Abstract
The objective of this study was to examine the correlation between serum interleukin-6 (IL-6) and IL-10 levels and outcome in chronic lymphocytic leukemia (CLL). Serum IL-6 and IL-10 levels were measured by enzyme-linked immunoabsorbent assays from 159 and 151 CLL patients, respectively, and from healthy control subjects (n = 55 [IL-6]; n = 37 [IL-10]). Cytokine levels were correlated with clinical features and survival. Serum IL-6 levels were higher in CLL patients (median, 1.45 pg/mL; range, undetectable to 110 pg/mL) than in control subjects (median, undetectable; range, undetectable to 4.30 pg/mL) (P < .0001). Serum IL-10 levels were higher in CLL patients (median, 5.04 pg/mL; range, undetectable to 74 pg/mL) than in normal volunteers (median, undetectable; range, undetectable to 13.68 pg/mL) (P < .00001). Assays measuring both Epstein-Barr virus-derived and human IL-10 yielded higher values than assays measuring primarily human IL-10 (P < .05). Patients with elevation of serum IL-6 or IL-10 levels, or both, had worse median and 3-year survival (log rank P < .001) and unfavorable characteristics (prior treatment, elevated β2-microglobulin or lactate dehydrogenase, or Rai stage III or IV). Elevated IL-6 and IL-10 levels were independent prognostic factors for survival when analyzed individually or in combination (Cox regression analysis). However, if β2-microglobulin was incorporated into the analysis, it was selected as an independent prognostic feature, and IL-6/IL-10 were no longer selected. In patients with CLL, serum IL-6 and IL-10 (viral and human) levels are elevated and correlate with adverse disease features and short survival. In multivariate analysis, however, β2-microglobulin is the most important prognostic factor.
Introduction
Interleukin-6 (IL-6) is a pleiotropic cytokine produced by a variety of cell types, including fibroblasts, endothelial cells, monocytes, normal hematopoietic cells, and lymphocytes.1-3 Several earlier studies suggested a possible role for dysregulated production of IL-6 in malignant lymphomas. For instance, IL-6 messenger RNA and protein expression has been found in lymphoma cells using immunohistochemical stains,4-9 and the degree of positivity correlates with the proliferative rate of the malignant cells.9Furthermore, acquired immunodeficiency syndrome (AIDS)-related B-cell lymphoma cell lines produce large amounts of IL-6.10Expression of an exogenous IL-6 gene in human Epstein-Barr virus (EBV)-infected cells confers growth advantage and tumorigenicity,11 and, in transgenic mice, IL-6 expression has been associated with the development of lymphomas.12
Serum IL-6 levels have been correlated with an increased risk for development of lymphoma in patients with AIDS10 and in renal transplant recipients.13 Serum IL-6 levels are increased in diffuse large cell lymphomas, associated with adverse prognostic features, and predictive of a poor failure-free and overall survival in multivariate analysis.14,15 Interestingly, serum IL-6 levels may also be elevated16,17 and correlate with poor prognostic features and an inferior outcome in Hodgkin disease,17,18 indolent non-Hodgkin lymphomas (NHLs),19 renal cell carcinoma,20 prostatic cancer,21 ovarian cancer,22 and multiple myeloma.23 24
Interleukin-10 (IL-10) is a pleiotropic cytokine produced by type 2 helper cells (Th2),25 as well as monocytes and macrophages, and normal and neoplastic B lymphocytes.26-28It is highly homologous to an open reading frame of EBV called BCRF1, and EBV infection of B cells up-regulates IL-10 production. IL-10 production has strong immunosuppressive effects via inhibition of Th1 type cytokines, including interferon-gamma and interleukin-2.25 IL-10 has a potent stimulating effect on B cells, inducing proliferation and differentiation. Interestingly, in cell lines derived from B-cell lymphomas, IL-10 has been found to serve as an autocrine growth factor.29-31 Serum IL-10 levels have been found to be important prognostic factors for Hodgkin lymphoma and, when assays that detect both human and viral IL-10 are employed, for NHLs.32-36
In this study, we measured the pretreatment serum IL-6 and IL-10 levels in a large cohort of patients with chronic lymphocytic leukemia (CLL) to determine if high IL-6 and/or IL-10 levels correlate with disease features and outcome.
Patients and methods
Patients
The study group consisted of 177 patients with a diagnosis of CLL in whom frozen sera (at −70°C) were obtained at diagnosis or relapse. All patients were off treatment for at least 3 months. The samples were derived from all consenting patients meeting the criteria for analysis who were seen in a 1.5-year time period. All histological material was reviewed at M. D. Anderson Cancer Center. The patients were followed every 3 to 6 months while off treatment and more often as necessary while on any treatment. Initial evaluation included a medical interview; complete physical examination; complete blood and platelet counts; serum chemistry; β2-microglobulin; serum protein electrophoresis; quantitative immunoglobulin levels; peripheral and bone marrow lymphocyte immunophenotype; chest x-ray; computerized tomography of chest, abdomen, and pelvis (when indicated); and bone marrow aspiration and biopsies. The follow-up evaluations included a medical interview, complete physical examination, bone marrow biopsies, and tests as appropriate. Seventy-five patients (42%) received treatment prior to referral to our institute. The initial treatment for 126 patients was a fludarabine-based regimen. The first treatment after sample collection at our institute was a fludarabine-based regimen in 103 patients. The initial therapy for the majority of other patients was a chlorambucil-based regimen. The decision to treat was based on the National Cancer Institute guidelines.37 All patients gave informed consent in accordance with institutional policy, for both treatment and sample collection.
Control subjects
Frozen serum samples from 55 healthy normal donors were analyzed for serum IL-6 levels. (Some of these control subjects had been used in an earlier study.15 However, different vials of serum from these volunteers were analyzed alongside the CLL serum samples in the current study.) Samples were collected from control subjects only if the subjects had not had fever within 1 week, were not receiving any medications, were not known to be pregnant, and did not have a history of any chronic or acute illnesses. Control samples were frozen and stored in a manner identical to patient samples. Thirty-seven normal control samples were obtained for the analysis of serum IL-10 levels. Samples from control subjects and from patients had not been previously thawed.
IL-6 assays
IL-6 levels were assayed in duplicate with all values expressed as a mean of the 2 determinations. A standard curve was generated, using known concentrations of recombinant human IL-6. The concentration of IL-6 in the patient and control serum samples was then determined from the standard curve. IL-6 was measured, using a validated commercial enzyme-linked immunosorbent assay (ELISA) (Quantikine, R&D Systems, Minneapolis, MN). This assay uses a solid-phase murine monoclonal anti–IL-6 antibody, bound to microtiters plates, that captures IL-6 in the sample assayed. Unbound protein is removed by washing, and a polyclonal anti–IL-6 antibody conjugated to horseradish peroxidase is added, with any excess conjugated antibody removed by further washing. The IL-6 bound is quantified by the addition of substrate solution (containing stabilized hydrogen peroxide and stabilized chromogen [tetramethylbenzidine]). If antibody-peroxidase conjugate is present, the substrate is oxidized, and the intensity of the color that develops is proportional to the amount of bound IL-6. The reaction is stopped after 20 minutes of incubation at room temperature, and the color intensity is quantified by using a microtiter plate reader at a wavelength of 450 nm. The manufacturer reports an intra-assay coefficient of variation of 2.7% to 4.2% at 5.2 to 107 ng/mL, and an inter-assay coefficient of variation 2.4% to 7% at 4.7 to 107 pg/mL. No cross-reactivity has been detected with any human cytokines detected to date. All values for IL-6 were converted to the NIBSC/WHO standard assay, using a correction factor according to the manufacturer's instructions. The assay has a lower sensitivity level of 0.9 pg/mL (corrected to WHO standard).38
IL-10 assays
Two IL-10 ELISAs were run. The reason for running 2 assays was based on previous studies which demonstrated that, in NHL, IL-10 levels are higher if measured by an ELISA that detects both viral and human IL-10 than if measured by an ELISA that discerns only human IL-10.35 Furthermore, correlation with prognosis is seen, using the former, but not the latter, assay.32-35
The ENDOGEN Inc (Cambridge, MA) assay detects both viral and human IL-10 (as per manufacturer) and was used to test 159 CLL serum samples and all the normal control samples. For comparison purposes, the second IL-10 assay (detecting predominantly human IL-10 [as per manufacturer]) was used in a smaller subgroup (Cytoscreen, Biosource International, Camarillo, CA).
IL-10 levels were assayed in duplicate with all values expressed as a mean of the 2 determinations. A standard curve was generated, using known concentrations of recombinant human IL-10. The concentration of IL-10 in the patient and control serum samples was then determined from the standard curve. As mentioned above, for most patients, IL-10 was measured, using a validated commercial ELISA test (Endogen). This assay uses an antihuman IL-10 biotinylated-antibody reagent and antihuman IL-10 antibody, bound to microtiters plates, that captures IL-10 in the sample assayed. Unbound protein is removed by washing, and a dilute streptavidin conjugated to horseradish peroxidase is added, with any excess conjugated antibody removed by further washing. The IL-10 bound is quantified by the addition of substrate solution. If antibody-peroxidase conjugate is present, the substrate is oxidized, and the intensity of the color that develops is proportional to the amount of bound IL-10. The reaction is stopped after 30 minutes of incubation at room temperature, and the color intensity is quantified by using a microtiter plate reader set at a wavelength of 450 nm. The manufacturer reports an intra-assay coefficient of variation of less than 10%, and a interassay coefficient of variation of less than 10%. No cross-reactivity has been detected with any human cytokines detected to date. The assay has a lower sensitivity level of 3 pg/mL.
As discussed above, serum from a small group of patients was assessed, using an IL-10 assay from Biosource International. In brief, the assay uses a mouse monoclonal antibody (MoAb) specific for human IL-10 coated onto microtiter wells into which samples were pipetted followed by the addition of a second mouse anti–IL-10 MoAb (which is biotinylated). Free antibody is removed by further washing, and streptavidin-peroxidase is added. A final wash removes unbound enzyme, and, subsequently, a substrate solution (tetramethyl benzidine) is added that acted upon by any bound enzyme to produce color. After 30 minutes, the reaction is stopped, and the absorbance is read on a microtiter plate reader at 450 nm. The intensity of the color is directly proportional to the IL-10 present in the assayed sample. The lower sensitivity of the assay is 5 pg/mL as per the manufacturer. There is no cross-reactivity of this assay with viral IL-10, or human IL-1a, IL-1b, IL-2, IL-6, or tumor necrosis factor α at a concentration of these cytokines of 800 to 1000 ng/mL. The percentage of cross-reactivity is estimated at ≤ 0.005% in each case. The recovery of IL-10 added to normal serum averages 93%. The intra-assay coefficient of variation is 2.6% to 5.3% at 60.2 to 498.9 pg/mL, and the interassay coefficient of variation is 2.8% to 6.1% at 61.8 to 499.9 pg/mL.
Determination of intracytoplasmic cytokine synthesis by B-CLL cells
Preparation for the detection of cytokines in the cellular cytoplasms was done as previously described.39 Blood samples from B-CLL patients with a white blood cell (WBC) count more than 15 × 109/L were diluted to 15 × 109/L to bring the WBC to within normal range. Blood (500 μL) was incubated at 37°C with 10 μg of brefeldin-A (a nontoxic but potent inhibitor of intracellular transport; Sigma Chemical, St Louis, MO) for 4 hours. To determine surface immunophenotype of the B cells, the cells were washed and stained with anti–CD19-peridinin chlorophyll protein (PerCP) and anti-CD5–fluorescein isothiocyanate (FITC) for 15 minutes in the dark at ambient temperature. Next, the cells were treated with a permeabilization solution (Becton Dickinson, Mountain View, CA) for 1 hour at 37°C to allow entry of the anti-cytokine monoclonal antibody. Finally, the cells were stained with a cytokine-specific MoAb conjugated with phycoerythrin to detect IL-6 or IL-10. All cell preparations were fixed in a solution of 1% paraformaldehyde and stored at 4°C until analysis by the FACSCalibur flow cytometer. (Antibodies for these experiments were obtained from Becton Dickinson, Franklin Lakes, NJ.)
The analysis was conducted by gating on CD19-PerCP and side scatter to identify CD19+ B cells among the 8000 total events acquired. List-mode multiparameter data files (each file with forward scatter, side scatter, and 3 fluorescence parameters) were analyzed, using the CellQuest software program (Becton Dickinson). Isotype controls were used to verify the staining specificity of experimental conditions and as a guide for setting markers to delineate positive and negative populations. Cytokine synthesis by CD19+ B cells was determined in cells reactive to versus those nonreactive to anti-CD5–FITC. This analytical approach permitted the determination of IL-6 and IL-10 syntheses in CD19+ CD5+ (B-CLL) and CD19+ CD5− (normal) B cells.
Statistical analysis
The statistical estimation of distribution of survival times was performed, using the Kaplan-Meier method.40 For survival analysis, all patients still alive were censored at the date of last follow-up. The starting time for all survival analyses was the date of sample collection. Comparisons between survivals were made, using the log-rank method.
Categoric data were compared, using the chi-square test or Fisher exact test as indicated,41 and continuous data were compared, using the Mann-Whitney test or Kruskal-Wallis test as appropriate.42 Paired sample data (for intracytoplasmic detection of cytokines in cell subsets) were compared, using the Wilcoxon signed rank test. Comparison of IL-6 and IL-10 levels between CLL patients and control subjects was done as continuous data. For statistical purposes, all IL-6 levels below the detection limit of the assay were regarded as equivalent to 0.9 pg/mL, and all IL-10 levels (Endogen) below the limit of detection were regarded as equivalent to 3pg/mL. All P values given are 2 sided.
Results
Control subjects
Thirty-nine (71%) of 55 normal volunteers had a serum IL-6 level below the limit of detection of the assay (< 0.9 pg/mL). The serum IL-6 levels in the entire group ranged from < 0.9 pg/mL to 4.30 pg/mL (median, < 0.9 pg/mL). We, therefore, defined any serum level of ≥ 4.30 pg/mL as elevated. (The same cutoff was used for intermediate-grade lymphomas in our previous publication.15)
Thirty-seven normal volunteers were tested for serum IL-10 levels (Endogen), with 36 (97%) of 37 having nondetectable values (range, < 3-13.68 pg/mL). A cut-point value greater than 10 pg/mL was defined as an elevated value.
Patients with CLL
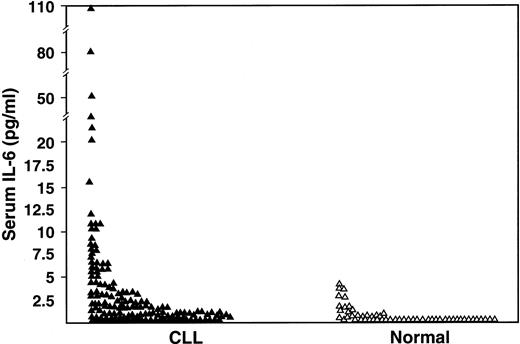
One-hundred seventy-seven patients with CLL were studied for serum IL-6 (n = 151), serum IL-10 (n = 159), or both cytokines (n = 133). Their clinical features are listed in Table1. The serum IL-6 levels were undetectable in 62 (41%) of 151 patients compared with 71% of control subjects (P < .0001), and 36 (24%) CLL patients had values above the upper limit of the normal range (4.3 pg/mL) (P < 0.0001) (Figure 1).
Patient characteristics
| No. | 177 |
| Median age (y) (range) | 60 (21-82) |
| Female/male | 66/111 |
| Rai stage | |
| 0 | 44 |
| I | 78 |
| II | 24 |
| III | 8 |
| IV | 23 |
| No. patients previously treated (%) | 75 (42) |
| β2-microglobulin (n = 153) | 2.7 mg/L |
| Median (range) | (0.9-16.8 mg/L) |
| No. previous treatments | |
| 0 | 102 |
| 1 | 43 |
| 2 | 14 |
| 3 | 10 |
| 4 or more | 8 |
| Median serum IL-10 (range) (pg/mL) | 5.04 (0-74) |
| Median serum IL-6 (range) (pg/mL) | 1.45 (0-110) |
| Median follow-up of survivors (mo) | 30 (1-40) |
| No. patients dead (%) | 42 (24) |
| Median survival (mo) | Not reached |
| No. | 177 |
| Median age (y) (range) | 60 (21-82) |
| Female/male | 66/111 |
| Rai stage | |
| 0 | 44 |
| I | 78 |
| II | 24 |
| III | 8 |
| IV | 23 |
| No. patients previously treated (%) | 75 (42) |
| β2-microglobulin (n = 153) | 2.7 mg/L |
| Median (range) | (0.9-16.8 mg/L) |
| No. previous treatments | |
| 0 | 102 |
| 1 | 43 |
| 2 | 14 |
| 3 | 10 |
| 4 or more | 8 |
| Median serum IL-10 (range) (pg/mL) | 5.04 (0-74) |
| Median serum IL-6 (range) (pg/mL) | 1.45 (0-110) |
| Median follow-up of survivors (mo) | 30 (1-40) |
| No. patients dead (%) | 42 (24) |
| Median survival (mo) | Not reached |
IL-10 indicates interleukin 10; IL-6, interleukin 6.
Comparison of serum IL-6 levels as measured by ELISA in CLL patients (n = 151) and in normal subjects (n = 55) (P < .001).
Comparison of serum IL-6 levels as measured by ELISA in CLL patients (n = 151) and in normal subjects (n = 55) (P < .001).
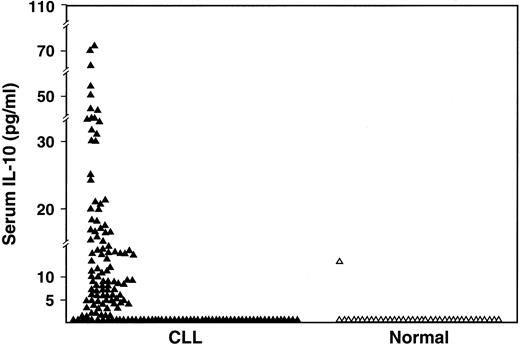
The serum IL-10 levels (Endogen; detects both viral and human IL-10) were undetectable in 60 (38%) of the 159 patients compared to 97% of normal volunteers (P < .0001) and elevated to greater than 10 pg/mL in 34% of patients compared to 3% of normal volunteers (P < .0001) (Figure2). The median value of serum IL-10 in the patients was 5.04 pg/mL (range, < 3-74 pg/mL).
Comparison of serum IL-10 levels as measured by ELISA in CLL patients (n = 159) and in normal subjects (n = 37) (P < .001).
Comparison of serum IL-10 levels as measured by ELISA in CLL patients (n = 159) and in normal subjects (n = 37) (P < .001).
In a small subset of patients (n = 20), a second IL-10 assay was used (Biosource). This assay detects human IL-10 only. Despite a level of sensitivity that was similar to that of the Endogen assay, the serum IL-10 level was detectable in only one patient using the Biosource assay. In contrast, the median serum IL-10 values for these 20 CLL patients was 8.1 pg/mL (range, undetectable to 44.3 pg/mL) by using the assay that detects both viral and human IL-10 (Endogen) (P < .01). These data suggest that in CLL, as in NHL,35 results may differ, depending on the assay method used, and that IL-10 levels are higher when assays capable of measuring both viral and human IL-10 are employed.
Correlation of serum IL-6 and serum IL-10 levels with disease characteristics/transformation
Higher serum IL-6 levels correlated with Rai stage III and IV (P = .0001), previous treatment (P = .0004), elevated β2-microglobulin level (≥2 mg/L) (P < .001), elevated serum lactate dehydrogenase (LDH) levels (P = .02), and age of greater than or equal to 60 years old (P = .003, Mann-Whitney test) (Table2).
IL-6 levels and disease characteristics (n = 151)
| Category . | No. patients . | Median IL-6 (pg/mL) (range) . | P value* . |
|---|---|---|---|
| β2-microglobulin (mg/L)1 | |||
| <2 | 24 | <0.91 (<0.91-11) | <.001 |
| ≥2 | 104 | 1.93 (<0.91-110) | |
| Previous treatment | |||
| Yes | 89 | 2.16 (<0.91-110) | .0004 |
| No | 62 | <0.91 (<0.91-37) | |
| Serum LDH level (IU/mL)† | |||
| ≥618 | 24 | 2.91 (<0.91-74) | .02 |
| <618 | 112 | 1.20 (<0.91-110) | |
| Rai stage | |||
| 0, I, II | 124 | 1.04 (<0.91-110) | .0001 |
| III, IV | 27 | 2.94 (<0.91-51) | |
| Age (y) | |||
| <60 | 77 | <0.091 (<0.91-80) | .003 |
| ≥60 | 74 | 2.01 (<0.91-110) |
| Category . | No. patients . | Median IL-6 (pg/mL) (range) . | P value* . |
|---|---|---|---|
| β2-microglobulin (mg/L)1 | |||
| <2 | 24 | <0.91 (<0.91-11) | <.001 |
| ≥2 | 104 | 1.93 (<0.91-110) | |
| Previous treatment | |||
| Yes | 89 | 2.16 (<0.91-110) | .0004 |
| No | 62 | <0.91 (<0.91-37) | |
| Serum LDH level (IU/mL)† | |||
| ≥618 | 24 | 2.91 (<0.91-74) | .02 |
| <618 | 112 | 1.20 (<0.91-110) | |
| Rai stage | |||
| 0, I, II | 124 | 1.04 (<0.91-110) | .0001 |
| III, IV | 27 | 2.94 (<0.91-51) | |
| Age (y) | |||
| <60 | 77 | <0.091 (<0.91-80) | .003 |
| ≥60 | 74 | 2.01 (<0.91-110) |
IL-6 indicates interleukin 6; LDH, lactate dehydrogenase.
Using the Mann-Whitney test.
Upper limit of normal for LDH at M. D. Anderson Cancer Center is 618 IU/mL.
Higher serum IL-10 levels were associated with elevated LDH (P = .000002), previous treatment (P = .005), elevated β2-microglobulin (P = .001), and advanced Rai stage (P = .001, Mann-Whitney test) (Table3). High serum IL-10 levels also correlated with high serum IL-6 levels (P = .01).
IL-10 levels and disease characteristics (n = 159)
| Category . | No. patients . | Median IL-10 (pg/mL) (range) . | P value3-150 . |
|---|---|---|---|
| β2-microglobulin (mg/L) | |||
| <2 | 30 | <3 (<3-42) | .001 |
| ≥2 | 112 | 6.60 (<3-70) | |
| Previous treatment | |||
| Yes | 91 | 3.27 (<3-64) | <.005 |
| No | 68 | 8.60 (<3-74) | |
| Serum LDH level (IU/mL)3-151 | |||
| ≥618 | 29 | 16.10 (<3-74) | .000002 |
| <618 | 112 | 4.43 (<3-55) | |
| Rai stage | |||
| 0, I, II | 131 | 4.29 (<3-64) | .001 |
| III, IV | 28 | 10.40 (<3-74) | |
| Age (y) | |||
| <60 | 84 | 4.57 (<3-74) | .068 |
| ≥60 | 75 | 6.81 (<3-70) |
| Category . | No. patients . | Median IL-10 (pg/mL) (range) . | P value3-150 . |
|---|---|---|---|
| β2-microglobulin (mg/L) | |||
| <2 | 30 | <3 (<3-42) | .001 |
| ≥2 | 112 | 6.60 (<3-70) | |
| Previous treatment | |||
| Yes | 91 | 3.27 (<3-64) | <.005 |
| No | 68 | 8.60 (<3-74) | |
| Serum LDH level (IU/mL)3-151 | |||
| ≥618 | 29 | 16.10 (<3-74) | .000002 |
| <618 | 112 | 4.43 (<3-55) | |
| Rai stage | |||
| 0, I, II | 131 | 4.29 (<3-64) | .001 |
| III, IV | 28 | 10.40 (<3-74) | |
| Age (y) | |||
| <60 | 84 | 4.57 (<3-74) | .068 |
| ≥60 | 75 | 6.81 (<3-70) |
IL-10 indicates interleukin 10; LDH, lactate dehydrogenase.
Using the Mann-Whitney test.
LDH of 618 is the upper value of normal at M. D. Anderson Cancer Center.
Richter transformation (n = 10) and progression to prolymphocytic leukemia (n = 5) occurred in a total of 15 patients (after sample collection). Transformation/progression was more common in patients with at least one cytokine elevation (13 of 74), compared to those patients without elevation (2 of 103) (P = .0005).
Finally, samples from 15 patients (with initially elevated IL-6 and IL-10 levels), who achieved complete remission and were at least 3 months after their last therapy, were available. Both IL-6 and IL-10 levels were within normal range in these patients at this time point.
Correlation of serum IL-6 and IL-10 levels and other prognostic features with survival
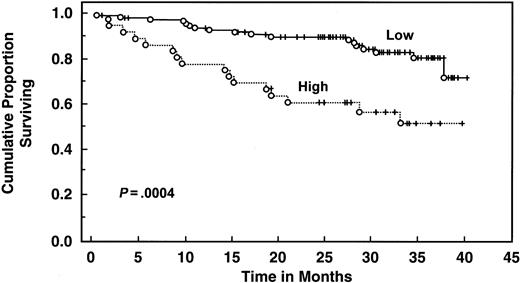
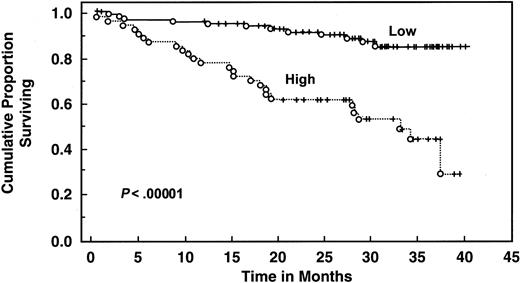
With a median follow-up of 30 months, 135 patients are still alive. Patients with elevated serum IL-6 levels (>4.30 pg/mL) had decreased 2- and 3-year survival (61% and 51%, respectively) compared with patients with low levels (median survival not reached, 2-year survival 90%, 3-year survival 81%, log rank P = .00043) (Table 4 and Figure3). Elevated serum IL-10 level (>10 pg/mL) was also associated with worse survival (median survival 32 months, 2-year and 3-year survival 62% and 45%, respectively) than patients with lower levels (median survival not reached, 2-year and 3-year survival of 92% and 85%, respectively;P < .000001) (Table 4 and Figure4). When both cytokines were used to predict survival, 3 groups were formed according to the number of cytokines increased: zero, one, or both elevated, with median survival not reached, 36 months, and 19 months (P < .00001) (Figure 5). Elevated Rai stage, elevated β2-microglobulin, and previous treatment correlated with shorter survival (Table 4).
Survival in patients with CLL according to characteristics
| Variable . | No. patients . | Dead (%) . | Median survival (mo) . | 2-y survival (%) (95% CI) . | 3-y survival (%) (95% CI) . | P value . |
|---|---|---|---|---|---|---|
| Age | ||||||
| ≤60 y | 92 | 16 (17) | NR | 87 (79-95) | 70 (60-80) | .065 |
| >60 y | 85 | 26 (31) | 37 | 75 (67-83) | 64 (51-77) | |
| Rai stage | ||||||
| 0 | 44 | 2 (5) | NR | 95 (89-100) | 95 (89-100) | |
| I | 78 | 11 (14) | NR | 93 (87-99) | 80 (67-93) | |
| II | 24 | 11 (46) | 30 | 60 (40-80) | 46 (22-70) | <0.00001 |
| III | 8 | 4 (50) | 25 | 63 (29-97) | 47 (11-83) | |
| IV | 23 | 14 (61) | 19 | 43 (22-64) | 37 (15-59) | |
| β2-microglobulin | ||||||
| <2 | 33 | 1 (3) | NR | 96 (89-100) | 96 (89-100) | |
| 2 to <3 | 55 | 6 (11) | NR | 93 (86-100) | 86 (75-96) | |
| ≥3 to <4 | 35 | 10 (29) | NR | 78 (63-93) | 63 (43-83) | <0.00001 |
| ≥4 | 30 | 19 (63) | 18 | 43 (25-61) | 25 (1-49) | |
| Prior therapy | ||||||
| No | 102 | 8 (8) | NR | 94 (89-97) | 92 (86-98) | <0.00001 |
| Yes | 75 | 34 (45) | 33 | 63 (52-74) | 46 (32-60) | |
| LDH | ||||||
| Normal | 125 | 20 (16) | NR | 86 (80-92) | 80 (71-89) | <0.00001 |
| Elevated | 34 | 20 (59) | 26 | 54 (36-72) | 33 (13-53) | |
| Serum IL-10 | ||||||
| ≤10 pg/mL | 105 | 12 (11) | NR | 92 (87-97) | 5 (77-93) | <0.00001 |
| >10 pg/mL | 54 | 26 (48) | 32 | 62 (49-75) | 45 (29-61) | |
| Serum IL-6 | ||||||
| ≤4.30 pg/mL | 115 | 18 (16) | NR | 90 (84-96) | 81 (72-91) | 0.0004 |
| >4.30 pg/mL | 36 | 16 (44) | NR | 61 (45-77) | 51 (33-69) | |
| IL-6 + IL-10 | ||||||
| Neither elevated | 75 | 6 (8) | NR | 96 (91-100) | 89 (81-97) | <0.00001 |
| One elevated | 42 | 14 (33) | 36 | 75 (62-88) | 50 (33-67) | |
| Both elevated | 16 | 10 (63) | 19 | 50 (25-75) | 27 (0-54) |
| Variable . | No. patients . | Dead (%) . | Median survival (mo) . | 2-y survival (%) (95% CI) . | 3-y survival (%) (95% CI) . | P value . |
|---|---|---|---|---|---|---|
| Age | ||||||
| ≤60 y | 92 | 16 (17) | NR | 87 (79-95) | 70 (60-80) | .065 |
| >60 y | 85 | 26 (31) | 37 | 75 (67-83) | 64 (51-77) | |
| Rai stage | ||||||
| 0 | 44 | 2 (5) | NR | 95 (89-100) | 95 (89-100) | |
| I | 78 | 11 (14) | NR | 93 (87-99) | 80 (67-93) | |
| II | 24 | 11 (46) | 30 | 60 (40-80) | 46 (22-70) | <0.00001 |
| III | 8 | 4 (50) | 25 | 63 (29-97) | 47 (11-83) | |
| IV | 23 | 14 (61) | 19 | 43 (22-64) | 37 (15-59) | |
| β2-microglobulin | ||||||
| <2 | 33 | 1 (3) | NR | 96 (89-100) | 96 (89-100) | |
| 2 to <3 | 55 | 6 (11) | NR | 93 (86-100) | 86 (75-96) | |
| ≥3 to <4 | 35 | 10 (29) | NR | 78 (63-93) | 63 (43-83) | <0.00001 |
| ≥4 | 30 | 19 (63) | 18 | 43 (25-61) | 25 (1-49) | |
| Prior therapy | ||||||
| No | 102 | 8 (8) | NR | 94 (89-97) | 92 (86-98) | <0.00001 |
| Yes | 75 | 34 (45) | 33 | 63 (52-74) | 46 (32-60) | |
| LDH | ||||||
| Normal | 125 | 20 (16) | NR | 86 (80-92) | 80 (71-89) | <0.00001 |
| Elevated | 34 | 20 (59) | 26 | 54 (36-72) | 33 (13-53) | |
| Serum IL-10 | ||||||
| ≤10 pg/mL | 105 | 12 (11) | NR | 92 (87-97) | 5 (77-93) | <0.00001 |
| >10 pg/mL | 54 | 26 (48) | 32 | 62 (49-75) | 45 (29-61) | |
| Serum IL-6 | ||||||
| ≤4.30 pg/mL | 115 | 18 (16) | NR | 90 (84-96) | 81 (72-91) | 0.0004 |
| >4.30 pg/mL | 36 | 16 (44) | NR | 61 (45-77) | 51 (33-69) | |
| IL-6 + IL-10 | ||||||
| Neither elevated | 75 | 6 (8) | NR | 96 (91-100) | 89 (81-97) | <0.00001 |
| One elevated | 42 | 14 (33) | 36 | 75 (62-88) | 50 (33-67) | |
| Both elevated | 16 | 10 (63) | 19 | 50 (25-75) | 27 (0-54) |
CLL indicates chronic lymphocytic leukemia; CI, confidence interval; NR, not reached; LDH, lactate dehydrogenase; IL-10, interleukin 10; IL-6, interleukin 6.
Survival of 151 CLL patients stratified by IL-6 levels.
Distributions were estimated, using the Kaplan-Meier method. Tick marks indicate the point of last follow-up for one or more patients who did not die. Low refers to IL-6 levels ≤4.3 pg/mL. High refers to IL-6 levels >4.3 pg/mL.
Survival of 151 CLL patients stratified by IL-6 levels.
Distributions were estimated, using the Kaplan-Meier method. Tick marks indicate the point of last follow-up for one or more patients who did not die. Low refers to IL-6 levels ≤4.3 pg/mL. High refers to IL-6 levels >4.3 pg/mL.
Survival of 159 CLL patients stratified by IL-10 levels.
Distributions were estimated, using the Kaplan-Meier method. Tick marks indicate the point of last follow-up for one or more patients who did not die. Low refers to IL-10 levels ≤10 pg/mL. High refers to IL-10 levels >10 pg/mL.
Survival of 159 CLL patients stratified by IL-10 levels.
Distributions were estimated, using the Kaplan-Meier method. Tick marks indicate the point of last follow-up for one or more patients who did not die. Low refers to IL-10 levels ≤10 pg/mL. High refers to IL-10 levels >10 pg/mL.
Survival of 133 CLL patients stratified by IL-6 and IL-10 levels.
Distributions were estimated, using the Kaplan-Meier method. Tick marks indicate the point of last follow-up for one or more patients who did not die. “None” means that neither IL-6 nor IL-10 were elevated. “One” means that either IL-6 or IL-10 were elevated. “Both” means that both IL-6 and IL-10 were elevated. Elevated IL-6 levels are those >4.3 pg/mL; elevated IL-10 levels are those >10 pg/mL.
Survival of 133 CLL patients stratified by IL-6 and IL-10 levels.
Distributions were estimated, using the Kaplan-Meier method. Tick marks indicate the point of last follow-up for one or more patients who did not die. “None” means that neither IL-6 nor IL-10 were elevated. “One” means that either IL-6 or IL-10 were elevated. “Both” means that both IL-6 and IL-10 were elevated. Elevated IL-6 levels are those >4.3 pg/mL; elevated IL-10 levels are those >10 pg/mL.
When Cox regression multivariate analysis was performed (with IL-6, IL-10, and β2-microglobulin categorized as ≤4.3 pg/mL versus >4.3 pg/mL [for IL-6], ≤10 pg/mL versus >10 pg/mL [for IL-10] and ≤2 mg/L versus >2 mg/L [for β2-microglobulin]) in Cox regression models, the combination of both cytokines (P = .001) was an independent prognostic variable as was previous treatment (P < .001) (Table 5). However, when β2-microglobulin was added to the Cox regression model, neither IL-6 nor IL-10 was selected as an independent variable (data not shown) (Figure 6). Similarly, IL-6 (as well as previous treatment, LDH, and RAI stage), and IL-10 (as well as previous treatment and LDH) were independent prognostic factors in multivariate analysis when analyzed individually unless β2-microglobulin was included in the analysis, in which case these cytokines were no longer selected. (If IL-6 and IL-10 were both entered into the multivariate analysis as individual factors, IL-10, but not IL-6, was selected as an independent prognostic factor.)
Multivariate analysis for overall survival
| Analysis . | Variable . | Multivariate P value . | Relative risk (95% confidence interval) . |
|---|---|---|---|
| Cox regression for overall survival | Combined IL-6 and IL-10 levels (none, one, or both elevated) | 0.001 | 2.6 (2.1-3.1) |
| Prior therapy (yes or no) | <0.001 | 6.7 (5.9-7.5) |
| Analysis . | Variable . | Multivariate P value . | Relative risk (95% confidence interval) . |
|---|---|---|---|
| Cox regression for overall survival | Combined IL-6 and IL-10 levels (none, one, or both elevated) | 0.001 | 2.6 (2.1-3.1) |
| Prior therapy (yes or no) | <0.001 | 6.7 (5.9-7.5) |
β2-microglobulin not included in the analysis.
IL-6 indicates interleukin 6; IL-10, interleukin 10.
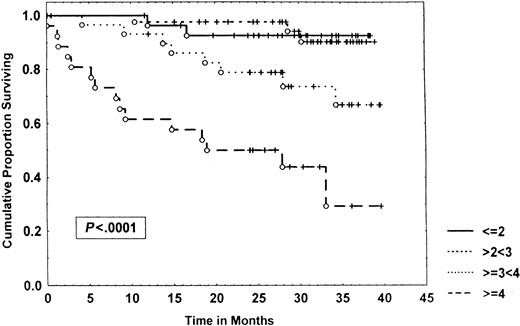
Survival of 153 CLL patients stratified by β2-microglobulin levels.
Data were analyzed, using the Kaplan-Meier method. Tick marks indicate the point of last follow-up for one or more patients who did not die. The numbers to the right of the graph refer to the β2-microglobulin level in milligram per liter.
Survival of 153 CLL patients stratified by β2-microglobulin levels.
Data were analyzed, using the Kaplan-Meier method. Tick marks indicate the point of last follow-up for one or more patients who did not die. The numbers to the right of the graph refer to the β2-microglobulin level in milligram per liter.
Determination of intracytoplasmic cytokine synthesis by B-CLL cells
In both previously treated and untreated CLL patients, the percentage of IL-10–positive B cells was significantly higher among the B cells that were not derived from the leukemic clone (CD19+, CD5−) compared to those derived from the leukemic clone (CD19+, CD5+) (mean ± SEM = 14.6% ± 8.7 versus 2.0% ± 1.9% [P = .03, Wilcoxon signed rank test] [untreated patients, n = 7] and mean ± SEM = 58.8% ± 18.6% versus 6.0% ± 2.2% [P = .02] [previously treated patients, n = 7]). In regard to IL-6, the source of IL-6 did not appear to be either the malignant or the normal B cells (mean ± SEM = 1.96% ± 1.0 [CD19+, CD5− cells] versus 0% ± 0% [CD19+, CD5+ cells] [P = not significant] [untreated patients, n = 9] and mean ± SEM = 0% ± 0% [CD19+, CD5− cells] versus 0% ± 0% [CD19+, CD5+ cells] [P = not significant] [previously treated patients, n = 7]).
Discussion
IL-6 in lymphoproliferative disorders
Several reports have demonstrated that serum IL-6 levels are elevated in patients with aggressive NHL14-17,24,43-46 and correlate with shorter failure-free survival.14,15,17 In our current study, we analyzed the serum levels of IL-6 in 151 CLL patients and demonstrated that a serum level of IL-6 above the range in our normal control subjects (4.30 pg/mL) is found in 24% of those individuals. A previous study reported that only 15% of patients with low-grade lymphoma have elevated serum IL-6 levels, but the cut point used in that study was higher (5 pg/mL)25 than the one in our study (4.30 pg/mL). In contrast, 35% to 78% of the patients with aggressive lymphomas have elevated serum IL-6 levels.14,15,17,23 43-47
We found that, in CLL, high serum IL-6 levels correlated with unfavorable phenotypic features of the disease such as advanced Rai stage, older age, previous treatment, and elevated β2-microglobulin and serum LDH. Similar results have been reported in diffuse large cell lymphomas,14,15 indolent lymphomas,19 and in Hodgkin disease.17The correlation between the elevation of serum IL-6 levels and β2-microglobulin may be secondary to increased proliferation of lymphoma cells because of stimulation by IL-6,4,48,49 causing an increase in the turnover of the membrane-bound β2-microglobulin. In addition, β2-microglobulin is a major histocompatibility complex (MHC) class I protein, and IL-6 can increase the expression of MHC class I genes in human fibroblasts50 and in colon carcinoma cells.51
Serum IL-6 levels show significant correlation with the overall survival. The 3-year survival for the group of patients with an elevated serum IL-6 was 51% (95% confidence interval [CI], 33%-69%), whereas the 3-year survival was 81% (95% CI, 72%-91%) in patients with normal serum IL-6 levels (P = .0004) (Figure 3). Similar correlation with survival has been seen in diffuse large cell lymphomas.14,15 However, in the latter disorder, serum IL-6 levels tend to be elevated in a much larger proportion of patients and also correlated with complete remission rate and with failure-free survival.15 It therefore appears that IL-6 levels are more often elevated in the more aggressive lymphomas, and it is conceivable that measurement of serum IL-6 levels may help to identify a subgroup of patients whose disease is beginning to evolve to a more aggressive form even though morphologic evidence of transformation is not yet evident. In keeping with this notion, Richter transformation and/or evolution to prolymphocytic leukemia was significantly more common in our CLL patients who had elevation of at least one cytokine (IL-6 and/or IL-10) compared to those without cytokine elevation.
The source and role of IL-6 in patients with NHL and CLL is currently being investigated. Evidence from the literature suggests that both the lymphoma cells themselves and benign reactive lymphocytes in lymphomatous nodes can produce IL-6.4-9,44,47,52 In CLL, however, leukemic cells do not appear to be the source of IL-6.53 As to the role of IL-6 in CLL, several investigators54 55 have suggested that this molecule inhibits proliferation but prolongs survival (by suppressing apoptosis) of CLL cells.
IL-10 in lymphoproliferative disorders
Several other reports have demonstrated that serum IL-10 levels are elevated in patients with aggressive NHL26,32,35 and Hodgkin disease36 and are associated with a shorter failure-free survival and poor prognostic features. The source of IL-10, like that of IL-6, appears to be both the malignant cells as well as immunoregulatory cells in NHL.52 In our current study, we analyzed the serum levels of IL-10 in 159 CLL patients by using a methodology that detects both viral and human IL-10. We demonstrate that a serum level of IL-10 greater than 10 pg/mL is found in 34% of our patients. High serum IL-10 levels correlated with unfavorable phenotypic features of the disease such as advanced Rai stage, previous treatment, and elevated β2-microglobulin and serum LDH. Serum IL-10 levels also show significant correlation with the overall survival. The 3-year survival for the group of patients with an elevated serum IL-10 was 45% (95% CI, 29%-61%), whereas the 3-year survival was 85% (95% CI, 77%-93%) in patients with normal serum IL-10 levels (P < .000001) (Figure 4).
The source of IL-10 in CLL appears to be polyclonal B cells rather than the leukemic cells.53 Study of the role of IL-10 in CLL has suggested that this molecule inhibits proliferation.56In regard to survival, some investigators57 suggest that IL-10 prevents apoptotic death of CLL cells, whereas58others suggest that it enhances it.
Interestingly, we have noted that assays measuring both human and viral IL-10 yield significantly higher serum IL-10 levels in CLL patients than assays measuring only human IL-10. A similar phenomenon has been described in NHL.35 In that disease, levels of IL-10, as assessed by assays recognizing both human and viral forms, were found to be an independent prognostic factor in multivariate analysis; human IL-10 levels showed little to no correlation with prognosis.32-35 Because viral IL-10 is an EBV-derived molecule, we are currently investigating whether EBV is present and plays a role in CLL, as well as other lymphoid malignancies.
Combined IL-6 and IL-10
When a combination of IL-6 and IL-10 levels was used, 3 different prognostic groups emerged: no elevation of these cytokines, elevation of one of them, or both, with a 3-year survival of 89%, 50%, and 27%, respectively (P < .00001) (Figure 5). The combination of these cytokines was also an independent prognostic variable in multivariate analysis that incorporated well-established factors such as Rai stage, prior treatment, age, and LDH. However, when β2-microglobulin was added to the analysis, it emerged as the most important prognostic factor, and IL-6/IL-10 were no longer selected. These results confirm a report by Keating et al59 that showed that β2-microglobulin is a potent prognostic factor for CLL. Similar results have been found in other malignancies. β2-Microglobulin is an MHC I-type molecule, and its levels may be regulated, at least in part, by endogenous cytokines. β2-Microglobulin levels may, therefore, reflect the convergence of several cytokines, as well as other pathways.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Razelle Kurzrock, Department of Bioimmunotherapy, Box 302, The University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal