Abstract
Moderately elevated plasma homocysteine levels are an important independent risk factor for arterial and venous thrombosis and for atherosclerosis. Some investigators have proposed that homocysteine's effects result from oxidant injury to the vascular endothelium or from an alteration in endothelial function. However, homocysteine may have other cellular targets. We now report that homocysteine, at physiologically relevant concentrations, induces the expression of tissue factor by monocytes. In response to homocysteine, monocytes express procoagulant activity in a dose-dependent and a time-dependent manner. This activity is attributable to tissue factor because it was dependent on factor VII and blocked by anti-tissue factor antibodies. Tissue factor mRNA levels were also increased in monocytes after homocysteine treatment. The effect was found to be specific because analogues of homocysteine (homocystine and homocysteine thiolactone) did not mimic homocysteine's activity, nor did other thiol compounds (cysteine, 2-mercaptoethanol, dithiothreitol). On the other hand, methionine, the metabolic precursor of homocysteine, was active though less potent than homocysteine. Catalase and superoxide dismutase (scavengers of H2O2 and O2− Radicals, respectively) were unable to block the expression of tissue factor induced by homocysteine, as was a 5-fold excess of the reducing agent 2-mercaptoethanol. We conclude that the induction of tissue factor expression by circulating monocytes is a plausible mechanism by which homocysteine may induce thrombosis and that a nonspecific redox process is not involved.
Tissue factor (CD 142) is now known to be the principal activator of the coagulation cascade in hemostasis. Recently, attention has begun to focus on the possible role of the aberrant expression of tissue factor within the vasculature as a cause of pathologic coagulation, that is, thrombosis. Of the cells within the vasculature with which blood plasma normally is in contact, the only ones shown to be capable of expressing tissue factor are monocytes and endothelial cells. Monocytes readily express tissue factor in response to inflammatory stimuli, and increased tissue factor expression has been documented on the monocytes of patients with cirrhosis or sepsis and after surgery.1-3 Induction of tissue factor expression on monocytes has been implicated in the thrombotic diathesis associated with antiphospholipid antibodies.4 5 Its involvement in other thrombogenic disorders has not been established.
Homocysteine is a thiol-containing amino acid derived from the metabolism of dietary methionine. Elevated plasma concentrations of homocysteine (hyperhomocystinemia) are now recognized as an important independent risk factor for atherosclerosis.6 Large, prospective, population-based studies have found that modestly elevated homocysteine levels are a risk factor for coronary, cerebral, and peripheral arterial disease and that as much as 10% of all cardiovascular risk may be attributable to homocysteine.7In patients with documented coronary artery disease, plasma homocysteine is a stronger risk factor for death than is plasma cholesterol,8 implying that homocysteine is linked pathophysiologically to the thrombus formation that is the terminal event in myocardial infarction, the main cause of death in these patients. Furthermore, elevated homocysteine levels are a risk factor for venous thromboembolism.9 10
Numerous mechanisms have been postulated by which hyperhomocystinemia may induce atherosclerosis and thrombosis.6 Most hypotheses involve injury to the vascular endothelium11,12 or some alteration in endothelial function, such as decreased expression of the anticoagulant regulatory protein thrombomodulin,13 of anticoagulant heparans,14 or of binding sites for tissue plasminogen activator.15,16 One study has reported that homocysteine can induce tissue factor expression by cultured umbilical vein endothelial cells.17 However, these studies have used very high concentrations of homocysteine (100-1000 μmol/L and higher). Total plasma concentrations of homocysteine can exceed several hundred μmol/L in patients who have rare, inborn errors of metabolism (homocystinuria), but the concentration in normal subjects is approximately 5 to 15 μmol/L. Homocysteine, like cholesterol, is a graded risk factor for cardiovascular disease through the normal range. Cardiac risk is estimated to increase 1.6- to 1.8-fold with each 5 μmol/L increment in plasma total homocysteine.7
It should be noted that most of the homocysteine in plasma exists in oxidized form, either as homocystine, as mixed disulfides, or as bound to free cysteinyl residues of proteins. A small percentage of reduced homocysteine is in exchangeable equilibrium with the oxidized species.
Several investigators have proposed that the toxic effects of homocysteine are caused by the oxidant species H2O2, which is generated when homocysteine auto-oxidizes to the disulfide homocystine or when it forms mixed disulfides with other thiols. Accordingly, the toxic effects of high concentrations of homocysteine on the endothelium can be mimicked by other thiol-containing molecules (eg, cysteine or mercaptoethanol) and blocked by the H2O2 scavenger catalase.12
Recently, some groups have reported effects of homocysteine at physiologically relevant concentrations. Wang et al18 found that low concentrations of homocysteine (10 μmol/L or more) specifically inhibited endothelial cell proliferation; however, homocystine was found to be even more potent. Homocysteine at concentrations as low as 20 μmol/L decreased endothelial cell synthesis of the vasodilator nitric oxide, a process not mimicked by cysteine.19 In chick embryos, homocysteine at 500 nmol/L is reported to activate transiently a mitogen-activated protein kinase-dependent signal transduction pathway.20 These mechanisms may contribute to the atherogenic effects of homocysteine but do not clearly explain the association with thrombosis.
We now report that homocysteine can induce tissue factor expression by human peripheral blood monocytes in a specific manner at physiologically relevant concentrations.
Materials and methods
Materials
DL-Homocysteine, L-homocystine,L-homocysteine thiolactone hydrochloride,L-cysteine, L-methionine, 2-mercaptoethanol,DL-dithiothreitol, lipopolysaccharide (LPS; fromEscherichia coli O55:B5), polymyxin B, catalase (bovine liver), and superoxide dismutase (bovine erythrocyte) were obtained from Sigma (St. Louis, MO). DL-Homocysteine was also obtained from Amersham (Piscataway, NJ). RPMI 1640 and RPMI 1640 without methionine were obtained from Life Technologies (Burlington, ON, Canada). Factor VII-deficient plasma was obtained from Sigma. Murine monoclonal antibodies against human brain tissue factor were obtained from American Diagnostica (Greenwich, CT). Human placental thromboplastin (Thromborel-S) was obtained from Behring (Deerfield, IL). Trizol RNA-isolation reagent and oligonucleotides were procured from Life Technologies. Titan 1-step reverse transcription–polymerase chain reaction (RT-PCR) kit, RNase inhibitor, and deoxynucleotides were obtained from Roche/Boehringer Mannheim Molecular Biochemicals (Laval, QC, Canada).
Monocyte isolation and culture
Blood was collected by venipuncture into heparin (final concentration, 10 U/mL) and immediately centrifuged at 150g for 20 minutes at room temperature. The buffy coat was collected, layered over Ficoll–sodium diatrizoate (Ficoll–Paque Plus 1.077; Pharmacia, Baie D'Urfé, QC, Canada) and centrifuged at 250g for 15 minutes at room temperature. The peripheral blood mononuclear cell (PBMC) layer was collected, and the cells were washed 3 times in Hanks balanced salt solution (HBSS) without phenol red. The cells were then resuspended in RPMI 1640 medium supplemented with 10% heat-inactivated calf serum (Cosmic Calf; Hyclone, Logan, UT). The total leukocytes were counted on a Coulter (Miami, FL) MaxM Analyzer, and the percentage of monocytes was determined by nonspecific esterase staining.21 The PBMC preparation contained 2.7 ± 0.8 × 106 cells/mL, of which 36.1% ± 3.3% were monocytes (n = 35). Viability of the cells was determined by trypan blue exclusion and, in all cases, was greater than 95% before and after incubation under all experimental conditions studied.
PBMCs, isolated as described above, were pipetted into the wells of 24-well tissue culture plates (Costar; Corning, Corning, NY), and 1 mL cell suspension (approximately 106 monocytes) was added per well. Only the center 8 wells of the plates were used. Edge wells were filled with sterile HBSS because we have previously observed discrepant rates of evaporation between center and edge wells. Plates were incubated at 37°C in 95% air/5% CO2 for 4 hours, unless otherwise specified. At the end of the incubation, the wells were scraped with a rubber policeman to remove adherent cells, and the cells were collected and frozen at −80°C until assay. After thawing, the cells were washed 3 times in HBSS and resuspended to a final volume of 200 μL in TBS (0.02 mol/L Tris, pH 7.5, 0.1 mol/L NaCl) supplemented with 11 mmol/L CaCl2, 0.02% azide, and 0.1% gelatin and placed on ice. Cell lysis was ensured by sonicating the cells on ice with 3 bursts of 10-second duration using a probe tip sonicator (Sonifer Cell Disruptor; Branson Sonic Power, Danbury, CT).
For most experiments, PBMCs isolated as above were used because other blood cells are not capable of expressing tissue factor. However, evidence shows that in some situations, tissue factor expression by monocytes is modified by signals from other cells.22 To examine this possibility, monocytes were further purified by allowing them to adhere to the dishes for 30 minutes at the beginning of the experiment. Then the wells were washed with media to remove nonadherent cells, fresh media were added, and experimental reagents were added. This procedure yields cells that consistently are composed of approximately 95% monocytes as determined by nonspecific esterase staining.
Tissue factor assay
A 1-stage clotting assay was used to determine tissue factor expression by monocytes. A modified prothrombin time assay was performed using a Coag-a-Mate (Warner–Lambert, Morris Plains, NJ) optical coagulometer as follows: 200μL cell lysate, or of a dilution of thromboplastin, was pipetted into the wells of the instrument's tray, and the reaction was started by the injection of 100μL citrated normal pool plasma. Human tissue thromboplastin (Thromborel-S; Behring) was used as a standard.
Analysis of tissue factor mRNA
Total RNA was prepared from PBMCs using Trizol (Life Technologies) either immediately after isolation or after 4-hour incubation with LPS or homocysteine. RT-PCR was performed as described previously.17 Total RNA (500 ng) was reverse transcribed and amplified by the Titan 1-Step RT-PCR kit using specific primers according to the package directions. This system uses AMV reverse transcriptase for first-strand formation and Taq polymerase and Pwo DNA polymerase for PCR, allowing semiquantitative determination of mRNA levels. As a control, mRNA for glyceraldehyde-3-phosphate dehydrogenase (GAPDH), a housekeeping gene, was also amplified in the same reaction mixture.
The primers used for tissue factor mRNA amplification were synthesized on the basis of the published sequence23 as follows: sense primer, 5′ GACAATTTTGGAGTGGGAACCC 3′, corresponding to nucleotides 267 to 287; antisense primer, 5′ CACTTTTGTTCCCACCTG 3′, corresponding to nucleotides 569 to 586, to give an amplified product of 310 bp. Primer sequences for GAPDH mRNA amplification were as follows: sense primer, 5′ CCACCCATGGCAAATTCCATGGCA 3′, corresponding to nucleotides 150 to 169; antisense primer, 5′ TCTAGACGGCAGGTCAGGTCCACC 3′, corresponding to nucleotides 720 to 743, to give an expected product of 650 bp. PCR was performed with 35 cycles (Perkin Elmer Gene Amp 9600 Thermocycler, PerkinElmer, Foster City, CA) under the following conditions: for tissue factor, 30 seconds at 94°C of denaturation, 1 minute of annealing at 52°C, and 2 minutes of elongation at 72°C; for GAPDH, 30 seconds at 94°C of denaturation, 2 minutes of annealing at 58°C, and 2 minutes of elongation at 72°C. PCR products were visualized on a 2% agarose gel stained with ethidium bromide, photographed, digitized, and subjected to densitometric analysis.
Endotoxin assay and removal
All reagents were assayed for the presence of endotoxin using a limulus amebocyte lysate assay (Sigma; BioWhittaker, Walkersville, MD). Unless otherwise specified, they contained less than 0.1 ng/mL. Catalase and superoxide dismutase preparations were found to contain significant levels of endotoxin and, correspondingly, induced tissue factor expression by the monocytes. To remove the contaminating endotoxin, a phase separation technique with Triton X-114 (Calbiochem, La Jolla, CA) was used.24 This procedure reduced the level of endotoxin by approximately 10 000-fold without significant loss of enzyme activity. Catalase and superoxide dismutase activities were determined by published methods.25 26
Data analysis
Thromboplastin activity was extrapolated from a standard curve made with dilutions of the standard human thromboplastin reagent; a plot of log(dilution) versus log(clotting time) was linear to a dilution of 1:1000. Thromboplastin activity was expressed in milliunits, where 1 mU gives the clotting time observed with a 1:1000 dilution of the standard thromboplastin reagent, and results are reported as milliunits per 106 monocytes. The skew in the data for thromboplastin activity was corrected by log transformation; thus, throughout this article geometric means are reported, with the SEM computed in the log domain, and statistical comparisons are made on the log-transformed data. Comparisons between groups were by t test or by 2-way random-block analysis of variance, with subsequent intergroup comparisons by the Scheffé test.27
Results
Tissue factor induction by homocysteine
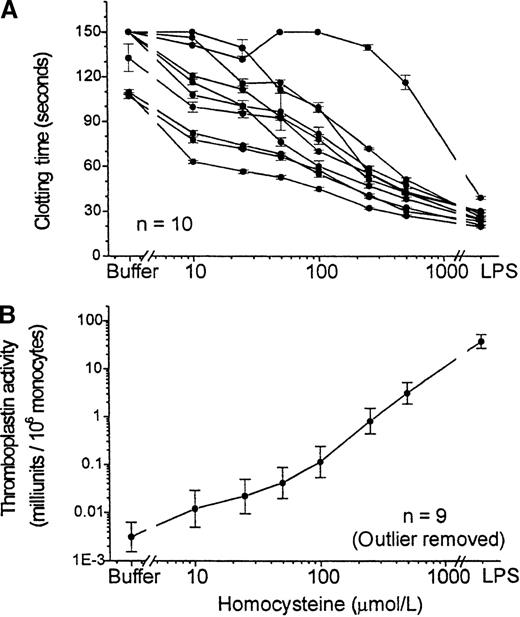
DL-Homocysteine, added to PBMCs and incubated for 4 hours, induced a dose-dependent expression of procoagulant activity (Figure 1). A significant shortening of the clotting time was observed even at a concentration of 10 μmol/L. LPS, a powerful activator of monocytes used as a positive control in each experiment, induced even higher levels of thromboplastin activity. Interestingly, of 10 normal subjects studied, 1 was clearly hyporesponsive to both homocysteine and LPS; the reason for this was unknown, but this effect was observed repeatedly. This subject was excluded from subsequent experiments. For some experiments, we used a single homocysteine concentration of 100 μmol/L at the approximate midpoint of the concentration–response curve.
Tissue factor expression by PBMC in response to homocysteine concentration–response curve.
Procoagulant activity of PBMC was assayed after 4 hour-incubation with various concentrations (10 μmol/L to 500 μmol/L) of homocysteine, with buffer or with LPS (100 ng/mL) as a positive control. (A) Clotting times in the 1-stage assay are shown for 10 different healthy volunteers. Data are shown as mean ± SEM of triplicate samples for each subject. (B) Thromboplastin activity, calculated by reference to a standard curve performed with each experiment and normalized to the number of monocytes in each sample. Results shown are geometric mean ± SEM of the results for 9 of the subjects shown in the top panel, after exclusion of the outlier.
Tissue factor expression by PBMC in response to homocysteine concentration–response curve.
Procoagulant activity of PBMC was assayed after 4 hour-incubation with various concentrations (10 μmol/L to 500 μmol/L) of homocysteine, with buffer or with LPS (100 ng/mL) as a positive control. (A) Clotting times in the 1-stage assay are shown for 10 different healthy volunteers. Data are shown as mean ± SEM of triplicate samples for each subject. (B) Thromboplastin activity, calculated by reference to a standard curve performed with each experiment and normalized to the number of monocytes in each sample. Results shown are geometric mean ± SEM of the results for 9 of the subjects shown in the top panel, after exclusion of the outlier.
The possibility that the effect of homocysteine was attributable to contamination with endotoxin was addressed in 3 ways. First,DL-homocysteine was purchased from 2 different suppliers (Sigma and Amersham). Both preparations exhibited nearly identical activity to induce tissue factor expression (data not shown). Second, the homocysteine preparations were tested for the presence of endotoxin using a sensitive chromogenic limulus amebocyte lysate assay (BioWhittaker). The Sigma preparation contained no detectable endotoxin, whereas the Amersham preparation contained a trace of endotoxin, though enough only to give a final endotoxin level of less than 0.01 ng/mL when the homocysteine concentration was 100 μmol/L. Finally, the endotoxin-binding compound polymyxin B (5 μg/mL) reduced the tissue factor expressed in response to LPS (1 ng/mL) by 95%, from 4.9 ± 0.511 to 0.23 ± 0.088 mU/106monocytes, but it had no effect on tissue factor expressed in response to homocysteine (100 μmol/L) from 0.43 ± 0.067 to 0.40 ± 0.065 mU/106 monocytes (n = 2).
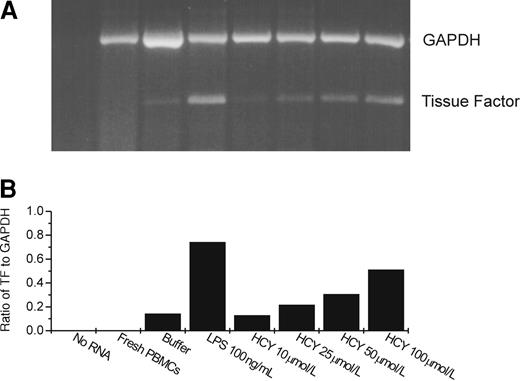
To determine whether the procoagulant effect induced by homocysteine was caused by tissue factor, we performed the clotting time assay with factor VII-deficient plasma or in the presence of blocking antibodies to tissue factor. Thromboplastin activity was reduced by more than 90% in the absence of factor VII and by more than 99% in the presence of the antibodies to tissue factor. Furthermore, a semiquantitative RT-PCR assay showed a dose-dependent increase in tissue factor mRNA after homocysteine exposure (Figure 2), showing that this induction occurred at the message level.
Tissue factor and GAPDH message expression after treatment of PBMC with homocysteine or LPS by semiquantitative RT-PCR.
(A) Gel. (B) Results of densitometric analysis of the gel, expressing tissue factor message as a ratio to that of the message for the housekeeping gene, GAPDH. HCY, homocysteine. In the lanes marked buffer, LPS, and HCY, PBMCs were incubated with the respective reagent for 4 hours before RNA was extracted. In the lane marked fresh PBMCs, RNA was extracted immediately after isolation of the monocytes.
Tissue factor and GAPDH message expression after treatment of PBMC with homocysteine or LPS by semiquantitative RT-PCR.
(A) Gel. (B) Results of densitometric analysis of the gel, expressing tissue factor message as a ratio to that of the message for the housekeeping gene, GAPDH. HCY, homocysteine. In the lanes marked buffer, LPS, and HCY, PBMCs were incubated with the respective reagent for 4 hours before RNA was extracted. In the lane marked fresh PBMCs, RNA was extracted immediately after isolation of the monocytes.
Homocysteine did not interfere directly with the clotting assay used. Even at high concentrations (1 mmol/L), homocysteine added to the coagulometer reaction cuvette had no effect on the clotting times obtained with the standard thromboplastin (data not shown).
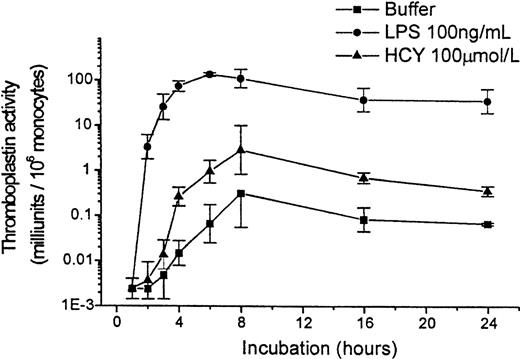
Expression of tissue factor by monocytes in response to homocysteine was time dependent. Peak expression occurred 6 to 8 hours after homocysteine was added, but substantial expression was observed at 4 hours (Figure 3). Similarly, LPS induced tissue factor expression over time, but at the concentration used the effect was more powerful and occurred more quickly. Control cells incubated without any agonist expressed tissue factor slowly and at lower levels. In subsequent experiments, the 4-hour time point was used to minimize the background level of tissue factor expression of the controls and to reduce the likelihood that the response to homocysteine would be mediated by secondary signals (such as tumor necrosis factor-α or IL-1β) that could be released by monocytes in response to activating stimuli.
Time course of tissue factor expression by PBMC in response to homocysteine or LPS.
Results shown are geometric mean ± SEM of the thromboplastin activity of PBMCs from 2 donors.
Time course of tissue factor expression by PBMC in response to homocysteine or LPS.
Results shown are geometric mean ± SEM of the thromboplastin activity of PBMCs from 2 donors.
Adherence-purified monocytes
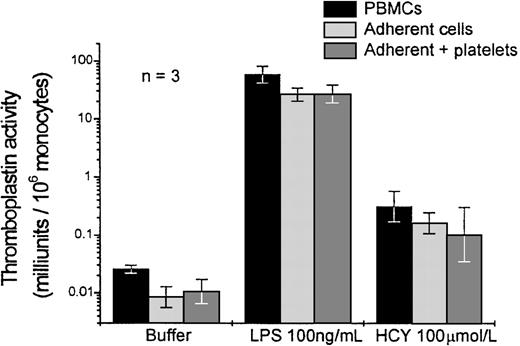
Experiments were performed to determine whether the expression of tissue factor by monocytes was dependent on the presence of platelets or other leukocytes that contaminated the PBMC preparation. In these experiments, PBMCs were added to the wells. Monocytes were allowed to adhere for 30 minutes, and nonadherent cells were removed by 3 washes with media. This procedure yielded monocytes that were approximately 95% pure, as determined by nonspecific esterase staining. Wells were refilled with medium or with medium supplemented with washed autologous platelets. Homocysteine, LPS, or buffer was added and incubated for 4 hours. In parallel wells, the PBMC suspension was incubated for the same period of time but without washing, according to our usual protocol. Tissue factor expression was only slightly reduced in the wells from which nonadherent cells had been removed (Figure 4). Addition of platelets had no significant effect. The ratio of homocysteine to LPS-induced tissue factor expression was the same in wells with or without nonadherent cells.
Effect of removal of nonadherent cells, with or without replacement of platelets, on the expression of tissue factor by PBMC in response to homocysteine or LPS.
Results shown are the geometric mean ± SEM of the thromboplastin activity of PBMC from 3 donors, normalized to the monocyte count of the cell preparation at the time of addition to the wells. PBMCs, adherent cells, and adherent cells supplemented with autologous platelets were prepared as described in Materials and Methods.
Effect of removal of nonadherent cells, with or without replacement of platelets, on the expression of tissue factor by PBMC in response to homocysteine or LPS.
Results shown are the geometric mean ± SEM of the thromboplastin activity of PBMC from 3 donors, normalized to the monocyte count of the cell preparation at the time of addition to the wells. PBMCs, adherent cells, and adherent cells supplemented with autologous platelets were prepared as described in Materials and Methods.
Specificity of homocysteine effect and role of reactive oxygen intermediates
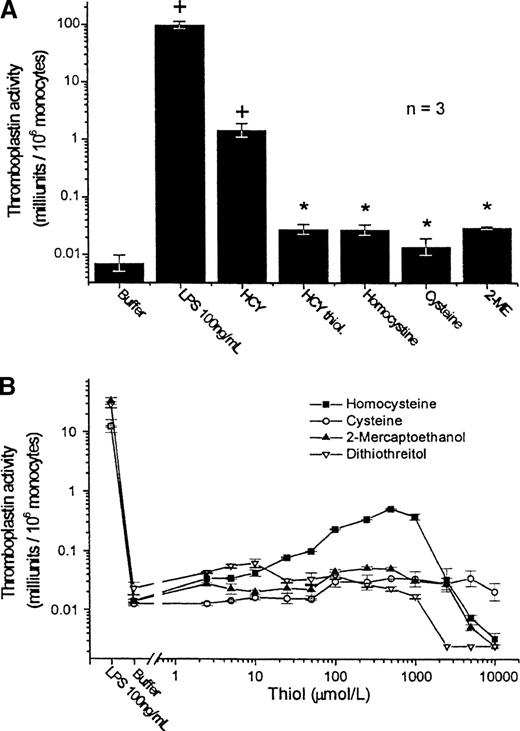
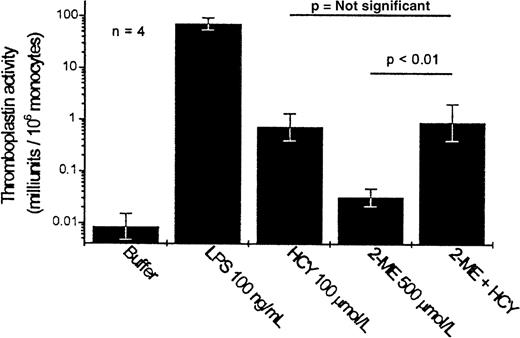
We examined whether related substances could exert effects similar to those of homocysteine. Neither L-homocystine (the oxidized form of homocysteine, in which 2 homocysteine residues are coupled by a disulfide bond) nor homocysteine thiolactone (a cyclic derivative of homocysteine, in which the thiol moiety is incorporated into a thiolactone bond with the carboxylic acid group) mimicked the activity of homocysteine (Figure5, top). These results suggested initially that the free thiol moiety was required for activity. However, 3 other free-thiol–containing compounds—cysteine (which differs structurally from homocysteine only in that the side chain contains 1 less carbon atom), dithiothreitol, and 2-mercaptoethanol—exhibited markedly less ability to induce tissue factor expression than an equimolar concentration of homocysteine (Figure 5, bottom). Furthermore, in the presence of a 5-fold excess of the reducing agent 2-mercaptoethanol, the ability of homocysteine (100 μmol/L) to induce tissue factor expression was undiminished (Figure 6).
Effect of analogues of homocysteine and other thiol compounds on the expression of tissue factor by PBMC.
(A) Homocysteine analogues. Each compound is used at a final concentration of 100 μmol/L; the response to LPS (100 ng/mL) is shown for comparison. Results shown are the geometric mean ± SEM of the thromboplastin activity of PBMC from 3 donors. Statistical comparisons shown are by Scheffé test. +P < .01 compared to buffer control; *P < .01 compared to homocysteine. HCY,DL-homocysteine; HCY thiol, L-homocysteine thiolactone hydrochloride; Homocystine, L-homocystine; Cysteine, L-cysteine; 2-ME, 2-mercaptoethanol. (B) Dose response to various thiol compounds. Results shown are the geometric mean ± SEM of the thromboplastin activity of triplicate samples of PBMC from 1 donor.
Effect of analogues of homocysteine and other thiol compounds on the expression of tissue factor by PBMC.
(A) Homocysteine analogues. Each compound is used at a final concentration of 100 μmol/L; the response to LPS (100 ng/mL) is shown for comparison. Results shown are the geometric mean ± SEM of the thromboplastin activity of PBMC from 3 donors. Statistical comparisons shown are by Scheffé test. +P < .01 compared to buffer control; *P < .01 compared to homocysteine. HCY,DL-homocysteine; HCY thiol, L-homocysteine thiolactone hydrochloride; Homocystine, L-homocystine; Cysteine, L-cysteine; 2-ME, 2-mercaptoethanol. (B) Dose response to various thiol compounds. Results shown are the geometric mean ± SEM of the thromboplastin activity of triplicate samples of PBMC from 1 donor.
Effect of 2-mercaptoethanol on the homocysteine-induced expression of tissue factor by PBMC.
Results shown are the geometric mean ± SEM of the thromboplastin activity of PBMC from 4 donors.
Effect of 2-mercaptoethanol on the homocysteine-induced expression of tissue factor by PBMC.
Results shown are the geometric mean ± SEM of the thromboplastin activity of PBMC from 4 donors.
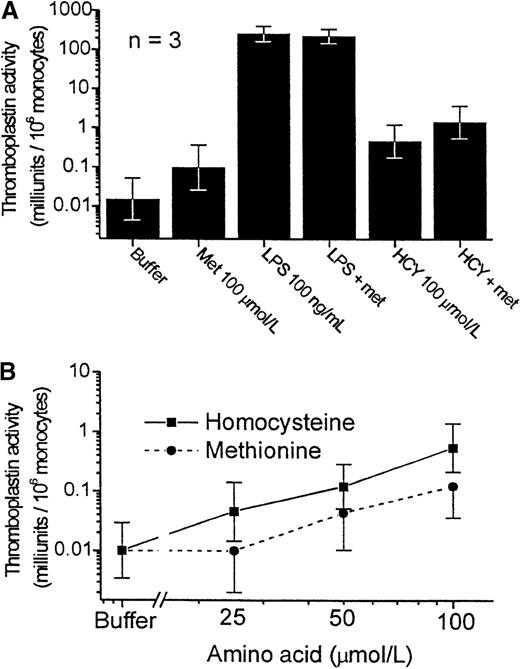
Homocysteine is derived metabolically from methionine. We therefore investigated whether methionine could also induce tissue factor expression. For these experiments, we used methionine-free media because standard RPMI 1640 contains nearly 100 μmol/L methionine. The use of methionine-deficient media did not alter the response of monocytes to LPS or to homocysteine (Figure 7, top). Methionine exhibited activity similar to that of homocysteine, though it was less potent at each concentration tested (Figure 7, bottom).
Comparative effects of homocysteine and methionine on the expression of tissue factor by PBMC.
Experiments in this figure were conducted with the PBMC cultured in RPMI 1640 lacking methionine. Results shown are the geometric mean ± SEM of the thromboplastin activity of PBMCs from 3 donors. (A) Effect of methionine replacement on the tissue factor expression in the presence of buffer, LPS, or homocysteine. Met, methionine. (B) Dose-response curve to homocysteine (▪)and methionine (•) (P < .001 and P < .02 for trend, respectively).
Comparative effects of homocysteine and methionine on the expression of tissue factor by PBMC.
Experiments in this figure were conducted with the PBMC cultured in RPMI 1640 lacking methionine. Results shown are the geometric mean ± SEM of the thromboplastin activity of PBMCs from 3 donors. (A) Effect of methionine replacement on the tissue factor expression in the presence of buffer, LPS, or homocysteine. Met, methionine. (B) Dose-response curve to homocysteine (▪)and methionine (•) (P < .001 and P < .02 for trend, respectively).
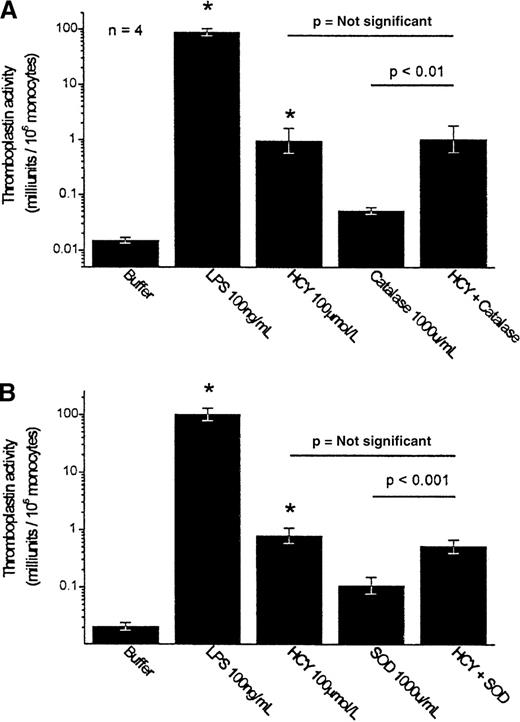
Because the production of H2O2 has been implicated in the toxic effects of homocysteine, such as endothelial injury, the ability of H2O2 to evoke tissue factor expression was investigated. Addition of authentic H2O2 to the PBMCs over a broad range of concentrations (10 pmol/L to 10 mmol/L) was able to evoke little expression of tissue factor. Maximal effect was observed at 100 μmol/L; higher concentrations actually suppressed tissue factor expression. Even at 100 μmol/L, H2O2 induced the expression of only 0.056 ± 0.030 mU thromboplastin activity/106 monocytes (n = 4), substantially less than the 0.48 ± 0.25 mU/106 monocytes observed with 100 μmol/L homocysteine or the 7.0 ± 3.3 mU/106monocytes observed with 500 μmol/L homocysteine (n = 10; P < .05). Quantitatively, therefore, the production of H2O2 could not account for homocysteine's activity. Furthermore, the H2O2 scavenger catalase (1000 U/mL) failed to inhibit the induction of tissue factor induced by homocysteine when added to the PBMC preparation (Figure 8, top).
Effect of catalase and superoxide dismutase on the homocysteine-induced expression of tissue factor by PBMC.
Results shown are the geometric mean ± SEM of the thromboplastin activity of PBMCs from 4 donors. Statistical comparisons shown are by Scheffé test. *P < .001 compared to buffer control. (A) Catalase. (B) SOD, superoxide dismutase.
Effect of catalase and superoxide dismutase on the homocysteine-induced expression of tissue factor by PBMC.
Results shown are the geometric mean ± SEM of the thromboplastin activity of PBMCs from 4 donors. Statistical comparisons shown are by Scheffé test. *P < .001 compared to buffer control. (A) Catalase. (B) SOD, superoxide dismutase.
To examine the direct role of superoxide anion radicals, superoxide dismutase, a scavenger of O2− radicals, was added to the PBMC suspension. Superoxide dismutase (1000 U/mL) also failed to inhibit the homocysteine-induced expression of tissue factor by monocytes (Figure 8, bottom).
Discussion
These results demonstrate a plausible mechanism by which homocysteine may contribute to thrombosis. Homocysteine induced procoagulant activity on monocytes in a dose-dependent and a time-dependent manner, with a significant effect at concentrations as low as 10 μmol/L. This seems consistent with the observation of vascular risk with even mild elevations of the plasma homocysteine concentration.28 It should be noted that only the racemic mixture, DL-homocysteine, is commercially available and was used in these experiments. If one speculates that only theL stereoisomer was active, the effective concentration would have been only half that reported. On the other hand, the concentrations of free (reduced) homocysteine are likely to be higher in our experimental conditions (with only 10% serum) than in plasma containing the same total homocysteine concentration.
Although in some circumstances monocytes may express other procoagulant activities, such as fgl2 prothrombinase29 or CD11b/CD18-dependent factor X activation,30 the procoagulant activity induced by homocysteine was attributable almost entirely to the expression of tissue factor because it was blocked by antibodies to tissue factor or by the absence of factor VII (for which tissue factor is a specific cofactor) in the clotting assay. Furthermore, though it is plausible that some of the variation in procoagulant activity could result from expression by the monocytes of tissue factor pathway inhibitor,31 the observation that tissue factor mRNA is up-regulated after homocysteine exposure confirms that tissue factor expression is activated either at the level of transcription23 or of message stability.32 33
Our results are supported by those of Durand et al.34,35 In a rat model, they have reported that the level of tissue factor expression by peritoneal macrophages is increased by a chronically folate-deficient diet34 or by parenteral methionine loading.35 Although plasma homocysteine concentrations were increased by these interventions, it is impossible to know in the in vivo model whether the effects on the macrophages were mediated by the change in homocysteine concentration or indirectly by some other perturbation induced by the interventions used. Furthermore, the reactivity of macrophages can differ in important ways from that of circulating monocytes, whereas it is the circulating monocytes that may contribute to thrombogenesis by the expression of tissue factor.
Expression of tissue factor by monocytes in response to endotoxin has been reported to be augmented by interactions with other blood cells, including platelets and neutrophils.36 Our PBMC preparation contained slightly more than one-third monocytes; the remaining cells were mostly lymphocytes, with scarce neutrophils and variable numbers of contaminating platelets. To determine whether homocysteine acts directly on the monocytes or requires cooperation with other cell types to be active, we purified the monocytes further by adherence. Although the expression of thromboplastin activity was reduced by approximately half in the wells from which nonadherent cells had been removed, the proportionate increase in tissue factor expression in response to LPS or homocysteine was unchanged. The modest decrease in tissue factor activity might have reflected a role for accessory cells, though re-adding platelets did not restore the expression. It might also have been caused in part by loss of some of the monocytes that failed to adhere to the dish. In any event, it is clear that the contribution of cell–cell communication in enhancing tissue factor response to homocysteine was minor at best; accessory cells are not mandatory for homocysteine's effects.
Interestingly, the effect of homocysteine was found to be highly specific. Analogues of homocysteine, such as its oxidized disulfide and cyclized thiolactone forms, were inactive. The role of free thiols was investigated using other thiol-containing compounds. Thiol reactivity correlates positively with the pKa of the thiol moiety.37 Compared with homocysteine, cysteine has a lower pKa, whereas 2-mercaptoethanol has a higher pKa.38Nonetheless, all the other compounds were less potent in inducing tissue factor expression, suggesting that homocysteine activity cannot be explained by nonspecific thiol redox effects.
These results are consistent with the fact that cysteine is 25-fold more abundant than homocysteine in plasma, yet the mass of clinical and epidemiologic evidence points to homocysteine rather than cysteine as a risk factor for vascular disease. Although a few case–control studies39-41 have reported that plasma cysteine is elevated in patients with vascular disease, cysteine concentrations were strongly correlated with homocysteine concentrations in each study, and in each study homocysteine elevations in patients were proportionately greater than cysteine elevations. Furthermore, in the study for which such analysis is reported,39 homocysteine remained a significant risk factor after the adjustment for plasma cysteine concentration. Thus, although the role of cysteine as a cause of vascular disease cannot be excluded from the clinical data, the available data suggest that homocysteine is the more important offending agent.
One possible mechanism by which homocysteine thiol moiety could be involved in its activity is the formation of disulfide bonds with other thiols, particularly free cysteine residues of proteins. Conceivably, homocysteine could alter the structure and function of a protein, such as a cell-surface signaling receptor, by covalently complexing with a free cysteine residue. Against this possibility, however, is the observation that the addition of a reducing agent, 2-mercaptoethanol, did not inhibit the tissue factor-inducing activity of homocysteine.
The fact that methionine was also able to evoke tissue factor expression is of considerable interest. It is likely that methionine exerts this effect by metabolism to homocysteine. In vivo, the oral administration of methionine results in a substantial increase in plasma homocysteine concentrations.42 Because the production of homocysteine can only occur intracellularly, this observation suggests that at least some cells and tissues take up and make more homocysteine in response to increased extracellular concentrations of methionine.
Oxidant injury caused by the generation of oxidative reactive intermediates (H2O2, O2−) has been postulated widely as a mechanism for homocysteine-mediated thrombogenesis. Our results showing that catalase and superoxide dismutase (scavengers of H2O2 and superoxide anion radical) were unable to block the homocysteine-induced tissue factor expression indicate that nonspecific redox stress is not involved in the response to homocysteine. These experiments do not rule out the possibility that redox mechanisms (as have been implicated in the activation of NF-κB43) play a role in downstream intracellular signaling in response to homocysteine.
These observations are the first evidence of a direct mechanism by which homocysteine, at physiologically and pathophysiologically relevant concentrations, may induce thrombosis. Although the biochemical pathway by which homocysteine activates monocytes is not yet clear, our findings suggest a highly specific effect and a probable intracellular site of action. Clinical correlation studies will be required to determine whether this mechanism explains the risk for thrombosis associated with hyperhomocystinemia.
Supported by grants from Manitoba Cancer Treatment and Research Foundation and the Heart and Stroke Foundation of Canada.
Reprints:Donald S. Houston, Manitoba Institute of Cell Biology and Department of Internal Medicine, University of Manitoba, 100 Olivia St, Winnipeg, Manitoba R3E 0V9, Canada; e-mail:houston@cc.umanitoba.ca.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal