates guanine phosphoribosyltransferase; BMT, bone marrow transplantation; PS, performance status.
Toxicities
Regimen-related toxicities are summarized in Tables3 and 4. Fludarabine/melphalan combinations were associated with 10 instances of grade 3-4 Bearman toxicities and 3 toxic deaths among the 29 nonrelapse deaths occurring before day 100. The main factors contributing to these deaths were bad risk category, active infection, and extensive prior therapy. The combination of cladribine/melphalan at the dose and schedule used was associated with 5 cases of grade 3-4 renal toxicity and 7 deaths before day 100. Three of these patients had undergone more than 2 prior regimens and had refractory disease. However, the occurrence of severe toxicities in 3 of 4 patients with untreated first relapse receiving this therapy led us to close this treatment arm to further patient accrual. The nonrelapse mortality rate at 100 days was 37.4% for patients receiving fludarabine/melphalan combinations and 87.5% for patients receiving cladribine/melphalan. For all patients, the nonrelapse mortality rate at 2 years was 44.7%. Patients in a bad risk category, recipients of unrelated donor cells, recipients of cladribine/melphalan, or patients with 3 or more prior therapies were at higher risk for nonrelapse death.
Bearman grade 3 or 4 toxicity
| . | N . | Mucosa . | Liver . | Lung . | Kidney . | Heart . |
|---|---|---|---|---|---|---|
| FM 140/180 | 78 | 0/0 | 3/2 | 3/2 | 6/1 | 1/1 |
| 2CDA/M | 8 | 0/0 | 2/1 | 0/2 | 2/3 | 1/1 |
| . | N . | Mucosa . | Liver . | Lung . | Kidney . | Heart . |
|---|---|---|---|---|---|---|
| FM 140/180 | 78 | 0/0 | 3/2 | 3/2 | 6/1 | 1/1 |
| 2CDA/M | 8 | 0/0 | 2/1 | 0/2 | 2/3 | 1/1 |
FM indicates fludarabine/melphalan; 2CDA/M, cladribine/melphalan.
Causes of death before day 100 after transplantation
| Toxicity . | GVHD . | Infection . | Relapse . | Other . | NRM d 100 . |
|---|---|---|---|---|---|
| 3 | 16 | 9 | 5 | 1 | 29 |
| 4 | 0 | 3 | 0 | 0 | 7 |
| Toxicity . | GVHD . | Infection . | Relapse . | Other . | NRM d 100 . |
|---|---|---|---|---|---|
| 3 | 16 | 9 | 5 | 1 | 29 |
| 4 | 0 | 3 | 0 | 0 | 7 |
GVHD indicates graft-versus-host disease; NRM, nonrelapse mortality.
Engraftment and chimerism
All 76 patients surviving more than 21 days achieved a neutrophil recovery of more than 0.5 × 109/L in a median of 14 days (range, 9-35 days). Fifty-five patients achieved platelet transfusion independence at a median of 21 days (range, 9-118 days), and the other 21 patients had relapses or died before achieving this endpoint. Seventy-five patients had chimerism studies using cytogenetics or molecular techniques, and the median percentage of donor cells at the time of first post-transplant bone marrow examination (approximately 30 days after stem cell transplantation) was 100% (range, 0%-100%). Five patients had less than 50% donor cells. Of these 5 patients, 2 failed to clear bone marrow blasts, one had full autologous reconstitution, another became fully chimeric after immunosuppression withdrawal, and one had secondary graft failure and died of infectious complications. At 3 months, 40 of 42 patients in remission had more than 90% donor cells using the same techniques. At 1 year all 17 patients in remission had 100% donor cell engraftment by the same techniques.
Acute and chronic GVHD
Thirty-four patients had grade 2-4 acute GVHD for a probability of 0.49 (95% CI, 0.38-0.60); of these, 19 patients had grade 3-4 acute GVHD for a probability of 0.29 (95% CI, 0.18-0.41). Sixteen of the 41 deaths before day 100 were related to acute GVHD. Patients receiving unrelated donor transplants had a trend toward a higher risk for acute GVHD (grade 2-4, 62% vs 41% [P = .05]; grade 3-4, 39% vs 19% [P = .10]). Deaths from acute GVHD were more common in recipients of unrelated donor transplants than in other donor types (11 of 40 vs 4 of 46; P = .026). Chronic GVHD developed in 21 of 46 evaluable patients, for an actuarial risk of 68% ± 9%.
Responses
Twenty-six of 43 patients with AML/MDS achieved complete remission. Of the 17 patients who did not, one did not clear bone marrow blasts, 10 died before day 30, 5 died before platelet transfusion independence from transplantation complications, and one died of progressive disease. Nineteen of the 27 patients with CML achieved complete remission. Of the 8 patients who did not, one patient had autologous reconstitution and 7 died of transplantation complications before platelet transfusion independence.
Among the 16 remaining patients, 1 of 3 patients with ALL, 1 of 4 patients with Hodgkin disease, and 2 of 9 patients with non-Hodgkin lymphoma achieved complete remission. All other patients died of transplantation-related complications.
Overall survival and disease-free survival
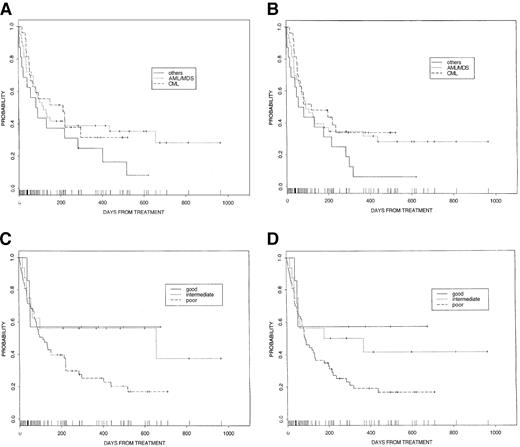
The overall 2-year survival probability was 0.28 (95% CI, 0.20-0.39) for all patients. On univariate analysis significant prognostic factors for survival included risk category, number of prior therapies, donor sex, and preparative regimen (fludarabine vs cladribine/melphalan). The probability of disease-free survival at 2 years was 0.23 (95% CI, 0.15-0.34) for all patients. Significant factors associated with improved disease-free survival on univariate analysis included better risk category, fewer prior therapies, better performance status, and male sex of donor (Table 5). Survival and disease-free survival according to diagnosis and risk group are demonstrated in Figure 1.
Univariate analysis for overall survival, disease-free survival, and transplantation-related mortality
| Variable . | OS at 1 y . | P . | DFS at 1 y . | P . | NRM at d 100 . | P . |
|---|---|---|---|---|---|---|
| Age < 52 (43) | 35.8 | 31.7 | 44.3 | |||
| Age > 52 (43) | 33.5 | NS | 25.4 | NS | 39.8 | NS |
| Male (52) | 33.3 | 29.6 | 39.0 | |||
| Female (34) | 34.8 | NS | 25.9 | NS | 47.1 | NS |
| AML (43) | 39.1 | 34.7 | 39.8 | |||
| CML (27) | 31.7 | 34.0 | 40.7 | |||
| Other (16) | 25.0 | NS | 6.25 | NS | 50.0 | NS |
| < 18 m to SCT (42) | 39.2 | 28.3 | 33.3 | |||
| > 18 m to SCT (44) | 29.9 | NS | 28.0 | NS | 50.1 | NS |
| Good risk (7) | 57.1 | 57.1 | 42.9 | |||
| Intermediate (16) | 56.2 | 50.0 | 37.5 | |||
| Bad risk (63) | 25.4 | NS | 19.0 | .04 | 32.2 | NS |
| Prior SCT | ||||||
| No (62) | 38.7 | 30.2 | 38.8 | |||
| Yes (24) | 23.4 | NS | 23.8 | NS | 40.6 | NS |
| Prior Rx | ||||||
| < 3 (43) | 53.0 | 45.2 | 32.6 | |||
| ≥ 3 (43) | 16.5 | .002 | 11.9 | .001 | 51.8 | .03 |
| PS < 2 (72) | 40.9 | 33.8 | 33.9 | |||
| PS ≥ 2 (14) | 0.0 | < .0001 | 0.0 | < .0001 | 81.0 | < .0001 |
| FM180/140 (78) | 36.4 | 29.8 | 37.4 | |||
| 2CDA/M (8) | 12.5 | .005 | 12.5 | .006 | 87.5 | .0001 |
| BM (52) | 26.0 | 22.3 | 46.4 | |||
| PB (34) | 50.0 | NS | 38.6 | NS | 35.3 | NS |
| Related (46) | 36.7 | 27.6 | 32.6 | |||
| Unrelated (40) | 31.8 | NS | 29.1 | NS | 53.0 | .05 |
| Male donor (44) | 49.5 | 42.0 | 36.4 | |||
| Female donor (42) | 14.4 | .006 | 11.4 | .003 | 48.1 | .06 |
| Variable . | OS at 1 y . | P . | DFS at 1 y . | P . | NRM at d 100 . | P . |
|---|---|---|---|---|---|---|
| Age < 52 (43) | 35.8 | 31.7 | 44.3 | |||
| Age > 52 (43) | 33.5 | NS | 25.4 | NS | 39.8 | NS |
| Male (52) | 33.3 | 29.6 | 39.0 | |||
| Female (34) | 34.8 | NS | 25.9 | NS | 47.1 | NS |
| AML (43) | 39.1 | 34.7 | 39.8 | |||
| CML (27) | 31.7 | 34.0 | 40.7 | |||
| Other (16) | 25.0 | NS | 6.25 | NS | 50.0 | NS |
| < 18 m to SCT (42) | 39.2 | 28.3 | 33.3 | |||
| > 18 m to SCT (44) | 29.9 | NS | 28.0 | NS | 50.1 | NS |
| Good risk (7) | 57.1 | 57.1 | 42.9 | |||
| Intermediate (16) | 56.2 | 50.0 | 37.5 | |||
| Bad risk (63) | 25.4 | NS | 19.0 | .04 | 32.2 | NS |
| Prior SCT | ||||||
| No (62) | 38.7 | 30.2 | 38.8 | |||
| Yes (24) | 23.4 | NS | 23.8 | NS | 40.6 | NS |
| Prior Rx | ||||||
| < 3 (43) | 53.0 | 45.2 | 32.6 | |||
| ≥ 3 (43) | 16.5 | .002 | 11.9 | .001 | 51.8 | .03 |
| PS < 2 (72) | 40.9 | 33.8 | 33.9 | |||
| PS ≥ 2 (14) | 0.0 | < .0001 | 0.0 | < .0001 | 81.0 | < .0001 |
| FM180/140 (78) | 36.4 | 29.8 | 37.4 | |||
| 2CDA/M (8) | 12.5 | .005 | 12.5 | .006 | 87.5 | .0001 |
| BM (52) | 26.0 | 22.3 | 46.4 | |||
| PB (34) | 50.0 | NS | 38.6 | NS | 35.3 | NS |
| Related (46) | 36.7 | 27.6 | 32.6 | |||
| Unrelated (40) | 31.8 | NS | 29.1 | NS | 53.0 | .05 |
| Male donor (44) | 49.5 | 42.0 | 36.4 | |||
| Female donor (42) | 14.4 | .006 | 11.4 | .003 | 48.1 | .06 |
OS indicates overall survival; DFS, disease-free survival; NS, not significant; SCT, stem cell transplantation; Rx, therapy; BM, bone marrow; PB, peripheral blood; for other abbreviations see Tables 1-4.
Survival rates.
(A) Overall survival for patients according to diagnosis. (B) Disease-free survival for patients according to diagnosis. (C) Overall survival for patients according to risk group (good, CR1 or chronic phase; intermediate, any remission greater than 1 or accelerated-phase CML; poor, all others). (D) Disease-free survival for patients according to risk group (good, CR1 or chronic phase; intermediate, any remission greater than 1 or accelerated-phase CML; poor, all others).
Survival rates.
(A) Overall survival for patients according to diagnosis. (B) Disease-free survival for patients according to diagnosis. (C) Overall survival for patients according to risk group (good, CR1 or chronic phase; intermediate, any remission greater than 1 or accelerated-phase CML; poor, all others). (D) Disease-free survival for patients according to risk group (good, CR1 or chronic phase; intermediate, any remission greater than 1 or accelerated-phase CML; poor, all others).
Twenty-three patients had relapses at a median of 120 days (range, 0-369 days). Nineteen were on immunosuppressive therapy at the time of relapse, and this therapy was withdrawn. Only one patient with CML responded to immunosuppression withdrawal and achieved complete remission, but this patient subsequently died of complications of chronic GVHD. Five patients received chemotherapy followed by donor lymphocyte infusion; 3 achieved complete remission. One has remained in complete remission for more than 1 year, and 2 had relapses within 3 months. Two patients received donor lymphocyte infusions in conjunction with immunosuppression withdrawal and failed to respond.
Cox regression model analyses
Multivariate Cox regression models for survival and nonrelapse mortality were obtained using the variable selection algorithm described in the “Statistical methods” section. Each of the following was associated with decreased relative risk for death: good or intermediate prognosis risk group, therapy with fludarabine/melphalan 180, and shorter time to transplantation (up to 4 years) (Table6). Although receiving a transplant from a matched, unrelated donor was moderately associated with increased mortality (RR [relative risk] = 1.85; P = .054) compared to receiving a transplant from a sibling donor (6 of 6 or 5 of 6) based on a univariate Cox model analysis, this association was insignificant after accounting for the covariates in Table 6. Patients whose donors were female had more prior therapies (62% with 3 or more prior treatments vs 39% for those with male donors; P = .0516), waited longer for transplantation (median, 21.5 months vs 15.5 months; P = .38), and had a higher probability of a bad risk score (83% vs 64%;P = .0052). Because the 3 variables—donor sex, 3 or more prior therapies, and risk category—were highly associated, at most one of these variables was allowed in each fitted multivariate Cox model. Starting with the model summarized in Table 6, if poor risk group is replaced by female donor, then RR = 1.72 for this new variable (P = .056), with the effects of treatment and time to transplantation essentially unchanged. Similarly, if poor risk group is replaced by 3 or more prior therapies, RR = 2.13 for the new variable (P = .007).
Multivariate analysis of survival
| Variable . | Relative risk . | P . |
|---|---|---|
| Poor risk group | 1.99 | .046 |
| Fludarabine/melphalan | .51 | .021 |
| Time to transplantation6-150 | 1.04/mo | .003 |
| Variable . | Relative risk . | P . |
|---|---|---|
| Poor risk group | 1.99 | .046 |
| Fludarabine/melphalan | .51 | .021 |
| Time to transplantation6-150 | 1.04/mo | .003 |
mo indicates month.
For patients whose time to transplantation was less than 4 years.
For nonrelapse mortality, the multivariate Cox model covariates were use of cladribine/melphalan (RR = 8.06, P < .001), 3 or more prior therapies (RR = 2.46, P = .011), and time to transplantation up to 4 years (RR = 1.04/mo, P = .004). Because only 8 of the 86 patients received cladribine/melphalan, separate Cox model fits were obtained for the subset of 78 patients who were treated with fludarabine/melphalan. In this subgroup, the multivariate Cox model included the 2 covariates time to transplantation up to 4 years (RR = 1.03/mo, P = .009) and 3 or more prior therapies (RR = 2.58, P = .002). In this model, if 3 or more prior treatments is replaced bypoor risk group, RR = 2.29 is the new variable (P = .026). A similar analysis of the 78 fludarabine/melphalan patients for nonrelapse mortality yielded a Cox model having the same covariates as for overall survival, with RR = 1.04/mo (P = .007) for time to transplantation and RR = 2.19 for 3 or more prior therapies (P = .032).
Discussion
Based on our initial experience with fludarabine-based nonmyeloablative conditioning regimens, we hypothesized that a reduced-intensity conditioning with melphalan/purine analog combinations could be well tolerated in patients considered poor candidates for conventional myeloablative therapies. This combination should also be adequately immunosuppressive to allow engraftment of allogeneic progenitor cells, including those procured from unrelated or mismatched donors. The rationale for this hypothesis was based on the broad-spectrum activity of melphalan in a variety of hematologic malignancies, the tolerability of high-dose melphalan in debilitated patients with myeloma, and the possibility that purine analogs could inhibit the mechanisms of DNA repair from alkylator-induced DNA damage, thus providing for a potential synergistic effect of purine analogs and melphalan.10-12 31
Melphalan is a bifunctional alkylating agent with broad-spectrum activity in a variety of hematologic malignancies.10 It has been used as a single agent for conditioning in allogeneic bone marrow transplantation. Singhal et al11 reported on 28 patients who underwent allogeneic BMT for acute leukemia or lymphoblastic lymphoma conditioned with melphalan 240 mg/m2followed by bone marrow from an HLA-identical sibling. Twenty-two patients had sustained engraftment. The 3-year probabilities of transplantation-related mortality and relapse rates were 35% and 62% with a 10-year disease-free survival rate of 25%.11 The myeloablative dose of melphalan has not been determined. Moreau et al31 treated 102 patients with 140 mg/m2melphalan. The duration of neutropenia was 23.5 days for patients receiving granulocyte-macrophage colony-stimulating factor and 29 days for patients not receiving growth factors. However, 10 of 102 patients experienced fatal complications with this therapy, suggesting that doses above 140 mg/m2 should not be explored without stem cell support.
The 100-day nonrelapse mortality rate for the fludarabine/melphalan combination was 37%. Fatal toxicities directly related to the preparative regimens occurred in 3 of 29 patients who died of nonrelapse causes before day 100. In contrast, the combination of cladribine and melphalan was significantly more toxic. Fatal regimen-related toxicities developed in 4 of 8 patients, leading to the early closure of this treatment arm. The toxicity of cladribine/melphalan was unexpected because, as a single agent, cladribine has been administered in doses up to 21 mg/m2with neurotoxicity in 4 of 8 patients being the dose-limiting toxicity.32,33 The combination of cladribine and cytarabine has been explored with and without stem cell support. Kornblau et al34 reported their results with cladribine and cytarabine combinations during a phase I/II trial. In the phase I portion of the trial, 6 of 25 patients had serum creatinine levels higher than 2 mg/dL, and 2 patients required dialysis at dose levels of 9 and 13 mg/m2 daily for 7 days. In phase II of their study, the same dose of cladribine was used as in this report (12 mg/m2 daily for 5 days). At this dose level, 6 of 17 patients had serum creatinine increases greater than 1.5 mg/dL, but none required dialysis.34 Similarly, no patients with renal failure requiring dialysis were observed when cladribine/cytarabine combinations were used with stem cell support.8 The reason for this increased renal toxicity is unknown, but it could have been caused by synergistic toxic effects on the kidneys by melphalan and cladribine.
The main causes of death before day 100 in patients receiving the fludarabine/melphalan combinations were infection and GVHD. The risk for grade III-IV GVHD in this patient population, whose median age was greater than 50, was 19% for transplants from related donors and 39% for transplants from unrelated donors (which were serologically matched for class I antigens and molecular matches for DRB1). Although this incidence is similar to what Petersdorf et al35 reported for younger patients receiving myeloablative therapies, it is still high, and efforts to reduce the risk for GVHD are being explored (ie, pretransplantation ATG and molecular matching for DRB2 and DQ, if possible). Kottaridis et al36 have reported on the preliminary results with CAMPATH-1H used before reduced conditioning with 140 mg/m2 fludarabine/melphalan and have shown that this agent can significantly reduce the risk for GVHD after related and unrelated marrow transplantation and possibly enhance engraftment. Further exploration of this and other approaches to reduce GVHD after nonablative stem cell transplantation are warranted because the risk for GVHD will remain substantial in these patients, and their tolerance to treatment with high-dose steroids may be reduced.
Our initial experience with patients who have refractory AML and undergo nonmyeloablative stem cell transplantation with either fludarabine, idarubicin and cytarabine, or cladribine and cytarabine demonstrated that this strategy was feasible and resulted in the engraftment of donor cells. However, patients with refractory disease had rapid recurrence, and the combination did not seem to be immunosuppressive enough to allow for the engraftment of unrelated donor cells, at least in patients with CML.8,37 Other groups have explored different combinations of agents to develop reduced-intensity or nonmyeloablative regimens with the same goal of achieving engraftment with minimal toxicity. The Seattle group has described the use of low-dose (200 cGy), single-fraction total body irradiation as a preparative regimen for nonablative stem cell transplantation; the true nonablative characteristics of this regimen are confirmed by the low incidence of myelosuppression.38The regimen was immunosuppressive enough to allow engraftment in 24 of the first 29 patients treated, toxicity was minimal, and 7 patients attained or maintained complete remission at the time of the report.38,39 Slavin et al9 pioneered the use of fludarabine in combination with 8 mg/kg busulfan. The initial report of this combination consisted of 28 patients with a variety of malignant and nonmalignant disorders. The combination was well tolerated, and, as in our experience, GVHD was the most important cause of early death. The disease-free survival rate of 77% at 14 months is encouraging, but it cannot be directly compared to the current report because the median age in the Hadassah report was 36 years whereas in our report it was 52 years.9 Comparative studies between different reduced-intensity conditioning regimens are needed to determine whether any regimen is superior or better tolerated than another.
This report demonstrates that melphalan/purine analog combinations allow for almost universal engraftment of allogeneic progenitor cells, including those procured from unrelated or mismatched related donors. Ninety-three percent of the patients showed between 80% and 100% donor cell engraftment by day 30, and all patients in continued remission at 6 months and 1 year showed primarily donor-derived hematopoiesis. Remissions in these patients were durable, particularly in patients without refractory or bulky disease. The disease-free survival rates of 57% at 1 year for the 7 patients in first remission or chronic phase and 49% for patients with untreated first relapse or in remission at the time of transplantation are comparable to results obtained in younger patients who have undergone myeloablative therapy.
In summary, reduced-intensity conditioning with fludarabine/melphalan combinations is feasible in older patients with associated comorbidities; the treatment allows the engraftment of unrelated and mismatched progenitor cells with acceptable levels of toxicity. Disease recurrence and GVHD continue to be the most important causes of treatment failure. Our study, however, demonstrates that long-term disease control can be achieved by allogeneic transplantation in older patients who have hematologic malignancies and that further development of these preparative regimens, including the treatment of patients in chronic phase or first remission, is warranted. For patients with very poor performance status or refractory disease, alternative strategies must be explored.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Sergio Giralt, Department of Blood and Bone Marrow Transplantation, University of Texas MD Anderson Cancer Center, Box 65, 1515 Holcombe Blvd, Houston, TX 77030; e-mail:sgiralt@notes.mdacc.tmc.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal