Abstract
Comparison of gene expression profiles in closely related subpopulations of primitive hematopoietic cells offers a powerful first step to elucidating the molecular basis of their different biologic properties. Here we present the results of a comparative quantitative analysis of transcript levels for various growth factor receptors, ligands, and transcription factor genes in CD34+CD38− and CD34+CD38+ cells purified from first trimester human fetal liver, cord blood, and adult bone marrow (BM). In addition, adult BM CD34+CD38− cells were examined after short-term exposure to various growth factors in vitro. Transcripts for 19 of the 24 genes analyzed were detected in unmanipulated adult BM CD34+CD38− cells. Moreover, the levels of transforming growth factor beta (TGF-β), gp130, c-fos, and c-jun transcripts in these cells were consistently and significantly different (higher) than in all other populations analyzed, including phenotypically similar but biologically different cells from fetal or neonatal sources, as well as adult BM CD34+ cells still in G0 after 2 days of growth factor stimulation. We have thus identified a subset of early response genes whose expression in primitive human hematopoietic cells is differently regulated during ontogeny and in a fashion that is recapitulated in growth factor-stimulated adult BM CD34+CD38− cells, before their cell cycle progression and independent of their subsequent differentiation response. These findings suggest a progressive alteration in the physiology of primitive hematopoietic cells during development such that these cells initially display a partially “activated” state, which is not maximally repressed until after birth.
Introduction
Analyses of mature blood cell differentiation from multipotent human hematopoietic progenitors at the population level has led to the widely held view that this process involves a series of ordered biologic changes that typically span many cell generations. These changes include modulation of the cell surface phenotype, nuclear transcription factor activity, growth factor responsiveness, proliferative activity, proliferative potential, and lineage restriction. Nevertheless, deviations from a single coordinated process are well documented from studies of both bulk 1-4 and clonal populations 5-8 derived from otherwise indistinguishable primitive hematopoietic progenitors. In addition, comparisons of functionally similar populations obtained from different stages of ontogeny have shown these cells to exhibit differences in their cell cycle transit times,9,10 in their biologic responses to various cytokines,11-18 in their in vivo turnover rates, 9,19-21 and in some of the lineage-specific genes they express.22-24 Previous studies have indicated that the majority of CD34+CD38− cells analyzed directly after their isolation from the bone marrow (BM) of healthy adults are deeply quiescent.25-27 Nevertheless, most of these cells, including those identified functionally as long-term culture-initiating cells (LTC-ICs) on the basis of their ability to generate colony-forming cell (CFC) progeny for at least 6 weeks on stromal feeder layers,6 will enter mitosis within 7 days of exposure in vitro to high concentrations of flt3-ligand (FL), Steel factor (SF), interleukin-3 (IL-3), IL-6, and granulocyte colony-stimulating factor (G-CSF), although a first division may not be seen before the third day.25,27-30 In contrast, the same cytokines will stimulate human cord blood CD34+CD38− cells to begin to divide one day earlier and most, including the LTC-ICs, will complete at least one mitosis within 5 days.10,31 Analogous experiments with human fetal liver CD34+CD38− cells suggest they can be recruited into cycle even faster.10 32 Because most phenotypic differences between cells will ultimately be dictated by their particular recent gene expression profiles, we hypothesized that ontogenically earlier populations of CD34+CD38− human hematopoietic cells might show quantitative differences in certain gene transcripts when compared with their counterparts in normal adult BM. Further, we anticipated that the expression of at least some of these genes would be affected by growth factor stimulation of adult BM CD34+CD38− cells.
To address this question required a semiquantitative procedure for measuring transcripts for multiple genes that would be applicable to the small numbers (less than 104) of highly purified cells obtainable. Previous studies had shown that these requirements could be met using a reverse transcriptase-polymerase chain reaction (RT-PCR) method originally developed by Brady et al,33 which, however, is limited to the detection of transcripts with unique 3′ terminal sequences. From the information available at the time this study was initiated, this constraint could be met for 6 growth factor genes, 7 growth factor receptor genes, and several transcription factor genes, all known to be relevant to hematopoiesis,7 34-36as well as for a number of less hematopoietic-specific, “early response” genes. A total of 24 genes were thus selected as an initial test group to determine which were expressed in adult BM CD34+CD38− cells and how their expression would be altered by growth factor stimulation, both in vivo (as indicated by analysis of the CD34+CD38+ subset of adult human BM cells) and in vitro. The results of such analyses have revealed a number of consistent and rapid changes in gene expression that occur in growth factor-stimulated adult BM CD34+CD38− cells even before their exit from G0. As predicted, similar differences in expression of many of the same genes were found to distinguish adult from neonatal or fetal sources of CD34+CD38− cells as well as adult BM CD34+CD38+ and CD34+CD38− cells.
Materials and methods
Cells
First trimester fetal liver tissue from scheduled abortions, umbilical cord blood from full-term deliveries obtained by cesarean section, and healthy adult BM obtained from cadaveric or allogeneic transplant donors were prepared as low-density (less than 1.077 gm/cm3) single-cell suspensions and cryopreserved in DMSO as previously described.37 Informed consent was obtained for all material according to institutional guidelines.
Cell purification
As required, cell aliquots were thawed and CD34+CD38− and CD34+CD38+ subsets isolated by FACS after removal of cells expressing CD2, CD3, CD14, CD16, CD19, CD24, CD56, CD66b, and glycophorin A using an immunomagnetic affinity column (StemSep, StemCell Technologies Inc, Vancouver, BC, Canada).38 Viable (propidium iodide [PI, Sigma Chemicals, St Louis, MO]-negative) CD34+ cells with low to medium forward light-scattering characteristics were subdivided into CD38+ and CD38− subpopulations using a gate that excluded more than 99% of cells stained with a similarly labeled isotype control antibody and collected into separate tubes containing Iscove medium (IMDM, StemCell), supplemented with a serum substitute (BIT, StemCell). For the cell cycle tracking experiments, lineage marker-negative (lin−) adult BM cells were first incubated for 45 minutes at 37°C in 10 μM Hoechst 33342 (Hst, Molecular Probes, Eugene, OR) to which 5 μg/mL Pyronin Y (Py, Sigma) was then added, and the incubation continued at 37°C for another 45 minutes. The cells were then labeled with anti-CD34–FITC for an additional 20 minutes, washed in the continuing presence of 10 μM Hst and 2.5 μg/mL Py plus 1 μg/mL PI, resuspended in the same solution without PI, and kept on ice in the dark until being sorted. Separate aliquots of cells stained with PI only, with PI and anti-CD34–FITC, and with PI, anti-CD34–FITC and Py were used to set the compensation. The PI− CD34+ cells expressing low levels of Hst and Py (HstloPylo) and considered to be in G0 (referred to as the initial G0[G0i] cells) were then collected as described in detail previously.39 To isolate G0 cells from 40-hour cultures initiated with these CD34+ G0i cells, the cells harvested from the culture were restained with Hst, Py, and PI, and then the PI− HstloPylo(G0′) cells were sorted. The remaining (G1/S/G2/M) PI− cells in the cultures (designated as non-G0 or NG0′ cells) were also collected and analyzed in parallel using the same protocol as for the cells used to initiate the cultures.
Liquid suspension cultures
CD34+CD38− or CD34+G0i cells were suspended in Iscove's medium supplemented with BIT, 40 μg/mL low-density lipoproteins (Sigma), 10−4 M 2-mercaptoethanol (Sigma) at 4 × 103cells/mL, and cultured at 37°C in 3 to 6 mL volumes with one of 5 different combinations of purified human recombinant growth factors (A, B, C, D, and thrombopoietin [TPO] only), as indicated. Combination A consisted of FL (Immunex Corporation, Seattle, WA) at 300 ng/mL, SF (Amgen, Thousand Oaks, CA) at 300 ng/mL, IL-3 (Novartis, Basel, Switzerland) at 60 ng/mL, IL-6 (Cangene, Mississauga, Ontario) at 60 ng/mL, and G-CSF (StemCell) at 60 ng/mL. Combination B contained the same growth factors but each at a 30-fold lower final concentration. Combination C consisted of FL, SF, and IL-3 only, each at the same concentration as in combination A. Combination D also consisted of these 3 growth factors, the IL-3 at the same concentration (60 ng/mL) but both the FL and SF reduced 30-fold to 10 ng/mL each. TPO (Genentech, CA) was added at 50 ng/mL. For single-cell cultures, individual cells were deposited by the single-cell deposition unit of the FACS directly into the round-bottomed wells of a 96-well microtiter plate, each of which had been preloaded with 100 μL of the complete serum-free medium described above plus growth factor combination A or 50 ng/mL TPO, as indicated. The wells were examined immediately after sorting to confirm the presence of a single cell in each well and then daily thereafter.
RNA extraction and reverse transcriptase-polymerase chain reaction analyses
For each sample, a minimum of 2 × 103 cells of each population to be analyzed were obtained and lysed in 50 μL of GIT buffer (5 M guanidine isothiocyanate, 20 mM 1,4-dithiothreitol [DTT], 25 mM sodium citrate [pH = 7.0], 0.5% sarcosyl), and the nucleic acids in the lysate were then precipitated in ethanol using 4 mg of glycogen as a carrier. For reverse transcription, the procedure of Brady et al40 as modified by Sauvageau et al41 and Jiang et al42 was used. Briefly, RNA was redissolved in 5.8 μL of RNase-free water plus 0.2 μL of oligo (dT) primer (1 μg/mL) (60 mer: 5′CATGTCGTCCAGGCCGCTCTGGACAAAATATGAATTCT24) and heated to 70°C for 10 minutes, quenched on ice, and mixed with 2 μL of 5X RT buffer (GIBCO/BRL, Grand Island, NY). One microliter of 0.1 M DTT, 0.2 μL of 25 mM (deoxynucleotide triphosphate) dNTPs (GIBCO/BRL), 0.5 μL of placental RNase inhibitor (GIBCO/BRL), 0.5 μg nuclease-free bovine serum albumin (BSA) (Boehringer Mannheim, Laval, Quebec), and 0.5 μL of Superscript II (GIBCO/BRL). The reaction mixtures were incubated at 42°C for 1 hour and heat inactivated at 70°C for 10 minutes. After ethanol precipitation, the pellet was resuspended in 5.5 μL of tailing solution (1 μL of 5X tailing buffer [GIBCO/BRL]), 0.5 μL of 100 mM dATP, 3.5 μL of water, and 0.5 μL of terminal deoxynucleotidyl transferase (15 U/mL, GIBCO/BRL) for 15 minutes at 37°C and heated to 70°C for 10 minutes. Aliquots of this solution were subjected to a PCR in a solution of 50 mM KCl, 5 mM MgCl2, 0.5 μg/mL BSA, 1 mM dNTPs, 1 μL of gene 32 (Pharmacia), and 5 units of Taq polymerase (GIBCO/BRL). The complementary DNAs (cDNAs) were initially amplified for 25 cycles, each consisting of 1 minute at 94°C, 2 minutes at 55°C, and 10 minutes at 72°C, except for the first cycle, in which the annealing temperature was 37°C instead of 55°C. An additional 5 units of Taq polymerase was then added and a further 25 cycles carried out. This procedure produces a semiquantitative amplification of the reverse transcribed total messenger RNAs (mRNAs) present in the original extract40,41 and has been used previously to discriminate different transcript levels in various cell populations in which the small numbers of cells available for analysis preclude the use of alternative methodologies of mRNA quantitation.8,40,41,43 44
Southern analysis
The amplified cDNAs were electrophoresed in 1% agarose gels and transferred to nylon membrane (Hybond-N, Amersham) for hybridization with specific cDNA probes as follows. A full-length cDNA for human FL was obtained from Immunex. The cDNAs for human IL-1β, SF, and TGF-β were provided by Dr K. Humphries (Terry Fox Laboratory, Vancouver, BC, Canada). The 3′ region of human c-kit cDNA corresponding to 4043-base pair (bp) to 4775-bp was cloned from human fetal liver by RT-PCR, using 5′ CAG TAT CTA TAT ATG TGT ATG TAC C 3′ as the forward primer and 5′ CTG AAG TAC CTA GAC ATC TAT AAC 3′ as the reverse primer. A 750-bp PCR product was then cloned into a PCR cloning kit (In-Vitrogen) using the EcoRI site created at the end of each primer, and the sequence confirmed by automatic DNA sequencing before being used as a probe. A full-length human flt-3 cDNA was a gift from Dr D. Birnbaum (Institut Paoli-Calmettes Marseille, France), and the EcoRI/PstI fragment was used as a probe. The cDNA for gp130 (pRMHA-3) was obtained from Dr M. Hall (University of Birmingham, Birmingham, UK) and the EcoRI/BamHI fragment was used as a probe. The cDNAs for the murine G-CSFR, c-myc, Id-1, c-fos, and c-jun were obtained from Dr P. Reddy (Fels Institute, Philadelphia, PA). For c-fos and c-jun, cDNAs corresponding to the nonhomologous regions N-terminal to the basic helix-loop-helix regions were PCR-amplified using 5′ ATG ACT GCA AAG ATG GA 3′ and 5′ TCA AAA TGT TTG CAA CT 3′ primers for c-jun and 5′ GAG ACA GAC CAA CTA GA 3′ and TCA CAG GGC CAG CAG CG 3′ primers for c-fos. The cDNAs for AML-1β and PU.1 were obtained from Dr S. Hiebert (Vanderbilt University, Nashville, TN) and Dr D. Tenen (Harvard Medical School, Boston, MA), respectively. The probe for SCL/tal-1 was prepared by RT-PCR using Tal-F2 (5′ ATA GAA TTC CTA AGC CCA TGG GAC AAA TTG C 3′) as the forward primer and Tal-R2 (5′ AAT GAA TTC ATA CTG TGG CTG CTT CTC ATT CC 3′) as the reverse primer for PCR amplification of the region between 2654-bp and 3180-bp, followed by DNA sequencing to verify the probe obtained. For each PCR-amplified probe used, the 3′ coding region was chosen to minimize nonspecific hybridization to repetitive genomic sequences as determined by hybridization to the products of control reactions to which no reverse transcriptase was added. The cDNA for murine c-myb was also obtained from Dr P. Reddy and the 3′ half of the nonhomologous region, excluding the N-terminal DNA binding domain, was prepared bySmaI/BamHI digestion. The cDNAs for ets-1 and ets-2 (also from Dr P. Reddy) were digested with EcoRI to obtain the fragments used as probes. The cDNA for Ki-67 (Kon-21) was obtained from Dr T. Scholzen (Borstel Research Institute, Borstel, Germany) and digested with BamHI/NotI to isolate the probe used. The cDNA for IL-3Rα was obtained from Dr T. Kitamura (University of Tokyo, Tokyo, Japan) and was digested withXhoI. The cDNA for IL-3 receptor beta chain (IL-3Rβc) was obtained from Dr A. Mui (University of British Columbia, Vancouver, BC, Canada) and was digested withBglII/XbaI. The cDNA for IL-6R was obtained from Immunex and was digested with SalI. The glyceraldehyde-3 phosphate dehydrogenase (GAPDH) probe was obtained from Dr G. Krystal (Terry Fox Laboratory) and prepared by PstI digestion.
For Southern analyses, each filter was hybridized with probes labeled with 32P using a random primer kit (GIBCO/BRL) and hybridized for 12 to 16 hours at 45°C in a solution of 40% formamide, 50 mM NaPO4, 0.5% SDS, 5X SSPE, 5X Denhardt's solution, 0.25 mg/mL denatured salmon sperm DNA, and 100 mg/mL sodium dextran. The hybridized filters were then washed at room temperature in 2X SSC plus 0.1% SDS, followed by consecutive washes in 0.2X SSC plus 0.1% SDS first at 50°C and then at 55°C.
To quantitate each gene-specific signal, the washed filters were exposed to a phosphoimager screen (Molecular Dynamics, Sunnyvale, CA) for 2 to 4 hours, scanned in the phosphoimager (Storm 860, Molecular Dynamics), or autoradiographed for 1 to 2 days, and the signal quantitated using Molecular Dynamics APPS software. Each measurement was corrected for the signal level obtained in the RT− control that was hybridized to the same filter under the same conditions. Filters were then stripped and rehybridized to the GAPDH probe to normalize for the amount of cDNA loaded onto each gel. For comparisons of transcript levels between fresh and/or cultured cells from the same tissue sample, each normalized transcript signal was expressed as a ratio relative to the normalized transcript signal measured in the reference population (either the corresponding freshly isolated CD34+CD38− cells or the CD34+ G0i cells in the cell cycle experiments with adult BM), which was obtained from the same gel and filter. The resultant ratios were then averaged, and the standard error of the mean (SEM) for each data set was calculated. For comparisons of transcript levels in populations obtained from different tissues (eg, CD34+CD38− cells from adult BM vs cord blood vs fetal liver samples), at least one sample of each of the populations being compared were run on the same gel to control for variations in the Southern blotting procedure. Normalized transcript signal values were then expressed as a ratio relative to the average normalized value for the same type of transcript in the adult BM CD34+CD38− populations assessed. The Student t test was then used to determine which transcript signals were significantly different from the reference population with a P < .05.
Results
Differences in gene expression in the CD38− and CD38+ subsets of adult bone marrow CD34+ cells
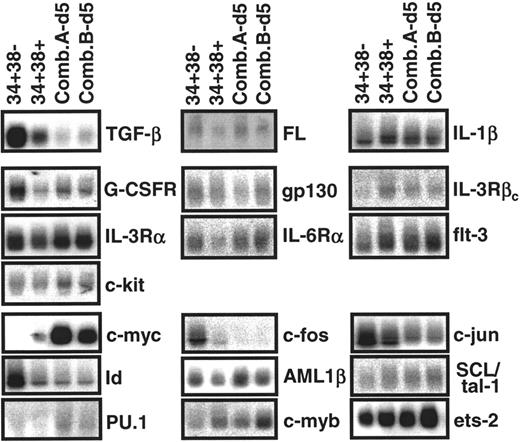
To obtain a reference pattern of gene expression in adult BM CD34+CD38− cells and to test the ability of the comparative procedure described in the “Materials and methods” to detect quantitative differences in transcript levels between closely related, but phenotypically or biologically distinct populations, we first compared the results obtained from paired CD38− and CD38+ subsets of CD34+ cells isolated from several different healthy adult BM samples. This comparison included a survey of transcripts for various growth factor receptors, ligands and transcription factors known or anticipated from previous studies to be involved in the regulation of CD34+ hematopoietic cell proliferation and differentiation.7 34-36 Transcripts for TGF-β, FL, IL-1β, G-CSFR, gp130, IL-3Rα, IL-6Rα, flt-3, c-kit, c-fos, c-jun, Id, AML-1β, SCL/tal-1, PU.1, c-myb, and ets-2 were readily detected, albeit at variable levels, in all CD34+CD38−adult BM cell isolates examined (n = 5). In contrast, extracts of these same cells contained very low levels of IL-3Rβc and c-myc transcripts. Figure 1shows the hybridization results for a representative experiment. Transcripts for IL-3, IL-6, SF, and ets-1 were not detectable in either CD34+ subset (data not shown). Comparison of the transcript levels between the matched pairs of CD34+CD38+and CD34+CD38− adult BM cells for 19 other genes analyzed showed a number of consistent differences. As can be seen in Figure 2, on average, transcripts for TGF-β, G-CSFR, gp130, c-fos, and c-jun appeared approximately 2-fold lower in the CD34+CD38+ cells, and for IL-3Rβcand c-myc were approximately 3- to 4-fold higher. Expression of Id in the CD34+CD38+ population also appeared slightly reduced, although this difference was not statistically significant (P > .05). Levels of transcripts for the other 16 genes surveyed appeared to be similar in both subsets of adult BM CD34+ cells.
Representative analysis of transcripts found in different populations of primitive adult BM cells.
RNA extracts from purified CD34+CD38−(34+38−) and CD34+CD38+ (34+38+) adult BM cells and their progeny obtained after 5 days of stimulation by 2 growth factor combinations (A and B, described in “Materials and methods”) were subjected to semiquantitative RT-PCR, followed by Southern blot analysis using gene-specific cDNA probes. Each filter was then stripped and reprobed with a GAPDH probe (not shown) to allow normalization for cDNA loading.
Representative analysis of transcripts found in different populations of primitive adult BM cells.
RNA extracts from purified CD34+CD38−(34+38−) and CD34+CD38+ (34+38+) adult BM cells and their progeny obtained after 5 days of stimulation by 2 growth factor combinations (A and B, described in “Materials and methods”) were subjected to semiquantitative RT-PCR, followed by Southern blot analysis using gene-specific cDNA probes. Each filter was then stripped and reprobed with a GAPDH probe (not shown) to allow normalization for cDNA loading.
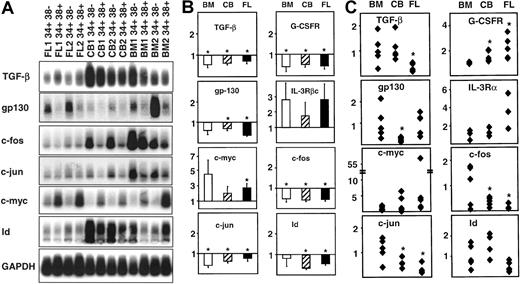
Comparative analysis of gene expression in CD34+ subpopulations from adult BM, cord blood (CB), and fetal liver (FL).
Panel A shows a representative Southern blot analysis of cDNAs for 7 genes expressed in the matching CD34+CD38− and CD34+CD38+ subpopulations isolated from 2 different samples of each tissue. Panel B shows the results of a quantitative comparison of the different levels of transcripts in the CD38− and CD38+ subsets of CD34+cells obtained from a total of 3 samples of each different tissue. In each case the value shown is the mean ± SEM of the normalized gene-specific transcript levels in the CD34+CD38+ subset expressed as a fraction of the levels measured in the matching CD34+CD38−population (set = 1.0) as described in the “Materials and methods.” Panel C shows a quantitative analysis of the relative levels of expression of the same genes as shown in panel B but, in this case, comparing the results for the CD34+CD38− subset in CB and FL with the CD34+CD38− data from adult BM examined on the same filter. The result for the CD34+CD38−cells from each sample (panel B) was thus first normalized and then expressed as a proportion of the average value obtained for all adult BM CD34+CD38− samples analyzed using the same probe (average value set = 1.0, n = 5). In panels B and C, significant differences between the test and the reference population (P < .05) are indicated by an asterisk (*).
Comparative analysis of gene expression in CD34+ subpopulations from adult BM, cord blood (CB), and fetal liver (FL).
Panel A shows a representative Southern blot analysis of cDNAs for 7 genes expressed in the matching CD34+CD38− and CD34+CD38+ subpopulations isolated from 2 different samples of each tissue. Panel B shows the results of a quantitative comparison of the different levels of transcripts in the CD38− and CD38+ subsets of CD34+cells obtained from a total of 3 samples of each different tissue. In each case the value shown is the mean ± SEM of the normalized gene-specific transcript levels in the CD34+CD38+ subset expressed as a fraction of the levels measured in the matching CD34+CD38−population (set = 1.0) as described in the “Materials and methods.” Panel C shows a quantitative analysis of the relative levels of expression of the same genes as shown in panel B but, in this case, comparing the results for the CD34+CD38− subset in CB and FL with the CD34+CD38− data from adult BM examined on the same filter. The result for the CD34+CD38−cells from each sample (panel B) was thus first normalized and then expressed as a proportion of the average value obtained for all adult BM CD34+CD38− samples analyzed using the same probe (average value set = 1.0, n = 5). In panels B and C, significant differences between the test and the reference population (P < .05) are indicated by an asterisk (*).
Differences in gene expression in CD34+CD38− and CD34+CD38+ cells as a function of their ontogenic state
We next examined the RT-PCR products obtained from paired CD34+CD38− and CD34+CD38+ cell subpopulations isolated independently by FACS from several different fetal liver and cord blood samples. A representative set of filters for 8 of the genes examined is shown in Figure 2A. Figure 2B shows the differences in transcript levels observed when the CD38+ and CD38−subsets for both of these tissues were compared using the matched CD38− subset as the reference population in each case. Cord blood and fetal liver CD34+CD38+ cells both showed lower levels of transcripts for TGF-β, G-CSFR, gp130, c-fos, and c-jun, and even lower levels of Id transcripts, but higher levels of IL-3Rβc and c-myc transcripts, relative to the CD34+CD38− cells in the same sample. These differences are similar to those seen when CD34+CD38− and CD34+CD38+ cells from adult BM are compared. Levels of transcripts that appeared to be similar in adult BM CD34+CD38− and CD34+CD38+ cells (ie, for FL, IL-1β, IL-3Rα, IL-6Rα, flt-3, c-kit, AML-1β, SCL/tal-1, PU.1, c-myb, and ets-2) also appeared similar in the corresponding subsets of CD34+ fetal liver and cord blood cells (data not shown).
Given the known differences in the biologic properties of CD34+CD38− cells from fetal liver, cord blood, and adult BM, it was of interest to also compare the transcript levels in these phenotypically similar populations. To minimize variability, RT-PCR products were analyzed on the same filters (eg, Figure 2A) and transcript levels first normalized to matched GAPDH transcript levels. A value of 1.0 was then assigned to the average value obtained from the adult BM CD34+CD38− cell data set and all other values expressed as a ratio of that value. Figure 2C shows the individual data points obtained from this analysis. Despite the considerable intersample variation still observed, significant differences between the levels of some transcripts in fetal or neonatal versus adult sources of CD34+CD38− cells could be discerned. For example, relative to CD34+CD38− adult BM cells, there were fewer TGF-β transcripts in CD34+CD38− fetal liver cells, fewer gp130 transcripts in CD34+CD38−cord blood cells, and fewer c-fos and c-juntranscripts in both. Similarly, transcripts for IL-3Rβcand c-myc appeared to be present at increasingly higher levels in CD34+CD38− cells from cord blood and fetal liver, although these latter differences were not statistically significant. In addition, the 16 genes whose expression had not been found to be different between the CD38− and CD38+ subsets of CD34 cells from any particular stage of development also showed no difference in transcript levels when the CD34+CD38− subsets from fetal, neonatal, and adult hematopoietic tissues were compared. The one exception to this was the increased level of G-CSFR transcripts typical of CD34+CD38− cord blood and fetal liver cells relative to their counterparts in adult BM.
Changes in gene expression induced in adult BM CD34+CD38− cells after growth factor stimulation
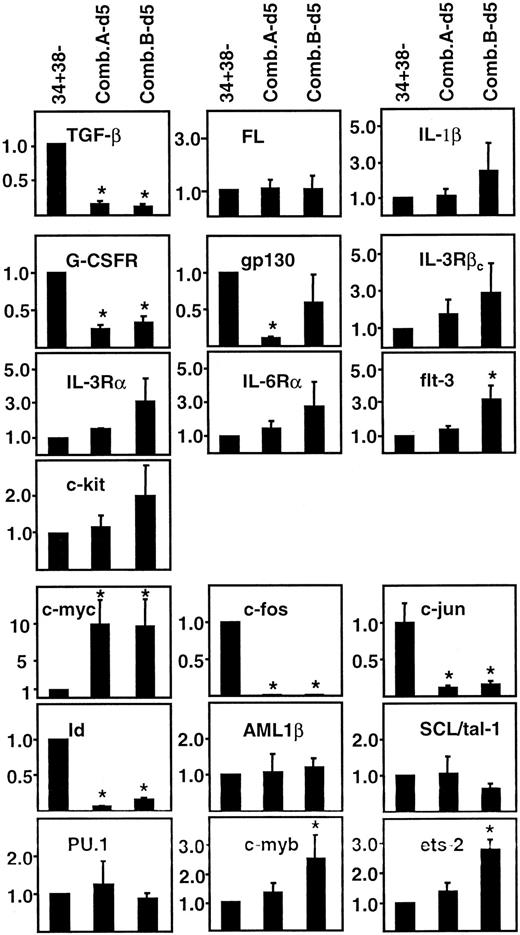
We next asked whether the same genes whose expression was different between ontogenically distinct sources of CD34+CD38− cells would also be affected by in vitro growth factor stimulation of adult BM CD34+CD38− cells. Accordingly, aliquots of CD34+CD38− cells from 3 of the same BM samples used for the ontogenic analyses were cultured in serum-free medium containing FL, SF, IL-3, IL-6, and G-CSF at 2 different concentrations. After 5 days, cells were harvested and RNA was extracted. The 5-day period of culture was chosen anticipating that this might be sufficient to allow a biologically relevant pattern of altered gene expression to be established before extensive cell division. The 2 growth factor combinations used (A and B, as described in “Materials and methods”) had been shown previously to have equivalent mitogenic activity on adult BM CD34+CD38− cells while differing by more than 50-fold in their ability to preserve LTC-IC activity among the progeny generated after 10 days.3Assessment of transcript levels in the cells that had been cultured under these conditions for 5 days (Figure 1) revealed similar quantitative changes in expression of many of the same genes whose transcript levels had been found to differ between freshly isolated and CD34+CD38− hematopoietic cells from adult versus fetal and neonatal sources (Figure 2C), as well as between internally compared CD34+CD38− and CD34+CD38+ cells from each of these tissues (Figure 2B). Specific changes seen included even greater decreases in TGF-β, gp130, c-fos, c-jun, and Id transcripts and greater increases in c-myc transcripts (Figure3). In most cases, these differences were statistically significant (P < .05). In the cultured cells, IL-3Rβc transcripts were also increased but transcript levels for FL, IL-1β, AML-1β, SCL/tal-1, and PU.1 were unchanged. Exposure of adult BM CD34+CD38−cells to the lower concentration of FL, SF, IL-3, IL-6, and G-CSF elicited a number of other changes not seen when freshly isolated CD34+CD38+ and CD34+CD38− cells were compared. These included detectable increases in IL-3Rα, IL-6Rα, flt-3, and c-kittranscripts and more pronounced increases in c-myb and ets-2 transcripts.
Quantitative analysis of gene-specific transcript levels in differently stimulated populations of primitive adult BM cells.
Values were calculated as described in the “Materials and methods” using adult BM CD34+CD38− cells as the reference population ( = 1.0). Shown are the mean of analyses of at least 3 independent BM samples ± SEM. Values that are significantly different from 1.0 (P < .05) are indicated by an asterisk (*).
Quantitative analysis of gene-specific transcript levels in differently stimulated populations of primitive adult BM cells.
Values were calculated as described in the “Materials and methods” using adult BM CD34+CD38− cells as the reference population ( = 1.0). Shown are the mean of analyses of at least 3 independent BM samples ± SEM. Values that are significantly different from 1.0 (P < .05) are indicated by an asterisk (*).
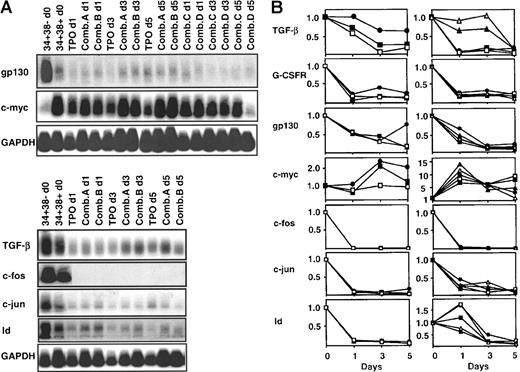
To assess whether the similarity in gene expression profiles of growth factor-stimulated CD34+CD38− cells and freshly isolated CD34+CD38+ cells from adult BM might lie in the differences in the proliferative activity of these 2 populations by comparison to freshly isolated CD34+CD38− adult BM cells,25,27 a time course experiment was performed. Two samples of adult BM CD34+CD38− cells were therefore cultured with the same growth factor combinations (ie, A and B), and the cells then harvested for growth transcript analysis after 1, 3, and 5 days of incubation. In addition, aliquots of the same original cells were cultured with 2 other growth factor combinations (C and D, described in the “Materials and methods”) and similarly followed. Growth factor combinations C and D are, like A and B, similarly mitogenic for adult BM CD34+CD38− cells but optimize and minimize, respectively, the retention of LTC-IC activity by the stimulated cells.3 As a further comparison, a time course experiment was simultaneously performed on adult BM CD34+CD38− cells cultured in serum-free medium for up to 5 days in the presence of TPO only. In contrast to the other 4 growth factor cocktails, TPO alone was confirmed by direct visual analyses of single cell cultures to be poorly mitogenic for adult BM CD34+CD38− cells over at least 5 days (data not shown), although during this period, TPO alone is quite efficient at preserving the viability45 and LTC-IC activity of these cells.39
Figure 4 shows the results only for the transcripts whose levels relative to adult BM CD34+CD38− cells were found to be modulated during ontogeny (Figure 2) and/or after growth factor stimulation (Figures 1 and 3). In both of the time course experiments performed, decreases in G-CSFR, gp130, c-fos, and c-juntranscripts were already apparent and (except for gp130) were maximal within 1 day, independent of the growth factor stimulus applied. Most changes were complete within 3 days; however, the timing of the decrease in TGF-β and Id transcripts and the increase in c-myc expression appeared more variable and in some instances delayed. Because very few adult BM CD34+CD38− cells enter mitosis before the third day of exposure to any of the growth factors used in these studies25 27-30 (and data not shown), these results suggested that the changes in gene expression seen could occur before cell division.
Time course of changes in gene expression in variously growth factor-stimulated primitive adult BM cells.
Panel A shows the Southern blot analyses of transcript cDNAs detected in 2 independent experiments in which FACS-sorted CD34+CD38− cells were stimulated with one of 5 different growth factor conditions (as shown) and the cells harvested for RT-PCR analysis at the times indicated. Panel B shows the same data normalized as described in the “Materials and methods” using the corresponding starting CD34+CD38− BM cells as the reference population in each case (values set = 1.0). Each symbol identifies the results obtained for a different growth factor condition (●, TPO; ■, Comb. A; □, Comb. B; ▴, Comb. C; Δ, Comb. D).
Time course of changes in gene expression in variously growth factor-stimulated primitive adult BM cells.
Panel A shows the Southern blot analyses of transcript cDNAs detected in 2 independent experiments in which FACS-sorted CD34+CD38− cells were stimulated with one of 5 different growth factor conditions (as shown) and the cells harvested for RT-PCR analysis at the times indicated. Panel B shows the same data normalized as described in the “Materials and methods” using the corresponding starting CD34+CD38− BM cells as the reference population in each case (values set = 1.0). Each symbol identifies the results obtained for a different growth factor condition (●, TPO; ■, Comb. A; □, Comb. B; ▴, Comb. C; Δ, Comb. D).
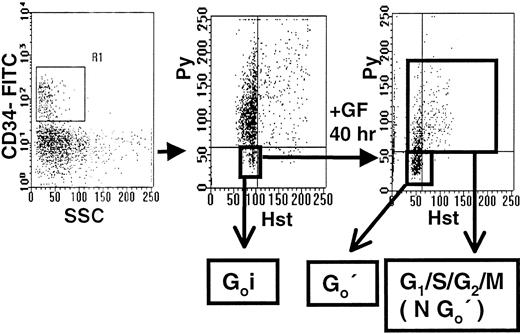
A next experiment was therefore undertaken to determine whether the changes in gene expression observed within 1 to 2 days of growth factor-stimulation of adult BM CD34+CD38−cells could be formally shown to occur even before these cells entered G1. For this, the method of dual Hst/Py labeling was used to allow the selective isolation by FACS of CD34+ cells in G0 both before (G0i) and after (G0′) they had been in culture with growth factor combination A for 40 hours (Figure5). In these experiments, staining for CD38 was omitted because isolation of G0(HstloPylo) cells from within the PI− CD34+CD38− population would have required a 2-step staining and sorting procedure. The 40-hour culture interval was chosen to increase the selectivity of the analysis for CD34+CD38− cells persisting in G0. Gene expression profiles for the G0populations isolated before and after culture were then compared.
Hst/Py staining of adult BM CD34+ cells before and after culture with growth factor combination A.
Lin− adult BM cells were labeled with CD34-FITC, Hst and Py as described in the “Materials and methods,” and then the gates shown were used to isolate the G0(HstloPylo) fraction (middle panel) from within the PI− CD34+ population (left panel). These G0i cells were then cultured in serum-free medium containing FL, SF, IL-3, IL-6, and G-CSF (Comb. A) for 40 hours. The cells were then restained as before to allow the isolation of the persisting G0 cells (G0′) by FACS separate from the remaining G1/S/G2/M (NG0′) cells.
Hst/Py staining of adult BM CD34+ cells before and after culture with growth factor combination A.
Lin− adult BM cells were labeled with CD34-FITC, Hst and Py as described in the “Materials and methods,” and then the gates shown were used to isolate the G0(HstloPylo) fraction (middle panel) from within the PI− CD34+ population (left panel). These G0i cells were then cultured in serum-free medium containing FL, SF, IL-3, IL-6, and G-CSF (Comb. A) for 40 hours. The cells were then restained as before to allow the isolation of the persisting G0 cells (G0′) by FACS separate from the remaining G1/S/G2/M (NG0′) cells.
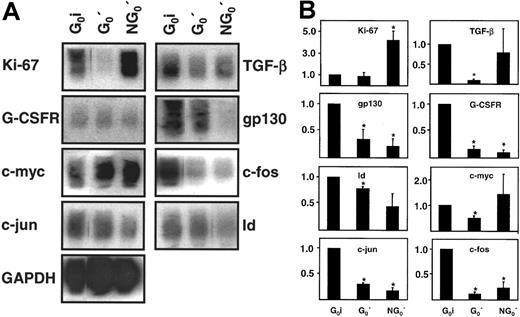
The blots obtained from a representative experiment of this design are shown in Figure 6A. Relative levels of all genes surveyed in these studies (using the initial CD34+ G0 cells as the reference population) are shown in Figure 6B. Low expression of Ki67 in both populations defined as G0cells on the basis of Hst/Py staining and increased expression of Ki67 (on average 4-fold, P < .05) in the cultured NG0′ fraction confirmed the validity of the FACS gates used to isolate both G0 populations. Interestingly, TGF-β, G-CSFR, gp130, c-myc, c-fos, c-jun, and Id transcript levels were all significantly (P < .05) reduced, albeit to variable degrees, in the persisting G0′ cells. Reductions in G-CSFR, gp130, c-fos, c-jun, and Id transcripts were also seen in the simultaneously harvested, cultured NG0′ cells. The lack of evidence of a similar response with respect to the expression of TGF-β and c-myc in the cultured NG0′ and G0′ cells is, however, difficult to interpret because of the wide intersample variation seen in the responses of these 2 particular genes in the NG0′ cells. Nevertheless, overall, many of the changes in gene expression that occur in growth factor-stimulated CD34+ adult BM cells could be seen to be induced independently of the timing of their exit from G0.
Analysis of changes in gene expression in growth factor-stimulated adult BM CD34+ cells remaining quiescent for 40 hours.
Panel A shows representative blots comparing transcript cDNAs for 9 genes expressed in freshly isolated CD34+ G0(G0i) cells, cells found to be still in G0after 40 hours in culture (G0′ cells), and the remaining (NG0′) cells. Cells were cultured and isolated as described in Figure 5 and then RNA extracts were prepared and subjected to semiquantitative RT-PCR analysis as described in the “Materials and methods.” Panel B shows the same data quantitated and normalized as in Figure 2, but using the G0i cells as the reference population in this case (value for each transcript = 1.0 for the G0i cells). Shown are the mean ± SEM of data from 3 independent experiments in each of which BM cells from a different individual were used. Values found to be significantly different from 1.0 (P < .05) are indicated by an asterisk (*).
Analysis of changes in gene expression in growth factor-stimulated adult BM CD34+ cells remaining quiescent for 40 hours.
Panel A shows representative blots comparing transcript cDNAs for 9 genes expressed in freshly isolated CD34+ G0(G0i) cells, cells found to be still in G0after 40 hours in culture (G0′ cells), and the remaining (NG0′) cells. Cells were cultured and isolated as described in Figure 5 and then RNA extracts were prepared and subjected to semiquantitative RT-PCR analysis as described in the “Materials and methods.” Panel B shows the same data quantitated and normalized as in Figure 2, but using the G0i cells as the reference population in this case (value for each transcript = 1.0 for the G0i cells). Shown are the mean ± SEM of data from 3 independent experiments in each of which BM cells from a different individual were used. Values found to be significantly different from 1.0 (P < .05) are indicated by an asterisk (*).
Discussion
It is generally accepted that many features of differentiation and development will eventually be understood by more complete descriptions of the changing patterns of gene expression that occur when these processes take place. Indeed, such studies have already led to the identification of a number of relevant genes for transcription factors, as well as genes that encode receptors for ligands that stimulate the transition of specific cell types from one state to another. In the hematopoietic system, examples of the former include genes like theHOX genes that appear to be stage- but not tissue-specific,46 as well as others like the GATA family, SCL-/tal-1, AML-1β, and PU.1 that are more unique to the hematopoietic system.35 36 Nevertheless, the mechanisms by which the products of such genes interact to allow lineage determining events to occur, and the dynamics of these processes are still poorly understood.
Progress in the development of technologies for detecting transcript levels in rare populations33,40,47-49 has begun to make possible the analysis of gene expression patterns in purified subsets of human hematopoietic cells that are highly enriched in cells with stem cell activity. In a study reported by Bello-Fernández et al,50 transcripts for GM-CSFRα and GM-CSFRβ ( = IL-3Rβc), c-kit, G-CSFR, flt-3, myeloperoxidase, and TNFRI and TNFRII were all consistently detected in freshly isolated CD34+Thy-1+ and CD34+Thy-1− subsets of cord blood cells. However, differences in levels of these transcripts between the 2 populations were not resolved. Evidence of GM-CSFR (α and β), c-kit, G-CSFR, and flt-3 on G-CSF mobilized adult CD34+CD38− cells and of c-kit, flt-3, and IL-1R expression by freshly isolated adult BM CD34+CD38− cells has also been obtained from ligand-binding studies.51,52 Scadden and colleagues43,53 surveyed the changes in the expression profiles of many of these as well as a number of other genes associated with hematopoietic cell differentiation. However, their initially isolated CD34+CD38− cells had been exposed to various growth factors for at least 2 days before the first analysis. Nevertheless, their studies were able to document the additional presence of transcripts for GM-CSFRα, c-kit, gp130, IL-6Rα, IL-1R, G-CSFR, SCL, GATA-1 and GATA-2, NF-E2, PU.1, AML1β, and C/EBPα, but not IL-3Rβc ( = GM-CSFRβ), erythropoietin receptor (EPOR), or c-MPL. In a recent report, we showed that neither freshly isolated adult BM CD34+CD45RA− CD71−cells nor the CD34+CD45+ and/or CD71+ cells contain detectable transcripts for IL-3, IL-6, SF, GM-CSF, G-CSF, or TPO.42 Thus, there is a growing body of information about which genes may (or may not) be expressed in primitive human hematopoietic cells from different sources and enriched to varying degrees in their stem cell content. Nevertheless, a systematic quantitative comparison of transcript levels in such samples has been lacking.
In the current study, we have used a semiquantitative procedure33 to address this question and to test the hypothesis that primitive hematopoietic cells from fetal and neonatal tissues might display a pattern of gene expression exhibited by their adult counterparts after a period of growth factor stimulation. Initially, we confirmed and extended previous surveys of gene expression in primitive human hematopoietic cells by demonstrating an absence of IL-3, IL-6, and SF transcripts, and the presence of low, but detectable levels of IL-3Rβc transcripts, in addition to readily detectable levels of transcripts for TGF-β, G-CSFR, gp130, IL-6Rα, flt-3, c-kit, SCL/tal-1, PU.1, and AML1β in highly purified CD38+ and CD38− subpopulations of CD34+ cells obtained from human fetal liver, cord blood, and adult BM. Our current findings also provide the first documentation of detectable levels of mRNA for the transcription factors: c-myb, c-fos, c-jun, Id, ets-2, and the growth factors: FL and IL-1β, and low to detectable levels of c-myc in each of these primitive human hematopoietic cell populations. Internal comparisons of the relative levels of particular transcripts in the CD38+ and CD38− fractions of CD34+ cells from human fetal liver, cord blood, and adult BM revealed remarkably consistent differences between the 2 populations in each case. In fact, most of the 23 genes studied showed little or no evidence of a change in the expression between these 2 subpopulations. However, transcripts for TGF-β, G-CSFR, gp130, c-fos, c-jun, and Id were generally lower in the CD38+ population, and for c-myc and IL-3Rβc, were usually higher. Taken together, these results demonstrate the presence of detectable transcripts in the most primitive hematopoietic cells of many genes generally considered specific to hematopoietic cell differentiation. In addition, they underscore the likely importance of quantitative changes in the expression of these genes in the mechanisms by which the biologic status of primitive hematopoietic cells is altered.8 43
Consistent with this prediction was the finding that the expression of many of the genes affected by CD34+CD38− cell differentiation into CD34+CD38+ cells in vivo is altered, and in a similar fashion, when adult BM CD34+CD38− cells are mitogenically stimulated by growth factors in vitro. Interestingly, this was found to be the case for all 5 growth factor cocktails tested, even though these vary markedly in their mitogenic and differentiation promoting activities on CD34+CD38− adult BM cells, although all support the viability of these cells.3 45 Moreover, the common changes in transcript levels seen in these variously stimulated CD34+CD38− adult BM cells could be detected before most of the original cells had completed a first division (ie, within 3 days) and were also detectable in CD34+ cells that had been exposed to growth factors for 2 days but were not yet ready to exit G0. Thus, genes whose level of expression in CD34+CD38− cells is modulated during ontogeny appear to overlap extensively with “early response” genes of growth factor-stimulated primitive adult hematopoietic cells.
These findings provide transcriptional evidence to support the concept that primitive fetal hematopoietic cells and, to a lesser extent, their neonatal derivatives, show features of an activated state relative to their CD34+CD38− counterparts in adult BM. The extent to which this is causally related to potential differences in the microenvironmental characteristics of fetal and adult hematopoietic tissues is not yet clear. Alternative, although not necessarily mutually exclusive, explanations would include the possibilities that fetal and adult gene expression programs are, at least to some extent, predetermined, or that the regulated expression of a CD34+CD38− phenotype during development may not be as tightly linked to the differentiation status of primitive hematopoietic cells as has been assumed. Nevertheless, the current observations do suggest that the different cytokine response behavior of primitive adult and fetal/neonatal hematopoietic cells is unlikely to be explained solely by differences in ligand and/or receptor expression. An alternative possibility would be that the signaling status of these cells, or key downstream targets such as c-fos, c-jun, and c-myc, are present in these cells at different levels. With the rapidly emerging capacity to extend the type of study performed here to all genes expressed in human hematopoietic stem cells, more extensive comparison of the differences between fetal and adult human cells should allow this concept to be more rigorously examined.
The nature of the current analysis unfortunately makes it difficult to infer a specific role for any of the changes in gene expression observed. However, in light of published evidence that endogenous TGF-β production may serve as an autocrine inhibitor of primitive hematopoietic cell proliferation,54-56 it might be speculated that a down-regulation of TGF-β transcripts could play a necessary, although perhaps not sufficient, role in promoting the exit of adult CD34+CD38− cells out of a G0 state. This would be consistent with our observation that TGF-β mRNA levels were highest in unmanipulated CD34+CD38− BM cells. It is also interesting to note that the forced expression of c-fos in primitive murine hematopoietic cells has been found to inhibit their cell cycle progression.57 These latter findings are consistent with the present observation that c-fos transcripts are highest in a subset of primitive hematopoietic cells that are relatively resistant to growth factor activation (ie, adult BM CD34+CD38− cells). Finally, it is interesting to note that our studies did not reveal any changes in gene expression that appeared to correlate with the retention/loss of the primitive functional status of the cells analyzed, as inferred from previously documented changes in the LTC-IC activity of adult BM CD34+CD38− cells cultured under the same conditions as used here.3 Accordingly, it might be argued that changes in the expression of the genes analyzed in this study do not represent steps in the process by which hematopoietic stem cell differentiation decisions are determined. This would not, of course, preclude a potentially important role of the products of these genes in such processes, or the likelihood that they may contribute to such cellular decisions through other mechanisms, which could include changes in expression of other genes whose products they might interact with.58-62 Indeed, there is now increasing evidence that the early differentiation process may involve quantitative changes in a complex portfolio of transcripts whose dynamics may have major consequences on the pace and direction of differentiation that primitive hematopoietic cells will undertake.63 64
The presently documented ability of deeply quiescent primitive hematopoietic cells to generate a rapid and integrated response to growth factor stimulation that can accompany, but does not require, progression of the cells into G1 and can be monitored by quantitative transcript analyses also has potentially interesting practical applications. For example, the changes observed (eg, up-regulation of c-myc or down-regulation of TGF-β, G-CSFR, c-fos, c-jun, or Id) may prove useful in future studies of neoplastic hematopoietic stem cell populations, or may serve as sensitive indicators of novel factors to which normal CD34+CD38− cells may be responsive. Further studies to identify the points in time when growth factor-induced differentiation of CD34+CD38− cells may be fixed irreversibly should also help to establish the relevance of the kinetics of changes in expression of TGF-β, G-CSFR, c-fos, c-jun, and Id in very primitive hematopoietic populations.
Acknowledgments
We thank Dianne Reid and Margaret Hale for expert technical assistance; the staff of the Stem Cell Assay Laboratory for assistance in the initial processing, cryopreservation, and isolation of lin− cells from fetal liver, cord blood, and adult BM samples; and Yvonne Yang for assistance in preparing the manuscript. We also thank Cangene, Genentech, Immunex, Novartis, StemCell, D. Birnbaum, M. Hall, S. Hiebert, K. Humphries, T. Kitamura, P. Lansdorp, A. Mui, P. Reddy, and D. Tenen for their generous provision of reagents and probes.
Supported by a grant from the National Cancer Institute of Canada (NCIC) with funds from the Terry Fox Run. I.O. held an NCIC Postdoctoral Fellowship and C.J.E. was a Terry Fox Cancer Research Scientist of the NCIC.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
C. J. Eaves, Terry Fox Laboratory, 601 W 10th Ave, Vancouver, BC V5Z 1L3, Canada; e-mail: connie@terryfox.ubc.ca.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal