Abstract
Stem cell proliferation induced by potent cytokines usually leads to a loss of primitive potential through differentiation. In this study, the ability of cytokines and murine MS5 stromal cells to independently regulate the proliferation and long-term culture-initiating cell (LTC-IC) activity of primitive CD34+CD38low/neg human bone marrow cells was evaluated. To compare populations with identical proliferation histories, cells were labeled with carboxy fluorescein diacetate succinimidyl ester, and LTC-IC activity was assessed 4 days later in cells that had accomplished the same number of divisions with or without MS5 cells. MS5 cells counteracted dramatically the loss of LTC-IC activity observed in the presence of cytokines alone. Thus, in the presence of MS5 cells, means of 1233 (n = 5) and 355 (n = 9) LTC-IC–derived colony-forming cells (CFCs) were generated by 1000 cells that performed 3 and 4 divisions respectively, whereas 311 (n = 5) and 64 (n = 5) CFCs were generated by 1000 cells cultured without MS5 cells. Interestingly, MS5 cells had no detectable effect on the LTC-IC activity of cells that divided only twice in 4 days—1606 CFCs (n = 6) and 1993 (n = 6) CFCs, respectively, without and with MS5 cells—and a 48 additional hours of coculture were necessary to unmask changes in the LTC-IC activity mediated by stromal cells. These results indicate that cytokines and stroma-derived signals can regulate independently the proliferation and differentiation of primitive cells and that these stroma-derived extracellular factors act directly on their target cells.
Introduction
The loss of stem cell activity that invariably occurs when primitive cells are induced to divide by cytokines compromises attempts to obtain a net increase in the number of stem cells for therapeutic purposes in transplantation or gene transfer with murine retroviral vectors. What can be demonstrated is an amplification of cells retaining similar functional properties, such as the ability to reconstitute long-term culture (LTC) in vitro1 or nonobese diabetic–severe combined immunodeficient (NOD-SCID) recipients in vivo.2,3 However, this is by no means proof of self-renewal because the molecular identities of daughter and parent cells cannot be demonstrated in the absence of known genetic mechanisms that control self-renewal versus differentiation. Thus, net increases in the number of long-term culture-initiating cell, (LTC-IC), human NOD-SCID–reconstituting cells (rc), and, more rarely, murine long-term repopulating cells4 have been described after a short-term exposure to cytokines, but usually the expansion does not exceed a factor of 10, and the low frequency of proliferating cells participating in the expansion of the stem cell compartment also complicates evaluation of quantitative analyses of these responses.
Although stromal cells have been assigned contradictory functions, they have often been viewed as important for the maintenance of primitive progenitor activity,5 particularly in the lymphoid lineages.6 Coculture with stromal cells is the basis of the LTC system. However, now that many purified cytokines are available, several groups have demonstrated an additional ability of stromal feeders to maintain primitive hematopoietic cell function in cultures used for expansion7-9 or gene transfer protocols.10 The contribution of cytokines, adhesion molecules,11 and other stroma-derived regulators, such as Notch ligands,12 in these effects has been reported, though it is unclear whether stromal cells act by affecting cell survival13 or by promoting or delaying cell-cycle entry.14 One helpful strategy to monitor variations in cell proliferation in these conditions has been to label the cells with fluorescent dyes such as PKH-2, PKH-26,15 or more recently carboxy fluorescein diacetate succinimidyl ester (CFSE).16-19 Because these dyes are diluted by cell division, they can be used to track the proliferative behavior of cells in vitro and are thus useful to correlate changes in cell function with successive cell divisions. This approach has been used recently to identify cells that remain quiescent for long periods in vitro, even when they are exposed to suboptimal concentrations of cytokines and stromal feeders.20,21 It has also allowed cell proliferation to be correlated with retention of primitive phenotypes and functions (cobblestone area-forming cells and NOD-SCID repopulation) by primitive progenitors exposed to cytokines for a short period.18 19 These data have confirmed that actively proliferating cells can retain LTC-IC or NOD-SCID-rc functions, but only transiently.
Others and we22-25 have reported that murine stromal cells can counteract the loss of stem cell activity of human bone marrow CD34+CD38low/neg samples exposed to high concentrations of cytokines. However, it remains unresolved whether this effect of stromal cells resulted from the rate of proliferation of an unidentified subpopulation of primitive cells. To address this question, we labeled adult bone marrow-derived CD34+CD38low/neg with CFSE to monitor simultaneously cell divisions and concomitant changes in the LTC-IC activity of cells exposed to high concentrations of cytokines in the presence of murine MS5 stromal cells. The presence of MS5 cells caused marked retention of a high LTC-IC potential by cells that completed 3 or 4 divisions in 4 days in comparison to cells cultured with cytokines alone, without feeders, that showed a rapid loss of LTC-IC function. These data demonstrate that the proliferation and differentiation of primitive human hematopoietic cells can be independently regulated by stroma-derived molecules.
Materials and methods
Cell preparation and labeling
Samples of bone marrow were obtained with informed consent from patients undergoing hip surgery and were subjected to a standard CD34 immunomagnetic bead separation (Miltenyi Biotec, Auburn, CA) exactly as previously described.25 For CFSE labeling, cells were resuspended at 1 × 106 cells/mL in phosphate-buffered saline (PBS), and CFSE (Molecular Probes, Eugene, OR) was added at 1 μmol/L. After 10 minutes at 37°C, further uptake of the dye was stopped by the addition of ice-cold PBS supplemented with 30% fetal calf serum (FCS; Stem Cell Technology, Vancouver, Canada). The cells were washed 3 times, the last wash in PBS without FCS, and then resuspended at a concentration of 5 to 10 × 105 cells/mL in αMEM supplemented with 10% FCS and 10 ng/mL PEG-recombinant human (rhu)-megakaryocyte growth and development factor (MGDF) (a kind gift from Amgen, Thousand Oaks, CA, and Kirin, Tokyo, Japan), 10 ng/mL rhu-Flt-3 ligand (kindly given by Immunex, Seattle, WA), and 20 ng/mL rhu-SCF (stem cell factor) (a gift from Amgen). Cells were cultured overnight at 37°C in 24-well plates to allow the efflux of all CFSE not stably bound to intracellular proteins.16 After they were washed in αMEM with 10% FCS, CFSE-labeled CD34+CD38low/neg (10% to 20% total CD34+ cells) or CD34+CD38neg (1% to 2% CD34+cells) fractions were sorted using a FACSVantage (Becton Dickinson, Le Pont de Claix, France) in a narrow gate (25 to 40 channels wide using a 1024-channel log amplifier). This narrow gate was required to increase the resolving power of subsequent analysis of CFSE-labeled cells. CFSE of itself did not compromise the expression of LTC-IC or the clonogenic potential of cultured CD34+ bone marrow-derived progenitor cells (data not shown).
Short-term cultures
In most experiments, 1 to 5 × 104CFSE+CD34+CD38low/neg cells were cultured during 4 days in 24-well plates precoated or not with murine MS5 stromal cells in 1 mL αMEM containing 10% FCS. In some experiments, serum-free Iscove minimum essential medium was used.26 The following cytokines were added: rhu-MGDF (10 ng/mL), rhu-IL-6 (100 IU/mL), rhu-granulocyte macrophage colony-stimulating factor (10 ng/mL), rhu-Flt-3 ligand (10 ng/mL), rhu-SCF (20 ng/mL), and rhu-IL-3 (2 ng/mL; a kind gift from Novartis, Basel, Switzerland). An aliquot of cells was cultured during 48 hours without MS5 cells in the presence of 0.1 μg/mL Colcemid (Sigma, St Quentin Fallavier, France) as a control for undivided cells. On day 4, cells corresponding to the second, third, and fourth generations were resorted (see “Results”). The LTC-IC activity of cells from each generation was assessed in standard assays. The 5-week LTC-IC assay using MS5 cells as feeders was performed exactly as previously described in detail25 27 in bulk conditions or in limiting dilution (10 to 200 cells/well).
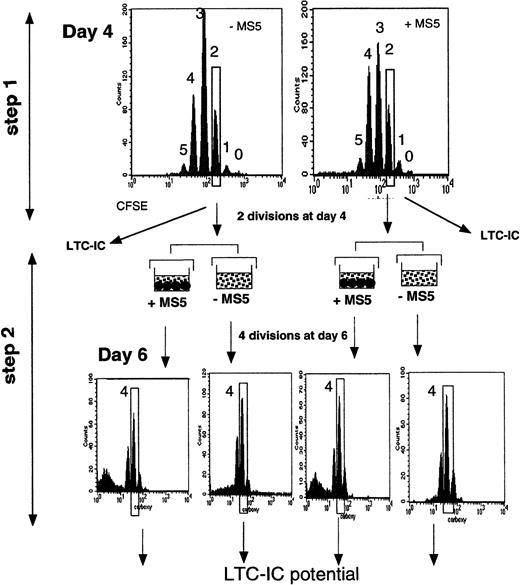
In 2 experiments, to increase the homogeneity of the input cells, we performed double sorting, as illustrated in Figure1. On day 4, we first selected cells that had divided twice (slow-dividing cells), an aliquot of which was kept to assay LTC-IC activity, with the remaining cells cultured 2 days later with or without MS5 cells. At the end of the second culture period, cells that had performed 2 more divisions (a total of 4 from day 0) were again sorted, and their LTC-IC potentials were analyzed.
Schematic representation of the experimental strategy.
CD34+CD38low cells labeled with CFSE were first incubated for 4 days in conditions with or without stromal cells (step 1). At this time, cells were analyzed for the expression of fluorescence. Each peak corresponds to a cell generation and was numbered by reference to the 0-division point defined by analyzing an aliquot of the cells cultured under the same conditions but in the presence of Colcemid to block mitosis. The additional CFSE-negative population, seen in the upper right graph, corresponds to the MS5 cells. Cells that had divided fewer than 2 times and 2, 3, and 4 times were sorted, and their LTC-IC activities were assessed. In some experiments, a 2-step procedure was followed. Two-division cells were sorted on day 4 and incubated for 2 more days with cytokines, with or without MS5 cells (step 2). After this period (6 days from day 0), cells that had achieved 2 more divisions (4 since day 0) were sorted, and their LTC-IC activity was assessed.
Schematic representation of the experimental strategy.
CD34+CD38low cells labeled with CFSE were first incubated for 4 days in conditions with or without stromal cells (step 1). At this time, cells were analyzed for the expression of fluorescence. Each peak corresponds to a cell generation and was numbered by reference to the 0-division point defined by analyzing an aliquot of the cells cultured under the same conditions but in the presence of Colcemid to block mitosis. The additional CFSE-negative population, seen in the upper right graph, corresponds to the MS5 cells. Cells that had divided fewer than 2 times and 2, 3, and 4 times were sorted, and their LTC-IC activities were assessed. In some experiments, a 2-step procedure was followed. Two-division cells were sorted on day 4 and incubated for 2 more days with cytokines, with or without MS5 cells (step 2). After this period (6 days from day 0), cells that had achieved 2 more divisions (4 since day 0) were sorted, and their LTC-IC activity was assessed.
In one experiment, we sorted cells that had completed 4 divisions (4-division cells) and those that had completed fewer than 4 divisions (0- to 3-division cells) after 4 days in culture. Four-division cells were assayed in LTC, whereas 0- to 3-division cells were cultured for another 24 hours with and without MS5 cells. The next day (day 5), we again sorted separately 4-division cells and 0- to 3-division cells, and both fractions were handled as described previously. This step was repeated again after 6 and 7 days in culture.
Statistical analysis
Statistical significance was determined by Studentt-test (paired data analysis).
Results
Murine MS5 stromal cells do not alter the initial rate of division of CD34+CD38low/neg cells in short-term culture
To investigate whether MS5 cells altered the function of CD34+CD38low/neg cells exposed to cytokines through a change in their proliferation rate, we labeled cells with CFSE16 and measured the LTC-IC activity of cells that underwent the same number of divisions in 4-day cytokine-supplemented cultures with or without MS5 cells. At the end of the 4 days, the nucleated cells were distributed in different peaks of fluorescence, each corresponding to one cell generation, as shown by FACS analysis (Figure 1, step 1). As would be expected from an equal partitioning of the dye between daughter cells at each division,28 there was a linear decrease in fluorescence from one peak to the next (not shown). The 0-division point was defined from the amount of fluorescence measured in input cells cultured with Colcemid.
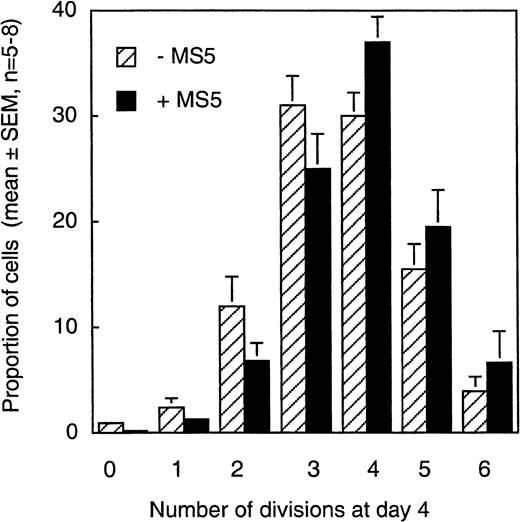
Our first observation was the heterogeneous rate of proliferation of individual CD34+CD38low/neg cells; 6 or 7 generations of daughter cells could be identified independent of the presence of MS5 cells (Figure 2). During the first 4 days, more than 98% of CD34+CD38low/neg cells exposed to the 6 cytokines had divided at least once and 60% at least 3 times, which fits with the 5- to 10-fold overall increase observed in the total number of nucleated cells. As shown in Figure 2, in the absence of MS5 cells, 81% ± 2% of the cells underwent 3 divisions or more, and 88% ± 3% underwent them in the presence of MS5 (mean ± SEM of 5 experiments). There was also no significant difference in the proportions of cells in the different CFSE peaks in MS5 cell-containing or MS5-free cultures, which further shows that the MS5 feeders had no effect on cell proliferation rate. A small population remained quiescent or divided only once, and, interestingly, this fraction was reproducibly lower in the presence of MS5 cells (0.5%) than in their absence (3.3%). Consequently, the proportion of cells that had accomplished more than 3 divisions appeared slightly higher on stromal cells (63% versus 50% without MS5 cells), though these latter differences did not reach significance.
Distribution of cells produced by CD34+CD38low cells in 4 days according to their proliferation rate.
CD34+CD38low cells labeled with CFSE and cultured for 4 days in the presence (black bars) or in the absence (stippled bars) of MS5 cells (as described in the Figure 1 legend and “Materials and methods”) were analyzed by FACS. Each histogram represents the mean (± SEM, n = 5-8) proportion of day 4 cells that fell in the peak corresponding to 0, 1, 2, 3, 4, 5, or 6 divisions (Figure 1). There were no significant differences in the proportions of cells that accomplished a given number of cell divisions with or without MS5 cells (P > .5).
Distribution of cells produced by CD34+CD38low cells in 4 days according to their proliferation rate.
CD34+CD38low cells labeled with CFSE and cultured for 4 days in the presence (black bars) or in the absence (stippled bars) of MS5 cells (as described in the Figure 1 legend and “Materials and methods”) were analyzed by FACS. Each histogram represents the mean (± SEM, n = 5-8) proportion of day 4 cells that fell in the peak corresponding to 0, 1, 2, 3, 4, 5, or 6 divisions (Figure 1). There were no significant differences in the proportions of cells that accomplished a given number of cell divisions with or without MS5 cells (P > .5).
Thus, the induction of cell division in CD34+CD38low/neg cells was primarily controlled by cytokines. This agrees with our previous results showing that few CD34+CD38low/neg cells with LTC-IC function, cultured as described above, survived a short exposure to 5-fluorouracil or a 16-hour exposure to high-specific activity H3-TdR (data not shown), an observation also reported by others.29
Thus, these results confirmed previous observations from this (data not shown) and other laboratories29 showing that most CD34+CD38low/neg cells proliferate rapidly, but asynchronously, in response to a combination of cytokines.1 The presence of MS5 cells did not change this pattern, though it might have accelerated entry into the cycles of a few cells.
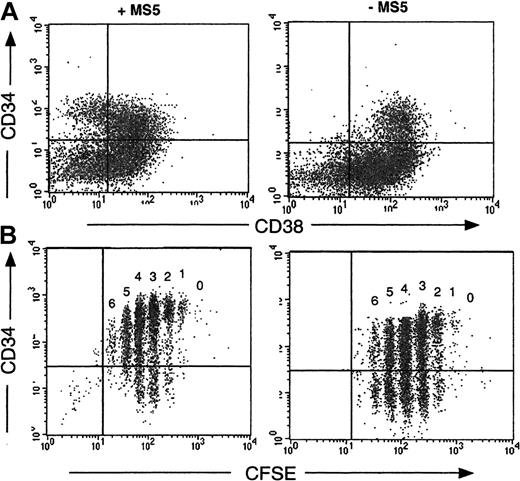
Coculture with MS5 cells influences the phenotype of cells produced from CD34+CD38low/neg cells that have undergone the same number of divisions
A recurrent finding in these experiments was the retention by cells cultured in the presence of MS5 feeders of high levels of expression of the CD34 antigen: thus, on day 7, the proportion of CD34+ cells was 72% ± 16%, and on day 10 it was 54% ± 4% (n = 16), whereas without MS5 cells these proportions fell to 48% ± 17% on day 7 and 20% ± 2% on day 10 (P < .01). Moreover, 20% ± 2% (n = 15) of the CD34+ cells derived from cocultures on MS5 cells were still CD38low/neg (Figure3A), whereas none of those without MS5 cells had a CD34+CD38low/neg phenotype (Figure3B). Analysis of cultured CFSE-labeled cells on day 4 confirmed that persistence of the CD34 antigen was independent of cell division. In the absence of MS5 cells more than 50% of cells in each generation peak were CD34−, but in the presence of MS5 cells more than 70% of cells were CD34+ (Figure 3B).
Retention of the CD34 antigen by cells grown on MS5 cells.
CD34+CD38low cells were cultured 4 days in the presence (left panels) or in the absence (right panels) of MS5 cells. The dot plot analysis of cells labeled concomitantly with monoclonal antibodies against CD34 and CD38 is presented in the upper panel (A). The quadrant limits have been set according to labeling with control antibodies. The lower panel (B) visualizes the proportion of CD34+ cells within each cell generation as defined by CFSE distribution. In the absence of MS5 cells (B, right) at least 50% of cells were CD34−, whereas more than 75% cells dividing on MS5 cells retained the CD34 antigen (B, left).
Retention of the CD34 antigen by cells grown on MS5 cells.
CD34+CD38low cells were cultured 4 days in the presence (left panels) or in the absence (right panels) of MS5 cells. The dot plot analysis of cells labeled concomitantly with monoclonal antibodies against CD34 and CD38 is presented in the upper panel (A). The quadrant limits have been set according to labeling with control antibodies. The lower panel (B) visualizes the proportion of CD34+ cells within each cell generation as defined by CFSE distribution. In the absence of MS5 cells (B, right) at least 50% of cells were CD34−, whereas more than 75% cells dividing on MS5 cells retained the CD34 antigen (B, left).
Influence of MS5 cells on the LTC-IC activity of CD34+CD38low/neg cells that have undergone the same number of divisions
In the next series of experiments, we analyzed whether cells cultured for 4 days with MS5 cells and cytokines would have a different LTC-IC activity than those cultured without MS5 cells. To this end, we sorted cells that had undergone 4 or fewer divisions after 4 days in the presence or in the absence of MS5 cells, and then we cultured the sorted populations in standard LTC assays (without added cytokines)30 either in bulk cultures (2000 cells/well) or at limiting dilution (10, 50, and 200 cells/well). At least 5 independent experiments were performed for each cell generation studied.
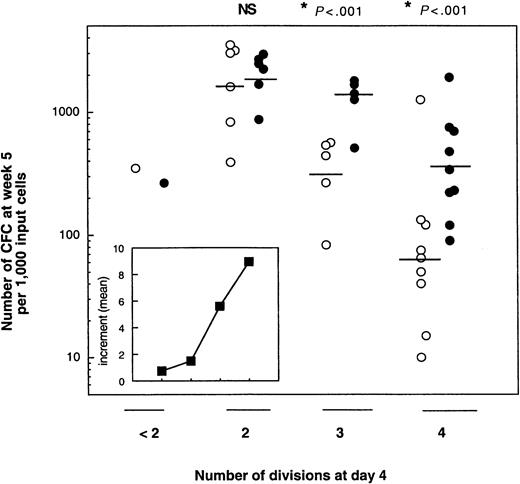
As shown in Figure 4, which summarizes the data obtained from the bulk cultures, the number of CFCs produced at week 5 was highly dependent on 2 parameters: the number of cell divisions completed during the 4 days of cultures with cytokines and the presence of MS5 cells. Independent of the culture conditions, the number of LTC-IC–derived CFCs was the highest for cells that had divided only twice. Thus, without MS5 cells, the geometric mean of week 5 CFCs per 1000 cells that had divided twice (2-division cells) on day 4 was 1606 (n = 5), and it decreased in cells that underwent more divisions (to 311 for 3-division cells [n = 5] and 64 for 4-division cells [n = 9]). Corresponding numbers for MS5 cell-containing cultures were 1993, 1223, and 355, respectively. In both conditions, with and without MS5 cells, the decrease in the number of LTC-IC–derived CFCs between 2- division and 4-division cells was highly significant (P < .001). Interestingly, in one experiment, cells that had divided either not at all or only once produced only 350 CFCs at week 5. Similar differences were observed in experiments seeded with 50 cells/well (data not shown).
Effect of MS5 cells on the LTC-IC potential of CD34+CD38low/neg exposed 4 days to cytokines with or without MS5 cells.
CFSE-labeled CD34+CD38low/neg were cultured with 6 growth factors (see “Materials and methods”) in the presence (closed circles) or absence (open circles) of stromal cells. On day 4, cells that had completed fewer than 2 divisions or 2, 3, or 4 divisions were sorted. One thousand cells from each were then assayed for LTC-IC activity. Each point represents the number of CFCs produced by 1000 cells derived from primary cultures containing MS5 cells (closed symbols) or not (open symbols). Each point is from a different experiment. The horizontal bar represents the geometric mean of the number of CFCs. Differences in the number of CFCs between cultures with and without MS5 cells were significant (*P < .01) for experiments performed with 3- and 4-division cells, but not for those with 2-division cells (NS). (Inset) Each point corresponds to the mean of the increments, calculated in each experiment, in the number of week 5 CFCs obtained from cells originally cultured for 4 days with or without MS5 cells.
Effect of MS5 cells on the LTC-IC potential of CD34+CD38low/neg exposed 4 days to cytokines with or without MS5 cells.
CFSE-labeled CD34+CD38low/neg were cultured with 6 growth factors (see “Materials and methods”) in the presence (closed circles) or absence (open circles) of stromal cells. On day 4, cells that had completed fewer than 2 divisions or 2, 3, or 4 divisions were sorted. One thousand cells from each were then assayed for LTC-IC activity. Each point represents the number of CFCs produced by 1000 cells derived from primary cultures containing MS5 cells (closed symbols) or not (open symbols). Each point is from a different experiment. The horizontal bar represents the geometric mean of the number of CFCs. Differences in the number of CFCs between cultures with and without MS5 cells were significant (*P < .01) for experiments performed with 3- and 4-division cells, but not for those with 2-division cells (NS). (Inset) Each point corresponds to the mean of the increments, calculated in each experiment, in the number of week 5 CFCs obtained from cells originally cultured for 4 days with or without MS5 cells.
A striking effect of the MS5 cells was to cause a high LTC-IC activity to be retained by cells that completed 3 and 4 divisions in the first 4 days. Thus, 1223 and 355 week 5 CFCs were produced by 3-division cells with and without MS5 cells, respectively, and 311 and 64 for 4-division cells. These differences were statistically significant for both 3-division and 4-division cells (P < .01). The retention of LTC-IC activity mediated by MS5 cells was seen in each experiment, and the insert in Figure 4 shows that the mean of the increments accounted for by the presence of MS5 cells increased almost linearly with the number of cell divisions. The higher frequency of LTC-IC in 4-division cells grown on MS5 cells (1 per 50, n = 4) compared to that of cells grown without MS5 cells (1 per 300, n = 4) confirmed the above data.
When CD34+CD38neg cells were used instead of CD38low cells, the numbers of week 5 CFCs were higher. This result was not surprising because the most immature progenitors lacked the CD38 antigen (Table 1). Nevertheless, in this population, the effect of MS5 cells was still clear.
Number of LTC-IC–derived CFCs generated at week 5 or week 12 by 1000 2- to 4-division cells sorted on day 4
| Experiment . | Cell divisions . | MS5 cells* . | Weeks in LTC . | |
|---|---|---|---|---|
| 5 . | 12 . | |||
| CD38lowSF | 2 | − | 830 | 278 |
| + | 870 | 180 | ||
| 4 | − | 50 | 0 | |
| + | 90 | 25 | ||
| CD38low+ S | 3 | − | 442 | 216 |
| + | 1250 | 910 | ||
| 4 | − | 73 | 1 | |
| + | 706 | 230 | ||
| CD38negSF | 4 | − | 133 | 79 |
| + | 225 | 102 | ||
| Experiment . | Cell divisions . | MS5 cells* . | Weeks in LTC . | |
|---|---|---|---|---|
| 5 . | 12 . | |||
| CD38lowSF | 2 | − | 830 | 278 |
| + | 870 | 180 | ||
| 4 | − | 50 | 0 | |
| + | 90 | 25 | ||
| CD38low+ S | 3 | − | 442 | 216 |
| + | 1250 | 910 | ||
| 4 | − | 73 | 1 | |
| + | 706 | 230 | ||
| CD38negSF | 4 | − | 133 | 79 |
| + | 225 | 102 | ||
CD34+CD38low or CD34+CD38neg cells were first exposed to cytokines for 4 days in the presence of serum (S) or in serum-free medium (SF) with or without MS5 cells, as indicated. Cells that had completed 2 or 4 divisions in each condition were then sorted and plated in a standard MS5-based LTC-IC assay and continued for either 5 or 12 weeks.
Numbers refer to the number of CFCs counted after 5 (column 4) or 12 (column 5) weeks in colony assays seeded with the progeny of 1000 cells on day 4. One experiment was performed for each condition.
A second striking observation in these experiments was the lack of a difference in the LTC-IC activity of 2-division cells cultured with or without MS5 cells (Figure 4). Thus 1000 cells grown without feeders generated 1606 CFCs; with feeders they generated 1993 CFCs. One possibility is that in these slow-dividing cells, which generated a high number of CFC at week 5, the decline in potential induced by cytokines was not sufficient to be translated into a decrease in the CFC output at week 5. Consequently, an effect of MS5 cells would not be revealed. A similar reasoning applies to cells with fewer than 2 divisions. Indeed, it has been reported that all the LTC-IC activity in such cultures is associated with mitotically active cells.17 If this were true, one might assume that extending the LTC period to week 12 might unmask an effect of MS5 cells on a more slowly recruited population. However, as shown in Table2, this was not the case, and the CFC output at week 12 was identical in LTC seeded with 2-division cells initially maintained with or without MS5 cells. This result could mean that MS5 cells have no effects on very primitive cells or that the LTC-IC assay was not sufficiently discriminating to discern the effects on them.
Effect of MS5 on the LTC-IC potential of CD34+CD38low/neg cells with the same mitosis history
ND, not done.
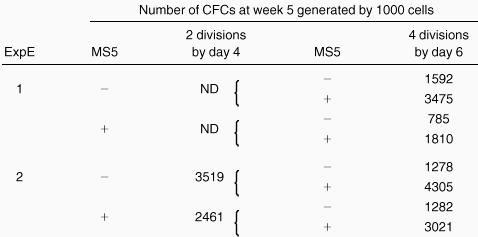
CD34+CD38low/neg cells were first exposed to cytokines for 4 days with or without MS5 cells. Cells that had completed 2 divisions in each condition were sorted and either assessed in LTC or cultured in the same medium with or without MS5 cells for an additional 2 days (see procedure in Figure 1). On day 6, cells that had divided 2 more times (4 divisions since day 0) were sorted and transferred to standard LTC-IC assays. Numbers refer to the number of LTC-IC–derived CFCs generated by 1000 cells sorted on day 4 (column 3) or on day 6 (column 5).
Coculture with MS5 cells retards differentiation by a direct effect on target cells
Results described above indicate that MS5 cells counteract the decline in LTC-IC activity in proliferating cells that have divided 3 to 4 times in 4 days. In contrast, no effect of MS5 cells was seen in 4 days on slow-dividing cells. Additional support for this was obtained from the following experiment. Day 4 cells that had completed 2 divisions, either with or without MS5 cells, were isolated by FACS and transferred to LTC-IC assays, and the remaining cells were cultured for another 2 days with cytokines, with or without MS5 cells, as shown in Figure 1. On day 6, cells that had divided 2 more times (a total of 4 divisions from day 0) were sorted and assayed for their LTC-IC activity. Table 2 and Figure 5 summarize the results, which were reproducible in both experiments. As in previous experiments, cells that had completed 2 divisions by day 4 had similar LTC-IC activity regardless of whether MS5 cells were present (Figures 4, 5, Table 2). However, 2 days later, an effect of MS5 was clearly seen (Figure 5, Table 2). The presence of stromal cells from days 4 to 6 increased significantly the number of LTC-IC–derived CFCs compared to wells cultured without MS5 from days 4 to 6 (compare closed and open symbols in Figure 5, panels B1 and B2). Fifty percent of the wells seeded with cells cultured with MS5 from day 4 to day 6 yielded more than 100 CFCs, whereas none of those cultured without MS5 had any LTC-IC activity. Moreover, whether MS5 cells were present during the first 4 days did not affect the results on day 6 (compare Figure 5, panels B1 and B2).
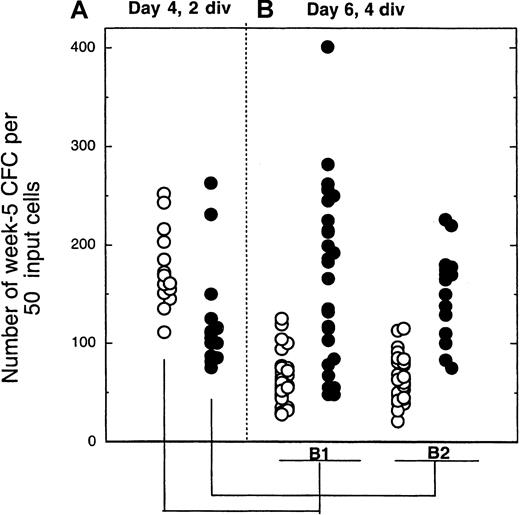
Number of colony-forming cells produced by cells sorted on day 4 after 2 divisions and exposed for another 2 days to cytokines ± MS5 cells.
The 2-step procedure is depicted in Figure 1. In panel A, 2-division cells from 4-day cultures with (closed symbols) or without (open symbols) MS5 cells were plated in LTC (50 cells/well). Each point from panel B indicates the number of CFCs produced by 50 cells that had completed a total of 4 divisions by day 6 and originated from the initially slowly dividing population (2 divisions by day 4). Panel B1 corresponds to cells cultured for 4 days without MS5 and then from day 4 to day 6 with (closed symbols) or without (open symbols) MS5 cells. Panel B2 corresponds to cells cultured for the first 4 days with MS5 cells. All cultures contained the same cytokines. Comparison of the number of CFCs generated by 4-division cells with or without MS5 yielded highly significant differences (*P < .01)
Number of colony-forming cells produced by cells sorted on day 4 after 2 divisions and exposed for another 2 days to cytokines ± MS5 cells.
The 2-step procedure is depicted in Figure 1. In panel A, 2-division cells from 4-day cultures with (closed symbols) or without (open symbols) MS5 cells were plated in LTC (50 cells/well). Each point from panel B indicates the number of CFCs produced by 50 cells that had completed a total of 4 divisions by day 6 and originated from the initially slowly dividing population (2 divisions by day 4). Panel B1 corresponds to cells cultured for 4 days without MS5 and then from day 4 to day 6 with (closed symbols) or without (open symbols) MS5 cells. Panel B2 corresponds to cells cultured for the first 4 days with MS5 cells. All cultures contained the same cytokines. Comparison of the number of CFCs generated by 4-division cells with or without MS5 yielded highly significant differences (*P < .01)
Retention of LTC-IC activity depends on the number of cell divisions more than on the time in culture
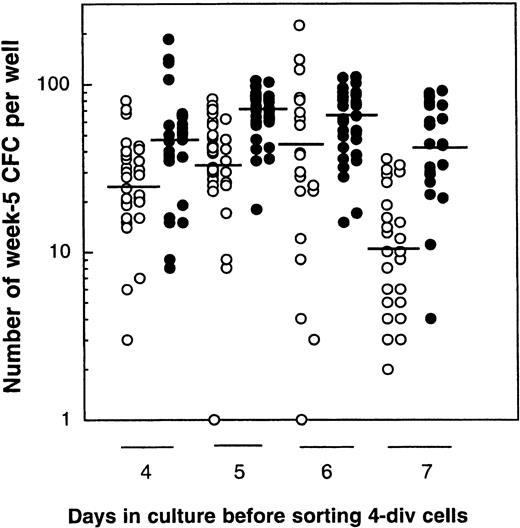
Finally, in a last series of experiments, we investigated whether it was the number of cell divisions completed or the time in culture that was the most important parameter causing a decline in LTC-IC activity. CD34+CD38low/neg cells were, therefore, cultured for 4 days with or without MS5 cells, and day 4 4-division cells and 0- to 3-division cells were then isolated. Four-division cells were then transferred to standard LTC (Figure6, left panel), and 0- to 3-division cells were kept in the cytokine-containing medium (±MS5 cells) for another 2 days, after which 4-division and 0- to 3-division cells were again sorted and separately assayed for LTC-IC activity. The procedure was repeated up to day 7. In this experiment, the geometric mean of the number of week 5 CFCs was 24, 31, 40, and 10 without MS5 cells, and 44, 66, 60, and 40 with MS5 for days 4, 5, 6, and 7, respectively (Figure6). Differences in the LTC-IC activity of cells cultured with or without MS5 cells were significant at each time point, but there was no difference in the LTC-IC activity of cells cultured for 4 to 6 days under the same conditions (ie, with or without MS5 cells). Such stability in the LTC-IC activity potential of cells that had accomplished 4 divisions either in the presence or absence of MS5 cells between day 4 and day 6 in the primary cultures indicates that, at least initially, the decline in LTC-IC activity was a function of the number of divisions completed rather that their time in culture. The decrease observed on day 7 may be partly explained by deleterious effects of repeated cell sorting and in vitro manipulation.
Comparison of the LTC-IC activity of cells that have completed 4 divisions in 4, 5, 6, or 7 days.
Results are from 1 experiment. CD34+CD38low/negcells were cultured with cytokines in the presence (closed circles) or absence (open circles) of MS5 cells. On day 4, 4-division cells were sorted, and their LTC-IC activity was assessed (50 cells/well). Cells that undertook 0 to 3 divisions during the same time were also sorted and kept in culture 1 more day. The next day (day 5), 4-division cells were sorted and assessed in LTC and 0- to 3-division cells were recultured for 1 more day. This procedure was repeated on days 6 and 7. Each point represents the number of CFCs produced after 5 weeks by 50 cells that had accomplished 4 divisions by the day indicated. Horizontal bars indicate the geometric mean of the number of CFCs produced. There were no statistically significant differences in the mean number of CFCs generated by 4-division cells grown on MS5 (closed symbols) on day 4, 5, or 6, or by 4-division cells grown without MS5 (open symbols) on day 4, 5, or 6 (P > .5). However, each day, the mean number of CFCs generated in the presence of MS5 cells was significantly higher than that produced by cells grown without MS5 cells (P < .01).
Comparison of the LTC-IC activity of cells that have completed 4 divisions in 4, 5, 6, or 7 days.
Results are from 1 experiment. CD34+CD38low/negcells were cultured with cytokines in the presence (closed circles) or absence (open circles) of MS5 cells. On day 4, 4-division cells were sorted, and their LTC-IC activity was assessed (50 cells/well). Cells that undertook 0 to 3 divisions during the same time were also sorted and kept in culture 1 more day. The next day (day 5), 4-division cells were sorted and assessed in LTC and 0- to 3-division cells were recultured for 1 more day. This procedure was repeated on days 6 and 7. Each point represents the number of CFCs produced after 5 weeks by 50 cells that had accomplished 4 divisions by the day indicated. Horizontal bars indicate the geometric mean of the number of CFCs produced. There were no statistically significant differences in the mean number of CFCs generated by 4-division cells grown on MS5 (closed symbols) on day 4, 5, or 6, or by 4-division cells grown without MS5 (open symbols) on day 4, 5, or 6 (P > .5). However, each day, the mean number of CFCs generated in the presence of MS5 cells was significantly higher than that produced by cells grown without MS5 cells (P < .01).
Discussion
The alteration of stem cell fate by external signals is well established in some cellular systems,31,32 but it is still a controversial issue in the hematopoietic system,5 though recent observations strongly suggest that environmental cues might induce tissue-specific differentiation programs in stem cells.33-35 However, whether it is possible, by manipulating the environment, to change, within a given tissue, the balance between self-renewal and differentiation is less clear. Our previous studies had shown that CD34+CD38low/neg cells cultured for 10 days on murine MS5 stromal cells retained a high LTC-IC activity.25 It was unclear whether MS5 stromal cells acted by directly changing the LTC-IC activity of cells, or by altering the rate of cell division of a rare subset of cells. In this study we used fluorescent dye labeling to correlate the variation in the LTC-IC activity of CFSE-labeled CD34+CD38low/neg cells with cell division and to investigate whether stromal (MS5) cells would alter this coupling.16,18,28 36
A striking and frequent observation was the dramatic effect of MS5 cells on the LTC-IC potential of CD34+CD38low/neg marrow cells that were exposed to cytokines and that achieved 3 or 4 divisions during the 4 days of the incubation period (Figures 4, 5, Table 2). Interestingly, MS5 cells had no detectable effect on the LTC-IC activity of cells that completed only 2 divisions. Of note, the presence of MS5 at the initiation of the culture had a dramatic effect on the size of the colonies generated at week 5 by LTC-IC–derived CFCs, which were much larger, and on their ability to generate BFU-E. All these findings support the conclusion that MS5 cells counteracted the differentiation of proliferating LTC-IC. This is in agreement with our previous observation that the main effect of MS5 cells in such cultures is to increase the absolute number of LTC-IC. This may be regarded as a functional measure of their self-renewal because the LTC-IC displayed CFC outputs similar to those of the parent LTC-IC.25
These differential effects of MS5 cells on CD34+CD38low/neg cells according to their mitotic rate deserve 2 comments. First, there was a decrease in the number of week 5 CFCs with each cell generation independent of the presence of stromal cells. Thus 2-division cells on day 4 generated more than 500 and up to 2500 CFCs 5 weeks later, whereas on average 10- to 100-fold lower numbers of CFCs were produced by other cells in the same primary cultures that had divided 4 times. This loss of LTC-IC activity by cells that divide rapidly, that is more than 3 to 4 times in 4 days, was expected because cells with the highest potential are quiescent or cycle slowly.13,15 Interestingly, in one experiment, cells that did not divide at all or that divided only once within 4 days showed little LTC-IC activity (Figure 4). This suggests that LTC-IC activity may be expressed transiently by primitive cells during their differentiation and is then lost after a critical number of cell divisions.17 Accordingly, events that dictate the retention by cells of an ability to form colonies 5 weeks later may be tightly controlled, perhaps by a molecular clock, as has been described for neuronal cells37 or for hematopoietic cells in which telomere shortening has been suggested as a mechanism that determines stem cell life span.38 39 As a consequence, there may be only a narrow window in the differentiation of primitive hematopoietic cells to assess changes in LTC-IC activity.
This is confirmed by data shown in Figures 4 and 5 in which the effect of MS5 cells was detectable only in cells that had gone through 3 to 4 divisions and not in more slowly dividing cells (0- to 2-division cells). Because the LTC-IC activity was very high in these 2-division cells, a subtle change induced by stromal cells may not have been translated into a change in the number of output CFCs 5 weeks later. Furthermore, in one experiment in which the culture was prolonged up to 12 weeks,40 the number of CFCs produced by cells grown with or without MS5 cells did not differ, indicating that the sensitivity of the assay was not the only parameter involved. Interestingly, the sizes and types of colonies seen in assays of LTC-IC from 2-division cells were identical, independent of the presence of MS5 cells in the primary cultures. This argues against a major functional difference in these slowly dividing LTC-ICs. Alternatively, because these cells divided only twice in 4 days, 2 mitoses might not have allowed sufficient time for MS5 cells to alter their fate. Nevertheless, it is remarkable that the increased CFC output obtained when MS5 cells were present was strongly correlated with the number of divisions completed (see Figure 4, insert). This suggests that MS5 cells act by changing the probability of a cell to retain or lose LTC-IC activity at each division.
To further exclude the contribution of a rare subset of input cells induced to proliferate by MS5 cells in the observed effect, we performed a 2-step experiment. Slowly dividing (2-division) cells were sorted on day 4, and these were then incubated for 2 more days either with or without MS5 cells before assessing the LTC-IC present. The results of these experiments clearly identified a striking effect of the MS5 cells in preventing a decrease in the LTC-IC activity seen in 4-division cells exposed to cytokines alone from day 4 to day 6 (P < .01; Figure 6). Thus, it appears that any strategy to influence the LTC-IC outcome will be best identified in cells that have gone through at least 3 divisions.
In summary, this study clearly shows that stromal cells can perturb the fate of primitive hematopoietic cells that actively proliferate when exposed to cytokines and that they allow some to retain primitive functions, such as those identified by the LTC-IC assay. The lack of differentiation could not be explained by a negative action of the feeder on cell proliferation.20,41 This raises the important question of the molecular mechanisms by which MS5 cells can affect the decisions of a primitive cell to lose (differentiate) or to retain (self-renew) its LTC-IC function. Increased self-renewal has been associated with increased Bcl-2 activity,42overexpression of HoxB4,43,44 inhibition of GATA-2,45 and increased telomerase activity.46 Activation of the Notch pathway should also be considered because the Jagged–Notch interaction promotes a 2- to 3-fold expansion of normal hematopoietic progenitors by preventing the differentiation of dividing cells.12 47-49 Because MS5 cells transcribe these DSL ligands (Jagged1-2 and Delta1) (data not shown), such a mechanism is a possibility. It will also now be of interest to determine whether MS5 cells can cause the retention of properties characteristic of primitive hematopoietic cells, such as their ability to engraft NOD-SCID mice or to generate lymphoid progeny.
Acknowledgments
We thank Brigitte Izac for her expert help with the LTC-IC assays and all the surgeons and nurses who helped provide bone marrow samples. We thank the staff at Amgen-France (rhu-SCF) and Amgen-Thousands Oaks (MGDF), Immunex (Flt3-l), Novartis (IL-3), and Cilag (Epo) for their generosity in providing cytokines. We also thank Yves Levy for reading the manuscript and Pierre Walrafen for help in statistical analysis.
Supported by INSERM, grants from ARC to L.C., and by Electricitéde France, Fondation de France (comité contre la leucémie). C.P. was funded by a fellowship from Fondation de France and Fédération des centres de lutte contre le Cancer.
A.B.G. and C.P. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Laure Coulombel, INSERM U 474, Maternité de Port Royal, Hôpital Port Royal, 123 Boulevard de Port Royal, Paris, France; e-mail: coulombel@cochin.inserm.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal