Abstract
γ-Glutamyl carboxylase (GC), a polytopic membrane protein found in the endoplasmic reticulum (ER), catalyzes vitamin K–dependent posttranslational modification of glutamate to γ-carboxyl glutamate. In an attempt to delineate the structure of this important enzyme, in vitro translation and in vivo mapping were used to study its membrane topology. Using terminus-tagged full-length carboxylase, expressed in 293 cells, it was demonstrated that the amino-terminus of the GC is on the cytoplasmic side of the ER, while the carboxyl-terminus is on the lumenal side. In addition, a series of fusions were made to encode each predicted transmembrane domain (TMD) followed by a leader peptidase (Lep) reporter tag, as analyzed by the computer algorithm TOPPRED II. Following in vitro translation of each fusion in the presence of canine microsomes, the topological orientation of the Lep tag was determined by proteinase K digestion and endoglycosidase H (Endo H) cleavage. From the topological orientation of the Lep tag in each fusion, the GC spans the ER membrane at least 5 times, with its N-terminus in the cytoplasm and its C-terminus in the lumen.
γ-Glutamyl carboxylase (GC) is a polytopic resident endoplasmic reticulum (ER) membrane protein1 that uses carbon dioxide, oxygen, and vitamin K hydroquinone to catalyze the carboxylation of a specific group of glutamate residues in the vitamin K–dependent proteins.2 This posttranslational modification is essential for the biological activities of vitamin K–dependent proteins in regulating blood coagulation, bone metabolism, and cell growth.
Despite purification3 and cloning4 of the carboxylase, our understanding of carboxylation is solely dependent on functional studies. For example, regions responsible for the active site and propeptide binding5-10 were accomplished by affinity labeling and site-specific mutagenesis studies. Inactivation of the carboxylase by sulfhydryl-reactive reagents11 indicates the presence of an essential cysteine(s); however, the topological orientations of the cysteines and their mechanistic functions remain to be identified.
In this study, we used in vitro translation/cotranslocation in an attempt to determine the membrane topology of the carboxylase. This method has been successfully used to identify the topogenic sequences of several integral membrane proteins.12-17 In addition, we determined the in vivo topological orientation of the amino- and carboxyl-termini of the carboxylase using an intact, fully active recombinant carboxylase.
We constructed a series of fusion molecules based on the TOPPRED II computer analysis of the carboxylase sequence. Fusions containing single putative transmembrane domains (TMDs) followed by the leader peptidase (Lep) reporter tag were used for the in vitro translation/cotranslocation study. Based on the signal-anchor potential of each putative TMD, we predict that human GC spans the membrane at least 5 times, with its N-terminus in the cytoplasm and its C-terminus in the lumen of the ER.
Materials and methods
Materials
The following materials were used: XL1 Blue bacterial strain and pSPUTK (Stratagene; San Diego, CA); the BacVector-3000 DNA kit (Novagen, Madison, WI); the in vitro transcription kit (Ambion, Austin, TX); rabbit reticulocyte lysate, pCl-neo expression vector, amino acid master mixture, proteinase K, and ribonuclease inhibitor (RNasin) (Promega Company, Madison, WI); 35S-methionine and the enhanced chemoluminescence (ECL) Western blotting detection kit (Amersham Life Sciences, Arlington Heights, IL); phenylmethylsulfonyl fluoride (PMSF) and mouse anti-FLAG M2 monoclonal antibody (mAb) (Sigma Chemical Co, St Louis, MO); endoglycosidase H (Endo H) (Boehringer Mannheim, Indianapolis, IN); biotinylated protein size markers (Bio-Rad Laboratories, Hercules, CA); avidin-HRP conjugate (Pierce, Rockford, IL); restriction endonucleases (New England BioLab, Beverly, MA); and HRP-conjugated secondary antibodies (Jackson Laboratories, West Grove, PA). Professor von Heijne (Stockholm University, Stockholm, Sweden) kindly provided rabbit anti-Lep antiserum and pING Lep-cDNA (complementary DNA).
DNA manipulation
A 735-nucleotide (nt) DNA fragment coding for the reporter tag, a peptide containing the C-terminal, 245 amino acid residues of the P2 domain of Lep, including a potential N-glycosylation acceptor site (Asn214), was generated by polymerase chain reaction (PCR) using pING-cDNA as the template. The PCR-amplified DNA fragment was cloned into the pSPUTK vector at the BamHI/ClaI(Bacillus amyloliquefaciens H/Caryophanon latum) sites to yield pSPUTK-Lep, which served as the recipient of all recombinant human (hGC) fragments.
Site-specific mutagenesis18 was performed to remove the endogenous NcoI (Nocardia corallina) site at 765 nt of the hGC cDNA. The resulting plasmid was used as a PCR template to generate various hGC fragments. A 5′-NcoI site and a 3′-BamHI site were introduced into each PCR-amplified hGC fragment. The PCR-amplified fragment containing a single TMD was cloned into NcoI/BamHI-cleaved pSPUTK-Lep. The 5′-NcoI site was immediately 3′ to the SP6 promoter and served as the in-frame initiation codon. The 3′-BamHIsite introduced 2 amino acid residues (glycine [G] and serine [S]) between the hGC fragment and the Lep tag.
We used site-specific mutagenesis18 to introduce appropriate restriction sites to accommodate the synthetic oligonucleotides encoding the FLAG tag. A 10–amino acid peptide (MDYKDDDDKG), including the FLAG epitope, was introduced to the amino-terminus of the full length of hGC to make FLAG-hGC, and an 8–amino acid peptide (DYKDDDDK) was attached to the carboxyl-terminus of the full length of hGC to make hGC-FLAG. The FLAG-tagged hGC cDNA was subcloned into the EcoRI (Escherichia coli RY13) site of the expression vector pCl-neo under control of the cytomegalovirus (CMV) promoter.
In vitro transcription and translation
Prior to in vitro transcription, the recombinant pSPUTKs were linearized at the ClaI site immediately 3′ to the Lep tag. Capped messenger RNA (mRNA) (Ambion) was synthesized according to the manufacturer's instructions. Dog pancreatic microsomal membranes were prepared as described.19 In vitro translation using rabbit reticulocyte lysate and cotranslocation using canine pancreatic microsomes were performed according to Shelness et al.15The reactions were performed at 25°C for 30 minutes in a final volume of 20 μL containing 10 μL rabbit reticulocyte lysate; 0.5 μL RNasin (40 U/μL); 0.4 μL of a 1 mmol/L amino acid mixture without methionine; 1.6 μL 35S-methionine (3.7 × 1013 Bq [1000 Ci/mmol]) at 370 MBq (10 mCi/mL); and 1 μg capped mRNA with or without 1 equivalent19 of canine pancreatic microsomes.
For further enzymatic cleavage, the translation products were chilled on ice and mixed with 30 μL incubation buffer containing 110 mmol/L potassium acetate, 2.5 mmol/L magnesium acetate, and 25 mmol/L K-HEPES (potassium 4-(2-Hydroxyethyl)-1-piperazineethanesulfonic acid) (pH 7.4). For deglycosylation, 1 μL Endo H (1 mU/μL) and 1 μL 5% CHAPS (3-[(3-cholamidopropyl)di-methylammonio]-1-propane sulfonate) were added to a 10-μL aliquot of the diluted translation products and incubated for 1 hour at 37°C. For protease digestion, 1 μL proteinase K (1 mg/mL) was added to a 10-μL aliquot of the diluted translation product in the presence or absence of 0.5% CHAPS. The reactions were carried out on ice for 30 minutes and terminated with 3 mmol/L PMSF. Before being subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) analysis, the samples were precipitated with 2 volumes of saturated (NH4)2SO4, washed with 5% trichloroacetic acid, and redissolved in 10 μL SDS-PAGE sample buffer.
Expression of FLAG-tagged carboxylase
Full-length carboxylase molecules with a FLAG tag at either the N- or C-termini were expressed in 293 cells. Transfection was performed using calcium phosphate precipitation,20 and the cells were selected with geneticin. Microsomes from cells expressing FLAG-hGC or hGC-FLAG were prepared as described.21 Protease digestion of the microsomes was performed as described above. After protease digestion, the microsomes were washed 3 times with normal saline buffer, and the pellet was resuspended and equally divided into 2 aliquots. One aliquot was directly subjected to SDS-PAGE without further treatment. The other was denatured in 1× denaturation buffer (0.5% SDS and 1% 2-mercaptoethanol) for 30 minutes at 45°C and then digested with Endo H for 2 hours at 37° before SDS-PAGE.
SDS-PAGE and Western blot analysis
SDS-PAGE analysis was performed according to Laemmli22under reducing conditions. Samples from the in vitro translation were subjected to 12% SDS-PAGE, and the full-length recombinant carboxylase from hEK-293 cells were subjected to 4%-20% SDS-PAGE. After electrophoresis, the proteins were transferred to a polyvinylidene difluoride membrane, and autoradiography was performed (Molecular Dynamics Storm 840 PhosphoImager; Molecular Dynamics, Sunnyvale, CA). For Western blot analysis, the proteins were probed with polyclonal rabbit anti-Lep antiserum or mouse anti-FLAG M2 mAb followed by an HRP-conjugated secondary antibody, and the proteins were detected using the ECL kit. The relative molecular weight of each peptide was estimated according to Weber and Osborn,23 using biotinylated protein as a standard.
Results
Prediction of membrane topology of human carboxylase using TOPPRED II
The vitamin K–dependent carboxylase is a 758–amino acid ER integral membrane protein. Figure 1A depicts the hydropathy plot of human carboxylase. The putative TMDs of the carboxylase were determined using the computer algorithm TOPPRED II24 with a default window of 21 amino acid residues. We predicted 7 TMDs, with the amino-terminus located in the cytoplasm and the carboxyl-terminus located in the lumen of the ER. For clarity, we denoted the TOPPRED II–predicted TMDs with parentheses until they were experimentally verified, after which we removed the parentheses. (Parenthetical coding is noted in the following text.)
Topological analysis of the human γ-glutamyl carboxylase.
(A) TOPPRED II analysis of human GC. The hydropathy plot generated by TOPPRED II with a default window of 21 is shown. We predicted 7 TMDs at amino acid residues 60-80 (TM1), 115-135 (TM2), 138-158 (TM3), 197-217 (TM4), 252-272 (TM5), 293-313 (TM6), and 361-381 (TM7). (B) Schematic representation of the GC and hGC-Lep constructs used to experimentally determine the topology. The solid bars indicate the TM segments predicted by TOPPRED II, and Y indicates potential N-linked glycosylation sites. The P2 domain of Lep was fused to the C-terminus of the hGC fragments in all constructs.
Topological analysis of the human γ-glutamyl carboxylase.
(A) TOPPRED II analysis of human GC. The hydropathy plot generated by TOPPRED II with a default window of 21 is shown. We predicted 7 TMDs at amino acid residues 60-80 (TM1), 115-135 (TM2), 138-158 (TM3), 197-217 (TM4), 252-272 (TM5), 293-313 (TM6), and 361-381 (TM7). (B) Schematic representation of the GC and hGC-Lep constructs used to experimentally determine the topology. The solid bars indicate the TM segments predicted by TOPPRED II, and Y indicates potential N-linked glycosylation sites. The P2 domain of Lep was fused to the C-terminus of the hGC fragments in all constructs.
Topological orientation of amino- and carboxyl-termini of carboxylase in vivo
To determine the topological orientation of the amino- and carboxyl-termini of human carboxylase, we constructed 2 full-length recombinant human carboxylase molecules tagged with a FLAG epitope at either end: FLAG-hGC and hGC-FLAG. hEK-293 cells expressing either construct displayed kinetic parameters characteristic of the native carboxylase isolated from bovine liver. By characterizing FLAG-antibody–purified GC from these 293 cells, we obtained results that demonstrate the equivalence of tagged and native carboxylases (data not shown). This indicates correct folding and topological orientation of the recombinant enzyme. Microsomes from the 293 cells expressing these constructs were subjected to proteinase K digestion in the presence or absence of CHAPS and subjected to SDS-PAGE analysis. The proteins were transferred to a PVDF membrane and probed with the anti-FLAG M2 mAb.
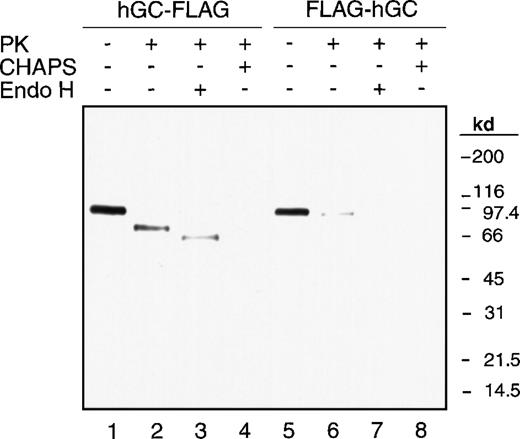
As shown in Figure 2, without proteinase K treatment, a 95-kd protein band of the size expected for the full-length carboxylase was demonstrated in both hGC-FLAG (lane 1) and FLAG-hGC (lane 5). Proteinase K digestion of the hGC-FLAG (lane 2) revealed a 60-kd fragment, indicating the lumenal location of the FLAG tag and therefore the carboxyl-terminus of the carboxylase. In contrast, FLAG-hGC (lane 6) did not show a proteinase K–resistant fragment except for the residual undigested full-length carboxylase, which indicates the cytoplasmic location of the FLAG tag and therefore the amino-terminus of the carboxylase. When 0.5% CHAPS was included in the proteinase K digestion, hGC-FLAG (lane 4) lost its 60-kd protease resistant fragment, further demonstrating the lumenal location of the FLAG tag. Still further proof of the lumenal location of the 60-kd fragment is provided by its shift in electrophoretic mobility after Endo H treatment (lane 3). These data are in agreement with the TOPPRED II prediction stating that the N-terminus of the carboxylase is in the cytoplasm, and the C-terminus is in the lumen of the ER. In addition, it provides evidence for the presence of an odd number of transmembrane domains.
Localization of the amino- and carboxyl-termini of the carboxylase.
Microsomes prepared from 293 cells expressing hGC-FLAG or FLAG-hGC were subjected to proteinase K digestion in the presence (+) or absence (-) of 0.5% CHAPS. Part of the protease digestion sample in the absence of CHAPS was further digested by Endo H. The anti-FLAG M2 mAb was used for Western blot analysis.
Localization of the amino- and carboxyl-termini of the carboxylase.
Microsomes prepared from 293 cells expressing hGC-FLAG or FLAG-hGC were subjected to proteinase K digestion in the presence (+) or absence (-) of 0.5% CHAPS. Part of the protease digestion sample in the absence of CHAPS was further digested by Endo H. The anti-FLAG M2 mAb was used for Western blot analysis.
Determination of the signal-anchor potential of the putative transmembrane sequences by in vitro translation/cotranslocation
Based on the TOPPRED II–predicted topology of hGC, the fusions were made to contain a single putative TMD followed by the reporter tag Lep, the P2 domain of the leader peptidase (amino acid residues 80-324) (Figure 1B). We chose the Lep tag because it is a well-characterizedE Coli inner membrane protein, and it has been successfully used to study the assembly of integral membrane proteins.17,25 Furthermore, it has been demonstrated that during in vitro translation, the Lep tag can be efficiently translocated into the lumen of canine pancreatic microsomes and glycosylated at Asn214, the potential glycosylation site.26 27 Therefore, the glycosylation state and the protease resistance of the Lep tag allow the determination its topological orientation and, therefore, the signal-anchor potential of a given amino acid sequence.
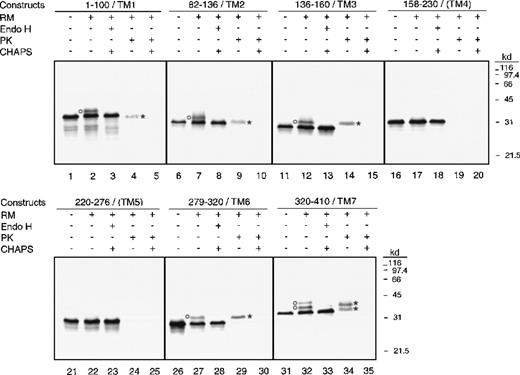
We made this series of constructs (Figure 1B) based on the assumption that if the Lep tag became glycosylated and resistant to proteinase K, then the given putative TMD functioned in vitro as a start-transfer sequence, and therefore it is likely to be an authentic transmembrane sequence in vivo.28-30 Figure 3is the autoradiograph of an SDS-PAGE analysis of the single-TMD fusions. As expected, without canine rough microsomes (RM−) all fusions yielded a single protein band, with the molecular weight expected of a nonglycosylated product (lanes 1, 6, 11, 16, 21, 26, and 31). In the presence of canine rough microsomes (RM+), fusions 158-230/(TM4) (transmembrane domain 4) and 220-276/(TM5) (lanes 17 and 22) yielded protein bands indistinguishable from those of their RM-minus counterparts (lanes 16 and 21). Fusions 1-100/(TM1), 82-136/(TM2), 136-160/(TM3), and 279-320/(TM6), however, yielded additional bands of higher molecular weight than those of their RM-minus counterparts (lanes 2, 7, 12, and 27). Interestingly, fusion 320-410/(TM7) yielded 2 additional bands compared to its RM-minus counterpart, and these bands were also of higher molecular weight than those of the RM-minus counterparts.
Analysis of fusions containing a single predicted transmembrane domain.
Phosphorimage of the 7 constructs containing a single TMD analyzed by SDS-PAGE is shown. In vitro translations were done in the presence (+) or absence (-) of canine pancreatic rough microsomes (RMs). Endo H treatment was done in the presence (+) of 0.5% CHAPS, while proteinase K treatment was done in the presence (+) or absence (-) of 0.5% CHAPS. Glycosylated products are indicated by circles, while proteinase K–resistant fragments are indicated by asterisks.
Analysis of fusions containing a single predicted transmembrane domain.
Phosphorimage of the 7 constructs containing a single TMD analyzed by SDS-PAGE is shown. In vitro translations were done in the presence (+) or absence (-) of canine pancreatic rough microsomes (RMs). Endo H treatment was done in the presence (+) of 0.5% CHAPS, while proteinase K treatment was done in the presence (+) or absence (-) of 0.5% CHAPS. Glycosylated products are indicated by circles, while proteinase K–resistant fragments are indicated by asterisks.
The extra additional band in fusion 320-410/(TM7) is likely due to the endogenous N-glycosylation site, Asn389, of the human carboxylase (lane 32). Endo-H digestion abolished all of the additional higher molecular weight bands (lanes 3, 8, 13, 28, and 33) indicating their glycosylated states. Fusions 158-230/(TM4) and 220-276/(TM5) yielded products indistinguishable from those of their RM-minus counterparts, and the electrophoretic mobility of their products remained unchanged after Endo-H treatment (lanes 18 and 23). In summary, these data indicate that TM1, TM2, TM3, TM6, and TM7 function as type II signal-anchor sequences in vitro, while (TM4) and (TM5) do not. The odd number of signal-anchor sequences determined in vitro agrees with the conclusion drawn from our in vivo studies described above.
We encountered relatively low glycosylation efficiency in our in vitro translation/cotranslocation system. Similar observations have been reported16,31 32 and attributed to the possible inefficiencies in both translocation and glycosylation. In our hands, most but not all of the nonglycosylated fusions were susceptible to proteinase K treatment (Figure 3, lanes 4, 9, 14, 29, and 34) and were removable from the membrane by alkaline extraction (data not shown). This indicates that inadequate membrane insertion of the fusion is the major cause of the apparent low glycosylation efficiency.
To confirm results obtained from the glycosylation state analysis of the fusions, we examined the proteinase K susceptibilities of each fusion. In Figure 3, fusions 158-230/(TM4) and 220-276/(TM5) were degraded by proteinase K whether CHAPS was present or not (lanes 19, 20, 24, and 25). These results indicate that these 2 fusions remain on the cytoplasmic side of the ER and therefore were not protected from proteinase K cleavage; thus (TM4) and (TM5) lack type II signal-anchor potential. In contrast, the remainder of the fusion constructs yielded protease K–resistant fragments (lanes 4, 9, 14, 29, and 34), thereby indicating the lumenal locations of their Lep tags. Since these putative TMDs possess type II signal-anchor activities in vitro, it is likely that they are authentic TMDs in vivo.
The size of the fusions and their proteinase K–resistant fragments was primarily determined by the size of the Lep tag, a 245–amino acid sequence, but the size was influenced by the number of amino acids surrounding the potential signal-anchor sequence. For example, TM1 functions in vitro as a type II signal-anchor sequence in the fusion 1-100/TM1, which has about 60 amino acids preceding the TMD. This fusion became significantly smaller after proteinase K digestion due to the proteinase K sensitivity of the first 60 amino acids residing on the cytoplasmic side of the ER membrane. Similarly, fusions 82-136/TM2 and 320-410/TM7, with approximately 30 and 40 amino acids preceding the TM2 and TM7 segments, respectively, also became noticeably smaller after proteinase K digestion (lanes 9 and 34). In contrast, fusion 136-160/TM3, with only 2 amino acids preceding the TM3 segment, appeared unchanged in size after proteinase K digestion (lane 14). Fusion 279-320/TM6, with 14 hydrophobic amino acids preceding TM6, exhibited unchanged electrophoretic mobility after proteinase K treatment (lane 29). Permeabilization of the microsomes with CHAPS prior to proteinase K treatment abolished all protease-resistant fragments from these fusions (lane 5, 10, 15, 30, and 35), indicating the lumenal location of the protected fusion fragment.
Consistent with the glycosylation state studies described above, Western blot analysis demonstrated that all the proteinase K–resistant fragments contained the Lep tag (data not shown), indicating the lumenal location of the Lep tag and therefore, the type II signal-anchor activity of these putative TMDs. These results indicate that TM1, TM2, TM3, TM6, and TM7 function as signal-anchor sequences in vitro and are likely to function as authentic TMDs in vivo. In contrast, (TM4) and (TM5) lack the type II signal-anchor activity in our in vitro system of single TMD construct.
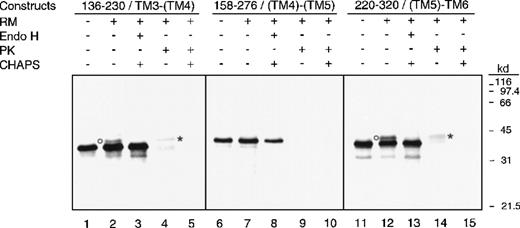
To further clarify the signal-anchor potential of (TM4) and (TM5), experiments were performed with fusions containing adjacent-paired putative TMDs: 136-230/TM3-(TM4), 158-276/(TM4)-(TM5), and 220-320/(TM5)-TM6 (Figure 1B). The phosphorimage analysis of the SDS-PAGE results of these fusions is shown in Figure4. As expected, without canine microsomes (RM-minus), all 3 fusions revealed a single protein band, with the relative molecular weight of that estimated for a nonglycosylated product (lanes 1, 6, and 11). In the presence of canine microsomes (RM-plus), fusions 136-230/TM3-(TM4) and 220-320/(TM5)-TM6 contained an additional minor protein band with a relative molecular weight slightly greater than that estimated for a nonglycosylated product (lanes 2 and 12). Endo-H treatment selectively abolished these additional protein bands indicating their glycosylated states (lanes 3 and 13). Proteinase K digestion of these 2 fusions (lanes 4 and 14) revealed protease-resistant fragments. Permeabilization of the microsomes with CHAPS (lanes 5 and 15) during proteinase K treatment eradicated the protected fusion fragments, which indicate their lumenal locations. In contrast, in the presence of canine microsomes, fusion 158-276/(TM4)-(TM5) yielded a product identical to its RM-minus counterpart (lane 7), and its translation product was not affected by Endo H treatment (lane 8).
Analysis of fusions containing adjacent pairs of predicted transmembrane domain.
Phosphorimage of the 3 constructs containing adjacent pairs of TMDs analyzed by SDS-PAGE is shown. In vitro translations were done in the presence (+) or absence (-) of canine pancreatic RMs. Endo H treatment was done in the presence (+) of 0.5% CHAPS, while proteinase K treatment was done in the presence (+) or absence (-) of 0.5% CHAPS. Glycosylated products are indicated by circles, while proteinase K resistant fragments are indicated by asterisks.
Analysis of fusions containing adjacent pairs of predicted transmembrane domain.
Phosphorimage of the 3 constructs containing adjacent pairs of TMDs analyzed by SDS-PAGE is shown. In vitro translations were done in the presence (+) or absence (-) of canine pancreatic RMs. Endo H treatment was done in the presence (+) of 0.5% CHAPS, while proteinase K treatment was done in the presence (+) or absence (-) of 0.5% CHAPS. Glycosylated products are indicated by circles, while proteinase K resistant fragments are indicated by asterisks.
In summary, fusions 136-230/TM3-(TM4) and 220-320/(TM5)-TM6 each contain one signal-anchor sequence in directing the Lep tag into the lumen of the ER. On the other hand, fusion 58-276/(TM4)-(TM5) either lacks or contains 2 signal-anchor sequences that cause the retention of the Lep tag on the cytoplasmic side of the ER. Combined with the results obtained from the single-TMD fusion, these data support the conclusion that TM1, TM2, TM3, TM6, and TM7 are functional type II signal-anchor sequences in vitro and therefore are likely to be authentic transmembrane sequences in vivo. In contrast, (TM4) and (TM5) do not function as a type II signal-anchor sequence in vitro; however, we cannot definitively rule out the possibility that they are authentic transmembrane sequences in vivo.
Discussion
In an attempt to obtain a basic understanding of the structure of the carboxylase, we used the in vitro transcription/cotranslation system to evaluate the putative TMDs predicted by TOPPRED II. This method has been proved accurate in identifying authentic transmembrane sequences in several integral membrane proteins.12-17 Thus, in the absence of concrete structural data, it has become a useful biochemical tool for identifying topogenic sequences of an integral membrane protein. In this study, we adopted the hypothesis that a signal-anchor sequence either functions as a start-transfer or a stop-transfer sequence depending on its order in the polytopic coding sequence.29,33 We made a series of single-TMD fusions with a carboxyl reporter tag, Lep. We postulated that if the Lep tag became glycosylated and resistant to proteinase K cleavage, the given putative TMD would be able to function, in vitro, as a type II signal-anchor sequence, that is a start-transfer, and therefore is likely to be an authentic transmembrane sequence in vivo.28-30
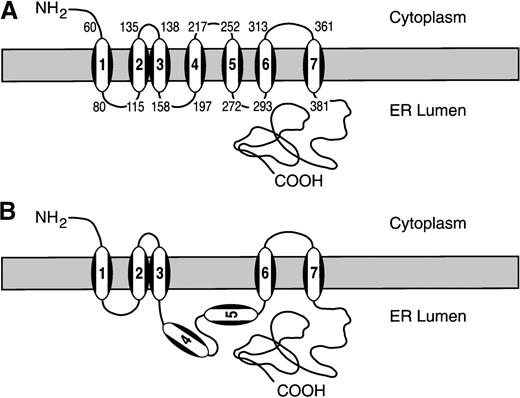
In Figure 5A,B, the membrane topology of the human GC derived from TOPPRED II is compared with that derived from our experimental data. In principle, our experimental data agree well with the TOPPRED II predictions; that is, the carboxylase contains an odd number of TMDs, a cytoplasmic amino-terminus, and a lumenal carboxyl-terminus. The only discrepancy between the TOPPRED II prediction (Figure 5A) and our results is that our data do not support (TM4) and (TM5) as authentic TMDs. Because the carboxylase contains an odd-number of TMDs, both (TM4) and (TM5) either are or are not TMDs. According to the hydropathy plot, (TM4) barely reaches the threshold to be defined as a transmembrane sequence. Furthermore, if (TM4) and (TM5) were both authentic TMDs, an intervening loop between (TM4) and (TM5), with a net charge of minus 2, would be placed in the cytoplasm, a situation violating the positive inside rule.17 24
The membrane topology of human GC.
(A) TOPPRED II–predicted topology. (B) Proposed membrane topology of hGC, based on our results.
The membrane topology of human GC.
(A) TOPPRED II–predicted topology. (B) Proposed membrane topology of hGC, based on our results.
Based on our model, the configuration of the carboxylase (Figure 5B) begins with a cytoplasmic amino-terminus, followed by 5 TMDs, and ends with a lumenal carboxyl-terminus. Because all 5 TMDs are in the first 400 residues of the carboxylase, this region contains the cytoplasmic amino-terminus, 2 cytoplasmic and 2 lumenal loops, thereby leaving the large hydrophilic carboxyl-terminus half uninterrupted in the lumen. Most of the carboxylase molecule (650 residues) is in the lumen (residues 81-114, 159-292, and 381-758), while only 108 residues (residues 1-59, 136-137, and 314-360) are in the cytoplasm. The net charge distribution is +3, +1, and +9 in the cytoplasmic segments and −1, −2, and −7 in the lumenal segments, which agrees with the experimental rules devised for charge distribution.17 24Although the theoretical deduction supports our model, the experimental approach only allows us to identify regions that will serve as a signal-anchor sequence in vitro.
Because the original sequential start-stop transfer model was proposed for polytopic transmembrane proteins,28 several factors have been demonstrated to affect the orientation of a signal-anchor sequence in the membrane. It has been reported that in addition to the flanking charged residues, the folding state of the amino-terminal segment, as well as the length and hydrophobicity of the signal-anchor sequence, are all important factors in determining its orientation in the membrane.34-38 It is possible that (TM4) and (TM5) both function as type I signal-anchor sequences and assume an N(out)-C(in) orientation in our TMD fusions. In that case, fusions 158-230/(TM4) and 220-276/(TM5) would have a small proteinase K–resistant fragment which contained the lumenal amino-terminus and the signal-anchor sequence. However, small peptides of that size are beyond the separation limit of most traditional electrophoresis systems. If we assume that (TM4) and (TM5) are authentic signal-anchor sequences, data from the 2-TM fusions would suggest that fusion 58-276/(TM4)-(TM5) would have an N(in)-C(in) orientation, while fusions 136-230/TM3-(TM4) and 220-320/(TM5)-TM6 would have an N(out)-C(out) orientation. However, the size of their proteinase K–resistant fragments strongly argues against the validity of this assumption. Structures from distant or flanking regions of the TM have been reported to affect the topological activity of a given TM.39-42 Therefore, it is possible that (TM4) and (TM5) are authentic TM sequences in the carboxylase, but their topogenic activities were suppressed in our in vitro system because other transmembrane sequences were not present. In the absence of high resolution structural data, any such interpretation remains a matter of conjecture.
Little is known about the structure-function relationship of the carboxylase, and by studying its membrane topology, we hope to gain insights into its functional and mechanistic properties. It has been reported that the enzymatic activity of the carboxylase resides in the lumen of the ER.1,43 Mutations at charged clusters 217/218, 234/235, and 359/361 have been reported to affect carboxylase activity.10 Using our 5-TMD model, we found that mutations 217/218 and 234/235 reside in the lumen of the ER. However, all 3 charged clusters are in the cytoplasmic region of the 7-TMD model. Comparing the cDNA sequences of human, bovine, and rat GC reveals that most sequence variations occur in the cytoplasmic segments of the carboxylase, while highly conserved sequences are found in the lumenal segments, as would be expected for segments of an active site.
We previously reported a propeptide binding site near residues 438-507,9 while Yamada et al8 reported a propeptide binding site between residues 184 and 225. According to the TOPPRED II 7-TMD model, residues 438-507 reside in the lumen of the ER, agreeing with our propeptide binding data but contradicting the Yamada et al data, which would have residues 184-197 in the lumen, residues 197-217 in the membrane, and residues 217-225 in the cytoplasm.8 However, if our 5-TMD model is used, both regions are located in the lumen of the ER. Because 2 linearly distant regions can be conformationally near one another, it is possible that the conditions used in the chemical cross-linking reaction to identify the propeptide binding site may have dictated the region where cross-linking occurred, thus explaining the apparent discrepancy.
One other structural feature important for understanding the carboxylase's function is the location of disulfide bonds and a free cysteine(s). Based on a series of simple step-by-step organic chemistry reactions, Dowd44 formulates an elegant mechanistic model for the carboxylation reaction. Using the published observation that sulfhydryl-reactive reagents inactivate carboxylase activity,11 he proposed that 2 essential cysteines are directly involved in the catalytic event. According to the 7-TMD and the 5-TMD models, both human and bovine carboxylases have 5 cysteines (C99, C288, C450, C598, and C700) in the lumen of the ER. However, it is unlikely that C700 is one of the critical cysteines proposed in Dowd's hypothesis because it is replaced by an arginine in the rat carboxylase. In addition, replacement of C700 with alanine does not affect carboxylase activity (V.M., unpublished data, May, 1999). We have previously demonstrated that the amino 30-kd and the carboxyl 60-kd tryptic fragments are linked by a disulfide bond(s) in the carboxylase. Because there is no apparent intermolecular disulfide linkage present in the purified carboxylase and because the enzyme is sensitive to sulfhydryl-reactive reagents, we conclude that there must be 2 free cysteines in the lumen of the ER. This agrees with Dowd's hypothesis that vitamin K hydroquinone and carbon dioxide (CO2) are coordinated with 2 individual cysteines that are directly involved in the catalytic event.
In summary, human GC spans the membrane at least 5 times, with its N-terminus in the cytoplasm and its C-terminus in the lumen of the ER. Because the method we used in this study does not allow us to unequivocally rule out that (TM4) and (TM5) function as authentic transmembrane sequences in vivo, their definite topogenic activities require further investigation.
Acknowledgments
We thank Dr von Heijne for kindly providing anti-Lep antibody and the Lep-containing plasmids, Pen-Jin Lin for technical support, and Dr David L. Straight for critically reviewing the manuscript.
Supported by grant HL48318-05 from the National Institutes of Health, Bethesda, MD.
Reprints:Darrel W. Stafford, Department of Biology, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599-3280; e-mail: dws@emailunc.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal