Abstract
Interleukin (IL)-1β and IL-6 are the 2 major inducers of a group of hepatic genes during acute inflammation; however, each cytokine uses different intracellular signaling molecules. In most instances, the 2 cytokines interact positively to enhance hepatic gene expression, but in one class of acute-phase reactants, which includes fibrinogen, IL-1β exerts a transient inhibitory effect over the IL-6 stimulatory signal. This study explored the effects of IL-1β/nuclear factor κB (NF-κB) and IL-6/signal transducer and activator of transcription 3 (STAT3) combinatory signaling on the transcriptional regulation of the rat γ fibrinogen gene. Northern blot and functional analyses employing luciferase reporter constructs driven by the rat γ fibrinogen promoter demonstrated that IL-1β inhibited the IL-6-mediated transcription of this gene. Exposing primary rat hepatocytes to IL-1β had no effect on IL-6-mediated STAT3 activation; instead, IL-1β-activated NF-κB associated with 2 IL-6 responsive elements (STAT3 binding site) on the rat γ fibrinogen promoter and blocked STAT3 binding to these regions. The competitive binding of NF-κB and STAT3 on the overlapping binding site provides a mechanism for the inhibition by IL-1β of the IL-6-mediated transactivation of rat γ fibrinogen.
Introduction
The mature fibrinogen molecule is composed of equal molar amounts of 3 pairs of nonidentical subunits.1Secretion of fibrinogen requires correct assembly of all 3 chains. Alterations in the production of any one subunit can influence assembly and secretion of the mature protein.2,3 Although fibrinogen is expressed constitutively, its production can be greatly increased during an acute-phase response by interleukin (IL)-6 and glucocorticoids4,5; therefore, it is regarded as an acute-phase reactant (APR).6
The initial proinflammatory cytokines produced in an acute-phase response are tumor necrosis factor α (TNFα) and IL-1β.7,8 These 2 cytokines not only affect the production of a number of hepatically derived APRs, but they are also responsible for the production of IL-6, the other major cytokine involved in regulating expression of hepatic genes.6,9TNFα and IL-1β both activate transcription factor complexes designated as nuclear factor κB (NF-κB).10 When IL-1β binds to its receptor, a cascade of signaling events results in the release of NF-κB subunits from IκB. NF-κB factors translocate to the nucleus and interact with κB DNA binding motifs (GGGAATTCCC).11-14 Signaling by IL-6 involves at least 2 types of transcription factors. One type includes the CCAAT-enhancer binding proteins (C/EBPs), composed of 2 isoforms C/EBPα and C/EBPβ (also known as NF-IL6), that bind to the consensus TT(N)nCAAT DNA motifs.15,16 The second type is known as STAT (signal transducer and activator of transcription).17-19Specifically, IL-6 activates STAT1 and STAT3. STAT3 binds to a DNA consensus sequence consisting of TTCTGGGAA. Activation of STAT3 is responsible for up-regulation of the fibrinogen genes during an acute-phase response.4 20
IL-1β and IL-6 act cooperatively to up-regulate transcription of several APR genes, such as serum amyloid A,21 C-reactive protein,22 and α1 acid glycoprotein.23 In these cases, the coordinated activation of NF-κB and NF-IL6 is important for the synergistic effect of IL-1β and IL-6.23 However, the IL-6-mediated expression of other APRs, such as fibrinogen, α1-antichymotrypsinogen, α2-macroglobulin, and thiostatin genes, is inhibited by IL-1β costimulation.23-25 The molecular basis of this inhibitory response has not been established. In the present study, we describe a mechanism whereby IL-1β-induced binding of NF-κB to its cognate site on the rat γ fibrinogen promoter directly inhibits binding of STAT3 to the IL-6 responsive element (IL-6RE). This novel interaction provides a plausible explanation for the IL-1β-mediated inhibition of the IL-6 signal on rat γ fibrinogen gene.
Materials and methods
Materials
The Klenow fragment was obtained from Boehringer Mannheim; radioactive [α-32P] dCTP and [α-32P] TTP were obtained from Amersham Life Science; double-strand poly(dI-dC) was purchased from Pharmacia; DNA oligonucleotides were synthesized by Biosyntheses, Inc.; polyclonal anti-STAT3 (sc-483), anti-NF-κB p65 (sc-372), anti-NF-κB p50 (sc-4191) antibodies and recombinant IκBα protein (sc-4094) were purchased from Santa Cruz Biotechnology, Inc; purified recombinant human NF-κB p50 protein and recombinant human IL-6 were purchased from Promega; recombinant mouse IL-1β was obtained from Pfizer, Inc. Rabbit polyclonal anti-fibrinogen antibodies were produced in our laboratory. Recombinant mouse IL-6 was expressed and purified as described previously.26 27
Cell culture and treatment with cytokines
We employed several different hepatoma cell lines as well as primary rat hepatocytes in this study, because some cell lines have defects in IL-1β or glucocorticoid signaling.28 Human hepatoma cell lines, HepG2 and Hep3B, and rat hepatoma cell lines, H35 and H4IIE, were obtained from American Type Tissue Collection. The cells were cultured in Eagle minimal essential medium with nonessential amino acids, Earle basic salt solution, sodium pyruvate, 10% fetal bovine serum, and 100 ng/mL ciprofloxacin. Primary hepatocytes were prepared by collagenase perfusion of rat livers and maintained in William E media as previously described.20 29 Recombinant human IL-6 was used at 500 U/mL for human hepatoma cells. Recombinant mouse IL-6 was used at 100 ng/mL for rat hepatocytes and the reconstituted system expressing rat IL-6 receptor. Recombinant mouse IL-1β was used at 10 ng/mL for all cells. Dexamethasone was used (10−6 mol/L) in all treatments to maximize the IL-6 induction.
Total RNA isolation and Northern blot analyses
Total RNA was prepared from cells treated with different cytokines, using TRIzol reagent (Gibco). Ten micrograms of total RNA samples were fractionated on 1.5% agarose gel and subjected to Northern blot assays. Rat γ fibrinogen gene complementary DNA (cDNA) or human γ fibrinogen gene cDNA was labeled with [α32P]-dCTP, using a random primer kit (Amersham Life Sciences) as the probe. Rat cyclophilin cDNA or human GAPDH cDNA was radiolabeled and used as the internal probe for rat or human RNA samples, respectively. Filters that had been hybridized and extensively washed were exposed to x-ray film or to a phosphoImage screen for quantification. Quantification of labeled bands was performed using the ImageQuant program (Molecular Dynamic).
Cell transfection and luciferase activity assay
Construct F2 contains the −800 base pair (bp) to +54 bp region of rat γ fibrinogen gene promoter in front of the luciferase gene in the pGL2 vector. Construct F3 contains the −300 bp to +54 bp of rat γ fibrinogen gene promoter region. In construct M1, the IL-6RE CTGGGA region was mutated.20 Construct M3 was made from F3 by site-directed mutagenesis, changing the κB site from GGGAATCCC to GGGAAGTAC. The plasmid DNA of each construct (5 μg) was transfected into H35, H4IIE, HepG2, or Hep3B cells for reporter analysis. To monitor the transfection efficiency, 1 μg of pCMV-βgal vector was always co-transfected with the reporter construct. For overexpression of rat IL-6 receptor, 5 μg of pCMV-RIL-6R plasmids were co-transfected with the reporter constructs into HepG2 cells. Transfection was performed in 100-mm dishes, using calcium phosphate precipitation procedures for HepG2 and Hep3B cells. Transfection of H35 or H4IIE cells was performed, using Lipofectin reagent (Gibco). After transfection for 48 hours, cells were split into 6-well plates, cultured overnight, and stimulated with cytokines (IL-1β and IL-6) for 6 hours. Cell lysates were prepared and luciferase activity was measured using Luciferase Assay System (Promega) on a Luminometer (Bio-Rad), and β-galactosidase activity was measured using β-galactosidase Assay System kit (Promega).
Nuclear protein extraction
Western blot analysis
Nuclear protein (50 μg/lane) were separated on 10% denatured sodium dodecyl sulfate–polyacrylamide gels and transferred onto a polyvinylidene fluoride membrane. The filter was blotted with anti-STAT3 antibodies, and the positive signal was developed with enhanced chemiluminescence reagents (Pierce).
Electrophoretic mobility shift assay (EMSA)
EMSAs were performed as described previously.20,29Radiolabeled double-stranded probes were prepared by filling in the 5′ overhanging ends of reannealed complementary oligonucleotides with appropriate radioactivity. The DNA sequences of used probes are (top strand only): site II probe, the site II IL-6RE from rat γ fibrinogen gene promoter, 5′ TGCAAAATCTGGGAATCCCT 3′; SIE probe, mutant 67 of serum inducible element on c-fos gene promoter, 5′ CGACATTTCCCGTAAATCG 3′; NF-κB probe, κB site from immunoglobulin κ chain enhancer, 5′ GATCCATGGGGAATTCCCC 3′; C/EBP probe, C/EBP binding site from IL-6 gene promoter, 5′ GACGTCACTTGCACAATCTTAA 3′; and α2MG probe: the IL-6RE of rat α2-macroglobulin gene promoter region, 5′ TTCTGGGAATTCCC 3′. For competition assays, unlabeled probes were added at 100-fold molar excess to 32P-labeled probes in Figure 5A or as indicated in Figure 5C. In EMSA that used antibodies for identification or verification of DNA binding proteins, nuclear extracts were incubated with 2 μg antibodies at 4°C for 1 hour prior to performing EMSA procedures. For DNA binding interference assay that used IκBα, 1 ng of purified recombinant IκBα protein was added into 20 μg nuclear extracts prior to carrying out EMSA procedures. For in vitro NF-κB and STAT3 DNA binding competition assays, different amounts (from 0.1 ng to 5 ng) of purified NF-κB p50 protein (Promega) were added into 20 μg nuclear extracts (control or IL-6 treated) before EMSA binding reaction using a labeled site II probe.29 Samples were analyzed on 6% non-denaturing polyacrylamide gels in 0.5 × TBE, 5% glycerol at 4°C for 2 to 3 hours. Dried gels were exposed to x-ray film overnight at −80°C with intensifying screens.
Results
IL-1 inhibits IL-6-induced γ fibrinogen gene expression
It is well established that IL-6 stimulates transcription of all 3 fibrinogen genes.5 In experiments performed in this study, the effects of IL-1β and IL-6 were examined only on the γ fibrinogen gene. Primary rat hepatocytes were exposed to IL-1β and IL-6 individually and together (Figure1A). IL-1β has no inducing effect on the rat γ fibrinogen gene, whereas IL-6 stimulates its expression (Figure 1A, lanes 1-3). When IL-1β was added simultaneously with IL-6, the stimulating signal of IL-6 was diminished (Figure 1A, lane 4). Similar findings were shown, using HepG2 human hepatoma cells (Figure 1B). These findings are consistent with the previously published observations.24 In addition, IL-1β treatment down-regulated the basal level of γ fibrinogen gene expression, which was more pronounced in HepG2 cells (Figure 1B, lane 2).
IL-1β inhibits IL-6-mediated γ fibrinogen gene expression.
(A) Total RNA was prepared from rat primary hepatocytes following treatment with IL-1β and IL-6 as indicated. The arrows on the right side indicate the migration positions of rat γ fibrinogen mRNA and cyclophilin mRNA, respectively. (B) Total RNA was prepared from human hepatoma HepG2 cells following treatment with IL-1β and IL-6. The arrows on the right side indicate the migration positions of human γ fibrinogen mRNA and GAPDH mRNA, respectively. The graphic represents the quantification results from one experiment of 2 independent studies.
IL-1β inhibits IL-6-mediated γ fibrinogen gene expression.
(A) Total RNA was prepared from rat primary hepatocytes following treatment with IL-1β and IL-6 as indicated. The arrows on the right side indicate the migration positions of rat γ fibrinogen mRNA and cyclophilin mRNA, respectively. (B) Total RNA was prepared from human hepatoma HepG2 cells following treatment with IL-1β and IL-6. The arrows on the right side indicate the migration positions of human γ fibrinogen mRNA and GAPDH mRNA, respectively. The graphic represents the quantification results from one experiment of 2 independent studies.
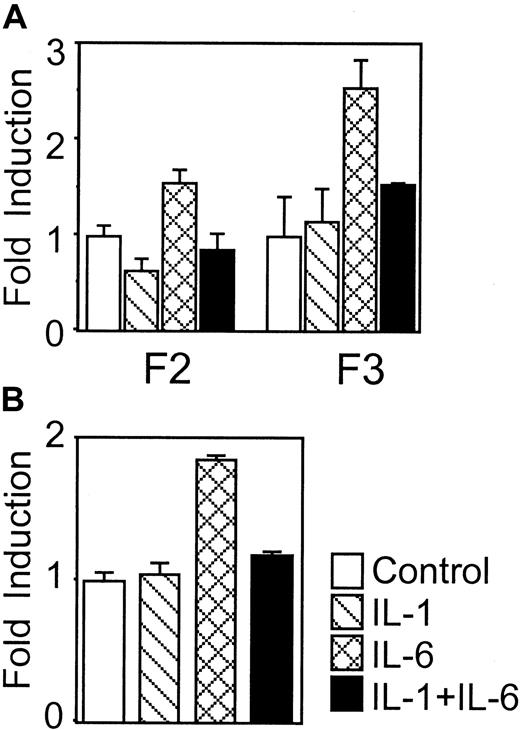
IL-1β inhibits IL-6-induced luciferase gene expression driven by the rat γ fibrinogen gene promoter
A luciferase reporter system was used to determine whether the rat γ fibrinogen gene promoter responded to the IL-1β inhibitory signal. We chose human HepG2 cells for the transfection studies because rat H35 and H4IIE cells are unable to respond to IL-1β stimulation (data not shown). Two constructs, F2 and F3, containing different lengths of rat γ fibrinogen promoter region were transfected into HepG2 cells and tested for responsiveness to IL-6 and IL-1β. In cells transfected with either the F2 or F3 construct, IL-6 stimulation induced a 2-fold increase of luciferase activity. The finding is similar to the reported IL-6 response of the Aα and γ human fibrinogen genes.30 31 The addition of IL-1β alone did not affect the luciferase activity. However, when IL-1β was co-administrated with IL-6, the inductive response of IL-6 was reduced. These findings demonstrated that IL-1β functions through the promoter region of rat γ fibrinogen gene to inhibit downstream gene transcription.
Overexpression of IL-6 receptor gp80 subunit does not affect the IL-1β inhibitory function
It has been postulated that IL-1β down-modulates IL-6 receptor subunit (gp80) expression.32 Thus inhibition of IL-6-mediated fibrinogen induction could be due, in part, to a reduction in the number of IL-6 receptors on the cell surface. To address this question, we developed a reconstitution system in which the rat IL-6 receptor (gp80) was overexpressed in HepG2 cells, and recombinant mouse IL-6 was used to stimulate these cells. Murine IL-6 binds only to the exogenously expressed rat IL-6 receptor and not to the endogenous human IL-6 receptor.32 When the reporter construct F3 was co-transfected with pCMV-RatIL-6R into HepG2 cells, exposure to IL-1β showed a similar inhibition of the murine IL-6-induced reporter gene expression (Figure2B). These results indicated that down-regulation of the IL-6 receptor expression is not a mechanism for the IL-1β inhibition of IL-6 signaling.
IL-1β inhibits IL-6-induced luciferase gene expression driven by rat γ fibrinogen promoter.
(A) Constructs F2 and F3 were transfected into human hepatoma HepG2 cells. Cytokine (IL-1β and IL-6) treatment is indicated. Results presented are means of fold induction above the control level from triplicate experiments. (B) Overexpression of IL-6 receptor does not affect the IL-1β inhibition. The reporter construct F3 was co-transfected with pCMV-RIL6R into human hepatoma HepG2 cells. After transfection, the cells were treated with recombinant mouse IL-6 or IL-1β as indicated. The results presented are means of fold induction over the control levels from triplicate experiments. The error bars in A and B indicate standard deviation.
IL-1β inhibits IL-6-induced luciferase gene expression driven by rat γ fibrinogen promoter.
(A) Constructs F2 and F3 were transfected into human hepatoma HepG2 cells. Cytokine (IL-1β and IL-6) treatment is indicated. Results presented are means of fold induction above the control level from triplicate experiments. (B) Overexpression of IL-6 receptor does not affect the IL-1β inhibition. The reporter construct F3 was co-transfected with pCMV-RIL6R into human hepatoma HepG2 cells. After transfection, the cells were treated with recombinant mouse IL-6 or IL-1β as indicated. The results presented are means of fold induction over the control levels from triplicate experiments. The error bars in A and B indicate standard deviation.
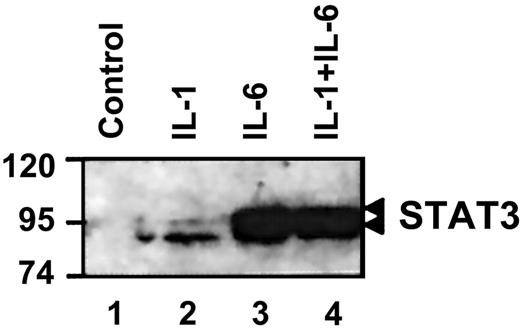
IL-1β does not affect IL-6-mediated STAT3 activation and nuclear accumulation
IL-6 controls rat γ fibrinogen gene expression through activation of transcription factor STAT3.20 Under normal conditions, STAT3 remains in a latent (nonphosphorylated) state in the cytoplasm. On activation, STAT3 dimerizes and translocates into the nucleus.17 19 To determine if IL-1β affects IL-6-induced STAT3 activation, we performed immunoblotting to analyze the nuclear distribution of STAT3 following co-stimulation with IL-6 and IL-1β (Figure 3). Under control conditions, little to no STAT3 could be detected in the nuclear extract (Figure 3, lane 1). IL-1β stimulation did not change the STAT3 nuclear distribution because it does not activate STAT3 (Figure 3, lane 2). With IL-6 stimulation, STAT3 was activated, translocated, and accumulated in the nucleus (Figure 3, lane 3). Co-stimulation with IL-1β did not affect the STAT3 nuclear accumulation mediated by IL-6 (Figure 3, lane 4). These findings demonstrated that IL-1β does not interfere with the IL-6-mediated STAT3 activation and nuclear accumulation.
IL-1β does not affect IL-6-mediated STAT3 activation.
Nuclear extracts were prepared from rat primary hepatocytes following exposure to the indicated cytokines. Western blot analysis was performed as described in “Materials and methods.” The migration position of STAT3 is indicated on the right side.
IL-1β does not affect IL-6-mediated STAT3 activation.
Nuclear extracts were prepared from rat primary hepatocytes following exposure to the indicated cytokines. Western blot analysis was performed as described in “Materials and methods.” The migration position of STAT3 is indicated on the right side.
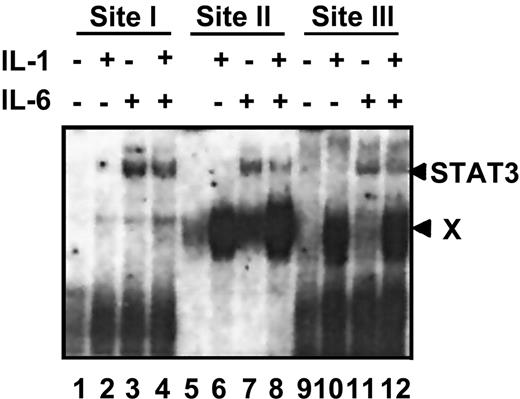
IL-1β-activated protein complex associates with the site II region of rat γ fibrinogen gene promoter
Within the rat γ fibrinogen gene promoter, there are 3 identified IL-6REs (containing the CTGGGAA motif). Binding of IL-6-activated STAT3 to these 3 elements is essential for the transactivation of rat γ fibrinogen gene.20 To further understand the molecular basis for the IL-1β inhibitory function, we performed EMSA, using the oligonucleotide probes that correspond to the 3 IL-6REs (designated site I, site II, and site III) and nuclear extracts prepared from rat primary hepatocytes following their treatment with different cytokines (Figure4). IL-6-activated STAT3 complexes associated with all 3 probes as described previously (Figure 4, lanes 3, 4, 7, 8, 11, and 12). IL-1β activated a distinct protein complex (complex X) that was strongly associated with the site II and site III probes (Figure 4, lanes 6, 8, 10, and 12) but was very weakly associated with the site I probe (Figure 4, lanes 3 and 4). Similar results were also obtained, using nuclear extracts prepared from HepG2 or Hep3B cells (data not shown).
IL-1β-activated protein complexes associate with the site II and site III probes.
EMSA was performed as described in “Materials and methods.” Nuclear extracts were prepared from rat primary hepatocytes following exposure to the indicated cytokines. Site I, site II, and site III probes are derived from the 3 IL-6REs of the rat γ fibrinogen gene. The arrows on the right side indicate the migration positions of 2 different protein complexes, the IL-6-induced STAT3 complex, and the IL-1-induced complex X.
IL-1β-activated protein complexes associate with the site II and site III probes.
EMSA was performed as described in “Materials and methods.” Nuclear extracts were prepared from rat primary hepatocytes following exposure to the indicated cytokines. Site I, site II, and site III probes are derived from the 3 IL-6REs of the rat γ fibrinogen gene. The arrows on the right side indicate the migration positions of 2 different protein complexes, the IL-6-induced STAT3 complex, and the IL-1-induced complex X.
IL-1β-induced protein complexes that associate with the site II probe contain NF-κB p65 and p50 factors
To characterize the IL-1β-induced protein complexes associated with the site II probe, we first performed competition EMSAs (Figure5A). The addition of unlabeled site II probe competes away both IL-6- and IL-1β-induced protein complexes (Figure 5A, lane 3), verifying the binding specificity of both complexes. The addition of unlabeled SIEm67 probe (a STAT3-specific probe17) only competes away the IL-6-induced protein complex that contains activated STAT3, but it has no effect on the formation of IL-1β-induced protein complex (Figure 5A, lane 4). The addition of unlabeled κB probe blocks IL-1β-induced protein complexes but does not affect IL-6-induced complex formation (Figure5A, lane 5). Unlabeled C/EBP probe used as a negative control showed no effect on any complex formation (Figure 5A, lane 6). These findings indicated that the IL-1β-induced protein complex formed on the site II probe contains factors that bind to NF-κB binding sequences.
IL-1β-activated protein complexes contain NF-κB p65 and p50 factors.
(A) Competition EMSA. Nuclear extracts were prepared from rat primary hepatocytes co-stimulated with IL-1β and IL-6. The different probes used in competition EMSAs are indicated as: Site II, γ fibrinogen site II probe; SIE, mutant 67 of the serum inducible element onc-fos gene promoter, which has a strong binding affinity for STAT3; NF-κB, NF-κB binding site on the immunoglobulin κ chain enhancer; C/EBP, CAAT-enhancer binding protein binding site on the IL-6 gene promoter. Competition EMSAs and the DNA sequences of these probes are described in “Materials and methods.” (B) Analyses of IL-1-induced protein complexes with antibodies and IκBα protein. Nuclear extracts were prepared from rat primary hepatocytes stimulated with IL-1β. The antibodies and reagents are indicated. The supershift assays were performed as described in “Materials and methods.” For IκBα treatment, nuclear extract was preincubated with 1 ng of recombinant IκBα prior to EMSA. (C) Cross-competition EMSA. Radio-labeled α2MG probe was used in the binding assay together with the indicated molar excess of unlabeled site I, site II, and site III probes for cross-competition. The arrows on the left indicate the migration position of STAT3 and NF-κB complexes.
IL-1β-activated protein complexes contain NF-κB p65 and p50 factors.
(A) Competition EMSA. Nuclear extracts were prepared from rat primary hepatocytes co-stimulated with IL-1β and IL-6. The different probes used in competition EMSAs are indicated as: Site II, γ fibrinogen site II probe; SIE, mutant 67 of the serum inducible element onc-fos gene promoter, which has a strong binding affinity for STAT3; NF-κB, NF-κB binding site on the immunoglobulin κ chain enhancer; C/EBP, CAAT-enhancer binding protein binding site on the IL-6 gene promoter. Competition EMSAs and the DNA sequences of these probes are described in “Materials and methods.” (B) Analyses of IL-1-induced protein complexes with antibodies and IκBα protein. Nuclear extracts were prepared from rat primary hepatocytes stimulated with IL-1β. The antibodies and reagents are indicated. The supershift assays were performed as described in “Materials and methods.” For IκBα treatment, nuclear extract was preincubated with 1 ng of recombinant IκBα prior to EMSA. (C) Cross-competition EMSA. Radio-labeled α2MG probe was used in the binding assay together with the indicated molar excess of unlabeled site I, site II, and site III probes for cross-competition. The arrows on the left indicate the migration position of STAT3 and NF-κB complexes.
The composition of the IL-1β-induced protein complexes was further determined by EMSA, using antibodies to 2 major NF-κB factors, NF-κB p50 and p65, and also by recombinant IκBα protein (Figure 5B). The addition of anti-p50 antibodies resulted in a super-shifted band (Figure 5B, lane 3), suggesting p50 is involved in the complex formation. The addition of anti-p65 antibodies reduced the intensity of the IL-1β-induced protein complex (Figure 5B, lane 4), indicating that IL-1β-induced protein complex also contains NF-κB p65. Furthermore, the addition of recombinant IκBα protein completely blocked IL-1β-induced protein complex formation (Figure5B, lane 6), further demonstrating that the IL-1β-induced protein complex contained NF-κB factors.
We had noted in earlier experiments that IL-1β had no effect on the IL-6 stimulatory signal in rat hepatoma H35 and H4IIE cells. When we treated H35 and H4IIE cells with IL-1β and then analyzed NF-κB DNA binding activity by EMSA, IL-1β was unable to activate NF-κB in these cells. TNFα-induced NF-κB DNA binding activity in these cells is normal (data not shown). These results demonstrated that the IL-1β signaling is altered in these cells. These observations correlate with the Northern blot and functional analyses, showing that IL-1β did not affect IL-6 function in these cells.
NF-κB binds to site II probe with a higher affinity than STAT3 does
The IL-1β-induced protein complexes have a higher affinity to the site II and site III probes than STAT3 does. This finding was confirmed by a cross-competition EMSA (Figure 5C). Previously, with the use of nuclear extracts prepared from rat hepatocytes co-stimulated with IL-1β and IL-6, we were able to show that both STAT3 and NF-κB formed complexes on α2MG probe (Figure 5C, lane 1), which has a perfect palindromic STAT3 binding motif overlapping with a κB site. By using increasing concentrations of unlabeled site I, site II, and site III probes, we tested the relative binding affinity of the 3 γ fibrinogen probes to both STAT3 and NF-κB by comparing their capacities to compete the formation of the STAT3 and NF-κB complexes on the α2MG probe. The addition of 100-fold molar excess of unlabeled site I probe only partially competed the STAT3 complex (Figure 5C, lane 4), whereas the addition of 100-fold molar excess of site II probe completely competed away the STAT3 complex (Figure 5C, lane 8). The addition of 100-fold molar excess of site III probes did not compete the STAT3 complex (Figure 5C, lane 12). These results allowed us to tentatively assign the binding affinity of STAT3 to these 3 probes in the following order: site II > site I > site III. In the case of NF-κB binding affinity, the addition of 100-fold molar excess of site I probes did not affect the NF-κB complexes (Figure 5C, lane 5). The addition of 10-fold molar excess of the site II probe competed away most of the NF-κB binding (Figure 5C, lane 7). The addition of unlabeled site III probes was somewhat less effective than that of unlabeled site II probes (Figure 5C, lanes 11-13). These findings demonstrated that NF-κB complexes associate with site II and site III probes with higher affinity than does the site I probe. Finally, a direct comparison of the binding affinity of STAT3 complex and NF-κB complex to site II probe can be seen in Figure 5C, lane 7. The addition of 10-fold molar excess of unlabeled site II probes competed away the majority of the NF-κB complex but only partially competed away the STAT3 complexes (Figure 5C, lane 7). This finding indicates that the binding affinity of NF-κB to the site II probe is higher than that of STAT3 to the site II probe.
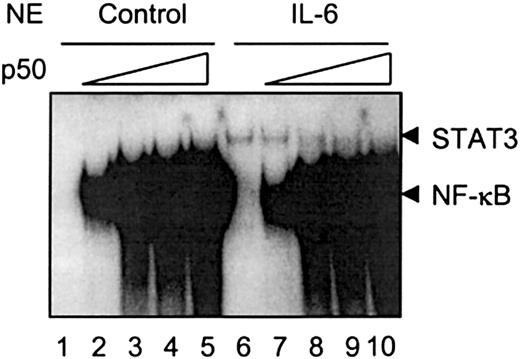
NF-κB p50 competes with STAT3 binding to the γ fibrinogen site II probe
Both NF-κB and STAT3 bind to sites II and III on the rat γ fibrinogen gene promoter region. IL-1β-activated NF-κB binding on this region does not promote transcription of rat γ fibrinogen gene, as shown in the Northern blot analysis (Figure 1) and in the transfection analysis in HepG2 cells (Figure 2). Instead, when IL-1β is added together with IL-6, IL-1β inhibits the IL-6 function. One possible explanation for the IL-1β inhibition is that NF-κB associates with the κB site and blocks STAT3 from binding to its DNA element. To test this hypothesis, we carried out in vitro binding assays, using purified recombinant NF-κB p50 protein (Figure6). As shown previously, IL-6-activated STAT3 weakly associates with the site II probe (Figure 6,lane 6). NF-κB p50 proteins form homodimers and strongly bind to the site II probe (Figure 6, lanes 1-5). The inclusion of p50 proteins into the IL-6-stimulated nuclear extracts resulted in the interference of STAT3 DNA binding (Figure 6, lanes 7 and 8), whereas higher concentrations of p50 protein completely blocked STAT3 association with the site II probe (Figure 6, lanes 9 and 10).
NF-κB competes with STAT3 binding to the γ fibrinogen site II probe.
The in vitro binding assays were performed as described in “Materials and methods.” Nuclear extracts prepared from rat primary hepatocytes stimulated with IL-6 were used as a crude source of activated STAT3 to show STAT3 DNA binding activity. Recombinant NF-κB p50 protein was premixed with nuclear extracts prepared from control cells or IL-6-stimulated cells at increasing amounts (0.1 ng to 5 ng) before normal EMSA procedures. The arrows on the right side indicate the migration positions of STAT3 and NF-κB complexes. The gel was exposed for 2 days.
NF-κB competes with STAT3 binding to the γ fibrinogen site II probe.
The in vitro binding assays were performed as described in “Materials and methods.” Nuclear extracts prepared from rat primary hepatocytes stimulated with IL-6 were used as a crude source of activated STAT3 to show STAT3 DNA binding activity. Recombinant NF-κB p50 protein was premixed with nuclear extracts prepared from control cells or IL-6-stimulated cells at increasing amounts (0.1 ng to 5 ng) before normal EMSA procedures. The arrows on the right side indicate the migration positions of STAT3 and NF-κB complexes. The gel was exposed for 2 days.
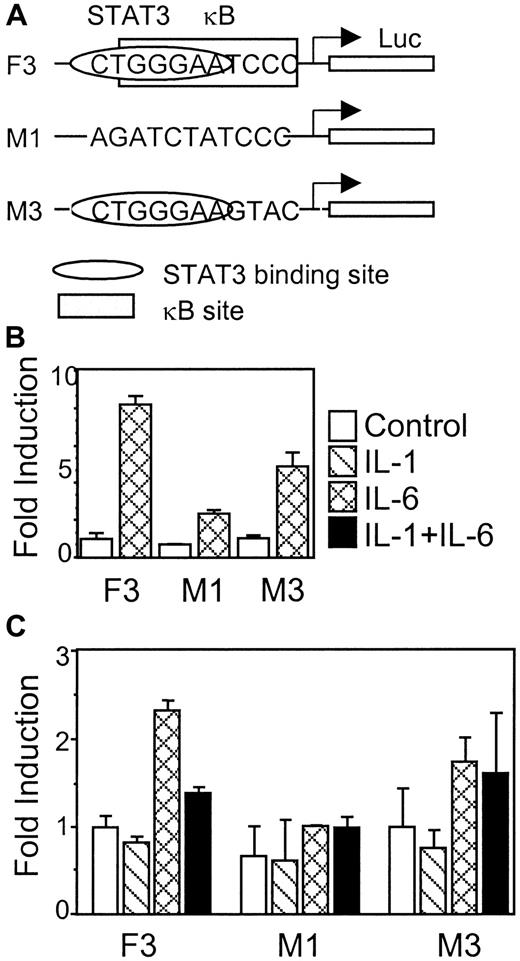
κB site that overlaps with the STAT3 binding site is required for IL-1β inhibition
To determine the function of the κB site on the γ fibrinogen promoter, functional assays were performed, using a construct (M3) in which half of the κB site was mutated (Figure7A). The different constructs were tested for their IL-6 response first in rat hepatoma H35 cells (Figure 7B). Construct F3 showed a normal IL-6 response in H35 cells (up to 8-fold induction). Mutating the site II CTGGGAA region in construct M1 almost completely abolished IL-6 response. Mutating the κB site that overlaps the STAT3 binding site in M3 caused a modulation of the IL-6 response; however, a reproducible 5-fold induction of luciferase activity on IL-6 stimulation was still evident. The 3 constructs were then transfected into HepG2 cells to analyze the IL-1β effects (Figure 7C). Construct F3 responds to IL-6, and IL-1β inhibits the IL-6 induction, as shown previously. Construct M1, lacking the CTGGGA region, shows no IL-6 response and no IL-1β inhibition. Construct M3, lacking the κB site, was still able to respond to IL-6. However, IL-1β failed to inhibit the IL-6 induction on this construct. These results suggest that the κB site overlapping the STAT3 binding site is important for IL-1β inhibition, although deletion of this region also affects the IL-6 response.
The κB site that overlaps with the STAT3 binding site is required for IL-1β inhibition.
(A) Diagrams of constructs F3, M1, and M3 show the relative position and modifications of the STAT3 and NF-κB binding sites. (B) Each construct was transfected into rat hepatoma H4IIE cells to test for its IL-6 response. (C) Each construct was transfected into human hepatoma HepG2 cells to test for the IL-1β-inhibitory function. The results presented are the means of fold induction above the control levels from triplicate experiments. The error bars indicate the standard deviations.
The κB site that overlaps with the STAT3 binding site is required for IL-1β inhibition.
(A) Diagrams of constructs F3, M1, and M3 show the relative position and modifications of the STAT3 and NF-κB binding sites. (B) Each construct was transfected into rat hepatoma H4IIE cells to test for its IL-6 response. (C) Each construct was transfected into human hepatoma HepG2 cells to test for the IL-1β-inhibitory function. The results presented are the means of fold induction above the control levels from triplicate experiments. The error bars indicate the standard deviations.
Discussion
We described a nucleotide sequence motif corresponding to a NF-κB binding element in the control region of the rat γ fibrinogen gene (Figure 8), which overlaps with a functional STAT3 binding motif.20 Because only IL-6 (and other members of this family) is capable of up-regulating fibrinogen expression, and IL-1β inhibits this signal, we reasoned that NF-κB binding might alter the STAT3 function and thus transiently block the positive IL-6 signal.
Sequence analyses of the IL-6–responsive regions of fibrinogen genes.
(A) Competing for DNA base contact on rat γ fibrinogen gene site II region by STAT3 and NF-κB. The contacting bases for STAT3 (open triangle) and NF-κB (closed triangle) within the site II probe are shown, based on the 3-dimensional structure of STAT3β homodimer34 or the NF-κB p50 homodimer36binding to its own consensus binding site. The directly competed bases were highlighted. (B) Conservation of the IL-6 responsive regions between human and rat fibrinogen genes. The identified IL-6 responsive regions of human Aα (−130 bp to −117 bp) and rat Aα (−131 bp to −118 bp) fibrinogen gene, human Bβ (−145 bp to −137 bp) and rat Bβ (−153 bp to −140 bp) fibrinogen gene, the putative human γ IL-6 responsive region (−160 bp to −147 bp), and the identified rat γ fibrinogen gene IL-6 responsive element site II (−154 bp to −141 bp) were aligned, using the Gendoc program. Identical bases were shaded as black.
Sequence analyses of the IL-6–responsive regions of fibrinogen genes.
(A) Competing for DNA base contact on rat γ fibrinogen gene site II region by STAT3 and NF-κB. The contacting bases for STAT3 (open triangle) and NF-κB (closed triangle) within the site II probe are shown, based on the 3-dimensional structure of STAT3β homodimer34 or the NF-κB p50 homodimer36binding to its own consensus binding site. The directly competed bases were highlighted. (B) Conservation of the IL-6 responsive regions between human and rat fibrinogen genes. The identified IL-6 responsive regions of human Aα (−130 bp to −117 bp) and rat Aα (−131 bp to −118 bp) fibrinogen gene, human Bβ (−145 bp to −137 bp) and rat Bβ (−153 bp to −140 bp) fibrinogen gene, the putative human γ IL-6 responsive region (−160 bp to −147 bp), and the identified rat γ fibrinogen gene IL-6 responsive element site II (−154 bp to −141 bp) were aligned, using the Gendoc program. Identical bases were shaded as black.
STAT factors recognize the palindromic DNA motifs (TTC–GAA).33,34 NF-κB factors contact DNA through the major groove of GGG and CCC regions35-38 (Figure 8A). Both factors can bind to the same 22-bp DNA probe (site II) individually as shown in Figure 6. However, when both factors are present at the same time, they compete for the GGA region as highlighted in Figure 8A. Competition for binding to this site was demonstrated in the in vitro binding assays, using purified recombinant NF-κB p50 proteins and activated STAT3 (from nuclear extracts). High concentrations of NF-κB p50 protein blocked STAT3 binding (Figure 6). The binding competition exhibited by these 2 transcription factors in the in vitro assay suggests that a similar competition occurs in vivo in which there is a limited number of the DNA binding sites but a relatively large excess of the activated transcription factors. Thus, the overlapping of these 2 binding sites likely leads to the partial inhibition of the IL-6-mediated response of fibrinogen gene expression. Given that the final outcome of an acute-phase response results in increasing fibrinogen expression, we recognize that the inhibition produced by IL-1β on the IL-6-mediated rat γ fibrinogen expression should be transient. However, how STAT3 displaces the NF-κB binding on the occupied site and leads to up-regulation of fibrinogen gene expression remains to be investigated.
In most cases, NF-κB factors act as transactivators for target genes. In this study, NF-κB binding to the rat γ fibrinogen promoter transactivates neither the endogenous rat γ fibrinogen gene nor the luciferase reporter gene driven by the rat γ fibrinogen gene promoter. It has been recognized that binding of NF-κB to the regulatory region of β interferon gene does not ensure transactivation of this gene. Additional factors, such as high-mobility group factors or the relative position of NF-κB to other regulatory factors are required for the function of NF-κB.39 Recent data40 showed that NF-κB binding to the 3′-flanking region of the human ξ-globin gene mediated developmental silencing of this gene. Here, our data show that NF-κB acts as a transient inhibitor for STAT3 binding on the rat γ fibrinogen gene promoter. The observation that mutating the κB site directly following the STAT3 binding site causes reduction of the IL-6 response in both H4IIE and HepG2 cells was unexpected (Figure 7). One possible explanation is that altering the surrounding nucleotide sequence of the STAT3 binding site may affect STAT3/DNA interaction. This idea is supported by the crystal structure, showing the direct contact between STAT1 homodimer and the bases surrounding the core binding region of STAT1 is essential.41
Sequence alignment of several potential STAT3 and NF-κB overlapping sites indicates that this configuration is not unique to the rat γ fibrinogen promoter (Table 1). The 3′ flanking regions of 2 CTGG(G)AA elements in rat α2-macroglobulin gene promoter also possess putative binding sites for NF-κB.42 As predicted, both NF-κB and STAT3 were able to bind to these regions, and NF-κB competed with STAT3 for binding.29 In addition, an overlapping binding site for STAT3 and NF-κB has been found within the downstream regulatory region of JunB gene.43 44 The identified, or potential, IL-6REs (CTGGGA motifs) for fibrinogen genes and their surrounding regions are highly conserved between rats and humans (Figure 8B). Potential overlapping STAT3/κB sites are also found in rat and human Aα, rat and human γ fibrinogen promoter regions. There are no κB sites near the Bβ fibrinogen gene IL-6RE for rats and humans. The finding of competitive binding between NF-κB and STAT3 reveals a novel interaction between the IL-1β and IL-6 signaling pathways and thus explains how IL-1β is able to inhibit IL-6-mediated rat γ fibrinogen expression.
Potential overlapping DNA binding sites for STAT3 and NF-κB
| Site . | DNA binding site . |
|---|---|
| Rat γ fibrinogen IL-6RE site II | CAAAATCTGGGAATCCCTCGG |
| Rat γ fibrinogen IL-6RE site III | CCAGACTGGGAATTCATATA |
| Rat α2-macroglobulin IL-6RE-1 | GCAGTAACTGGAAAGTCCTTA |
| Rat α2-macroglobulin IL-6RE-2 | ATCCTTCTGGGAATTCTGGCT |
| Rat cystatin S promoter region | AGAATTTCTGGAACACCAAGG |
| JunB gene downstream region | CAGATTCCGGGAATCCCCTCC |
| Site . | DNA binding site . |
|---|---|
| Rat γ fibrinogen IL-6RE site II | CAAAATCTGGGAATCCCTCGG |
| Rat γ fibrinogen IL-6RE site III | CCAGACTGGGAATTCATATA |
| Rat α2-macroglobulin IL-6RE-1 | GCAGTAACTGGAAAGTCCTTA |
| Rat α2-macroglobulin IL-6RE-2 | ATCCTTCTGGGAATTCTGGCT |
| Rat cystatin S promoter region | AGAATTTCTGGAACACCAAGG |
| JunB gene downstream region | CAGATTCCGGGAATCCCCTCC |
STAT3 binding regions are shown in the square; κB sites are underlined.
Acknowledgments
We thank our colleagues Drs Mitchell Olman and James Hagood for critical review of the manuscript.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Gerald M. Fuller, MCLM 680, Department of Cell Biology, University of Alabama at Birmingham, Birmingham, AL 35294; e-mail: gmfuller@bmg125.cmc.uab.edu.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal