Abstract
Unrelated cord blood (UCB) is being used as a source of alternative hematopoietic stem cells for transplantation with increasing frequency. From November 1994 to February 1999, 30 UCB transplant procedures were performed for both malignant and nonmalignant diseases in 27 children, aged 0.4 to 17.1 years. Patients received either HLA-matched (n = 3) or 1- or 2-antigen–mismatched (n = 27) UCB following 1 of 2 standardized preparative and graft-versus-host disease regimens (hyperfractionated total body irradiation, cyclophosphamide, and antithymocyte globulin [ATG] with cyclosporine A and methotrexate; or busulfan, melphalan, and ATG with cyclosporine A and prednisone). The median time to neutrophil and platelet engraftment was 27 days (12-60 days) and 75 days (33-158 days) posttransplantation, respectively. No correlation was noted between neutrophil and platelet engraftment and nucleated cells per kilogram, CD34+ cells per kilogram infused, or cytomegalovirus status of recipient. The cumulative probability of acute grade 2 or greater graft-versus-host disease (GVHD) was 37.2%, and of grade 3 or greater GVHD was 8.8%. No patients developed chronic GVHD. CD4, CD19, and natural killer cell recovery was achieved at a median of 12, 6, and 2 months, respectively. CD8 recovery was delayed at a median of 9 months. Normal mitogen response was achieved at 6 to 9 months. The probability of survival, disease-free survival, and event-free survival at 1 year was 52.3% (34.1%-70.5%), 54.7% (34.5%-74.9%) and 49.6% (29.9%-69.4%), respectively. This series of 30 UCB transplants suggests that although CD8 cell recovery is delayed, the pattern of immune reconstitution with UCB is similar to that reported for other stem cell sources.
Introduction
Bone marrow transplantation has become a curative therapy for an increasing number of malignant and nonmalignant diseases.1 Allogeneic donor identification is often the greatest challenge to stem cell transplantation. Although matched related donors offer superior outcomes, a family donor is often unavailable, and an alternative donor must be sought. The unrelated bone marrow transplant can be hampered by donor acquisition delays, donor complications, and significant morbidity in the transplant recipient during and following the transplant procedure. Additionally, the high incidence of acute and chronic graft-versus-host disease (GVHD) in the unrelated bone marrow transplant has yet to be resolved.2 Unrelated donor umbilical cord blood (UCB) transplants offer another option and have been reported with variable success for a variety of diseases.3 The initial reports of related and unrelated donor UCB transplantation have been generally successful. These initial reports, however, have been limited in their broad interpretation, as they are largely anecdotal and often are not the result of controlled clinical trials. The present study used both a standardized preparative and a GHVD regimen in an attempt to address this issue.
Unrelated donor UCB transplantation has been facilitated by the creation of public UCB banks, the first and largest being that established at the New York Blood Center.4-6 Results of the first 562 transplants facilitated by the New York Blood Center's Placental Blood Bank have been reported. An 81% actuarial estimate of neutrophil engraftment by day 42 and an 85% estimate of platelet engraftment by day 180 following transplantation was noted.4 These engraftment data are similar to those reported by others.7-9 Whereas there are no data on controlled clinical trials, the reported incidence of acute grade 3 or greater GVHD with UCB appears to be less than anticipated with T-cell–replete matched unrelated bone marrow in children (bone marrow, 23% to 37%; UCB, 11% to 23%).7-12 Additionally, the incidence of chronic GVHD appears to be less than anticipated in matched unrelated bone marrow transplantation in children (bone marrow, 60%; UCB, 25%).4,10 Finally, the rate and completeness of immune recovery following UCB transplantation have been minimally described.13 We have reported engraftment, survival, incidence of GVHD, and immune recovery following a standardized treatment approach.
Patients and methods
Eligibility
Patients younger than 18 years of age with advanced stage leukemia and lymphoma, inborn errors of metabolism, medically refractory histiocytosis, bone marrow failure syndrome, or severe primary immunodeficiencies were eligible for this study (Table1). No patient had an existing HLA-identical related donor. Unrelated donor UCB units were HLA-identical or mismatched at 1 or 2 loci, and contained at least 1 × 107 nucleated cells per kilogram at the time of cryopreservation. Patients and/or guardians consented to the transplantation procedure. Protocols were approved by the Institutional Review Board of Indiana University.
Patient characteristics
| (n = 27: one UCB transplant) | |
| (n = 3: two UCB transplants) | |
| Sex | |
| Male | 14 |
| Female | 13 |
| Age at time of procedure | |
| <2 yr | 4 |
| 2-5 yr | 11 |
| 5-11 yr | 8 |
| 11-17 yr | 6 |
| >17 yr | 1 |
| Body weight at time of procedure (kg) | |
| <10 kg | 2 |
| 10-19 kg | 14 |
| 20-39 kg | 10 |
| 40-59 kg | 2 |
| >60 kg | 2 |
| Diagnosis at time of procedure | |
| AML | |
| 1st CR | 1 |
| 2nd CR | 7 |
| 3rd CR | 2 |
| Refractory | 1 |
| ALL | |
| 1st CR | 1 |
| 2nd CR | 8 |
| 3rd CR or greater | 2 |
| Refractory | 2 |
| MDS refractory | 3 |
| CML | 0 |
| JMML | 1 |
| Histiocytosis | 1 |
| Wiskott-Aldrich | 1 |
| (n = 27: one UCB transplant) | |
| (n = 3: two UCB transplants) | |
| Sex | |
| Male | 14 |
| Female | 13 |
| Age at time of procedure | |
| <2 yr | 4 |
| 2-5 yr | 11 |
| 5-11 yr | 8 |
| 11-17 yr | 6 |
| >17 yr | 1 |
| Body weight at time of procedure (kg) | |
| <10 kg | 2 |
| 10-19 kg | 14 |
| 20-39 kg | 10 |
| 40-59 kg | 2 |
| >60 kg | 2 |
| Diagnosis at time of procedure | |
| AML | |
| 1st CR | 1 |
| 2nd CR | 7 |
| 3rd CR | 2 |
| Refractory | 1 |
| ALL | |
| 1st CR | 1 |
| 2nd CR | 8 |
| 3rd CR or greater | 2 |
| Refractory | 2 |
| MDS refractory | 3 |
| CML | 0 |
| JMML | 1 |
| Histiocytosis | 1 |
| Wiskott-Aldrich | 1 |
UCB indicates umbilical cord blood; AML, acute myeloid leukemia; ALL, acute lymphoblastic leukemia; CR, complete remission; MDS, myelodysplasic syndrome; CML, chronic myeloid leukemia; JMML, juvenile myelomonocytic leukemia.
Patients
Twenty-seven patients underwent 30 unrelated donor UCB transplantation procedures for malignant (n = 28) or nonmalignant diseases (n = 2) (Table 1). Of the patients, 3 underwent 2 unrelated donor UCB transplantations for recurrence of their malignancy. For purposes of engraftment analysis, the 3 patients who underwent 2 transplantations were evaluated as separate transplantation procedures. There were 4 patients who underwent a UCB transplant subsequent to a relapse following a purged autologous bone marrow transplant. Transplantation procedures were performed between November 1994 and February 1999 at the James W. Riley Hospital for Children at Indiana University. The median age at the time of transplantation was 4.85 years (range: 0.4-17.1 years), and the median weight was 18.4 kg (range: 5.65-71.4 kg). There were 14 males and 13 females (Table 1).
Cord blood selection
Individual patients underwent initial HLA typing for class I HLA-A, HLA-B, and HLA-C antigens by means of standard serological methods.14 Class II, HLA-DRB1 typing was performed with the use of high-resolution DNA techniques.15 Following identification of a possible UCB matched unit, confirmatory typing of all UCB was performed at Indiana University, prior to transplantation. The units were matched serologically at HLA-A and HLA-B loci and with molecular typing at HLA-DRB1. Three patients had a no-antigen mismatched donor; 17 had a 1-antigen mismatched donor; and 10 had a 2-antigen mismatched donor (Table 2). No UCB graft had 2 mismatches at the same HLA loci. Of the 30 transplants, 13 were ABO compatible. The median time from the initiation of search to identification of a compatible UCB unit was 46.5 days (range: 4-295 days). The median time from identification of a suitable UCB unit to transplantation was 91.5 days (range: 25-343 days). Patients, when possible, underwent either autologous peripheral blood progenitor cell apheresis or bone marrow harvest prior to conditioning therapy as an autologous stem cell source to be used in the event of nonengraftment.
Cord unit characteristics
| No. of HLA mismatches | |
| 0 | 3 |
| 1 | 17 |
| 2 | 10 |
| HLA mismatches | |
| A | 6 |
| B | 8 |
| DR | 3 |
| A & B | 7 |
| A & DR | 3 |
| B & DR | 0 |
| Nucleated cells/kg (×107) collected | |
| <1 | 0 |
| 0.1 | 17 |
| 0.2 | 8 |
| >10.1 | 5 |
| Nucleated cells/kg (×107) infused | |
| <1 | 1 |
| 1.1-5 | 21 |
| 5.1-10 | 7 |
| >10.1 | 1 |
| CD 34/kg (×105) infused | |
| <1 | 9 |
| 1.1-2 | 11 |
| 2.1-5 | 4 |
| >5 | 4 |
| missing | 2 |
| CD3/kg (×106) infused | |
| <5 | 6 |
| 0.1 | 7 |
| 10.1-20 | 9 |
| >20 | 4 |
| missing | 4 |
| CFU-GM (×104) infused | |
| 0 | 0 |
| 0.1-5 | 15 |
| 5.1-10 | 4 |
| 10.1-20 | 4 |
| >20 | 6 |
| missing | 1 |
| BFU-E (×104) infused | |
| 0 | 4 |
| 0.1-5 | 16 |
| 5.1-10 | 3 |
| 10.1-20 | 1 |
| >20 | 5 |
| missing | 1 |
| CFU-GEMM (×104) infused | |
| 0 | 18 |
| 0.1-5 | 8 |
| 5.1-10 | 1 |
| 10.1-20 | 0 |
| >20 | 2 |
| missing | 1 |
| No. of HLA mismatches | |
| 0 | 3 |
| 1 | 17 |
| 2 | 10 |
| HLA mismatches | |
| A | 6 |
| B | 8 |
| DR | 3 |
| A & B | 7 |
| A & DR | 3 |
| B & DR | 0 |
| Nucleated cells/kg (×107) collected | |
| <1 | 0 |
| 0.1 | 17 |
| 0.2 | 8 |
| >10.1 | 5 |
| Nucleated cells/kg (×107) infused | |
| <1 | 1 |
| 1.1-5 | 21 |
| 5.1-10 | 7 |
| >10.1 | 1 |
| CD 34/kg (×105) infused | |
| <1 | 9 |
| 1.1-2 | 11 |
| 2.1-5 | 4 |
| >5 | 4 |
| missing | 2 |
| CD3/kg (×106) infused | |
| <5 | 6 |
| 0.1 | 7 |
| 10.1-20 | 9 |
| >20 | 4 |
| missing | 4 |
| CFU-GM (×104) infused | |
| 0 | 0 |
| 0.1-5 | 15 |
| 5.1-10 | 4 |
| 10.1-20 | 4 |
| >20 | 6 |
| missing | 1 |
| BFU-E (×104) infused | |
| 0 | 4 |
| 0.1-5 | 16 |
| 5.1-10 | 3 |
| 10.1-20 | 1 |
| >20 | 5 |
| missing | 1 |
| CFU-GEMM (×104) infused | |
| 0 | 18 |
| 0.1-5 | 8 |
| 5.1-10 | 1 |
| 10.1-20 | 0 |
| >20 | 2 |
| missing | 1 |
CFU-GM indicates colony forming unit-granulocyte macrophage; BFU-E, burst forming unit-erythroid; and CFU-GEMM, colony forming unit-granulocyte erythroid monocyte macrophage.
Collection and processing of UCB
Of the 30 UCB units, 27 were obtained from the New York Placental Blood Center, New York, NY; 2 were from the St Louis Cord Blood Bank, St Louis, MO; and 1 was from the Milano Cord Blood Bank, Milan, Italy. All units were collected and processed as previously reported.6
Preparative regimens
All patients received 1 of 2 preparative regimens. Total body irradiation (TBI) was used for all transplantations except for patients with nonmalignant diseases and patients receiving a second transplant after a prior TBI-containing transplantation procedure. Twenty-three transplants were performed following hyperfractionated TBI, 1440 cGy delivered in 12 total doses over 4 days, 1.2 Gy per fraction; cyclophosphamide (CY), 60 mg/kg per day for 2 consecutive days; and antithymocyte globulin (ATG), 30 mg/kg per day for 2 consecutive days (ATGAM, Upjohn, Kalamazoo, MI) (TBI/CY/ATG). Seven transplants were performed following a preparative regimen consisting of oral busulfan (Bu), 16 mg/kg total dose delivered in 16 doses over 4 days; melphalan (Mel), 60 mg/m2 per day for 3 consecutive days; and ATG, 30 mg/kg per day for 3 consecutive days (Bu/Mel/ATG). The 3 patients who received 2 UCB transplants were conditioned with TBI/CY/ATG followed by Bu/Mel/ATG for their second UCB transplant following relapse after their first UCB transplant. Additionally, 2 patients received Bu/Mel/ATG as the preparative regimen because they had nonmalignant diseases, and 2 patients received Bu/Mel/ATG as they had received TBI in a prior autologous transplant. Busulfan levels were obtained with the first dose of busulfan and assayed at the Fred Hutchinson Cancer Research Center.16 Subsequent busulfan doses were modified on the basis of pharmacokinetic data to maintain a steady state of 600 to 900 ng/dL.16 The patients who failed to engraft following the UCB transplant and received an autologous reinfusion did not receive an additional preparative regimen prior to their unpurged autologous reinfusion.
GVHD prophylaxis
GVHD prophylaxis for the 23 transplants performed with the TBI/CY/ATG regimen consisted of cyclosporine A plus a short course of methotrexate (days 1, 3, 6, and 11).17 The 7 patients who underwent a preparative regimen of Bu/Mel/ATG received cyclosporine A plus methylprednisolone (days +5, +6: 10 mg/kg per day; days +7 through +9: 5 mg/kg per day; days +10 through +17: 2 mg/kg per day; day +17 onward: 10% taper per week to off).
Supportive care
All UCB units were negative for cytomegalovirus (CMV). Following engraftment, all CMV-positive patients underwent weekly CMV screening with CMV culture, antigenemia, or polymerase chain reaction. Ganciclovir was used only for patients with a positive CMV test result. No routine intravenous immunoglobulin (IVIG) was used unless serum immunoglobulin G (IgG) levels were less than 400 mg/dL and the patient developed an infectious complication. Of the 30 patients, 8 received IVIG for low IVIG levels and concurrent infections. All patients received trimethoprim/sulfamethoxazole prophylaxis (or pentamidine intravenously every 2 weeks if allergic to trimethoprim/sulfamethoxazole) pretransplantation and following neutrophil engraftment until phytohemaglutinin mitogen responses were 30% of normal or greater. All patients received granulocyte colony stimulating factor (Amgen, Thousand Oaks, CA) starting at day 0, at 5 μg/kg per day until the absolute neutrophil count was greater than 1500/μL for 2 consecutive days.
Hematologic recovery
The day of neutrophil engraftment was defined as the first of 3 consecutive days of an absolute neutrophil count 500/μL or greater. Platelet engraftment was defined as the first of 7 consecutive days when the platelet count exceeded 50 × 106/μL (untransfused). Disease status and percent chimerism was obtained on days 30, 60, 90, 180, 270, and 360 posttransplantation (unless clinically indicated at other times). Chimerism studies were performed for sex-mismatched and sex-matched donor/recipient pairs with fluorescent in situ hybridization (FISH) and restriction length fragment polymorphism (RFLP) assays, respectively. Patients were defined as having donor engraftment if they had more than 95% donor cells by these chimerism studies. Patients were evaluated for donor engraftment if they were alive 28 days or more and had not received an autologous reinfusion for failure to engraft following their UCB transplant.
Acute GVHD
Patients were evaluated daily while hospitalized and weekly through day 100 posttransplantation. Evaluation of acute GVHD was based upon clinical findings and histopathological confirmation when possible. Grading was based upon established Seattle criteria.18
Chronic GVHD
Patients were evaluated weekly, then monthly through day 365 post-transplantation. Evaluation of chronic GVHD was based upon clinical findings and confirmed with histopathology. Grading was based upon established Seattle criteria.18 Patients were evaluable for chronic GVHD if alive at day 100 with evidence of donor engraftment by previously defined chimerism studies.
Immunologic parameters
Immunologic evaluation occurred for all patients who had demonstrated donor engraftment. Immune reconstitution assays were performed at days 30, 60, 90, 180, 270, and 360 posttransplantation.
Immunoglobulins
Immunoglobulin levels were assessed by means of standard laboratory techniques. IgG, IgA, and IgM assays were performed with a nephelometer (Beckman, Berea CA). IgG subclasses were determined by means of radial immunodiffusion (Binding Site, Edinburgh, Scotland).
Immunophenotypic studies
Peripheral blood was examined by means of 2-color flow cytometric methods with Coulter XL (Coulter Immunology, Hialeah, FL). The pan-leukocyte antigen CD45 was used to exclude red blood cells. A panel of antigens was used to define T-, B-, and natural killer (NK)–cell numbers and subsets: CD3+, CD3+/CD4+, CD3+/CD8+, CD19+, CD3−/16+/56+(CD45, CD3, CD56: Coulter Immunology; CD4, CD8, CD19, and CD16: Becton Dickson Immunocytometry Systems, San Jose CA). White blood cell counts were obtained by means of an automated cell counter (Coulter STK 4, Coulter Immunology). Values obtained were compared with age-adjusted normal values.19
Proliferative response
Lymphocytes were separated from heparinized whole blood by Ficoll gradient centrifugation. After 3 washes, lymphocytes were resuspended in RPMI 1640 supplemented with 10% fetal calf serum and antibiotics. Cells were then transferred to 96-well flat-bottom plates. Phytohemaglutinin (PHA), Concanalavin A (Con A) and Pokeweed (PWM) mitogens (PHA = Difco PHA-P: final concentration 6.25 μg/mL, Fischer Scientific, Pittsburgh, PA; Con A: final concentration 5 μg/mL,, Roche Molecular Biochemicals, Indianapolis, IN; PWM: final concentration 2.5 μg/mL, Sigma, St Louis, MO) were added and the plates incubated at 37°C, in humidified atmosphere containing 5% CO2. Incubation for PHA and Con A was 3 days, and PWM incubation was 5 days.20At 16 to 18 hours before the end of the incubation period,3H-thymidine was added, and the plates were reincubated and then harvested onto filter paper by means of a Skatron cell harvester (Skatron, Sterling VA). Individual filter discs were transferred to plastic scintillation vials containing scintillation fluid and counted in a beta counter (Model LS7500, Beckman-Coulter, Fullerton, CA). Normal controls, age- and sex-matched if available, were run simultaneously.21 Results were reported as the percentile response of normal, with normal defined in the Immunology Laboratory at Indiana University on the basis of the mitogen response of volunteer normal donors.
NK cell assay
51Cr-labeled target cells (TCs) were added to partially purified NK cells at 2 or 3 NK/TC ratios (10:1, 20:1, 40:1) in 96-well round-bottom microtiter plates and incubated for 4 hours at 37°C in 5% CO2. After incubation, the plates were centrifuged at 4000g for 5 minutes, and the supernatant from each well was harvested by means of a supernatant collection system (Skatron, Sterling, VA). The percentage of cytotoxicity was determined with the formula: cytotoxicity = (sample cpm–spontaneous cpm)/(maximum cpm–spontaneous cpm) × 100, where maximum cpm was determined by measuring the radioactivity in 0.1 mL labeled TCs, and spontaneous cpm was determined by measuring the radioactivity in the supernatant of wells containing only 51Cr-labeled TCs. Each measurement was done in triplicate. Results were then compared with simultaneously run normal controls.20 21
Statistical analysis
Overall survival was defined from date of diagnosis until date of death or of last follow-up. Disease-free survival was defined from date of diagnosis until date of death or relapse or date of last follow-up. Event-free survival was defined from date of diagnosis until date of death, relapse, autologous reinfusion, or date of last follow-up. All survival estimates were based on Kaplan-Meier estimates.22 Corresponding 95% confidence intervals were calculated by means of Greenwood's method.23 Nonrelapse mortality estimates were calculated by means of cumulative incidence estimates that treat relapse as a competing risk event.24For engraftment analysis, patients who had received an autologous reinfusion for failure to engraft were excluded.
Correlative, time to engraftment, and time to immune recovery analyses were analyzed by means of the log-rank test.25Kaplan-Meier estimates were used to summarize engraftment data and time to immune recovery.
Results
UCB units
Following processing, the median volume of cells cryopreserved was 63.8 mL (range: 19.8-220.6 mL). The median volume of cells infused after thawing and processing, according to a published method, was 96 mL (range: 45-308 mL).6 The median number of nucleated cells per kilogram collected was 4.45 × 107/kg (range: 1.26-20.2 × 107/kg), with the median number of nucleated cells per kilogram infused being 3.63 × 107/kg (range: 0.95-13 × 107/kg). The median number of CD3 cells infused was 9.45 × 106/kg (range: 1.1-30.3 × 106/kg), and the median number of CD34 cells infused was 1.5 × 105/kg (range: 0.05-7.0 × 105/kg) (Table 2).
Hematologic recovery
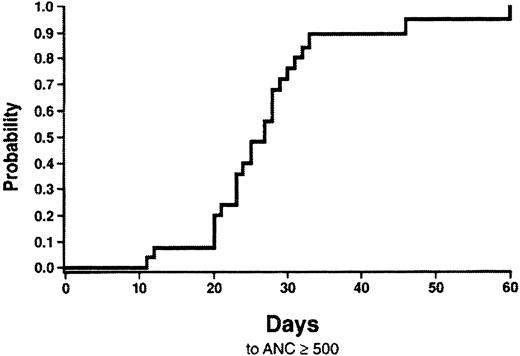
The cumulative probability of neutrophil engraftment was 89.3% (95% confidence interval [CI], 76.5%-100%) at day 42 and 100% by day 60 (Figure 1). Four patients received an autologous reinfusion of peripheral blood progenitor cells on days 30, 38, 42, and 54 posttransplantation, respectively, and were excluded from data analysis. The median time to neutrophil engraftment was 27 days (range: 12-60 days) posttransplantation. Among the entire cohort of patients, the cumulative probability of platelet engraftment (50 000/μL or greater) was 28.6% by day 60 (95% CI, 9.1%-48.1%) (Figure 2). The median time to platelet engraftment was 75 days (range: 33-158 days). There was no statistical relationship between the probability of neutrophil or platelet engraftment and nucleated cells per kilogram infused, CD 34+ cells per kilogram infused, or CMV status of the recipient. Additionally, neutrophil engraftment was not correlated to time of UCB storage.
Cumulative probability of neutrophil engraftment.
Neutrophil engraftment is defined as the first of 3 consecutive days with an absolute neutrophil count of 500/μL or more.
Cumulative probability of neutrophil engraftment.
Neutrophil engraftment is defined as the first of 3 consecutive days with an absolute neutrophil count of 500/μL or more.
Cumulative probability of platelet engraftment.
Platelet engraftment is defined as the first of 7 consecutive days with a platelet count of 50 000/μL or more, untransfused.
Cumulative probability of platelet engraftment.
Platelet engraftment is defined as the first of 7 consecutive days with a platelet count of 50 000/μL or more, untransfused.
Chimerism
Engraftment was defined by recovery of donor neutrophil and platelets. Donor host status was confirmed by RFLP or FISH chimerism studies in all patients who engrafted. A minimum of 95% donor cells was documented in these patients. In patients who relapsed posttransplantation, the chimerism studies heralded the return of donor cells. The 4 patients who received an autologous reinfusion underwent chimerism studies. In 3 of these 4 patients, donor cells were documented in their bone marrow at day 30 posttransplantation (Table3). These patients received the autologous reinfusion secondary to failure to demonstrate timely neutrophil engraftment.
Chimerism data of patients who received an autologous re-infusion
| Patient number . | Chimerism study . | Day of autologous reinfusion . | Donor cells at day 30 posttransplant (%) . | Day of neutrophil recovery . | Patient outcome . |
|---|---|---|---|---|---|
| 003 | FISH | 54 | 100 | Not achieved | Died day 58 posttransplant of multi-organ failure |
| 013 | RFLP | 30 | 0 | 40 | Alive NED |
| 021 | FISH | 42 | 3 | 53 | Relapsed at day 158 and died of disease |
| 025 | FISH | 38 | 5 | 47 | Relapsed day 328, alive with disease |
| Patient number . | Chimerism study . | Day of autologous reinfusion . | Donor cells at day 30 posttransplant (%) . | Day of neutrophil recovery . | Patient outcome . |
|---|---|---|---|---|---|
| 003 | FISH | 54 | 100 | Not achieved | Died day 58 posttransplant of multi-organ failure |
| 013 | RFLP | 30 | 0 | 40 | Alive NED |
| 021 | FISH | 42 | 3 | 53 | Relapsed at day 158 and died of disease |
| 025 | FISH | 38 | 5 | 47 | Relapsed day 328, alive with disease |
FISH indicates fluorescent in-situ hybridization; RFLP, restriction length fragment polymorphism; NED, no evidence of disease.
Graft-versus-host disease
Among the 30 transplant procedures, the cumulative probability of any GVHD by day 100 was 57.1% (95% CI, 37.7%-76.6%); of grade 2 or greater acute GVHD, 37.2% (95% CI, 16.7%-57.7%); and of grade 3 or greater acute GVHD, 8.8% (95% CI, 0%-20.6%) (Figure3). There was only one case of grade 3 GVHD (liver) and no grade 4 GVHD. Of the 15 patients who developed grade 2 or greater GVHD, all but 1 patient responded to therapy, with 1 patient dying at day 58 of multisystem organ failure. Two patients required ATG therapy, in addition to prednisone and cyclosporine. At day 100, 15 patients were alive with donor engraftment and were assessed for chronic GVHD. Neither limited nor extensive chronic GVHD was detected in any patient.
Cumulative probability of GVHD.
(A) Probability of grade 2 or greater GVHD. (B) Probability of grade 3 or greater GVHD.
Cumulative probability of GVHD.
(A) Probability of grade 2 or greater GVHD. (B) Probability of grade 3 or greater GVHD.
Immune recovery and infections
In our series of 30 UCB transplants, we have attempted to describe and correlate both quantitative and qualitative immune recovery. Using a Kaplan-Meier estimate of the cumulative probability of the time at which 50% of the children achieved an age-appropriate normal value, we describe quantitative T-, B- and NK-cell recovery as follows:
Numerical immune recovery.
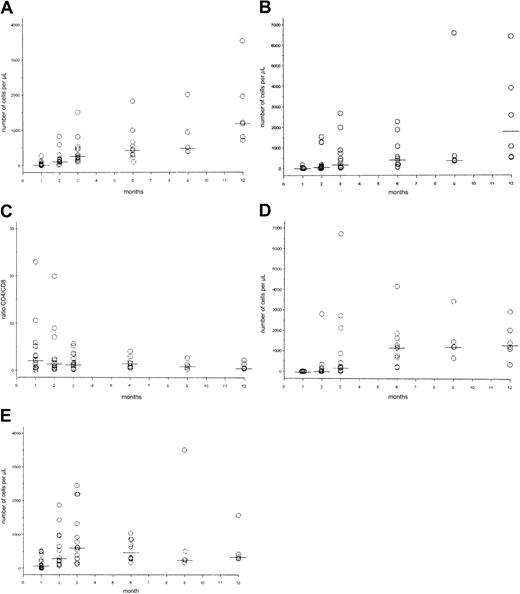
CD4+ T cells recovered at a median of 12 months, whereas CD8+ T cells recovered at a median of 9 months posttransplantation. CD 19+ B cells recovered at a median of 6 months posttransplantation, and NK cells recovered at a median of 2 months posttransplantation. The CD4-CD8 ratio was within the normal range by 2 months posttransplantation (Figure4). The actual patient values are also shown (Figure 5).
Kaplan-Meier estimates of numerical immune reconstitution.
(A) Cumulative probability of time to achieve an age-appropriate normal cell count for CD3+/CD4+ cells (dotted line), CD3+/CD8+ cells (dashed line), and CD4+–CD8+ ratio (solid line). Median time for CD4 and CD8 cell recovery was 12 and 9 months, respectively. (B) Cumulative probability of time to achieve an age-appropriate normal cell count for CD19+ cells (solid line) and NK (CD56+) cells (dashed line). Median time for CD19 and NK cell recovery was 6 and 2 months, respectively.
Kaplan-Meier estimates of numerical immune reconstitution.
(A) Cumulative probability of time to achieve an age-appropriate normal cell count for CD3+/CD4+ cells (dotted line), CD3+/CD8+ cells (dashed line), and CD4+–CD8+ ratio (solid line). Median time for CD4 and CD8 cell recovery was 12 and 9 months, respectively. (B) Cumulative probability of time to achieve an age-appropriate normal cell count for CD19+ cells (solid line) and NK (CD56+) cells (dashed line). Median time for CD19 and NK cell recovery was 6 and 2 months, respectively.
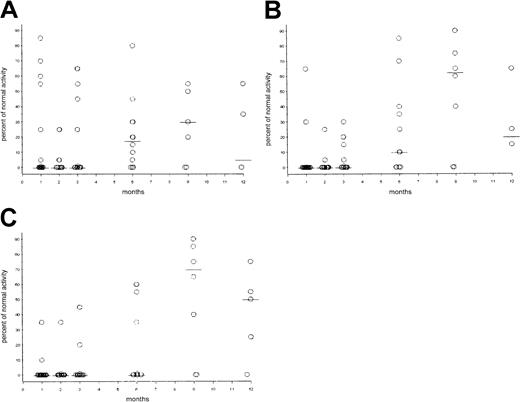
Dot plots of numerical immune reconstitution.
Circles represent patient values in cells per microliter; horizontal bars indicate median value. (A) CD3+/CD4+cells; normal value: 562 to 3908 cells/μL. (B) CD3+/CD8+ cells; normal value: 331 to 2479 cells/μL. (C) CD4+–CD8+ ratio (normal value: greater than 0.9). (D) CD19+ cells; normal value: 200 to 3345 cells/μL. (E) CD3−/CD16+/CD56+ cells (or NK cells), normal value: 90 to 140 cells/μL.
Dot plots of numerical immune reconstitution.
Circles represent patient values in cells per microliter; horizontal bars indicate median value. (A) CD3+/CD4+cells; normal value: 562 to 3908 cells/μL. (B) CD3+/CD8+ cells; normal value: 331 to 2479 cells/μL. (C) CD4+–CD8+ ratio (normal value: greater than 0.9). (D) CD19+ cells; normal value: 200 to 3345 cells/μL. (E) CD3−/CD16+/CD56+ cells (or NK cells), normal value: 90 to 140 cells/μL.
Functional immune recovery.
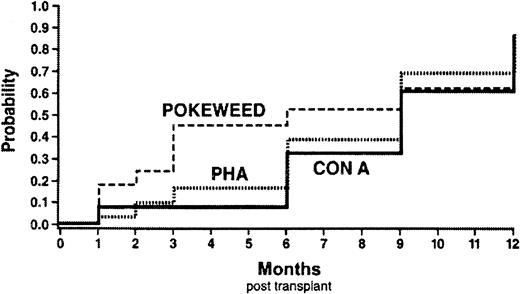
Functional recovery of T cells was assayed with mitogen stimulation. By means of the same Kaplan-Meier cumulative probability estimate method, a normal T-cell response to PHA, Con A, and PWM was reached at a median of 6 to 9 months posttransplantation (Figure6). Additionally, the actual patient values are shown (Figure 7). B-cell recovery was assessed by immunoglobulin production. Importantly, 22 of 30 patients did not receive any IVIG. For these 22 patients, IgG, IgM, and IgA and the subclasses of IgG remained within the normal range post-transplantation, with the lowest levels occurring between 1 and 3 months. NK lytic function was assessed with K562 as a target cell. NK function was detectable at 1 month posttransplantation in all children evaluated.
Kaplan-Meier estimates of functional immune reconstitution.
Cumulative probability of time to achieve an age-appropriate normal mitogen response to PHA (dotted line), Con A (solid line), and PWM (dashed line). Median time to recovery of normal mitogen response was 6 to 9 months posttransplantation.
Kaplan-Meier estimates of functional immune reconstitution.
Cumulative probability of time to achieve an age-appropriate normal mitogen response to PHA (dotted line), Con A (solid line), and PWM (dashed line). Median time to recovery of normal mitogen response was 6 to 9 months posttransplantation.
Dot plots of functional immune reconstitution.
Circles represent patient values as percentage of normal activity; horizontal bars indicate median value. Normal response is defined as more than 25% activity. (A) Response to PWM mitogen. (B) Response to Con A. (C) Response to PHA.
Dot plots of functional immune reconstitution.
Circles represent patient values as percentage of normal activity; horizontal bars indicate median value. Normal response is defined as more than 25% activity. (A) Response to PWM mitogen. (B) Response to Con A. (C) Response to PHA.
Clinically relevant immune recovery as determined by the incidence of severe infections was evaluated. In our series of 30 UCB transplant procedures, there were 16 grade III or grade IV infections, as graded by the National Institutes of Health toxicity scale. The grade IV infections were secondary to Aspergillus fumigatus,Candida parapsilosis, and Parainfluenzae, type III. In 3 patients (10%), the grade IV infection was a significant contributing factor to their death.
To further assess immune recovery in our patients, correlative analysis of pertinent clinical variables was performed. No relationship was determined for time to recovery of T-, B-, or NK-cell numbers and nucleated cells per kilogram or CD 34+ cells per kilogram. Among the functional assessments of immune recovery, a trend was noted between recovery of responses to PHA and PWM and nucleated cells per kilogram infused (P ≤ .04 andP ≤ .06, respectively). Finally, there was no correlation between either numerical or functional immune recovery and incidence or grade of infectious complications.
Survival
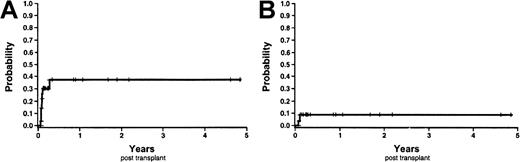
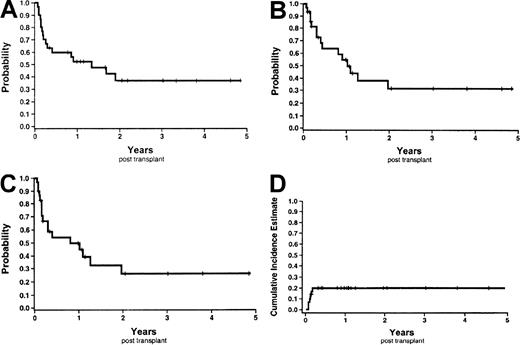
The probability of overall survival at 6 months and 1 year was 59.8% (95% CI, 42.2%-77.4%) and 52.3% (95% CI, 34.1%-70.5%) (Figure 8A). Causes of death were relapse (n = 10), multisystem organ failure (n = 3), infections (n = 2:A fumigatus and Parainfluenzae), and veno-occlusive disease (n = 1). The probability of disease-free survival and event-free survival at 1 year was 54.7% (95% CI, 34.5%-74.9%) and 49.6% (95% CI, 29.9%-69.4%) respectively (Figure 8B-C). Finally, the probability of nonrelapse mortality at 1 year was 20% (95% CI, 4%-36%) (Figure 8D). All nonrelapse deaths occurred before day 66. Of note, one patient died 15 days following the second UCB transplant of multisystem organ failure; this was 175 days following the first UCB transplant.
Survival curves and nonrelapse mortality Kaplan-Meier estimates.
(A) Overall survival. (B) Disease-free survival. (C) Event-free survival. (D) Nonrelapse mortality.
Survival curves and nonrelapse mortality Kaplan-Meier estimates.
(A) Overall survival. (B) Disease-free survival. (C) Event-free survival. (D) Nonrelapse mortality.
Discussion
Since the first cord blood transplant for Fanconi anemia in 1988, UCB has increasingly been used as an alternative source of hematopoietic stem cells.26 The current literature regarding unrelated cord blood transplantation has described engraftment, GVHD, and survival following an array of treatment approaches for children and adult patients with both malignant and nonmalignant diseases. In the current study, we report engraftment, survival, incidence and severity of GVHD, and immune reconstitution with the use of standardized transplantation conditioning and GVHD regimens.
Time to neutrophil and platelet engraftment in our series of patients was similar to previously reported data. In our series, the median time to achieve an absolute neutrophil count greater than 500/μL was 27 days. This can be compared to other reports of unrelated cord blood transplants, which ranged from 22-30 days.4 7-9 Of note, 23 patients (76%) received methotrexate as GVHD prophylaxis, which did not appear to inhibit engraftment. Although the number of patients is small, there is no obvious prolongation of the time to engraftment in comparison with other published trials that have, in general, used high doses of steroids in addition to cyclosporine rather than methotrexate.
Platelet recovery (greater than 50 000/μL) was achieved at a median of 75 days. Again, this is comparable to prior reports of 56 to 71 days.4,7-9 We did not discern a relationship between the tempo of neutrophil or platelet engraftment and the number of nucleated cells per kilogram infused. This compares to the experience of Wagner et al7 with unrelated cord blood transplants and contrasts with that of Kurtzberg et al8 and Rubenstein et al,4 who found a direct correlation with neutrophil engraftment and nucleated cell dose per kilogram. Additionally, we found no relationship between myeloid or platelet recovery and CD34+ cells per kilogram infused.8 This observation has not been extensively reported. Our evaluation of colony forming units, viability of unit, age of recipient, CMV status, and degree of HLA mismatch had no detectable relationship to time to neutrophil or platelet engraftment. Our relatively small sample size may have precluded detection of the correlations.
A significant contributing factor to nonrelapse mortality in allogeneic transplantation is both acute and chronic GVHD. Our data are discordant with the unrelated bone marrow donor experience with regard to acute and chronic GVHD. Our cumulative probability of grade 2 or greater GVHD was 37.2% by day 100. This contrasts with 83% and 98% in a comparable pediatric population undergoing matched and mismatched unrelated donor bone marrow transplantation, respectively.10 Prior reports indicated a 32% to 46% incidence of GVHD in unrelated cord blood transplantation; we experienced a comparable incidence (15 out of 30 or 50%) of grade 2 or greater acute GVHD.4,8,9 We did find a smaller probability of grade 3 or greater GVHD, 8.8%, than previously reported with UCB transplantation, 23%.4 Finally, we found no evidence of chronic GVHD in our patients. This finding was consistent with the lower probability of chronic GVHD previously reported.4This low probability and severity of chronic GVHD may be due to the young age of our recipients, the GVHD prophylaxis method, or perhaps the unique immunologic properties of UCB. Cord blood cells do not respond to IL-4, produce lower levels of IL-2, are less responsive to IL-2, and have lower antigen and mitogen proliferation.27,28 Additionally, cord blood serum appears to have significantly fewer soluble factors that enhance mitogen and IL-2–specific T-cell growth when compared with adult serum.29 These differences may contribute to the lower incidence of GVHD in cord blood transplantation.
All of the patients enrolled in this study underwent unrelated donor stem cell transplantation for a variety of life-threatening malignant or nonmalignant diseases. Therefore, comparisons with survival statistics is difficult, as survival is often disease-dependent. Upon comparison of our nonrelapse mortality with unmanipulated, unrelated donor bone marrow transplantation, we found a 20% probability of nonrelapse mortality at 1 year posttransplantation, with all deaths occurring prior to day 66 posttransplantation. Transplantation-related mortality in a comparable population of pediatric recipients of matched and mismatched unrelated donor bone marrow was 28% by 84 days post-transplantation.10 All but 3 of the 25 deaths in that series were associated with GVHD.
The recovery of immune function following any myeloablative transplantation is critical to patient survival. The immunological recovery following UCB has been minimally described.13,30,31 We have attempted to summarize the numerical, functional, and in vivo immune recovery in our series of UCB transplant recipients. The accurate reporting of long-term immune recovery is confounded by relapse and/or nonrelapse mortality. To provide a more precise measure of immune recovery, we used the Kaplan-Meier estimate to account for patient censorship. Using this method, we found numerical recovery of T cells between 9 and 12 months, B cells at 6 months, and NK cells at 2 months posttransplantation. This tempo of immune recovery differs from previous reports of other stem cell sources, specifically with regard to the marked delay in CD3+/CD8+ recovery at 9 months in our series.32-36 This contrasts with the rapid return of CD8+ cells with normal numbers between 1 and 3 months posttransplantation for the majority of other stem cell sources, including 3 UCB recipients.30,32-36 The delayed recovery of CD8+ cells in our patient population created an uncharacteristic normalization of the CD4–CD8 ratio at 2 months posttransplantation. We therefore did not observe the typical inversion of the CD4–CD8 ratio described following transplantation from other hematopoeitic sources.33,34,36,37 The delayed numeric recovery of CD8+ cells may reflect the unique immunologic properties of cord blood. Immature T cells CD4+ and CD8+ are found in higher proportions in UCB (0.6%-25%).38-40 This immature population in unrelated cord blood transplantation may not be capable of the rapid CD8 recovery observed in marrow and peripheral blood stem cell transplants from mature donors. An alternative explanation is that in spite of the absolute numbers of cells infused, the cord blood cells undergo a normal physiologic decline of CD8. The median percentage of CD3+/CD4+ and CD3+/CD8+in neonates is 41% and 24%, respectively. At 9 to 15 months of age, the median percentage of CD3+/CD4+ has risen to 44%, whereas the percentage of CD3+/CD8+ cells has declined to 18%.15 Finally, numeric recovery of B and NK cells in our patients was comparable to other stem cell sources.30-37,41 42
Regarding the functional immune recovery in our patients, we identified no difference in unrelated cord blood recipients' recovery of a normal mitogen response when compared with recipients of unrelated blood from other stem cell sources.32,41 This is of significant interest as the immune status of cord blood cells is considered to be immature or naı̈ve.3Interestingly, in all but 8 of our patients, immunoglobulin production remained within the normal range without the infusion of IVIG. This is a departure from the standard practice for a majority of unrelated allogeneic transplants8 and may offer a unique cost savings opportunity in allogeneic transplantation. Upon evaluation of pertinent clinical correlates and immune recovery, we found a relationship between time to recovery of a normal response to the mitogen PHA and nucleated cells per kilogram infused. The lack of other significant correlations may be due to patient censure, evaluations at discrete time intervals, and the relatively small patient population.
The most practical evaluation of immune reconstitution, the incidence of life-threatening infections, was characterized in this series of unrelated donor UCB transplants. Of our patients, 53% experienced either grade III or IV infections (16 grade III and 3 grade IV) in the first 100 days posttransplantation. This compares with a series of 60 adult patients who underwent unrelated donor T-cell–depleted bone marrow transplants43 in which it was reported that more than 50% of patients experienced a severe life-threatening infection (grade III). Of the patients in that series, 31.6% developed a systemic fungal infection with a 28.3% incidence of Aspergillus species infections. In a series of pediatric patients who underwent either autologous (n = 65) or allogeneic (n = 58) bone marrow transplants, 31% had a documented bacterial infection, and 3.4% had a fatal fungal infection.44 These data compare and contrast with our experience of 53.3% of the patients with either a grade III or IV infection, and a 6.6% incidence of systemic fungal infection. Prolonged immune suppression and a significant incidence of serious infections in unrelated donor UCB transplantation did not appear to be excessive in our UCB recipients. It is possible that the perceived increase in infectious complications after UCB transplantation is a result of the use of high doses of steroids rather than the stem cell source.
In summary, we have reported a complete assessment of engraftment, GVHD, and immune reconstitution in a series of 30 pediatric patients who underwent unrelated UCB transplantation under a standardized treatment approach. The probability of engraftment and incidence and the severity of acute GVHD were comparable to previously reported series of UCB transplantation. In addition, our data suggest that immune reconstitution after unrelated UCB transplantation has unique features, including delayed CD8+ recovery, normal CD4+-CD8+ ratio, and immunoglobulins that remain within the normal range. Ongoing studies using immunization with the T-cell–dependent neo-antigen φ X174, will further define the pattern of immune recovery after UCB transplantation.45
Acknowledgments
We thank Patricia Smith and Vicki Graves for their assistance in the preparation of this manuscript and the nurses of the Pediatric Stem Cell Unit for their diligence in caring for these special children.
Supported in part by grants from the Riley Memorial Association, The Clarian Health Values Research Fund, the National Institutes of Health (grant P30DK49218), and Centers of Excellence in Molecular Hematology.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Blythe G. Thomson, James W. Riley Hospital for Children, 702 Barnhill Dr, Rm 2720, Indianapolis, IN 46202; e-mail:bthomson@iupui.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal