Abstract
The interaction between the endocytic receptor low density lipoprotein receptor–related protein (LRP) and either coagulation factor IX or its active derivative factor IXa was studied. Purified factor IX was unable to associate with LRP when analyzed by surface plasmon resonance. By contrast, factor XIa–mediated conversion of factor IX into factor IXa resulted in reversible dose- and calcium-dependent binding to LRP. Active-site blocking of factor IXa did not affect binding to LRP, whereas LRP binding was efficiently inhibited in the presence of heparin or antibodies against factor IX or LRP. The factor IXa–LRP interaction could be described by a 2-site binding model with equilibrium dissociation constants of 27 nmol/L and 69 nmol/L. Consistent with this model, it was observed that factor IXa binds to 2 different recombinant receptor fragments of LRP (denoted cluster II and cluster IV) with equilibrium dissociation constants of 227 nmol/L and 53 nmol/L, respectively. The amount of factor IXa degraded by LRP-deficient cells was 35% lower than by LRP-expressing cells, demonstrating that LRP contributes to the transport of factor IXa to the intracellular degradation pathway. Because ligand binding to LRP is often preceded by binding to proteoglycans, the contribution of proteoglycans to the catabolism of factor IXa was addressed by employing proteoglycan-deficient cells. Degradation of factor IXa by proteoglycan-deficient cells proceeded at a 83% lower rate than wild-type cells. In conclusion, the data presented here indicate that both LRP and proteoglycans have the potential to contribute to the catabolism of factor IXa.
Introduction
Factor IX is a vitamin K–dependent serine protease precursor protein that, upon activation, participates in the blood coagulation process.1 The physiologic importance of factor IX is apparent from the notion that a deficiency or dysfunction is associated with the severe bleeding disorder hemophilia B.2 In plasma, factor IX circulates as an inactive, single-chain polypeptide (Mr56 000).3 Activation of factor IX is achieved by limited proteolysis, mediated by factor XIa or factor VIIa, resulting in the active enzyme factor IXa.4,5 Factor IXa (Mr 46 000) consists of a heavy and a light chain that are covalently linked. Factor IXa catalyzes the activation of factor X in a complex that includes Ca++ ions, the activated protein cofactor factor VIIIa, and a membrane surface,6 all of which are essential for optimal factor X activation. When assembled into the membrane-bound complex, factor IXa may also associate with other molecules present at the cellular surface. In this respect, in vitro binding studies employing arterial endothelial cells demonstrated the presence of a factor IXa binding site.7,8 Recently, the matrix protein collagen type IV has been proposed to serve as an endothelial cell receptor for factor IXa.9 Factor IXa shares this binding site with its precursor, the factor IX zymogen.7-9 In vivo clearance studies have confirmed the presence of a combined binding site for both factor IX and IXa.10 Interestingly, the same studies revealed the presence of an alternative site that recognizes factor IXa but not its precursor. Removal of factor IXa from the circulation, but not of factor IX, is markedly delayed by preinfusion of thrombin/antithrombin or trypsin/α1-protease inhibitor complexes.10 More recent studies revealed that both enzyme/inhibitor complexes are ligands for the low density lipoprotein receptor–related protein (LRP).11
LRP (Mr 600 000), also known as the α2-macroglobulin (α2M) receptor, is a membrane glycoprotein that is a member of the low density lipoprotein (LDL) receptor family of endocytic receptors.12,13 LRP is abundantly present in various tissues, such as liver, placenta, lung, and brain,14 and is expressed in an array of cell types, including parenchymal cells, Kupffer cells, neurons, astrocytes, smooth muscle cells, monocytes, adipocytes, and fibroblasts.14,15Also, commonly used cell lines like monkey kidney COS cells and Chinese hamster ovary (CHO) cells express LRP.16,17 LRP is involved in the transport of ligands from the cell surface to the endosomal degradation pathway in a Ca++-dependent manner. At present, a remarkable spectrum of structurally unrelated ligands has been identified. These include apolipoproteins, lipases, proteinases, proteinase-inhibitor complexes, Kunitz-type inhibitors, matrix proteins, and several others.12,13 In addition, we recently identified the coagulation cofactor factor VIII as a ligand of LRP.18 The broad range of ligands suggests a role for the receptor in diverse physiologic and pathophysiologic processes ranging from lipoprotein metabolism, fibrinolysis, hemostasis, cell growth, and migration to thrombosis, atherosclerosis, and Alzheimer's disease.13 The receptor contains 4 putative ligand-binding domains consisting of clusters of LDL receptor class A repeats, generally referred to as clusters I, II, III, and IV. Recently, we showed that clusters II and IV play a prominent role in ligand binding to the receptor.19
Various mechanisms have been proposed that describe the interaction between LRP and its ligands. First, ligands may directly bind from the circulation to cell surface–exposed LRP, as exemplified by α2-M.20,21 Second, ligand binding to LRP may be promoted by an accessory protein. An example of accessory proteins is the urokinase-type plasminogen activator receptor, which mediates degradation of urokinase complexed with plasminogen activator inhibitor-1 by LRP.22-24 In addition, it has been proposed that binding to proteoglycans preceeds LRP-mediated degradation of, for example, β-amyloid precursor protein, lipoprotein lipase, tissue factor pathway inhibitor, and thrombospondin.25-28
In the present study, we assessed the binding of factor IX and factor IXa to LRP. This was examined by surface plasmon resonance (SPR) employing purified components. Our data reveal that activation of factor IX into factor IXa results in exposure of a binding site for LRP that is located outside the exposed active site. Furthermore, we observed binding of factor IXa to the separate cluster II and cluster IV fragments of LRP, providing support for a 2-site binding model. In addition, degradation of factor IXa was studied using LRP-expressing, LRP-deficient, and proteoglycan-deficient cells. These experiments indicate that catabolism of factor IXa involves both LRP and cell-surface proteoglycans.
Materials and methods
Materials
The BIACORE2000 biosensor system and reagents, including an amine-coupling kit and CM5 sensor chips (research grade), were from Biacore AB (Uppsala, Sweden). Cell culture plates, fetal calf serum, penicillin, and streptomycin were from Gibco BRL (Breda, The Netherlands). Dulbecco modified Eagle medium/F12 (DMEM/F12) was from BioWittaker (Verviers, Belgium). Unfractionated heparin (grade 1-a), chondroitin sulfate, and benzamidine were purchased from Sigma (Zwijndrecht, The Netherlands). Na125I was from Amersham Pharmacia Biotech ('s Hertogenbosch, The Netherlands). Glu-Gly-Arg (EGR)-chloromethyl ketone was from CalBiochem (Bierges, France). Low molecular weight (LMW) heparin (Fragmin) was from Pharmacia and Upjohn (Woerden, The Netherlands).
Proteins
Placenta-derived, purified, full-length human LRP consisting of both the α and the β chain, was kindly provided by Dr S. K. Moestrup (Institute of Medical Biochemistry, University of Aarhus, Aarhus, Denmark). Factor IX, factor IXa, EGR–factor IXa, and recombinant LRP fragments were prepared as described.19,29Purified factor XIa was obtained from Enzyme Research Laboratories (South Bend, IN). Urokinase was a gift from Dr J. Henkin (Abbott Laboratories, Abbott Park, IL). Purified antibody CLB-FIX 14 has been described previously.30 Purified anti–factor IX polyclonal antibodies were prepared as described.29Purified anti-LRP polyclonal antibody was kindly provided by Dr D.A. Owensby (Center for Thrombosis and Vascular Research, University of New South Wales, Sydney, Australia). All proteins were homogenous as determined by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). Factor IXa was iodinated by using IODO-GEN (Pierce). Nonincorporated radiolabeled material was removed by dialysis against 20-mmol/L HEPES (pH 7.4), 150-mmol/L NaCl, and 5-mmol/L CaCl2 at 4°C. After radio-iodination, factor IXa had a specific radioactivity of approximately 103 cpm/fmol. Nonradiolabeled factor IXa and 125I-labeled factor IXa displayed similar activity. In addition, both were similar in binding to purified LRP as assessed by a solid-phase assay (data not shown). Human albumin was from CLB Products (Amsterdam, The Netherlands). Bovine serum albumin (BSA) was from Merck (Darmstadt, Germany).
Protein concentrations
SPR analysis
Binding studies were performed employing a BIACORE2000 biosensor system, and SPR analysis was done essentially as described.32 LRP or factor IXa was immobilized on a CM5 sensorchip at the indicated densities using the amine-coupling kit as instructed by the supplier. Routinely, a control channel was activated and blocked using the amine-coupling reagents in the absence of protein. Binding to coated channels was corrected for binding to noncoated channels (< 5% of binding to coated channels). For qualitative measurements, SPR analysis was performed in 20-mmol/L HEPES (pH 7.4), 150-mmol/L NaCl, 2-mmol/L CaCl2, and 0.005% (vol/vol) Tween 20 at 25°C with a flow rate of 5 μL/min. For quantitative measurements of factor IXa binding to immobilized LRP, experiments were performed in duplicate at 5 different concentrations (n = 5) of factor IXa or EGR–factor IXa. The concentrations were chosen at an appropriate range (around Kdvalues), and the proteins were passed at 25°C with a flow rate of 20 μL/min over 3 separate channels with immobilized LRP and over 1 control (noncoated) channel. Quantitative measurements of cluster II and IV binding to immobilized factor IXa were performed in a similar manner as for intact LRP. Regeneration of the surface of the LRP sensorchip and of the factor IXa sensorchip was performed with 100-mmol/L H3PO4 and 10 mmol/L ethylenediaminetetraacetic acid (EDTA), respectively.
Analysis of quantitative SPR data
For analysis of the association and dissociation curves of the sensorgrams, BIA evaluation software was used (Biacore AB, Uppsala, Sweden). Interaction constants were determined by performing nonlinear global fitting of data corrected for bulk refractive index changes. Data were fitted according to various models available within the BIA evaluation software. A model describing a 1:1 interaction was found to provide the best fit for data regarding the binding of LRP cluster II or IV to immobilized factor IXa. For the binding of factor IXa to immobilized LRP, a model describing the interaction between factor IXa and 2 independent binding sites (heterologous ligand, parallel reactions) was found to provide the best fit of the experimental data. Goodness of the fits was judged from residual plots and statistical parameters employing previously described equations.32 The data were further validated by subjecting them to tests of se lf-consistency.33
Factor XIa–mediated factor IX activation
Factor IX (3.2 μmol/L) and Factor XIa (16 nmol/L) were incubated at 37°C in 20-mmol/L HEPES (pH 7.4), 150-mmol/L NaCl, 2-mmol/L CaCl2, and 0.005% (vol/vol) Tween 20. At indicated timepoints, aliquots (5 μL) were taken and diluted 10-fold in the same buffer containing 10-mmol/L benzamidine to prevent further activation. Samples were analyzed by SPR using immobilized LRP (13 fmol/mm2 at a flow of 5 μL/min). Simultaneously with SPR samples, aliquots (25 μL) were taken and added to 8 μL of 0.25-mol/L Tris (pH 6.8), 8% (wt/vol) SDS, 40% (vol/vol) glycerol, and 0.04% (wt/vol) bromophenol blue and subjected to electrophoresis on a 7.5% (wt/vol) SDS-polyacrylamide gel. Proteins were visualized by staining with Coomassie blue brilliant.
Solid-phase binding assays
LRP (75 ng/well) was immobilized onto to microtiter wells in a volume of 50 μL. Remaining binding sites were blocked with 3% (wt/vol) BSA in 20-mmol/L HEPES (pH 7.4), 150-mmol/L NaCl, 5-mmol/L CaCl2, and 0.05% (vol/vol) Tween 20 (HBST) in a volume of 300 μL. Factor IXa (70 nmol/L) was then added for l hour at 37°C in 1% (wt/vol) BSA in HBST in the absence or presence of various components as indicated. After washing the wells 3 times with HBST, wells were incubated for 1 hour at 37°C in 1% (wt/vol) BSA in HBST with the monoclonal anti–factor IX antibody CLB-FIX 14. Bound CLB-FIX 14 was quantified using biotinylated rat antibodies against mouse immunoglobin Gκ chains (CLB Products, Amsterdam, The Netherlands). Bound complexes were then detected using horseradish peroxidase–labeled streptavidine (Amersham Pharmacia Biotech).
Cellular degradation assays
Three derivatives of CHO cells were used in the present study. CHO-K1 (ATCC CCL-61; American Type Culture Collection, Manassas, VA) is a wild-type cell line that constitutively expresses LRP, as do CHO-745 cells (ATCC CRL-2242). The latter cell line, however, is deficient in xylosyltransferase.34 The cell line CHO 13-5-1 (kindly provided by Dr D. J. FitzGerald) is deficient in LRP and has been prepared by toxin-mediated selection of mutagenized CHO-K1 cells.17 Cells were seeded into 24-well plates in DMEM/F12 medium supplemented with 10% (vol/vol) fetal calf serum, 100-U/mL penicillin, and 100-μg/mL streptomycin. The cells were grown to 90% to 100% confluence, which was accomplished in 2 days. Prior to incubation, cultured cells were extensively washed with DMEM/F12 medium. Degradation assays were initiated by adding 200 μL of 125I-labeled factor IXa (40 nmol/L) in DMEM/F12 containing 1% (wt/vol) BSA and 5-mmol/L CaCl2 (assay medium). Cells were washed 3 times with 1 mL of DMEM/F12 medium after 30 minutes of incubation at 37°C to remove nonbound ligand. Subsequently, the incubation was allowed to proceed for 5 hours at 37°C in 200 μL of assay medium. Then, 100-μL aliquots of the conditioned medium were taken to determine the amount of 10% (wt/vol) trichloroacetic acid–soluble factor IXa degradation products. Radioactivity was measured in a Packard γ counter (Hewlett-Packard, Amstelveen, The Netherlands). Total ligand degradation was corrected for the amount of degradation that occurred in control wells lacking cells.
Results
Binding of factor IXa and factor IX to immobilized LRP
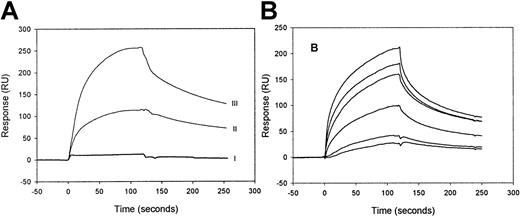
Binding of factor IXa and its precursor, factor IX zymogen, to LRP was investigated by SPR analysis using purified components. When 100-nmol/L factor IX zymogen was passed over LRP, virtually no increase of the resonance signal was observed (Figure1A). Even in the presence of very high concentrations of factor IX (up to 2 μmol/L), no binding could be detected (not shown), demonstrating that factor IX is unable to bind to LRP. In contrast, efficient binding was observed using 100 nmol/L of factor IXa. Upon replacement of factor IXa solution by buffer, the resonance signal gradually declines, indicating that factor IXa dissociates from immobilized LRP and that binding is reversible (Figure1A). Because the highest resonance signal is detected with the highest density of immobilized LRP (Figure 1A) and the level of the signal is a function of the concentration of the injected factor IXa (Figure 1B), the binding appears to be dose-dependent. These data indicate that factor IXa, but not its inactive precursor, binds to LRP in a reversible and dose-dependent manner.
SPR analysis of factor IXa and factor IX binding to immobilized LRP.
(A) LRP, immobilized onto a CM5 sensorchip at a density of 9 fmol/mm2 (II) and of 26 fmol/mm2 (I, III), was incubated with 100-nmol/L factor IX (I) or 100-nmol/L factor IXa (II, III) in 20-mmol/L HEPES (pH 7.4), 150-mmol/L NaCl, and 0.005% (vol/vol) Tween 20 at 25°C at a flow rate of 5-μL/min for 2 minutes. To initiate dissociation, the buffer was replaced by buffer devoid of ligand. (B) Six different concentrations (10, 20, 49, 60, 98, and 142 nmol/L) of factor IXa were passed at 25 °C with a flow rate of 20 μL/min over immobilized LRP (16 fmol/mm2). The subsequent association and dissociation are represented by the 6 data curves shown. The signal is indicated in resonance units (RU) and is corrected for aspecific binding, which was less than 5% of the binding to LRP-coated channels.
SPR analysis of factor IXa and factor IX binding to immobilized LRP.
(A) LRP, immobilized onto a CM5 sensorchip at a density of 9 fmol/mm2 (II) and of 26 fmol/mm2 (I, III), was incubated with 100-nmol/L factor IX (I) or 100-nmol/L factor IXa (II, III) in 20-mmol/L HEPES (pH 7.4), 150-mmol/L NaCl, and 0.005% (vol/vol) Tween 20 at 25°C at a flow rate of 5-μL/min for 2 minutes. To initiate dissociation, the buffer was replaced by buffer devoid of ligand. (B) Six different concentrations (10, 20, 49, 60, 98, and 142 nmol/L) of factor IXa were passed at 25 °C with a flow rate of 20 μL/min over immobilized LRP (16 fmol/mm2). The subsequent association and dissociation are represented by the 6 data curves shown. The signal is indicated in resonance units (RU) and is corrected for aspecific binding, which was less than 5% of the binding to LRP-coated channels.
Effect of factor XIa–mediated factor IX activation on LRP binding
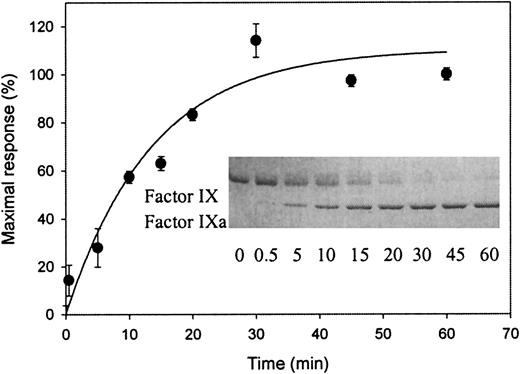
To investigate whether an LRP binding site is exposed during factor IX activation, factor IX was incubated in the presence or absence of its activator factor XIa. As expected, incubation of factor IX in the presence of factor XIa resulted in a time-dependent appearance of factor IXa when determined by SDS-PAGE (inset of Figure2). Samples simultaneously taken were also tested by SPR analysis for the ability to associate with immobilized LRP. As shown in Figure 2, no association could be observed when factor IX was incubated in the absence of factor XIa (referred to as t = 0). In contrast, in the presence of factor XIa, a time-dependent increase in association with LRP appeared. Control experiments did not show binding to LRP of the catalyst factor XIa (data not shown). Apparently, an LRP binding site within factor IX is exposed upon conversion to factor IXa.
Exposure of a binding site for LRP upon factor XIa–mediated activation of factor IX.
Factor XIa (final concentration of 16 nmol/L) was added to 3.2 μmol/L-factor IX in 20-mmol/L HEPES (pH 7.4), 150-mmol/L NaCl, and 0.005% (vol/vol) Tween 20 at 37°C. Aliquots (5 μL) were drawn at indicated intervals and diluted 10-fold in the same buffer containing 10-mmol/L benzamidine. These diluted samples were subjected to SPR analysis using immobilized LRP (13 fmol/mm2) equilibrated in 20-mmol/L HEPES (pH 7.4), 150-mmol/L NaCl, 2-mmol/L CaCl2, 0.005% (vol/vol) Tween 20, and 10 mmol/L benzamidine at a flow rate of 5 μL/min. Maximal response (RU), corrected for bulk refractive index changes and nonspecific binding, is shown for the samples obtained at different time intervals. Data represent the mean ± SD of 3 experiments. (Inset) Aliquots (25 μL) were drawn simultaneously with those used for SPR analysis and added to 8 μL of 0.25-mol/L Tris (pH 6.8), 8% (wt/vol) SDS, 40% (vol/vol) glycerol, and 0.04% (wt/vol) bromophenol blue. Nonreduced samples (12 μL, corresponding to 1.6 μg of factor IX) were subjected to electrophoresis on a 7.5% (wt/vol) SDS-polyacrylamide gel. Proteins were visualized by staining with Coomassie blue brilliant. The positions of factor IX and factor IXa are indicated. The sample at t = 0 for both SPR and gel electrophoresis analysis refers to 90 minutes of incubation at 37°C without the addition of factor XIa.
Exposure of a binding site for LRP upon factor XIa–mediated activation of factor IX.
Factor XIa (final concentration of 16 nmol/L) was added to 3.2 μmol/L-factor IX in 20-mmol/L HEPES (pH 7.4), 150-mmol/L NaCl, and 0.005% (vol/vol) Tween 20 at 37°C. Aliquots (5 μL) were drawn at indicated intervals and diluted 10-fold in the same buffer containing 10-mmol/L benzamidine. These diluted samples were subjected to SPR analysis using immobilized LRP (13 fmol/mm2) equilibrated in 20-mmol/L HEPES (pH 7.4), 150-mmol/L NaCl, 2-mmol/L CaCl2, 0.005% (vol/vol) Tween 20, and 10 mmol/L benzamidine at a flow rate of 5 μL/min. Maximal response (RU), corrected for bulk refractive index changes and nonspecific binding, is shown for the samples obtained at different time intervals. Data represent the mean ± SD of 3 experiments. (Inset) Aliquots (25 μL) were drawn simultaneously with those used for SPR analysis and added to 8 μL of 0.25-mol/L Tris (pH 6.8), 8% (wt/vol) SDS, 40% (vol/vol) glycerol, and 0.04% (wt/vol) bromophenol blue. Nonreduced samples (12 μL, corresponding to 1.6 μg of factor IX) were subjected to electrophoresis on a 7.5% (wt/vol) SDS-polyacrylamide gel. Proteins were visualized by staining with Coomassie blue brilliant. The positions of factor IX and factor IXa are indicated. The sample at t = 0 for both SPR and gel electrophoresis analysis refers to 90 minutes of incubation at 37°C without the addition of factor XIa.
Role of the factor IXa catalytic center in LRP interaction
Conversion of the zymogen factor IX into its active derivative factor IXa is associated with the exposure of its active-site residues. To examine to what extent the factor IXa active-site residues contribute to LRP binding, the catalytic triad was irreversibly blocked with the inhibitor EGR–chloromethyl ketone, yielding EGR–factor IXa. Subsequently, the binding of EGR–factor IXa to LRP was compared with that of active factor IXa by measuring the association and the dissociation rate constants kon andkoff, respectively (Table1). Binding of either factor IXa or EGR–factor IXa to LRP could be adequately described by employing a 2-site binding model, and similar kon andkoff values were obtained for factor IXa and EGR–factor IXa. The resulting Kd values are 27 nmol/L and 26 nmol/L and are 69 nmol/L and 71 nmol/L for factor IXa and EGR–factor IXa, respectively. Thus, both factor IXa and EGR–factor IXa display similar affinity for LRP, suggesting that the binding site for LRP is located outside the catalytic center of factor IXa. These data further indicate the presence of 2 independent binding sites for factor IXa within the LRP molecule. Alternatively, the possibility exists that LRP is heterogenous as a result of the immobilization procedure.
Kinetics of factor IXa and EGR-factor IXa binding to LRP and recombinant cluster II and IV fragments
| Injected protein . | Immobilized protein . | koff(s−1) . | kon (s−1M−1) . | Kd (nmol/L) . |
|---|---|---|---|---|
| Factor IXa | LRP | (1) 4.6 (± 0.6) × 10−3 | (1) 1.7 (± 0.2) × 105 | (1) 27.1 ± 4.8 |
| (2) 8.3 (± 1.4) × 10−2 | (2) 1.2 (± 0.2) × 106 | (2) 69.2 ± 16.4 | ||
| EGR-factor IXa | LRP | (1) 3.7 (± 0.3) × 10−3 | (1) 1.4 (± 0.2) × 105 | (1) 26.4 ± 4.3 |
| (2) 9.9 (± 1.0) × 10−2 | (2) 1.4 (± 0.2) × 106 | (2) 70.7 ± 12.4 | ||
| Cluster II | Factor IXa | 5.7 (± 1.2) × 10−3 | 2.5 (± 0.3) × 104 | 227.4 ± 55.1 |
| Cluster IV | Factor IXa | 9.6 (± 0.5) × 10−3 | 1.8 (± 0.2) × 105 | 53.3 ± 6.5 |
| Injected protein . | Immobilized protein . | koff(s−1) . | kon (s−1M−1) . | Kd (nmol/L) . |
|---|---|---|---|---|
| Factor IXa | LRP | (1) 4.6 (± 0.6) × 10−3 | (1) 1.7 (± 0.2) × 105 | (1) 27.1 ± 4.8 |
| (2) 8.3 (± 1.4) × 10−2 | (2) 1.2 (± 0.2) × 106 | (2) 69.2 ± 16.4 | ||
| EGR-factor IXa | LRP | (1) 3.7 (± 0.3) × 10−3 | (1) 1.4 (± 0.2) × 105 | (1) 26.4 ± 4.3 |
| (2) 9.9 (± 1.0) × 10−2 | (2) 1.4 (± 0.2) × 106 | (2) 70.7 ± 12.4 | ||
| Cluster II | Factor IXa | 5.7 (± 1.2) × 10−3 | 2.5 (± 0.3) × 104 | 227.4 ± 55.1 |
| Cluster IV | Factor IXa | 9.6 (± 0.5) × 10−3 | 1.8 (± 0.2) × 105 | 53.3 ± 6.5 |
Association and dissociation between either factor IXa or EGR-factor IXa and LRP was determined by passing 6 different concentrations (5-150 nmol/L) over 3 separate channels with immobilized LRP (16 fmol/mm2) and 1 control channel (no immobilized LRP) as described in the legend to Figure 1B. Association and dissociation between cluster II or cluster IV of LRP and factor IXa was assessed in a similar manner except that 5 different concentrations of cluster II or cluster IV (50-150 nmol/L) were passed over immobilized factor IXa. To this end, 122 fmol/mm2 or 32 fmol/mm2 of factor IXa was immobilized to determine cluster II or cluster IV binding constants, respectively. The data, obtained from duplicate experiments, were analyzed by performing nonlinear global fitting of data corrected for bulk refractive index changes to calculate association rate constants (kon) and dissociation rate constants (koff).32 Data represent average values ± SD.
Binding of factor IXa to separate, recombinant receptor fragments
Previously, we have demonstrated that 2 different domains of LRP, designated cluster II and cluster IV, encompass the predominant sites for ligand binding.19 Therefore, we used purified, recombinant receptor fragments (cluster I, II, III, and IV) to investigate whether these fragments bind to immobilized factor IXa when tested by SPR analysis. Whereas no binding could be detected to cluster I and cluster III even at very high concentrations (up to 1 μmol/L) of the injected fragments (data not shown), both cluster II and IV bound to factor IXa. The kinetic constants that describe the interactions between factor IXa and either cluster II or IV were determined and are summarized in Table 1. The calculatedKd values were 53 nmol/L and 227 nmol/L for cluster IV and cluster II, respectively. These data are compatible with the view that clusters II and IV represent 2 independent binding sites for factor IXa within LRP.
Effect of anti–factor IXa or anti-LRP polyclonal antibodies on the factor IXa–LRP interaction
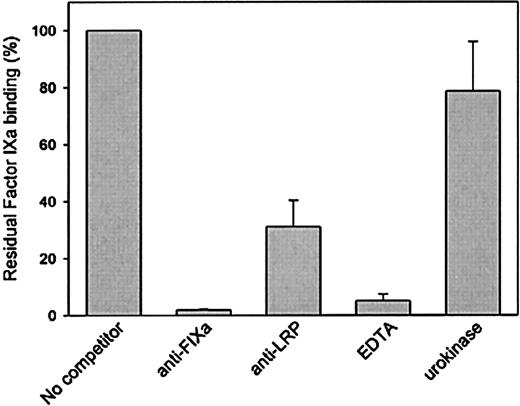
The interaction between factor IXa and LRP was further investigated to address specificity. LRP immobilized onto microtiter wells was incubated with various concentrations of factor IXa, and bound factor IXa was detected using the monoclonal anti–factor IX antibody CLB-FIX 14. Using this approach, a saturable and dose-dependent binding was observed (not shown). Subsequently, binding of factor IXa to LRP was tested in the presence of polyclonal antibodies directed against factor IXa or LRP. As presented in Figure3, both antibody preparations inhibited binding of factor IXa to LRP. Binding was also found to be impaired in the presence of EDTA (Figure 3). Because similar data were obtained when binding was assessed in the presence of EDTA using SPR analysis (not shown), these data demonstrate that the interaction between factor IXa and LRP is calcium dependent. Binding of factor IXa to LRP was also studied in the presence of another LRP ligand, urokinase. However, urokinase proved unable to interfere in the interaction between LRP and factor IXa (Figure 3). Apparently, factor IXa and urokinase have different binding sites within LRP.
Binding of factor IXa to LRP in a solid-phase binding assay in the absence or presence of various components.
Factor IXa (70 nmol/L) was incubated with immobilized LRP (75 ng/well) in a volume of 50 μL in 1% (wt/vol) BSA in HBST in the absence or presence of 1-μmol/L anti–factor IX polyclonal antibodies, 1-μmol/L anti–LRP polyclonal antibodies, and 10-mmol/L EDTA or 0.5-μmol/L urokinase for 1 hour at 37 °C. After washing with HBST, wells were incubated with 2-μg/mL monoclonal anti–factor IX antibody CLB-FIX 14. Bound CLB-FIX 14 was quantified using biotinylated rat antibodies against mouse immunoglobin Gκ chains. Bound complexes were then detected using horseradish peroxidase–labeled streptavidine. Binding is expressed as the percentage of binding in the absence of competitor and is corrected for nonspecific binding (5%-10% relative to binding to LRP coated wells). Data represent the mean and range of 2 independent experiments.
Binding of factor IXa to LRP in a solid-phase binding assay in the absence or presence of various components.
Factor IXa (70 nmol/L) was incubated with immobilized LRP (75 ng/well) in a volume of 50 μL in 1% (wt/vol) BSA in HBST in the absence or presence of 1-μmol/L anti–factor IX polyclonal antibodies, 1-μmol/L anti–LRP polyclonal antibodies, and 10-mmol/L EDTA or 0.5-μmol/L urokinase for 1 hour at 37 °C. After washing with HBST, wells were incubated with 2-μg/mL monoclonal anti–factor IX antibody CLB-FIX 14. Bound CLB-FIX 14 was quantified using biotinylated rat antibodies against mouse immunoglobin Gκ chains. Bound complexes were then detected using horseradish peroxidase–labeled streptavidine. Binding is expressed as the percentage of binding in the absence of competitor and is corrected for nonspecific binding (5%-10% relative to binding to LRP coated wells). Data represent the mean and range of 2 independent experiments.
Effect of heparin on the factor IXa–LRP interaction
Other studies have established that most LRP ligands bind to heparin, a property that is usually mediated by a series of positively charged amino acid residues.13 Furthermore, binding of these ligands to the receptor is inhibited by heparin, indicating that amino acid residues that constitute the heparin binding site may be involved in LRP binding as well.23 35 Because factor IXa is also a heparin-binding protein, we investigated whether heparin inhibits the binding of factor IXa to LRP by SPR. As expected, efficient factor IXa binding was observed in the absence of heparin (Figure 4). In the presence of increasing concentrations of heparin (both LMW and unfractionated), however, a decrease of the resonance signal was observed. The binding of factor IXa to LRP was fully suppressed in the presence of 100 U/mL of both LMW and unfractionated heparin (Figure 4). In contrast, little if any inhibition was observed in the presence of 100-μg/mL chondroitin sulfate (Figure 4). Similar data were obtained when binding of factor IXa to LRP was tested in the presence of these heparins or chondroitin sulfate in the above-described solid-phase assay (data not shown). These data indicate that the heparin-binding domain of factor IXa may contribute to the interaction with LRP.
Inhibition of factor IXa binding to LRP by heparins.
Binding of 100 nmol/L of factor IXa to immobilized LRP (26 fmol/mm2) was analyzed by SPR in the absence or presence of heparin (●) or fragmin (■) (0-100 U/mL) or 100-μg/mL chondroitin sulfate (▴). Binding is expressed as the percentage of binding in the absence of competitor and is corrected for nonspecific binding (< 5%). Data represent the mean ± SD of 3 experiments.
Inhibition of factor IXa binding to LRP by heparins.
Binding of 100 nmol/L of factor IXa to immobilized LRP (26 fmol/mm2) was analyzed by SPR in the absence or presence of heparin (●) or fragmin (■) (0-100 U/mL) or 100-μg/mL chondroitin sulfate (▴). Binding is expressed as the percentage of binding in the absence of competitor and is corrected for nonspecific binding (< 5%). Data represent the mean ± SD of 3 experiments.
Role of LRP and cell-surface proteoglycans in factor IXa degradation by CHO cells
To assess whether LRP is involved in mediating transport of factor IXa to the intracellular degradation pathway, cellular degradation of125I-labeled factor IXa both in LRP-deficient CHO cells17 and in wild-type CHO cells that constitutively express LRP was addressed. As shown in Figure5, the amount of factor IXa that was degraded by LRP-deficient cells was reduced by approximately 35% compared with wild-type CHO cells. This observation demonstrates that LRP indeed contributes to the transport of factor IXa to the intracellular degradation pathway. It has previously been shown that various LRP ligands may bind to cell-surface proteoglycans prior to transfer and binding to LRP.25-28 Therefore, the role of proteoglycans in cellular degradation of 125I-labeled factor IXa was also analyzed employing xylosyltransferase-deficient CHO cells. This mutant cell line is well suited to establish the role of proteoglycans because xylosyltransferase deficiency results in inability to generate proteoglycans.34 Significantly, the amount of factor IXa that was degraded by proteoglycan-deficient CHO cells was reduced by more than 80% compared with wild-type cells (Figure 5). Taken together, these data strongly suggest that both LRP and cell-surface proteoglycans have the potential to contribute to the catabolism of factor IXa.
Role of LRP and proteoglycans in factor IXa degradation by CHO cells.
Degradation was assessed by incubating wild-type (WT, black bar), LRP-deficient, (LRP-def, white bar), and proteoglycan-deficient (PG-def, gray bar) CHO cells with 125I-radiolabeled factor IXa (40 nmol/L) for 30 minutes at 37°C. After washing, bound material was incubated for an additional 5 hours, and degradation was determined as described in “Materials and methods.” Degradation of factor IXa by wild-type CHO cells is referred to as 100% and corresponds to 0.12 pmol/2.5 × 105 cells after 5 hours of incubation. The data represent the mean ± SEM of 3 experiments.
Role of LRP and proteoglycans in factor IXa degradation by CHO cells.
Degradation was assessed by incubating wild-type (WT, black bar), LRP-deficient, (LRP-def, white bar), and proteoglycan-deficient (PG-def, gray bar) CHO cells with 125I-radiolabeled factor IXa (40 nmol/L) for 30 minutes at 37°C. After washing, bound material was incubated for an additional 5 hours, and degradation was determined as described in “Materials and methods.” Degradation of factor IXa by wild-type CHO cells is referred to as 100% and corresponds to 0.12 pmol/2.5 × 105 cells after 5 hours of incubation. The data represent the mean ± SEM of 3 experiments.
Discussion
An essential step of the coagulation cascade consists of the activation of factor X by factor IXa in conjunction with the cofactor factor VIIIa. Consequently, the extent of factor X activation, and ultimately the amount of thrombin, depends on the regulation of factor IXa activity. It has been recognized that factor IXa activity may be controlled by the serine protease inhibitors (serpins) antithrombin and protease nexin-2 that may form inactive complexes.36,37Alternatively, the concentration of factor IXa may be determined by a catabolic pathway that selectively removes factor IXa from the circulation. In the present study, the contribution of cell surface–associated molecules to the catabolism of factor IXa was investigated. By using purified components, it is demonstrated that factor IXa binds to LRP in a reversible dose- and calcium-dependent manner (Figures 1 and 3). In contrast, the factor IX zymogen is unable to associate with LRP, demonstrating that LRP discriminates between the inactive and active species of factor IX. This is consistent with the previous observation that administration of competitive LRP ligands to mice (ie, thrombin/antithrombin or trypsin/α1-protease inhibitor complexes) delays the clearance of subsequently infused factor IXa but not that of factor IX.10
At present, little is known about the in vivo efficiency of factor IXa inhibition by antithrombin or protease nexin-2, but it seems likely that part of the factor IXa molecules that are generated during coagulation are subject to inhibition by these serpins. It would be of relevance, therefore, that such complexes like other enzyme-serpin complexes (eg, thrombin/antithrombin) are removed from the circulation by LRP. Indeed, it has previously been reported by Kounnas and coworkers that LRP is involved in the cellular uptake of factor IXa/protease nexin-2 complexes.25
The interaction between LRP and factor IXa differs in a number of aspects from LRP interactions with other serine protease-comprising ligands. First, factor IXa binds to LRP as a free enzyme, whereas most serine proteases interact with LRP exclusively upon formation of a complex with a serpin or with α2M.11,38 For instance, both thrombin and factor Xa bind to LRP only if complexes have been formed with antithrombin or α2M, respectively. Factor IXa shares the ability to bind to LRP as a free enzyme with the serine proteases tissue-type plasminogen activator and urokinase.22,39 However, both the single- and 2-chain variants of tissue-type plasminogen activator and urokinase bind to LRP. In contrast, only the 2-chain form of factor IX (ie, factor IXa) selectively interacts with LRP, whereas the single-chain form does not bind to LRP (Figures 1 and 2). Another aspect in which factor IXa differs from other LRP ligands relates to its interaction with the ligand-binding domains of LRP. We have recently shown that, from a number of LRP ligands tested, all showed similar affinity for both cluster II and cluster IV.19 With regard to factor IXa, however, the binding parameters for the interaction with cluster II and IV were dissimilar in that factor IXa displayed a 4-fold higher affinity for cluster IV compared with cluster II (Table 1). The difference in affinity was mainly due to a marked difference in the association rate constant. These data indicate that factor IXa preferentially binds to cluster IV over cluster II.
The transition of factor IX into its activated derivative factor IXa is associated with the exposure of a binding site for LRP (Figure 2). This site is located apart from the catalytic center, because active site–blocked factor IXa (ie, EGR–factor IXa) and active factor IXa bind equally efficiently to LRP (Table 1). Binding of factor IXa to LRP is efficiently inhibited in the presence of heparin (Figure4). Several mechanisms may explain this observation. First, heparin may interfere with binding of factor IXa to LRP by sterical hindrance. This seems less likely, however, because both unfractionated heparin and LMW heparin appeared to be equally effective in the inhibition of the factor IXa/LRP interaction. Alternatively, a heparin-binding region within factor IXa may be involved in LRP binding. Such heparin-binding regions in other LRP ligands have indeed been reported to be involved in the interaction with LRP.13 It should be mentioned in this respect that factor IX and factor IXa appear to bind heparin in a similar manner,40 yet only factor IXa is able to bind LRP (Figure 1). It seems conceivable, therefore, that the heparin-binding region within factor IXa contributes to LRP binding in an indirect manner.
Efficient activation of factor X requires the assembly of factor IXa and its cofactor factor VIII into a membrane-bound complex. Interestingly, we and others have recently identified factor VIII as a ligand for LRP as well.18,41 Thus, both enzyme and cofactor of the same complex may be catabolized by the same receptor. Whether this indicates that LRP is able to bind both ligands simultaneously or promotes dissociation of the complex remains to be investigated. It seems likely, however, that factor VIIIa/factor IXa–mediated factor X activation is modulated when proceeding at membranes enriched in LRP. It is of interest that the amount of factor Xa generated by the factor VIIIa/factor IXa complex at the surface of endothelial cells is markedly increased compared with the amount generated at the surface of monocytes or fibroblast.42This may be explained by the notion that monocytes and fibroblasts constitutively express LRP at their surface and, consequently, have the ability to remove factor VIII and factor IXa, whereas human umbilical vein endothelial cells lack this receptor.14 43
A common pathway for many LRP-internalized ligands seems to be emerging that involves cell-surface proteoglycans and possibly other accessory proteins. It has been proposed that several ligands bind to cell-surface proteoglycans prior to internalization by LRP.25-28,44-46 The rationale of this event will be sequestering of ligands to locally increase the concentration on the cell surface beyond Kd values and to accelerate the interaction with LRP. Our current data reveal a similar process for the catabolism of factor IXa because degradation of factor IXa proceeds inefficiently by proteoglycan-deficient CHO cells (Figure 5). Furthermore, it should be noted that in CHO cells degradation of factor IXa is only partially (35%) mediated by LRP. This may be explained by the observation that factor IXa binds efficiently to cluster IV but less efficiently to cluster II, resulting in a reduced rate of degradation of factor IXa compared with ligands that bind to both clusters with similar affinity. This slow rate of degradation via LRP would then ultimately lead to a small contribution of LRP in the total factor IXa degradation. Alternatively, another proteoglycan-dependent mechanism may contribute to factor IXa degradation as well. It is possible that ligand transfer may involve other receptors, including the other members of the LDL receptor family, which are known to share ligands with LRP.13 Alternatively, proteoglycans may directly mediate internalization of ligands without the participation of a surface-bound receptor. This has been reported previously for the syndecan family of proteoglycans, which are able to mediate internalization of atherogenic lipoproteins.47
Supported in part by the Netherlands Organization for Scientific Research, Council for Medical Research, Medical Sciences grant 902-26-175 (J.G.N.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Peter J. Lenting, Department of Haematology, University Medical Center Utrecht, G.03.647, Heidelberglaan 100, 3584 CX Utrecht, The Netherlands; e-mail:p.j.lenting@lab.azu.nl.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal