Abstract
All-trans retinoic acid (tRA) and arsenic trioxide (As2O3) induce non–cross-resistant complete clinical remission in patients with acute promyelocytic leukemia with t(15;17) translocation and target PML-RARα, the leukemogenic protein, by different pathways suggesting a possible therapeutic synergism. To evaluate this possibility, this study examined the effect of As2O3 on tRA-induced differentiation and, conversely, the effect of tRA on As2O3-induced apoptosis. As2O3 at subapoptotic concentrations (0.5 μM) decreased tRA-induced differentiation in NB4 cells but synergized with atRA to induce differentiation in tRA-resistant NB4 subclones MR-2 and R4 cells as measured by nitroblue tetrazolium reduction and tRA-inducible genes (TTGII, RARβ, RIG-E). tRA cleaved PML-RARα into distinct fragments in NB4 but not in tRA-resistant MR-2 or R4 cells, whereas As2O3 completely degraded PML-RARα in all 3 cell lines. As2O3-induced apoptosis was decreased by tRA pretreatment of NB4 cells but not of R4 cells and was associated with a strong induction of Bfl-1/A1 expression, a Bcl-2 protein family member. Severe combined immunodeficient mice bearing NB4 cells showed an additive survival effect after sequential treatment, but a toxic effect was observed after simultaneous treatment with tRA and As2O3. These data suggest that combined As2O3 and tRA treatment may be more effective than single agents in tRA-resistant patients. Although in vitro data do not always translate to in vivo response, toxicity and potential drug antagonism may be diminished by decreasing the concentration of As2O3 when given at the same time with therapeutic levels of tRA.
Introduction
Acute promyelocytic leukemia (APL) is a specific type of acute myeloid leukemia characterized by the t(15;17) translocation that fuses the PML gene on chromosome 15 to the retinoic acid receptor α (RARα) gene on chromosome 17 to form the fusion gene and leukemogenic protein PML-RARα.1,2 The specific sensitivity of APL cells to all-trans retinoic acid (tRA)-induced differentiation has been exploited to achieve a 90% remission rate with a projected 60% to 70% cure of patients with APL when combined with chemotherapy.3-7 However, the disease may relapse with resistance to further tRA and chemotherapy treatment.8 The recent discovery that treatment with As2O3induces durable remission in APL patients in relapse after tRA or chemotherapy has provided a novel therapy for APL patients.9-11 However, the reported chronic toxicities and carcinogenicity of As2O3 has hampered its acceptance as a first-choice drug,12 even though limited side effects were found in relapsed APL patients successfully treated with As2O3.10 11 Consideration of the facts that the toxicity of As2O3 is dose dependent and reversible and that small amounts of As2O3 have been used in traditional Chinese medicine suggests that it may be possible to use As2O3 as a first-choice drug if low concentrations prove effective.
Arsenic trioxide induces both apoptosis and partial differentiation in APL cells in vitro and in vivo.11,13-15 More than 0.5 μM As2O3 is required for induction of H2O2-mediated apoptosis in NB4 cells derived from t(15;17) APL.16 The H2O2concentration in cells is regulated mainly by selenium-dependent glutathione peroxidase (Gpx) activity. Because standard tissue culture conditions are deficient in selenium, the H2O2-removing activity of Gpx is low.17 By increasing selenium to physiologic concentration in the tissue culture medium the activity of Gpx increases; consequently, the H2O2 content is lowered and the apoptotic activity of As2O3 (at low concentration) is diminished.16 In contrast to NB4 cells, APL cells in primary culture are less sensitive and variable to 1 μM As2O3-induced apoptosis.11,13,14 18 These observations suggest that differentiation-induced terminal cell division rather than direct apoptosis may play a major role in As2O3-induced remission in patients with APL.
Patients with APL expressing PML-RARα by reverse transcriptase-polymerase chain reaction (RT-PCR) are responsive to As2O3, whereas patients with undetectable PML-RARα are not.19 This observation, along with the fact that As2O3 at concentrations as low as 0.1 μM degrades PML-RARα,13 suggests that this degradative process contributes to the therapeutic effect of As2O3. Because PML-RARα protein has been shown to function as a dominant-negative transcriptional repressor of RARα, As2O3-induced degradation of PML-RARα may allow physiologic tRA concentrations to trigger APL cell differentiation in vivo. tRA and As2O3 appear to degrade PML-RARα through different pathways,20-22suggesting that the combination may be synergistic therapeutically. Because controversial effects have been reported on tRA and As2O3 interactions,14 23 the present studies were developed to define their combined effects on apoptosis and differentiation in tRA-sensitive and tRA-resistant APL cells at the molecular and cellular level.
Materials and methods
Reagents
The tRA was purchased from Sigma Chemical Co (St. Louis, MO). As2O3 (0.1%) was provided by Dr Ting-tong Zhang (Harbin, China). Anti-RARα (F region) antibody RPα(F) was kindly provided by Dr P Chambon (Strasbourg, France).
Cell lines
The NB4 t(15;17) cells were obtained from Dr M. Lanotte (Paris, France)24 and tRA-resistant NB4 derivatives, R4 and MR-2, were developed as reported previously.25HL-60/Res cells were obtained from Dr R. Gallagher (Bronx, NY).26 The cells were cultured in RPMI-1640 medium supplemented with 100 U/mL penicillin, 100 μg/mL streptomycin, 1 mMl-glutamine, and 10% fetal bovine serum. Cells in logarithmic growth were seeded at 1 × 105 cells/mL for studies done in duplicate and repeated at least 3 times. The nitroblue tetrazolium (NBT) reduction assay was performed as previously reported.27
Western blot analysis
Protein extracts (50 μg) prepared with RIPA lysis buffer (50 mM Tris-HCl, 150 mM NaCl, 0.1% sodium dodecyl sulfate [SDS], 1% NP-40, 0.5% sodium deoxycholate, 1 mM PMSF, 100 mM leupeptin, and 2 μg/mL aprotinin, pH 8.0) were separated on an 8% SDS-polyacrylamide gel and transferred to nitrocellulose membranes. The membranes were stained with 0.2% Ponceau S red to ensure equal protein loading and transfer. After blocking with 5% nonfat milk, the membranes were incubated with anti-RARα antibody (Dr. P Chambon). The immunocomplex was visualized by enhanced chemiluminescence (ECL kit, Amersham, Parsipanny, NJ).15
Northern blot analysis
Total RNA was isolated with messenger RNA (mRNA) isolation kit (Gentra, Minneapolis, MN) from 106 cells. Twenty micrograms RNA was sized fractionated on a 1.2% agarose-2.2 M formaldehyde gel, transferred to hybrid-N+ membrane (Amersham) in 20 × standard sodium citrate (SSC), and UV-crosslinked (Stratalinker; Stratagene, La Jolla, CA). Whole length complementary DNA (cDNA) of human myeloperoxidase (MPO),28 RARβ, tissue transglutaminase II (TTGII),29 RIG-E,30Bfl-1/A1,31 and a commercial GAPDH cDNA probe (Ambion, Austin, TX)32 were used for probing. The probes were labeled with a 32P dCTP by random priming to a specific activity of 0.5 to 1 × 109 cpm/μg. The membranes were prehybridized for 4 hours at 42°C in 50% formamide, 6 × sodium chloride, sodium phosphate, EDTA (SSPE), 5 × Denhardt reagent, and 0.2 mg/mL salmon sperm DNA, and hybridized with radiolabeled probe. Membranes were then washed twice in 6 × SSC, 0.1% SDS, followed by a stringent wash with 0.2 × SSC, 0.1% SDS at 65°C.32
Animals
Female severe combined immunodeficient (SCID) mice, 4 to 8 weeks old, were bred and maintained under pathogen-free conditions. The NB4 SCID mouse ascites model was established as in our previous report.33 SCID mice were inoculated with 1 × 106 NB4 cells at passage less than 12. Mice injected with NB4 cells were allowed to develop a tumor burden for 7 days before treatment with various drug regimens. As2O3 was diluted appropriately with phosphate-buffered saline (PBS), pH 7.4, and injected intraperitoneally in tumor-bearing animals. Control (untreated) animals received PBS. The main end point of this study was survival, with each treatment group containing 10 to 20 tumor-bearing animals. The survival of all mice was followed, and a median survival time (MST) was calculated. A percent increase over the life span of control animals was calculated by dividing the MST of a treatment group by the MST of control group. Statistical analysis was performed by a log-rank test and the Wilcoxon test at the 5% significance level.
Results
Effects of As2O3 on NB4 cells and primary culture of APL cells
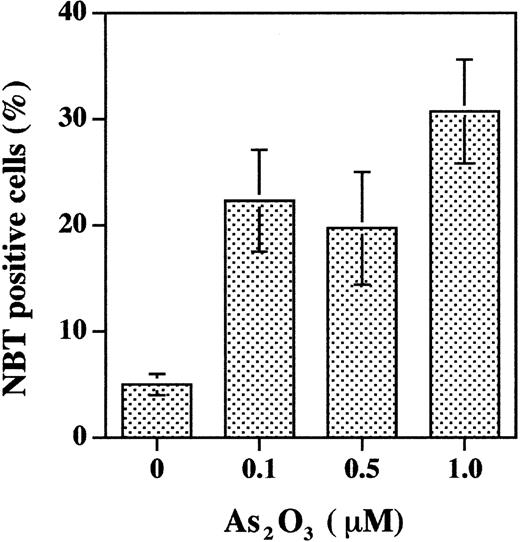
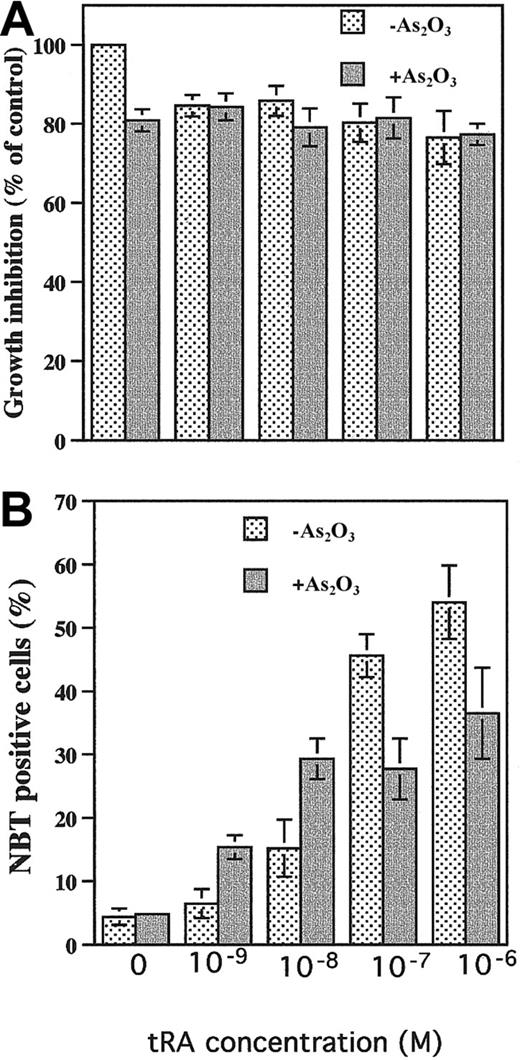
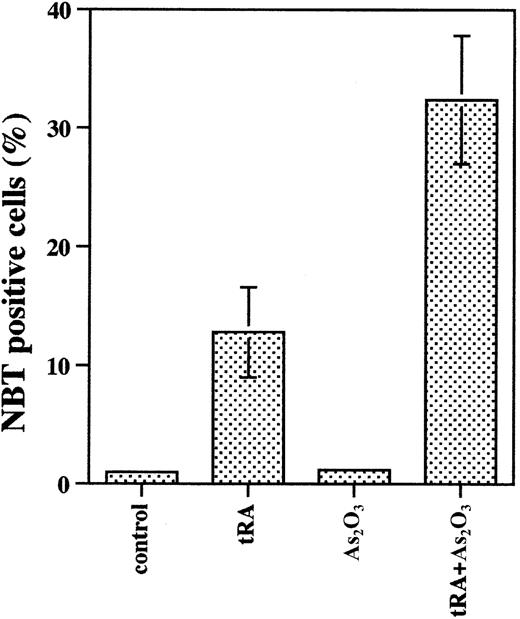
Studies were designed to clarify the observation that As2O3 treatment induces apoptosis with minimal differentiation of NB4 cells, whereas it induces partial differentiation but not apoptosis in primary cultures of APL cells. Treatment of APL cells in primary culture for 6 days with 0.1 to 1 μM As2O3 induced 20% to 30% differentiation (Figure 1) and less than 10% apoptosis (as measured by fluorescence microscopic morphologic change, data not shown). Under standard culture conditions, As2O3 (0.5 μM) did not induce differentiation in NB4 cells (Figure 2B). As2O3 slightly enhanced tRA-induced differentiation when physiologic concentrations of tRA were used (1-10 nM) but decreased tRA-induced differentiation when pharmacologic tRA concentrations were used (0.1-1 μM) (Figure 2B). There was no significant effect of As2O3 on cell number (Figure 2A) or cell viability (> 90% cell viability was observed in all groups). However, the differentiation was less than when tRA was used at therapeutic doses (1 μM). To create in vitro conditions more relevant to in vivo for evaluating the effect of As2O3 on NB4 cells, NB4 cells were precultured in the presence of 100 nM selenium (physiologic concentration) to induce Gpx activity.16 In NB4 cells grown in the presence of selenium for several passages, As2O3treatment (1 μM), which by itself did not induce significant apoptosis16 or differentiation (Figure3), enhanced physiologic (10 nM) tRA-induced differentiation 2-fold (Figure 3).
As2O3 treatment of primary cultured APL blasts induces differentiation.
APL cells in primary culture were treated with indicated concentrations of As2O3 for 6 days. NBT reduction was used to evaluate differentiation. The data are obtained from 3 separate studies.
As2O3 treatment of primary cultured APL blasts induces differentiation.
APL cells in primary culture were treated with indicated concentrations of As2O3 for 6 days. NBT reduction was used to evaluate differentiation. The data are obtained from 3 separate studies.
Effects of As2O3 on tRA-induced differentiation in NB4 cells.
(A) Growth inhibition. NB4 cells were treated with 0.5 μM As2O3 with or without tRA at indicated concentrations for 4 days. (B) Differentiation. The NB4 cells were treated with indicated concentrations of tRA with or without 0.5 μM As2O3 for 4 days. NBT reduction was used to measure differentiation.
Effects of As2O3 on tRA-induced differentiation in NB4 cells.
(A) Growth inhibition. NB4 cells were treated with 0.5 μM As2O3 with or without tRA at indicated concentrations for 4 days. (B) Differentiation. The NB4 cells were treated with indicated concentrations of tRA with or without 0.5 μM As2O3 for 4 days. NBT reduction was used to measure differentiation.
Selenium supplementation enhanced differentiation of NB4 cells treated with As2O3 combined with tRA.
NB4 cells were cultured in medium with 100 nM selenium for several passages and then treated with 1 μM As2O3plus 10 nM tRA for 4 days. NBT reduction was used to determine differentiation induction.
Selenium supplementation enhanced differentiation of NB4 cells treated with As2O3 combined with tRA.
NB4 cells were cultured in medium with 100 nM selenium for several passages and then treated with 1 μM As2O3plus 10 nM tRA for 4 days. NBT reduction was used to determine differentiation induction.
Degradation of PML-RARα and induction of differentiation
Both tRA and As2O3 degrade PML-RARα, whereas RARα levels may be decreased by tRA and not As2O3 treatment. As2O3degrades PML-RARα without an accumulation of an intermediate, whereas tRA treatment results in the accumulation of a truncated product termed ΔPML-RARα (Figure 4A). The cleavage product is not detectable in NB4 cells treated with low concentrations of tRA alone or when combined with As2O3 but is formed following therapeutic (pharmacologic) concentrations of tRA, even in the presence of As2O3. As2O3 partially inhibits tRA induction of Bfl-1/A1, RIG-E, RARβ, TTGII, and the down-regulation of MPO expression, which are known to be modulated by tRA in NB4 cells (Figure4B). The inhibition by As2O3 could be abrogated by increasing the concentration of tRA so that at a concentration of 1 μM the expression of these genes was only minimally diminished (Figure 4B).
Effects of As2O3 on tRA-induced PML-RARα degradation and gene modulation.
(A) PML-RARα and RARα protein levels. Anti-RARα antibody was used to detect PML-RARα and RARα. (B) Northern blot analysis of tRA inducible gene expression. NB4 cells were treated with different concentrations of tRA with or without 0.5 μM As2O3 for 4 days.
Effects of As2O3 on tRA-induced PML-RARα degradation and gene modulation.
(A) PML-RARα and RARα protein levels. Anti-RARα antibody was used to detect PML-RARα and RARα. (B) Northern blot analysis of tRA inducible gene expression. NB4 cells were treated with different concentrations of tRA with or without 0.5 μM As2O3 for 4 days.
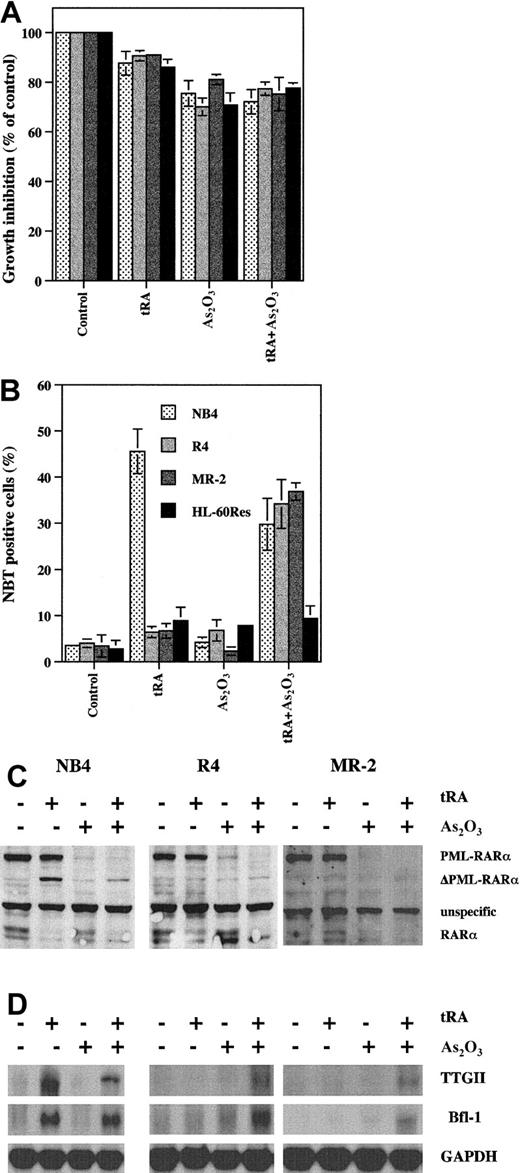
As2O3 increases tRA-induced differentiation in tRA-resistant APL cells
MR-2 and R-4 are NB4 subclones that are resistant to tRA-induced differentiation.25 Treatment with tRA at pharmacologic concentrations in these cells does not induce differentiation as determined by NBT reduction or the expression of retinoic acid responsive genes and does not cleave PML-RARα (Figure5). On the other hand, As2O3 completely degrades PML-RARα in these cells but does not induce cell differentiation or the expression of retinoic acid target genes. However, combining As2O3 with tRA induced differentiation and the expression of TTGII and Bfl-1/A1 in the resistant cells as in the parental sensitive NB4 cells. These data support the concept that tRA resistance can be at least partially overcome by As2O3-induced degradation of PML-RARα, diminishing the dominant effect of PML-RARα over RARα. Interestingly, RARα levels appear to be decreased in the presence of tRA or tRA with or without As2O3, whereas As2O3 had a minimal effect (Figure 5C). In contrast, As2O3 does not overcome tRA differentiation resistance in HL-60/Res cells with mutated RARα.
The combined effect of tRA and As2O3 on differentiation induction, gene modulation, and PML-RARα degradation in tRA-resistant APL cells.
(A) Growth inhibition. R4, MR-2, and HL-60/Res were treated with 0.5 μM As2O3 with or without 10−7 tRA for 4 days. (B) Differentiation. NBT reduction was used to measure differentiation ability. R4, MR-2, and HL-60/Res were treated with 0.5 μM As2O3 with or without 10−7 tRA for 4 days. (C) PML-RARα and RARα protein levels. NB4, R4, and MR-2 cells were treated with 0.5 μM As2O3 with or without 10−7 tRA for 4 days. Anti-RARα antibody was used to detect PML- RARα and RARα. (D) Gene expression. NB4, R4, and MR-2 cells were treated with 0.5 μM As2O3 with or without 10−7 tRA for 4 days. Northern blot analysis was used to measure gene expression.
The combined effect of tRA and As2O3 on differentiation induction, gene modulation, and PML-RARα degradation in tRA-resistant APL cells.
(A) Growth inhibition. R4, MR-2, and HL-60/Res were treated with 0.5 μM As2O3 with or without 10−7 tRA for 4 days. (B) Differentiation. NBT reduction was used to measure differentiation ability. R4, MR-2, and HL-60/Res were treated with 0.5 μM As2O3 with or without 10−7 tRA for 4 days. (C) PML-RARα and RARα protein levels. NB4, R4, and MR-2 cells were treated with 0.5 μM As2O3 with or without 10−7 tRA for 4 days. Anti-RARα antibody was used to detect PML- RARα and RARα. (D) Gene expression. NB4, R4, and MR-2 cells were treated with 0.5 μM As2O3 with or without 10−7 tRA for 4 days. Northern blot analysis was used to measure gene expression.
Pretreatment with tRA decreases As2O3-induced apoptosis in tRA-sensitive NB4 but not tRA-resistant cells
The induction of differentiation in APL cells treated with combined As2O3 and tRA is dose dependent and may be antagonistic or additive (Figure 2). Similar considerations are necessary when evaluating the effect of the drug combination on the induction of apoptosis. As2O3-induced apoptosis in NB4 cells is diminished by pretreatment with tRA and is time related (the longer the pretreatment with tRA the less apoptosis is induced by As2O3 treatment) (Figure6A). This antagonism is not demonstrated in the tRA-resistant R4 cell line. One possible explanation is that Bfl-1/A1 expression, a member of the Bcl-2 family, is induced by tRA in NB4 cells despite a fall in Bcl-2 protein level and may account for the inhibition of As2O3-induced apoptosis (Figure6B,C). In contrast, Bfl-1 is not induced by tRA in R4 cells. Thus, despite maintenance of Bcl-2 protein, apoptosis is significant following As2O3 treatment. Thus, the induction of Bfl-1 may contribute to a greater extent than Bcl-2 to the inhibitory effect of tRA on As2O3-induced apoptosis.
Effects of tRA pretreatment on As2O3-induced apoptosis.
(A) Apoptosis. NB4 and R4 cells were treated with 1 μM tRA for indicated times and washed with PBS to remove tRA; then the cells were treated with 2 μM As2O3 for 24 hours. Apoptotic cells were measured by morphologic changes using fluorescence microscopy. The values were mean plus SE of 3 independent experiments. (B) Western blot analysis of Bcl-2 levels after treatment with 1 μM tRA for indicated times. (C) Northern blot analysis of Bfl-1/A1 expression after treatment with tRA for indicated times.
Effects of tRA pretreatment on As2O3-induced apoptosis.
(A) Apoptosis. NB4 and R4 cells were treated with 1 μM tRA for indicated times and washed with PBS to remove tRA; then the cells were treated with 2 μM As2O3 for 24 hours. Apoptotic cells were measured by morphologic changes using fluorescence microscopy. The values were mean plus SE of 3 independent experiments. (B) Western blot analysis of Bcl-2 levels after treatment with 1 μM tRA for indicated times. (C) Northern blot analysis of Bfl-1/A1 expression after treatment with tRA for indicated times.
Combined effect of tRA and As2O3 in SCID mice bearing NB4 cells
The in vivo effect on survival in SCID mice bearing NB4 cells following treatment with tRA or As2O3 given alone, sequentially, or simultaneously is shown in Table1. As2O3 (8 mg/kg) or tRA (10 mg/kg), administered every other day for a total of 8 courses, increased the life span of the host to a similar extent (35%-39%). Sequential treatment of SCID mice with either agent first for 8 courses followed by the other for an additional 8 courses resulted in an additive outcome (70%-80% increase in life span), regardless of the sequence of treatment. However, combined treatment with As2O3 and tRA caused toxicity with weight loss and early treatment-associated death.
Effects of combined or sequential use of As2O3 and ATRA on survival time of SCID mouse–NB4 cell ascites model
| Protocol no. . | Sample no. . | Mean survival (d ± SD) . | % Prolongation compared with control . |
|---|---|---|---|
| A | 15 | 21.2 ± 1.6 | |
| B | 15 | 29.6 ± 3.5 | 39.9%* |
| C | 9 | 28.6 ± 3.1 | 35.2%* |
| D | 11 | 36.4 ± 5.1 | 71.9%* |
| E | 12 | 38.5 ± 4.8 | 81.9%* |
| F | 11 | 18.8 ± 6.5 |
| Protocol no. . | Sample no. . | Mean survival (d ± SD) . | % Prolongation compared with control . |
|---|---|---|---|
| A | 15 | 21.2 ± 1.6 | |
| B | 15 | 29.6 ± 3.5 | 39.9%* |
| C | 9 | 28.6 ± 3.1 | 35.2%* |
| D | 11 | 36.4 ± 5.1 | 71.9%* |
| E | 12 | 38.5 ± 4.8 | 81.9%* |
| F | 11 | 18.8 ± 6.5 |
Protocol numbers A, B, and C correspond to normal saline, As2O3 (8 mg/kg) or tRA (10 mg/kg) injected intraperitoneally (IP), once every 2 days, totally 10 times, respectively; protocol D: As2O3 (8 mg/kg) was used first once every 2 days, totally 8 times, followed by tRA (10 mg/kg) injected IP once every 2 days, totally 8 times; protocol E, tRA (10 mg/kg) was used first once every 2 days, totally 8 times, followed by As2O3 (8 mg/kg) injected IP once every 2 days, totally 8 times; protocol F: As2O3(8 mg/kg) combined with tRA (10 mg/kg) was injected IP once every 2 days, totally 10 times.
P < .01.
Discussion
It has been reported that tRA induces differentiation of APL cell lines and APL blasts in vivo and in vitro,3,34 whereas As2O3 induces apoptosis in both tRA-sensitive and tRA-resistant APL cell lines in vitro.13,14 However, we and others have shown that As2O3 induces NB4 cell apoptosis through a redox-mediated pathway16 that is PML-RARα independent15,35 and is diminished by either increased serum,23 selenium supplementation,16 or addition of granulocyte-macrophage colony-stimulating factor.36 Therefore, the dramatic in vitro induction of apoptosis by As2O3 may be minimized by physiologic growth and antiapoptotic factors that exist in vivo. Indeed, although As2O3 is capable of inducing apoptosis in primary APL cell cultures,13,14recent reports indicate that primary cultured human APL cells18 and transgenic mice APL cells37 are less sensitive to 1 μM As2O3-induced apoptosis than serially cultured NB4 cells. These considerations prompt the question of which is the main mechanism underlying As2O3-induced remission in APL patients. We report here, in agreement with the results of others,18,37that primary cultured APL cells undergo partial differentiation in response to 0.1 to 1 μM As2O3 (Figure 1). Although As2O3 does not induce differentiation in NB4 cells (Figure 2), even in presence of selenium (Figure 3), our results show that it enhanced differentiation induction by near-physiologic tRA concentrations (1 to 10 nM tRA) (Figures 2and 3). Note that in Figure, 3 As2O3 was tested at 1 μM, which normally induces NB4 cell apoptosis, but with the addition of selenium this concentration caused differentiation (Figure3) instead of apoptosis (data not shown). Taken together, these findings suggest that the As2O3 potentiation of physiologic tRA-induced differentiation may account in large part for the induction of APL remission by As2O3. Such potentiating effect of As2O3 may be explained by As2O3-induced PML-RARα degradation, as previously suggested by others.38
We found that As2O3 potentiated NB4 cell differentiation by 1 to 10 nM tRA but suppressed differentiation by 0.1 to 10 μM tRA (Figure 2; differentiation was scored using the NBT reduction assay). What might be the basis for this biphasic effect of As2O3? As2O3 induced extensive PML-RARα degradation at all concentrations of tRA tested (Figure 4A, right), indicating that the explanation lies elsewhere. When we monitored the tRA induction of differentiation target genes,39 we found that the tRA dose dependence for gene induction (in absence or presence of As2O3, Figure 4) did not agree well with the NBT reduction score. For instance, in contrast to a dose-dependent increase in NBT reduction (Figure 2), the extent of gene modulation by 10 nM and 1 μM tRA was about the same (Figure 4). In addition, As2O3 inhibited gene induction by 10 nM tRA but not 1 μM tRA (Figure 4). We do not understand the basis for this discrepancy between the 2 differentiation assays. However, as explained below, we have a hypothetical explanation for the observed relationship between tRA dose and target gene modulation.
The tRA treatment of NB4 cells results in partial PML-RARα proteolysis, with formation of a stable ∼90-kd product, which we refer to as ΔPML-RARα (Figure 4). This is in marked contrast to As2O3 treatment, which results in extensive PML-RARα proteolysis without accumulation of ΔPML- RARα (Figure4). On the basis of its recognition by an anti-C terminal RARα antibody and its apparent size, we estimate that ΔPML-RARα may lack the RING finger and B-boxes of the PML moiety, while retaining the coiled-coil domain. PML, through its RING finger, interacts with key regulatory proteins targeting them to nuclear bodies, and these events are disrupted by PML-RARα,40,41 suggesting that the predicted loss of the RING finger may allow the resumption of normal PML function (from the remaining allele). The coiled coil domain, which we predict is retained in ΔPML-RARα, has been shown to be essential for the PML-RARα-mediated enhancement of tRA-induced differentiation in hematopoietic cell lines.42 Therefore, we hypothesize that ΔPML-RARα does not act as a dominant-negative receptor and that it functions instead as wild-type RARα, contributing positively to tRA-induced cell differentiation. This is a testable hypothesis that offers an explanation for the observed effects of tRA on differentiation target gene expression. Thus, Figure 4 shows that there is a marked correlation between the amount of ΔPML-RARα formed and the extent of target gene modulation in response to different tRA doses (in absence or presence of As2O3), suggesting that ΔPML-RARα plays a positive role in target gene modulation. Moreover, Figure 5 demonstrates that in tRA-resistant R4 and MR-2 cells, ΔPML-RARα was not formed and cell differentiation (NB4 reduction and target gene induction) was not achieved. As2O3 treatment could partially overcome tRA resistance in R4 and MR-2 cells, presumably due to the induction of extensive PML-RARα breakdown (Figure 5). In these instances, differentiation by 0.1 μM tRA was attained without a detectable increase in ΔPML-RARα formation.
Although As2O3 induction of apoptosis may be diminished by conditions existing in vivo, apoptotic cells have been found in APL patients (whether apoptosis is a first event or follows As2O3-induced differentiation is not known).11 Because As2O3 is used to treat patients with APL who have been or will be treated with tRA, it is important to evaluate As2O3-induced apoptosis after pretreatment with tRA in sensitive or resistant APL cells. tRA pretreatment for 48 hours decreased As2O3-induced apoptosis in tRA-sensitive NB4 cells, but not in tRA-resistant R4 cells (Figure 6). Consistent with previous reports,43,44 we found that Bcl-2 was degraded by tRA in NB4 cells but not in R4 cells (Figure 6B). The degradation of Bcl-2 induced by tRA would be expected to sensitize NB4 cells to As2O3-induced apoptosis; however, as noted above, tRA was inhibitory (Figure 6A). Thus, other antiapoptotic genes modulated by tRA may compensate for the tRA-induced Bcl-2 degradation and protect against cell death. Several antiapoptotic genes identified by differential display, including Bfl-1 and Dad1, are induced by tRA in NB4 cells.39,45 We found that Bfl-1/A1 was rapidly and highly induced by tRA in NB4 cells, but not in R4 cells (Figure 6C). Bfl-1/A1 transfected cells have been shown to be resistant to several apoptotic inducers.31,46 47 Thus, tRA induction of Bfl-1 may in part account for the tRA inhibition of As2O3-induced apoptosis in NB4 cells.
Studies using the SCID mouse NB4 cell ascites model (Table 1) suggest an improved survival when the combination of tRA and As2O3 is used sequentially as compared to either agent alone. These data are not conclusive because the sequential treatments were longer in duration than when individual agents were used; however, they confirm the results obtained in transgenic mice models of APL.37 Sequential treatment where tRA is used prior to As2O3 would be predicted to be antagonistic according to the data in Figure 6A. However, at the concentrations used, the in vivo studies did not demonstrate this antagonism. There was toxicity and decrease in survival when tRA and As2O3 were used simultaneously at maximal therapeutic concentrations. This may be due to the high concentration of As2O3 and tRA used as compared to other in vivo animal studies.37 Therefore, we suggest that reducing the concentrations of tRA and As2O3 may allow therapeutic efficacy with tolerable toxicity. Note that, in vitro, differentiation induction by the combination of low tRA and low As2O3 (10 nM and 0.5 μM, respectively) was optimal, that is, could not be improved by increasing either the tRA or the As2O3concentration (Figures 2 and 3). Clinical studies using these in vitro predicted dose modifications when using combination tRA and As2O3 are required to determine efficacy in the treatment of tRA-resistant patients in relapse or toxicity in de novo patients.
Acknowledgments
We thank the following investigators for sharing their reagents: Pierre Chambon (anti-RARα antibody and mRARβ plasmid); H. Phillip Koeffler (mMPO plasmid); Peter Davies (TTGII plasmid); G. Chinnadurai (Bfl-1/A1 plasmid). We also appreciate the critical reading by Drs Geoge Acs and Rafael Mira-y-Lopez.
Supported by the Gloria and Sidney Danziger Foundation, the Samuel Waxman Cancer Research Foundation, and National Natural Sciences Foundation of China.
Y.J. and L.W. contributed equally to this paper.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Yongkui Jing, Department of Medicine, Division of Medical Oncology, Box 1178, Mount Sinai School of Medicine, 1 Gustave L. Levy Pl, New York, NY 10029-6547; e-mail: jing@msvax.mssm.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal