Abstract
Factor XIII on activation by thrombin cross-links fibrin. A common polymorphism Val to Leu at position 34 in the FXIII A subunit is under investigation as a risk determinant of thrombosis. Because Val34Leu is close to the thrombin cleavage site, the hypothesis that it would alter the function of FXIII was tested. Analysis of FXIII subunit proteolysis by thrombin using sodium dodecyl sulfate-polyacrylamide gel electrophoresis and high-performance liquid chromatography showed that FXIII 34Leu was cleaved by thrombin more rapidly and by lower doses than 34Val. Mass spectrometry of isolated activation peptides confirmed the predicted single methyl group difference and demonstrated that the thrombin cleavage site is unaltered by Val34Leu. Kinetic analysis of activation peptide release demonstrated that the catalytic efficiency (kcat/Km) of thrombin was 0.5 for FXIII 34Leu and 0.2 (μmol/L)−1× sec−1 for 34Val. Presence of fibrin increased the catalytic efficiency to 4.8 and 2.2 (μmol/L)−1 × sec−1, respectively. Although the 34Leu peptide was released at a similar rate as fibrinopeptide A, the 34Val peptide was released more slowly than fibrinopeptide A but more quickly than fibrinopeptide B generation. Cross-linking of γ- and -chains appeared earlier when fibrin was incubated with FXIII 34Leu than with 34Val. Fully activated 34Leu and 34Val FXIII showed similar cross-linking activity. Analysis of fibrin clots prepared using plasma from FXIII 34Leu subjects by turbidity and permeability measurements showed reduced fiber mass/length ratio and porosity compared to 34Val. The structural differences were confirmed by electron microscopy. These results demonstrate that Val34Leu accelerates activation of FXIII by thrombin and consequently affects the structure of the cross-linked fibrin clot.
On cleavage of fibrinopeptides A and B by thrombin, fibrin spontaneously polymerizes into a network of multimeric strands, initially held together by noncovalent interaction. Blood coagulation factor XIII (FXIII) is activated by thrombin, and activated FXIII (FXIIIa) covalently cross-links the fibrin clot to increase resistance to chemical, mechanical, and proteolytic insults. FXIII is a tetrameric protransglutaminase consisting of 2 A subunits, which contain the active site, and 2 B subunits, which serve a carrier function for the A subunit in plasma.1,2 FXIII is also found in platelets as an A-subunit dimer.2 Platelets do not release FXIII on activation, but lysis of platelets entrapped in the blood clot may increase local concentrations of FXIII.3
FXIIIa catalyzes the introduction of γ-glutamyl-ε-lysine peptide bonds between fibrin γ- and α-chains. Other substrates of FXIIIa are α2-antiplasmin,4 von Willebrand factor,5 thrombospondin,6 and fibronectin.7,8 Cross-linking of these substrates into the clot further contributes to the mechanical strength, viscosity, and resistance to proteolysis of fibrin. Activation of FXIII involves thrombin-induced cleavage of the peptide bond between Arg37 and Gly38 of the A subunit,2,9 resulting in the release of an amino-terminal activation peptide. In a second step, calcium induces dissociation of the A-subunit dimer from the B subunit.10 11 Both steps are essential for the activation of FXIII; the activation peptide release induces a conformational change in the A subunit and enables the dissociation of the subunits in the presence of calcium to unmask the catalytic site.
A common polymorphism with an allele frequency of around 25% has been identified in the FXIII A subunit (Val34Leu), 3 amino acids from the thrombin activation site.12 Recent studies have reported that the prevalence of the Leu encoding allele is lower in patients with myocardial infarction,13,14 deep vein thrombosis,15,16 and cerebral infarction17 when compared with matched control groups. These clinical studies suggest that this polymorphism may be a risk determinant of thrombosis in both the arterial and venous systems. Paradoxically, ex vivo and in vitro studies have suggested that possession of the Leu allele leads to increased cross-linking rates by FXIIIa.18-20 The mechanisms by which this occurred remained unclear. In view of the close proximity of the Val34Leu polymorphism to the activation site, we tested the hypothesis that this polymorphism alters the activation rate of FXIII by thrombin and affects fibrin structure.
Materials and methods
Blood sampling and processing
Venous blood was obtained from individuals with the homozygous FXIII 34Val, homozygous 34Leu, and heterozygous genotypes after an overnight fast. Blood was collected in 0.1 mol/L trisodium citrate, 9 parts of blood to 1 part of trisodium citrate. Within 1 hour after collection, the samples were centrifuged at 2560g for 20 minutes at room temperature to obtain platelet-poor plasma, frozen in aliquots in liquid nitrogen, and stored at −40°C until analysis.
Determination of the FXIII Val34Leu genotype
Genomic DNA was extracted with the BACC3 DNA extraction kit (Nucleon Biosciences Ltd, Glasgow, Scotland) from 10 mL venous blood that was anticoagulated with 1.6 mg/mL EDTA and from 5 mL of buffy coat obtained from the regional blood transfusion center. The FXIII Val34Leu genotype was determined by polymerase chain reaction and single-stranded conformational polymorphism analysis as previously described.13
Purification of the FXIII Val34Leu variants
Buffy coats from 34 outdated donations of human platelet-poor plasma were obtained from the regional blood transfusion center; genomic DNA was extracted and genotyped for the Val34Leu polymorphism. There were 19 homozygous FXIII 34Val samples, 1 homozygous 34Leu sample, and 14 heterozygous samples. FXIII 34Val was purified from a pool of 10 of the homozygous plasma donations (vol = 2.170 L) and homozygous FXIII 34Leu from a single plasma donation (vol = 0.205 L). In addition, FXIII was purified from the plasma of 40 outdated, mixed (unknown) genotype donations (vol = 11.895 L). Purification of FXIII was performed using a method adapted from previous publications.21,22 In brief, plasma was subjected to repeated precipitations with ammonium sulfate: 20% saturation at room temperature pH 7.0, 16% saturation at 4°C pH 5.4, 16% saturation at 4°C pH 7.0, and 36% saturation at 4°C pH 7.5. EDTA was added at 1 mmol/L to all the buffers used to prevent inopportune activation of FXIII. The final precipitate was resuspended in 1/1000 plasma volume of 0.05 mol/L Tris-HCl pH 7.5, 1 mmol/L EDTA, dialyzed against the same buffer and further purified by gel filtration on a Sepharose 6B column (2.6 × 40 cm), equilibrated and developed with 0.05 mol/L Tris-HCl pH 7.5, 1 mmol/L EDTA. Peak fractions containing both FXIII A and FXIII B subunit were pooled, concentrated on aquacide (Calbiochem Corp, La Jolla, CA) and extensively dialyzed against 0.05 mol/L Tris-HCl pH 7.5, 1 mmol/L EDTA. Purity and activity of the preparations were tested with sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), in-house FXIII A- and B-subunit sandwich enzyme-linked immunosorbent assays,23 and 5-(biotinamido) pentylamine incorporation assay.23,24 Concentration of the preparations was measured by absorbance at 280 nm using an extinction coefficient of= 1.38.22
Cross-linking assay for FXIII
The FXIIIa-specific cross-linking activity was determined with a microtiter assay using fibrinogen and 5-(biotinamido) pentylamine as substrates, as previously described.23,24 The assay is based on the incorporation of 5-(biotinamido) pentylamine by FXIIIa into microtiter plates coated with fibrinogen, following activation with thrombin. The amount of cross-linked 5-(biotinamido) pentylamine is detected by measuring phosphatase activity after incubation with a streptavidin-alkaline phosphatase conjugate. FXIIIa activity was measured before and after preincubation with human α-thrombin (Sigma Chemical, St Louis, MO) in plasma samples from subjects homozygous for FXIII 34Val, FXIII 34Leu, heterozygous, and in pooled normal plasma obtained from 47 healthy donors. Plasma samples (150 μL) were preincubated with 150 μL α-thrombin at a final concentration of 5 U/mL in microtiter plates, after which FXIIIa activity was measured with the pentylamine-incorporation assay in a separate plate. Samples were diluted 1/10 in Tromethamine-buffered saline (TBS) (40 mmol/L Tris-HCl, 140 mmol/L NaCl, 0.02% ([w/v]) NaN3, pH 8.3) containing 0.3 mg/mL of the synthetic peptide Gly-Pro-Arg-Pro-Ala-amide (Sigma) to prevent inopportune polymerization of fibrin in the preincubation mixture, which would interfere with the following subsampling procedure. After 30 minutes of preincubation, aliquots of 20 μL were subsampled into the pentylamine-incorporation plate containing 80 μL of the reaction mixture of 1.25 mmol/L 5-(biotinamido) pentylamine, 0.63 mmol/L dithiothreitol, and 0.12 mol/L CaCl2 in TBS. The pentylamine-incorporation assay was further performed as described.23
FXIII proteolysis studies using SDS-PAGE
The SDS-PAGE procedure was performed using a Miniprotean 3 (Biorad, Hercules, CA) electrophoresis unit. Gels were cast at a polyacrylamide concentration of 8% (bis/acrylamide ratio of 1:37.5) in 1.5 mol/L Tris-HCl, pH 8.8, and run at 150 V for 80 minutes. Gels were stained with Coomassie blue (2.5 g/L in 45% methanol, 10% acetic acid) for 30 minutes at room temperature and destained for 1 to 2 hours in 45% methanol, 10% acetic acid with 3 changes of destaining solution. Dose response of FXIII proteolysis by thrombin was studied by incubating 4.0 μmol/L of purified FXIII variants with 0 to 10 U/mL human α-thrombin (Sigma) in 0.05 mol/L Tris-HCl, 1 mmol/L EDTA, pH 7.5 for 1 hour at 37°C. The reactions were stopped by the addition of equal (1:1) volume of reducing loading buffer 100 mmol/L Tris-HCl, 0.1 mol/L DTT, 4% SDS, 0.2% bromophenol blue, 20% glycerol, pH 6.8 with immediate boiling for 5 minutes, and 25 μL was loaded on the gel. The time course of FXIII proteolysis was studied by incubating 4.0 μmol/L of purified FXIII variants with 0.4 U/mL human α-thrombin at 37°C. The reaction was stopped at 0 to 240 minutes as described for the dose-response reactions, and 25 μL was loaded on the gel.
Reverse-phase high-performance liquid chromatography (HPLC)
Reverse-phase HPLC was performed to analyze the FXIII activation peptide release using a 0.46 × 25 cm silica C18 (5 μm, 300Å) column (Pepmap C18; Perseptive Biosystems Inc) on a Biocad Sprint automated chromatography system (also from Perseptive Biosystems, Framingham, MA), according to a method previously described by Janus and coworkers.25 The column was equilibrated with 1 column volume of 85% buffer A/15% buffer B. The sample was applied and eluted with a linear gradient from 85% buffer A/15% buffer B to 100% buffer B in 7 column volumes. The column was further washed with 2 column volumes of 100% buffer B followed by a linear gradient from 100% buffer B to 85% buffer A/15% buffer B in 1 column volume. Flow-rate was set at 1 mL/min throughout the experiment. Buffer A consisted of 10% acetonitrile/90% 0.083 mol/L sodium phosphate pH 3.1 and buffer B of 40% acetonitrile/60% 0.083 mol/L sodium phosphate pH 3.1. All reagents were HPLC grade and solutions were filtered through 0.22 μm to eliminate particulates. Elution of peptides was detected by absorbance at 205 nm.
Kinetics of activation peptide release from FXIII
Molar quantities of the activation peptides analyzed by reverse-phase HPLC were calculated from the chromatograms by integration of the respective peak areas. The area under the curve was converted into molar quantity by comparison to that of a calibration FXIII activation peptide loaded at known concentration. Purified FXIII was dialyzed against 9.47 mmol/L sodium phosphate, 137 mmol/L NaCl, 2.5 mmol/L KCl, 0.1% PEG, pH 7.4. Dose-response kinetics of FXIII activation peptide release by thrombin were performed by incubating 4.0 μmol/L of dialyzed FXIII with 0, 0.01, 0.05, 0.1, 0.2, 0.3, 0.4, 0.5, 0.75, 1, and 10 U/mL (1 U/mL = 9.16 nmol/L) human α-thrombin (Sigma) for 1 hour at 37°C. The reactions were stopped by the addition of 1:10 (vol/vol) of 3 mol/L HClO4, centrifuged for 10 minutes at maximum speed in an Eppendorf centrifuge and 180 μL injected onto the C18 column. Time course of the activation peptide release reaction was studied by incubation of 4.0, 2.0, and 1.0 μmol/L of FXIII with 0.5 U/mL human α-thrombin at 37°C. The reactions were stopped at 0, 2, 5, 10, 20, 30, 60, 120, 180, and 240 minutes by subsampling 10 volumes into 1 volume of 3 mol/L HClO4. The samples were centrifuged and 180 μL injected onto the C18 column. A similar method was used for studying the time course of FXIII activation peptide release in the presence of fibrin or fibrin and Gly-Pro-Arg-Pro-Ala-amide. In the presence of fibrin alone, 2.7 μmol/L of FXIII was incubated with 3.1 μmol/L human fibrinogen (Sigma) and 0.2 U/mL human α-thrombin. At this concentration of fibrinogen a clot formed, which was defined in size and did not interfere with the subsampling procedure. The reaction was stopped after 0.25, 0.5, 1, 2, 3, 4, 8, and 16 minutes of incubation and 270 μL injected onto the C18 column. In the presence of fibrin and the antipolymerizing peptide Gly-Pro-Arg-Pro-Ala, 2.7 μmol/L of FXIII was incubated with 3.1 μmol/L human fibrinogen (Sigma), 2 mg/mL Gly-Pro-Arg-Pro-Ala-amide (Sigma), and 0.5 U/mL human α-thrombin. In these experiments, the reaction was stopped at 2, 4, 6, 8, 10, 12, 14, 16, 18, 20, and 22 minutes and 270 μL injected.
Catalytic efficiency
Catalytic efficiencies of the FXIII activation peptide release reaction by thrombin were calculated by fitting the data from the time-course and dose-response experiments analyzed with reverse-phase HPLC to the following equation25:
where [AP] is the concentration of FXIII activation peptide at a given time point, [APf] the concentration of FXIII activation peptide at full activation, e the concentration of thrombin, and t time. Fitting of the data to this equation was performed using the Enzfitter for Windows software version 2.0.8 (Biosoft, Cambridge, UK). The data obtained for activation in the presence of fibrin was also fitted to the equation, although it was recognized that this was an approximation due to the complex nature of enhanced FXIII activation by fibrin.25-28
Mass spectrometry
Fractions from reverse-phase HPLC containing the FXIII activation peptides were collected for each purified FXIII Val34Leu variant. Molecular weights were analyzed with a single quadrupole, bench top mass spectrometer (Platform II, Micromass, Cheshire, UK). Samples were infused into the ionization source at a flow rate of 4 mL/min using a Harvard syringe pump. The mass spectrometer was fitted with a standard electrospray ionization source. Positive electrospray ionization was affected with a probe tip voltage of 3.5 kV, and a counter electrode voltage of 0.5 kV. Nitrogen was used as both the nebulizing and the drying gas; typical flow rates were a nebulizing gas flow rate of 20 L/h and a drying gas flow rate of 200 L/h. The sampling cone voltage was set to 50 volts. Data were acquired over the m/z range 500 to 2500. Spectra were transposed onto a true molecular mass scale using Maximum Entropy techniques. An external calibration was applied on a separate introduction of horse heart myoglobin (molecular weight, 16 951.49 d).
Analysis of rates of - and γ-chain cross-link formation
Rates of α- and γ-chain cross-link formation were studied by incubating 6.1 μmol/L human fibrinogen (Sigma) with 0.1 μmol/L purified FXIII, 0.5 U/mL human thrombin (Sigma) and 10 mmol/L calcium in 0.05 mol/L Tris-HCl, 0.15 mol/L NaCl, pH 7.5. The reaction was stopped by the addition of equal (1:1) volume of reducing loading buffer with immediate boiling for 5 minutes, and 25 μL was loaded on an 8% polyacrylamide gel. SDS-PAGE was further performed as described for the FXIII proteolysis studies.
Turbidity measurements at 350 nm
Plasma samples from subjects homozygous for either FXIII 34Leu (n = 3) or FXIII 34Val (n = 3) were diluted 2/3 with 0.05 mol/L Tris-HCl, 0.15 mol/L NaCl, pH 7.5 and incubated with 1 U/mL human thrombin (Sigma) and 16 mmol/L calcium in a 0.5-mL cuvette. Immediately on addition of thrombin/calcium, absorbancy was read every 3 seconds at 350 nm for 5 minutes with a Perkin-Elmer Lambda 4B spectrophotometer. Parameters such as lag phase before start of fibrin polymerization, slope of the polymerization curve (A/min) and maximum absorbancy at full polymerization were recorded. Three replicate measurements were performed for each sample.
Clot permeation measurements
Plasma samples (100 μL) from subjects homozygous for either FXIII 34Leu (n = 3) or FXIII 34Val (n = 3) were incubated to allow clot formation with 1 U/mL human thrombin (Sigma) and 20 mmol/L calcium in open tubes for 2 hours at room temperature in a wet chamber. The tubes containing the clots were connected via plastic tubing to a reservoir containing 0.05 mol/L Tris-HCl, 0.15 mol/L NaCl, pH 7.5 with a pressure drop of 4 cm. After washing the clots, flow rates of buffer through the fibrin gels were measured by timing 6 drops for each tube and weighing each drop for exact volume. Four replicate clots of each sample were analyzed in this way. The permeation coefficient or Darcy constant, which represents the surface of the gel allowing flow through a network and thus provides information on the pore structure, was calculated using the following formula29:
where Q is the volume of liquid (mL) with the viscosity η (10−2 poise) flowing through a clot with length L (1.3 cm) and a cross-sectional areaA (0.049 cm2) in time t (seconds) under pressure ΔP (dyne/cm2). The unit of the resulting Darcy constant is cm2.
Scanning electron microscopy of fibrin clots
After permeation experiments, the clots were fixed by permeating them with a 2% (v/v) glutaraldehyde solution overnight. Clots were recovered from the tubes and further processed by dehydration using a stepwise ethanol gradient, critical point drying, and sputter coating with gold-palladium as previously described.30 Plasma clots from 3 homozygous FXIII 34Leu subjects and 3 homozygous FXIII 34Val subjects were observed and photographed digitally in at least 6 different areas using a scanning electron microscope (XL 20, Philips Electron Optics, Eindhoven, The Netherlands).
Results
Effect of preactivation on FXIIIa cross-linking activities
Plasma samples from subjects homozygous for FXIII 34Leu showed greater activity in a pentylamine-incorporation assay, in which there is thrombin activation during the course of the assay, than plasma samples from subjects homozygous for FXIII 34Val or heterozygous subjects. Cross-linking rates in this assay were typically higher for FXIII 34Leu than for FXIII 34Val, with an intermediate response to heterozygous samples and pooled normal plasma (Figure1A). After preincubation with 5 U/mL human α-thrombin for 30 minutes at 37°C, however, this difference disappeared, and cross-linking activities of plasma samples from subjects homozygous for FXIII 34Leu or FXIII 34Val and heterozygous subjects were similar (Figure 1B).
Effect of preactivation with thrombin on FXIII cross-linking rates.
Cross-linking rates of plasma samples from a subject homozygous for FXIII 34Leu (circles), FXIII 34Val (triangles), a heterozygous subject (squares), and pooled normal plasma (stars) were analyzed in a pentylamine-incorporation assay. FXIII 34Leu cross-linking activity was higher than that of FXIII 34Val, with intermediate response from heterozygous FXIII and pooled normal plasma (A), but this difference was abolished by preactivation with thrombin (B).
Effect of preactivation with thrombin on FXIII cross-linking rates.
Cross-linking rates of plasma samples from a subject homozygous for FXIII 34Leu (circles), FXIII 34Val (triangles), a heterozygous subject (squares), and pooled normal plasma (stars) were analyzed in a pentylamine-incorporation assay. FXIII 34Leu cross-linking activity was higher than that of FXIII 34Val, with intermediate response from heterozygous FXIII and pooled normal plasma (A), but this difference was abolished by preactivation with thrombin (B).
Increased proteolysis of FXIII 34Leu by thrombin
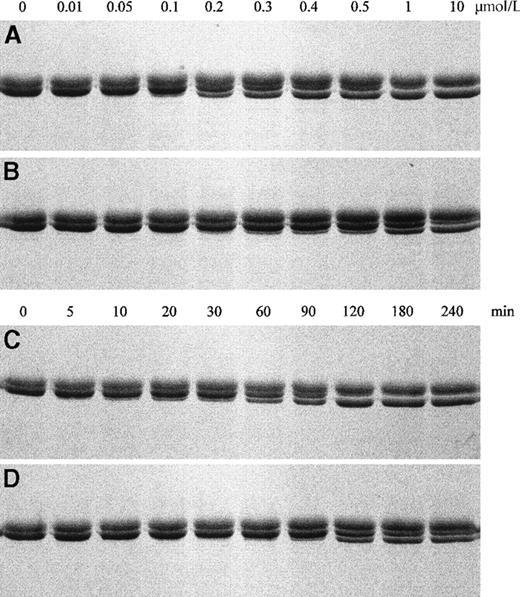
Proteolysis of FXIII subunits by thrombin was studied using SDS-PAGE. Electrophoresis of FXIII on SDS-polyacrylamide gels under reducing conditions showed 2 bands for the A and B subunit migrating very closely together. Activation by thrombin produced a distinct band for the activated A subunit that migrated faster in the gel. We first examined activation of purified FXIII 34Leu and FXIII 34Val by increasing doses of human α-thrombin. Figure2 shows that FXIII 34Val (panel B) proteolysis started with 0.3 to 0.4 U/mL of thrombin after 1 hour incubation at 37°C, whereas FXIII 34Leu (panel A) proteolysis started with 0.1 to 0.2 U/mL of thrombin and was complete with 1 U/mL. We next investigated FXIII cleavage by adding 0.4 U/mL human α-thrombin and stopping the reaction after increasing times. Proteolysis of FXIII 34Val (panel D) started after 60 to 90 minutes, whereas FXIII 34Leu cleavage (panel C) by the same dose of thrombin started after 10 to 20 minutes and was complete after 120 minutes.
Proteolysis of purified FXIII by thrombin.
The effects of 1 of hour incubation at 37°C with increasing amounts of human α-thrombin on 4.0 μmol/L FXIII 34Leu (A) and FXIII 34Val (B), and proteolysis of 4.0 μmol/L FXIII 34Leu (C) and FXIII 34Val (D) by 0.4 U/mL thrombin after increasing incubation times were analyzed by reducing SDS-PAGE. Formation of a distinct band for the cleaved A subunit shows activation by thrombin.
Proteolysis of purified FXIII by thrombin.
The effects of 1 of hour incubation at 37°C with increasing amounts of human α-thrombin on 4.0 μmol/L FXIII 34Leu (A) and FXIII 34Val (B), and proteolysis of 4.0 μmol/L FXIII 34Leu (C) and FXIII 34Val (D) by 0.4 U/mL thrombin after increasing incubation times were analyzed by reducing SDS-PAGE. Formation of a distinct band for the cleaved A subunit shows activation by thrombin.
Analysis of FXIII activation peptides
The preactivation and SDS-PAGE studies suggested that release of the activation peptide from FXIII 34Leu is more rapid than that from FXIII 34Val, but the incomplete resolution of the bands in SDS-PAGE did not allow quantification. Reverse-phase HPLC was used to quantify the amount of peptide released from each form of FXIII by thrombin. When activating purified FXIII 34Val with 10 U/mL human α-thrombin for 1 hour at 37°C, one distinct peak for the activation peptide was detected. Activating FXIII purified from a mixed genotype, however, produced 2 peaks, and analysis of activated purified FXIII 34Leu indicated that the 2nd peak represented the 34Leu activation peptide (Figure 3). This separation of the 2 species of activation peptides on the C18 column was confirmed by mass spectrometry. HPLC fractions containing the respective peptide peaks were collected and analyzed for the molecular weight of their peptides. The first peak contained a peptide of molecular weight of 3951.5 d and the second peak a peptide of 3965.0 d. These results confirmed that the first peak contains the FXIII 34Val activation peptide and the 2nd peak the FXIII 34Leu activation peptide. The difference in molecular weight between the peptides can be accounted for by the expected single methyl group (14.0 d) difference between the Leu and Val side chains. They also demonstrated that the thrombin cleavage site between Arg37 and Gly38 is unaltered by the Val34Leu polymorphism.
Separation of the 34Leu and 34Val activation peptides on reverse-phase HPLC.
Purified FXIII 34Val (0.9 nmol/0.18 mL), FXIII from a mixed genotype (34Val/Leu; 0.5 nmol/0.18 mL), and FXIII 34Leu (0.06 nmol/0.02 mL) were analyzed by HPLC after activation by 10 U/mL human α-thrombin for 1 hour at 37°C. Peptides were detected by absorbance at 205 nm.
Separation of the 34Leu and 34Val activation peptides on reverse-phase HPLC.
Purified FXIII 34Val (0.9 nmol/0.18 mL), FXIII from a mixed genotype (34Val/Leu; 0.5 nmol/0.18 mL), and FXIII 34Leu (0.06 nmol/0.02 mL) were analyzed by HPLC after activation by 10 U/mL human α-thrombin for 1 hour at 37°C. Peptides were detected by absorbance at 205 nm.
Kinetics of FXIII activation
The separation of the FXIII 34Val from the FXIII 34Leu activation peptide on reverse-phase HPLC allowed us to carry out kinetic analysis of the activation reaction for each form of FXIII using purified FXIII from a mixed genotype. Purified FXIII was incubated at 4.0 μmol/L with increasing concentrations of human α-thrombin. HPLC was performed on the incubation mixtures. Peak areas of the FXIII 34Val and FXIII 34Leu activation peptides were calculated from the chromatograms and transformed in molar quantities. More FXIII 34Leu activation peptide was released than FXIII 34Val activation peptide after incubation with thrombin concentrations between 0.01 and 0.5 U/mL for 1 hour at 37°C (Figure4A), confirming the findings from the gel electrophoresis studies that FXIII 34Leu is activated by lower doses of thrombin than FXIII 34Val. Time-course experiments were also performed. Purified FXIII at 4.0, 2.0, and 1.0 μmol/L was activated with 0.5 U/mL human α-thrombin at 37°C and the reaction was stopped at increasing times. More FXIII 34Leu activation peptide was released than FXIII 34Val activation peptide early in the reaction (Figure 4B). This faster initial rate was observed regardless of the relative concentrations of FXIII and thrombin; kinetic data are summarized in Table 1.
Activation of FXIII 34Val and FXIII 34Leu by human -thrombin as analyzed by HPLC.
(A) FXIII (4.0 μmol/L) was incubated with increasing doses of thrombin for 1 hour at 37°C. (B) FXIII (4.0 μmol/L) was incubated with 0.5 U/mL thrombin at 37°C for increasing periods of time. Squares represent FXIII 34Val and circles represent FXIII 34Leu. Results shown for each experiment are mean values ± SD of 3 replicates. Release of the activation peptides was expressed as the ratio between the molar quantity of the peptides ([AP]) and molar quantity of the peptides at full activation ([AP]f).
Activation of FXIII 34Val and FXIII 34Leu by human -thrombin as analyzed by HPLC.
(A) FXIII (4.0 μmol/L) was incubated with increasing doses of thrombin for 1 hour at 37°C. (B) FXIII (4.0 μmol/L) was incubated with 0.5 U/mL thrombin at 37°C for increasing periods of time. Squares represent FXIII 34Val and circles represent FXIII 34Leu. Results shown for each experiment are mean values ± SD of 3 replicates. Release of the activation peptides was expressed as the ratio between the molar quantity of the peptides ([AP]) and molar quantity of the peptides at full activation ([AP]f).
Catalytic efficiencies of the FXIII 34Val and 34Leu activation peptide release by thrombin in the presence or absence of fibrin (FI) or Gly-Pro-Arg-Pro-amide (GPRP)
| kcal/Km (μmol/L)−1 × sec−1 . | Time courses . | Dose response . | Mean ± SD (n = 4) . | +FI +GPRP (n = 3) . | +FI −GPRP (n = 4) . | ||
|---|---|---|---|---|---|---|---|
| 4.0 μmol/L . | 2.0 μmol/L . | 1.0 μmol/L . | |||||
| Valine 34 | 0.22 | 0.20 | 0.17 | 0.21 | 0.20 ± 0.02 | 0.40 ± 0.06 | 2.15 ± 0.13 |
| Leucine 34 | 0.56 | 0.41 | 0.40 | 0.57 | 0.49 ± 0.09 | 0.65 ± 0.11 | 4.81 ± 0.31 |
| kcal/Km (μmol/L)−1 × sec−1 . | Time courses . | Dose response . | Mean ± SD (n = 4) . | +FI +GPRP (n = 3) . | +FI −GPRP (n = 4) . | ||
|---|---|---|---|---|---|---|---|
| 4.0 μmol/L . | 2.0 μmol/L . | 1.0 μmol/L . | |||||
| Valine 34 | 0.22 | 0.20 | 0.17 | 0.21 | 0.20 ± 0.02 | 0.40 ± 0.06 | 2.15 ± 0.13 |
| Leucine 34 | 0.56 | 0.41 | 0.40 | 0.57 | 0.49 ± 0.09 | 0.65 ± 0.11 | 4.81 ± 0.31 |
Effect of fibrin on the activation peptide release reaction
Fibrinogen was added at a concentration of 3.1 μmol/L to 2.7 μmol/L purified FXIII and 0.2 U/mL human α-thrombin. The inset of Figure 5 shows a typical chromatogram from these experiments, with defined peaks for fibrinopeptide A, fibrinopeptide B, and FXIII 34Val and 34Leu activation peptides. In the presence of fibrin(ogen), the release of both FXIII activation peptides by thrombin was greatly accelerated. FXIII 34Leu activation peptide release remained significantly faster than that of FXIII 34Val. The FXIII 34Leu activation peptide was released at a similar rate as fibrinopeptide A, followed by release of the FXIII 34Val peptide and finally fibrinopeptide B (Figure 5).
Time-course of activation of FXIII by thrombin in the presence of polymerizing fibrin.
The activation of 3.1 μmol/L purified FXIII 34Val (squares) and FXIII 34Leu (circles) by 0.2 U/mL human α-thrombin with the addition of 2.7 μmol/L fibrinogen was analyzed by HPLC. Diamonds represent fibrinopeptide A and triangles fibrinopeptide B. Release of the peptides was expressed as the ratio between the molar quantity of the peptides ([peptide]) and molar quantity of the peptides at full activation ([peptide]f). Results shown are mean values ± SD of 4 experiments. The inset shows a typical chromatogram with 4 defined peaks for fibrinopeptide A, fibrinopeptide B, FXIII 34Val activation peptide, and FXIII 34Leu activation peptide.
Time-course of activation of FXIII by thrombin in the presence of polymerizing fibrin.
The activation of 3.1 μmol/L purified FXIII 34Val (squares) and FXIII 34Leu (circles) by 0.2 U/mL human α-thrombin with the addition of 2.7 μmol/L fibrinogen was analyzed by HPLC. Diamonds represent fibrinopeptide A and triangles fibrinopeptide B. Release of the peptides was expressed as the ratio between the molar quantity of the peptides ([peptide]) and molar quantity of the peptides at full activation ([peptide]f). Results shown are mean values ± SD of 4 experiments. The inset shows a typical chromatogram with 4 defined peaks for fibrinopeptide A, fibrinopeptide B, FXIII 34Val activation peptide, and FXIII 34Leu activation peptide.
Catalytic efficiency of FXIII activation
Catalytic efficiencies of thrombin-dependent activation peptide release from the different polymorphic forms of FXIII were calculated in the absence of fibrin, in the presence of fibrin alone, and in the presence of both fibrin and Gly-Pro-Arg-Pro-amide. The 34Leu form showed twice the catalytic efficiency, regardless of the experimental conditions (Table 1). The presence of fibrin greatly enhanced FXIII activation peptide release by thrombin and this catalytic effect was reduced when polymerization of fibrin was inhibited with Gly-Pro-Arg-Pro-amide. The difference in catalytic efficiency between FXIII 34Val and FXIII 34Leu remained in the presence of polymerizing fibrin.
Effect of FXIII 34Leu on - and γ-chain cross-linking
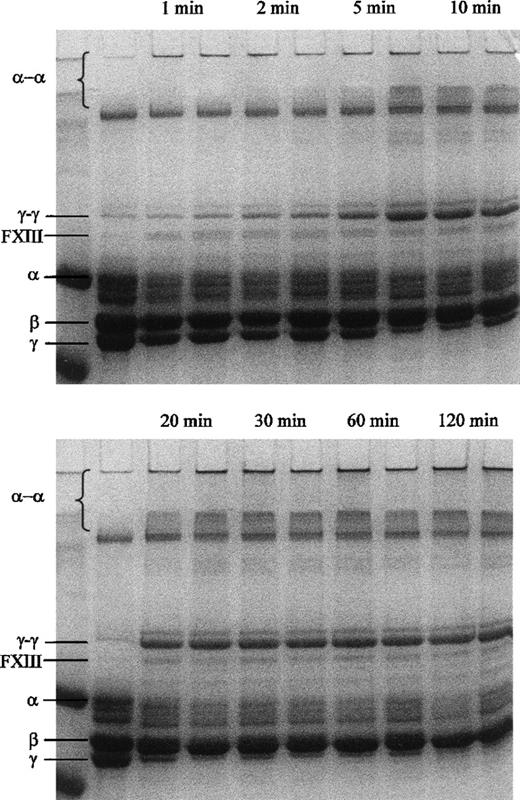
Cross-linking of the fibrin α- and γ-chains by the 2 forms of FXIII was studied using reducing SDS-PAGE. Cross-linking of both γ-chains and α-chains appeared earlier when incubating a mixture of fibrinogen and thrombin with purified FXIII 34Leu than with FXIII 34Val (Figure 6). The fibrinogen preparation used for these experiments had some contamination with fibronectin and FXIII. Due to traces of FXIII in the preparation, some γ-chain dimers were already present without incubation with the isolated FXIII variants. Nonetheless, more γ-chain dimers and α-chain polymers were formed by incubation with FXIII 34Leu than FXIII 34Val after 5 minutes. After 20 minutes incubation FXIII 34Leu produced more of high molecular weight α-chain polymers that did not enter the gel and remained in the loading well (Figure 6).
Formation of fibrin - and γ-chain cross-links as analyzed by SDS-PAGE.
First lane of each gel shows a molecular weight marker (102.0 and 81.0 kd) and second lane shows the fibrinogen preparation before incubation with thrombin and FXIII. Following lanes show pairwise incubations of 6.1 μmol/L fibrinogen with 0.5 U/mL human thrombin and 0.1 μmol/L FXIII 34Val (left-hand lanes) or FXIII 34Leu (right-hand lanes) for the times as indicated in the figure.
Formation of fibrin - and γ-chain cross-links as analyzed by SDS-PAGE.
First lane of each gel shows a molecular weight marker (102.0 and 81.0 kd) and second lane shows the fibrinogen preparation before incubation with thrombin and FXIII. Following lanes show pairwise incubations of 6.1 μmol/L fibrinogen with 0.5 U/mL human thrombin and 0.1 μmol/L FXIII 34Val (left-hand lanes) or FXIII 34Leu (right-hand lanes) for the times as indicated in the figure.
FXIII Val34Leu and fibrin structure
Plasma samples from subjects homozygous for either form of FXIII Val34Leu were used to study effects of this polymorphism on fibrin structure. The lag phase in formation of turbidity at 350 nm was shorter for homozygous FXIII 34Leu (1.08 ± 0.11 minutes) than for homozygous FXIII 34Val samples (1.55 ± 0.15 minutes). The slope of the turbidity curves did not differ, but maximum absorbancy after 5 minutes was slightly lower for the FXIII 34Leu (1.83 ± 0.04) than FXIII 34Val (1.93 ± 0.03), indicating the presence of thinner fibrin fibers in the FXIII 34Leu samples. Consistent with these results, permeation of fibrin clotted for 2 hours was found to be slower in clots from FXIII 34Leu samples when compared with FXIII 34Val. The Darcy constant, which reflects the surface area of the clot available for flow, was 3.6 ± 0.5 × 10−9 cm2 for FXIII 34Leu and 8.7 ± 4.4 × 10−9cm2 for FXIII 34Val. These structural differences were confirmed by scanning electron microscopy. Clots prepared from plasma samples homozygous for FXIII 34Val (Figure7A) showed a fibrin meshwork with thick fibers and large pores, whereas clots prepared from plasma samples homozygous for FXIII 34Leu showed a finer fibrin meshwork with thinner fibers and reduced space between the fibrin strands (Figure 7B).
Scanning electron micrographs of fibrin clots prepared from plasma samples of subjects homozygous for FXIII 34Val and FXIII 34Leu.
Clots were prepared from plasma samples from subjects homozygous for FXIII 34Val (A) and homozygous for FXIII 34Leu (B). Magnification is × 5000 for both micrographs. The electron micrographs are representative of 6 samples scanned in 6 different areas showing similar results.
Scanning electron micrographs of fibrin clots prepared from plasma samples of subjects homozygous for FXIII 34Val and FXIII 34Leu.
Clots were prepared from plasma samples from subjects homozygous for FXIII 34Val (A) and homozygous for FXIII 34Leu (B). Magnification is × 5000 for both micrographs. The electron micrographs are representative of 6 samples scanned in 6 different areas showing similar results.
Discussion
The FXIII gene is known to be highly polymorphic, with several common nucleotide sequence variations within the population, which encode amino acid substitutions. Although these amino acid sequence changes could alter the levels and activity of FXIII, to date there has been little formal examination of their effects. We report here the functional characterization of the Val34Leu polymorphism in the A subunit. This polymorphism is located adjacent to the thrombin cleavage activation site of FXIII. Presence of the 34Leu encoding allele has been associated with a protective effect against thrombotic disease in the arterial and venous systems.13-17
In this study, it is demonstrated that the nature of the amino acid substitution at position 34 influences the activation rate of FXIII. Using SDS-PAGE, the rate of proteolysis of the FXIII A subunit is shown to be faster and to occur at lower thrombin concentrations when Leu rather than Val is at position 34. HPLC analysis demonstrates that proteolysis of FXIII occurs at the activation cleavage site (by direct determination of the precise Mr of the activation peptide) and that the catalytic efficiency of thrombin cleavage of the activation peptide is increased approximately 2-fold by substitution of Val by Leu.
Prior studies of the kinetics of activation peptide release from FXIII have used pooled donations of plasma to prepare the protein, and this was carried out without knowledge of the genotype. The estimates of catalytic efficiency obtained in those studies, kcat/Km 0.13 to 0.16 (μmol/L)−1 × sec−1,25agree with that derived in the present study for FXIII 34Val. We now find that the 34Val and 34Leu activation peptides separate on a C18 column, with the 34Val peptide eluting before 34Leu. Also in the study by Janus and coworkers,25 a second eluting peak was observed when the FXIII activation was analyzed by HPLC. Amino acid analysis on the collected fractions demonstrated 1.8 and 2.7 leucine residues in the first and second peaks, respectively. The authors concluded that the second peptide was FXIII related, but were unable to identify the presence of the Val34Leu polymorphism in the variants. The second peak was ignored in the reported kinetic analysis, and in retrospect, the catalytic efficiency reported by Janus and colleagues, therefore, appears to be related solely to FXIII 34Val.
Characterization of the later eluting peak in the present study has enabled its identity to be determined as the activation peptide from FXIII 34Leu. This information has allowed an estimate to be made of the catalytic efficiency of its cleavage by thrombin, kcat/Km 0.49 (μmol/L)−1 × sec−1. The estimates obtained were robust in relation to the relative ratio of thrombin to its substrate over a range of concentrations of both thrombin and its substrate. In the presence of fibrin, the catalytic efficiencies of FXIII activation peptide release were further increased to 2.15 and 4.81 (μmol/L)−1 × sec−1 for FXIII 34Val and FXIII 34Leu, respectively, maintaining the polymorphism-related differences. These fibrin-induced increases are again compatible with previous reports, despite the lack of a rigorous kinetic approach here compared with the prior studies.25-28Polymerization of fibrin appears to play a role in the FXIII activation process, because inhibition of polymerization with Gly-Pro-Arg-Pro-amide significantly reduced the catalytic function of fibrin in the FXIII activation peptide release reaction.
The finding of an accelerated activation rate of FXIII 34Leu suggests that the pentylamine-incorporation assay, as it is routinely used for screening of FXIII activity levels, is particularly sensitive to the FXIII activation step. The result also provides an explanation for the relatively poor correlation between FXIII activity levels and FXIII A-subunit antigen levels,19,20,23 because the wide variability in activity in relation to the Val34Leu polymorphismwould largely override the effect of relatively minor fluctuations in the A-subunit levels under normal conditions. Kangsadalampai and Board expressed the 34Val and 34Leu forms of FXIII in vitro and also found that FXIII 34Leu showed higher activity in a pentylamine-incorporation assay.19 However, activation kinetics were not investigated, and their results could have also been related to the increased activation rate of FXIII 34Leu. We found that, once activated, the specific cross-linking activities of the 2 forms of FXIII do not differ.
When analyzing the sequence of the release of the FXIII activation peptides in relation to fibrinopeptides A and B, it was found that the FXIII 34Leu activation peptide was released at approximately the same rate as fibrinopeptide A, whereas release of the FXIII 34Val activation peptide was slower, with a rate in-between those of the 2 fibrinopeptides. These findings suggest that cross-linking activity by FXIII 34Leu is generated at the time of desA fibrin formation, whereas cross-linking activity by FXIII 34Val is generated more at a time when both fibrinopeptides A and B are cleaved from fibrinogen. It has to be borne in mind, however, that the experimental conditions of this experiment are different from those encountered in vivo. Whereas thrombin and fibrinogen concentrations could be considered to resemble those attainable under in vivo conditions, the FXIII concentration of 3.1 μmol/L, necessary to obtain measurable peak areas of the activation peptide by HPLC, was significantly higher than the physiologic plasma concentration of about 0.07 μmol/L.
The observed differences in cleavage of the 2 species of FXIII activation peptides in temporal relation to fibrinopeptide A and B release prompted us to investigate the effect of the FXIII Val34Leu polymorphism on the structure of the cross-linked fibrin clot. In agreement with the accelerated activation of FXIII 34Leu, analysis by SDS-PAGE showed that cross-linking of fibrin α- and γ-chains by FXIII 34Leu occurred earlier than that by FXIII 34Val. At later time points, both forms of FXIII generated similar amounts of cross-links. Polymerization, as followed by turbidity, was also faster in clotting plasma samples homozygous for FXIII 34Leu than in those homozygous for FXIII 34Val. After the initial more rapid rise of turbidity in the FXIII 34Leu samples, however, total turbidity at 5 minutes was lower than that in FXIII 34Val samples, indicating the presence of thinner fibrin fibers.
Biophysical and structural properties of fibrin in homozygous plasma samples clotted for 2 hours, allowing cross-linking by FXIII to reach completion, were analyzed using permeation characteristics of the fibrin gel in addition to electron microscopy. Fibrin that was fully cross-linked by FXIII 34Leu showed a finer structure with thinner fibers and smaller pores, than fibrin cross-linked by FXIII 34Val. Previous studies have shown that the delay in fibrinopeptide B release compared to the relatively fast release of fibrinopeptide A appears to be necessary for enhanced lateral aggregation and thicker fibers.31 32 Our present findings suggest that the accelerated generation of covalent cross-linking activity by FXIII 34Leu influences the lateral aggregation process and affects the molecular structure of the cross-linked fibrin clot. It appears that early cross-linking of fibrin by FXIII 34Leu, which is activated at the time of fibrinopeptide A release, inhibits lateral aggregation of the fibrin fibers, whereas delayed cross-linking by FXIII 34Val, which is activated at the time of desAB fibrin formation, allows for more lateral aggregation.
This study has demonstrated that the Val34Leu polymorphism in the A subunit affects the function of FXIII by increasing the rate of FXIII activation by thrombin and by altering the molecular structure of the cross-linked fibrin gel to one with reduced mass/length ratio of the fibers. The potential consequences of fibrin cross-linked by FXIII 34Leu, with a finer meshwork, thinner fibers, and altered permeation characteristics, on clinical (vascular) outcome remain to be elucidated.
Acknowledgments
The authors wish to thank Dr S. MacLennan and Mr E. Lee from the Regional Blood Transfusion Centre of Yorkshire, Leeds, UK, for the provision of outdated transfusion plasma and buffy coat, and Dr A. E. Ashcroft for mass spectrometry. Mr I. Moss and Ms K. Standeven, Dr Y. Veklich, and Dr R. Marchi are gratefully acknowledged for their assistance with the Biocad Sprint, turbidity measurements, and permeation measurements, respectively. We also wish to thank Dr J. P. Collet for helpful discussion of the effects of FXIII Val34Leu on fibrin structure.
Supported by the British Heart Foundation (PG/98104), the Special Trustees of Charing Cross Hospital and the National Institutes of Health (HL30954).
Reprints:Robert A. S. Ariëns, Unit of Molecular Vascular Medicine, University of Leeds, G-Floor, Martin Wing, Leeds General Infirmary, Leeds LS1 3EX, UK; e-mail: r.a.s.ariens@leeds.ac.uk.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.



![Fig. 4. Activation of FXIII 34Val and FXIII 34Leu by human -thrombin as analyzed by HPLC. / (A) FXIII (4.0 μmol/L) was incubated with increasing doses of thrombin for 1 hour at 37°C. (B) FXIII (4.0 μmol/L) was incubated with 0.5 U/mL thrombin at 37°C for increasing periods of time. Squares represent FXIII 34Val and circles represent FXIII 34Leu. Results shown for each experiment are mean values ± SD of 3 replicates. Release of the activation peptides was expressed as the ratio between the molar quantity of the peptides ([AP]) and molar quantity of the peptides at full activation ([AP]f).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/3/10.1182_blood.v96.3.988/5/m_bloo01557004x.jpeg?Expires=1765914120&Signature=2O3rkt3mNWjSJNHnKeOgLlW1oi6PLj4YIl6tktRj0LH5wZEQNQkAcKeLPnvFFikVAaNIU6DnsKFyV~fVpcjuIeonBgB9bnC4465BZ6eYk4ndUrXssDTZeEYpK3WqZeTROMak3ujCTonmU-539Gkv2w0BxJwUMkxR3H8BM8uYzdihXdaTwwaqgy~gw~o~ae5LDI8myXyFq-pho6ODaCsVrDEL5TAZnBbYR-bRfUCTwT6wl2v6PLoW6c-IVfuW9pguaR5VJv7~M8W3FcuRopkivSnNg7DxUQbo7rK0RdCdROwByexBIO0-uNcLH1v5wgevgrehBY5S57YTJKlvG2NAQQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Time-course of activation of FXIII by thrombin in the presence of polymerizing fibrin. / The activation of 3.1 μmol/L purified FXIII 34Val (squares) and FXIII 34Leu (circles) by 0.2 U/mL human α-thrombin with the addition of 2.7 μmol/L fibrinogen was analyzed by HPLC. Diamonds represent fibrinopeptide A and triangles fibrinopeptide B. Release of the peptides was expressed as the ratio between the molar quantity of the peptides ([peptide]) and molar quantity of the peptides at full activation ([peptide]f). Results shown are mean values ± SD of 4 experiments. The inset shows a typical chromatogram with 4 defined peaks for fibrinopeptide A, fibrinopeptide B, FXIII 34Val activation peptide, and FXIII 34Leu activation peptide.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/3/10.1182_blood.v96.3.988/5/m_bloo01557005y.jpeg?Expires=1765914120&Signature=YC-XydxJGwrAeRh-5ta0n38RrLMMwaHuvm5NZ0XIvoSKRgY9ane7coOvaBG5K1P~WDh8U6g6P8FeGa4Edodif3EoqXRbTAHbf341aMmNQ6f15jiMamTQt~0PLNSLohQ~dDBBJ4bK3Gzs12wl04c47zibrlHf~zSYu4L8nO4cN9Di-7WyHyXNm6jVh2NIcOPRMSzdcGJfCtwxUVAP4-yYK1vl3dUzXb6pnEuk3AU9yMdXkUU878MS93smQf8yzn95yIjNDxKcHRwN52kWbKLpLMrA2~N6Nhi7FGbEAX-2q1wNZGXHjLdrYJnoX9SrjZSBvHX-kDzzHcRTzwsKBHVyUA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal