Abstract
Congenital homozygous dysfibrinogenemia was diagnosed in a man with a history of 2 thrombotic strokes before age 30. His hemostatic profile was characterized by a dramatically prolonged plasma thrombin clotting time, and no clotting was observed with reptilase. Complete clotting of the abnormal fibrinogen occurred after a prolonged incubation of plasma with thrombin. The release of fibrinopeptides A and B by thrombin and of fibrinopeptide A by reptilase were both normal. Thrombin-induced fibrin polymerization was impaired, and no polymerization occurred with reptilase. The polymerization defect was characterized by a defective site “a,” resulting in an absence of interaction between sites A and a, indicated by the lack of fragment D1 (or fibrinogen) binding to normal fibrin monomers depleted in fibrinopeptide A only (Des-AA fm). By SDS-PAGE, the defect was detected on the γ-chain and in its fragment D1. The molecular defect determined by analysis of genomic DNA showed a single base change (A→T) in exon VIII of the γ-chain. The resulting change in the amino acid structure is γ 330 aspartic acid (GAT) → valine (GTT). It is concluded that the residue γ-Asp330 is essential for the normal functioning of the polymerization site a on the fibrinogen γ-chain.
Introduction
Fibrinogen is a disulfide-bridged protein of 340 000 d comprised of 2 symmetric half-molecules, each having 3 different polypeptides, designated Aα-, Bβ-, and γ-chains. During coagulation, thrombin cleaves the amino-termini of the Aα- and Bβ-chains of fibrinogen, releasing fibrinopeptides A and B, respectively, and converting fibrinogen to fibrin monomers. The spontaneous polymerization of fibrin monomers initiates fibrin clotting with the formation of protofibrils. The newly exposed chain amino-terminus (GPR) of one fibrin molecule, which is referred to as the “A” polymerization site, binds noncovalently to a complementary polymerization pocket, termed the “a” site, in the carboxyl terminus region of the γ-chain of a second fibrin(ogen) molecule. This A-a interaction aligns the fibrin monomers into half-staggered, 2-stranded protofibrils. Subsequently, the growing fibrils aggregate in a lateral fashion to form fibers, and this presumably involves interactions between the amino terminus of the β-chain, the “B” polymerization site, and a complementary site, “b,” whose location is uncertain.1-5 Under physiological conditions, the release of fibrinopeptide B follows the release of fibrinopeptide A and correlates with lateral aggregation.1 This sequential release of fibrinopeptides, so that the A site appears before the B site, may control the final clot structure.6
During the early stages of the fibrin polymerization process, protofibrils are stabilized by the factor XIIIa-catalyzed formation of cross-links between the γ-chains of the assembling fibrin molecules, and, in a slower process, multiple cross-links form between α-chains.7
More than 240 cases of inherited dysfibrinogenemia have been reported,8 and most were detected because of a prolonged thrombin clotting time related to a defect in the conversion of fibrinogen to fibrin. Many of these molecules have abnormalities located in the carboxyl terminus of the γ-chain and are characterized by a defective fibrin polymerization.9
In this study, we report a homozygous case of congenital dysfibrinogenemia, designated fibrinogen Alès, which is characterized by amino acid substitution of Asp for Val at position 330 in the γ-chain. This mutation is responsible for impaired fibrin polymerization owing to defective fibrin polymerization site a, which does not interact with its complementary polymerization site A.
Materials and methods
Routine hemostasis studies
Blood was collected into 0.13 mol/L trisodium citrate (1 part citrate and 9 parts blood). Plasma was obtained by centrifugation at 3000g for 20 minutes at 4°C and stored at −80°C.
Whole blood clotting time, recalcification time, 1-stage prothrombin time, and coagulation factor II, V, and VII + X quantification were performed by conventional methods reported in Caen et al.10 Thrombin and reptilase clotting times were determined using several concentrations of reagents diluted either in 150 mmol/L NaCl or in 25 mmol/L CaCl2. Fibrinogen cofactor activity in adenosine diphosphate–induced platelet aggregation was determined using normal washed platelets prepared according to Patscheke and Worner.11 Plasma fibrinogen concentration was determined by a functional assay according to Clauss,12 by an immunologic assay method according to Mancini et al,13 and by heat precipitation at 56°C as described previously.14 Fibrinogen-related antigen in serum was determined by enzyme-linked immunosorbent assay as described by Soria et al.15 Levels of known risk factors recommended by the International Society of Thrombosis and Haemostasis (ISTH)16 (protein C, protein S, antithrombin III, homocysteine, lupus anticoagulant, lipid analysis) were determined in routine laboratories. Genetic analysis for factor V Leiden was performed as described by Bertina,17 and prothrombin gene 20210 mutation was conducted as described by Poort et al.18 Apolipoprotein genotype was performed by gene amplification according to Hixson and Vernier.19
Fibrinogen purification
Fibrinogen was purified according to Blombäck and Blombäck.20 Fibrinogen preparations were undegraded as judged by polyacrylamide gel electrophoresis in the presence of sodium dodecyl sulfate (SDS-PAGE) under reducing conditions.21 The purified fibrinogen (98% purity) was dissolved in 150 mmol/L NaCl and stored at −80°C.
Analysis of the prolonged thrombin time
Fibrin polymerization curves were made according to Bouvier and Gruedlinger.22 Aggregation of fibrin monomer was studied as described by Belitser et al.23 The release of FpA and FpB after thrombin addition to purified fibrinogen (and that of FpA after reptilase addition) was examined by high-performance liquid chromatography (HPLC) as described by Kehl et al.24Cross-linking of fibrin (γ dimerization and α polymerization) was analyzed by SDS-PAGE according to Weber and Osborn.21Fibrin stabilized by factor XIIIa was obtained according to Schwartz et al.25
Plasmin digests of fibrinogen
Binding of purified fragment D1 (or of fibrinogen) to immobilized normal fibrin monomers depleted in fibrinopeptide A (Des-AA fm)
Purification of fragment D1.
Purified normal fibrinogen and fibrinogen Alès were degraded with plasmin (final concentration, 2 U/mL) in the presence of 5 mmol/L CaCl2 for 4 hours at 37°C. The digests were chromatographed on Sepharose–Lysine (Pharmacia, Uppsala, Sweden) to remove plasmin. After an extensive dialysis against 100 mmol/L Tris-HCl, pH 8.6, the fragment D1 was isolated by DEAE cellulose chromatography as described by Nilehn.28 The purity of fragment D1 was tested by SDS-PAGE, and its concentration was calculated from absorbance at 280 nm (A, 1%; 1 cm = 11.20).
Preparation of Des-AA fm.
Normal human fibrinogen (Kabi AB, Stockholm, Sweden) at 5 mg/mL was clotted with 0.2 U/mL reptilase in the presence of 5 mmol/L EDTA for 30 minutes at 37°C. The fibrin clot was collected, washed extensively with 150 mmol/L NaCl, and dissolved in 4 mol/L urea or in 25 mmol/L acetic acid. The concentration of Des-AA fm was calculated from absorbance at 280 nm (A, 1%; 1 cm = 15.0).
Binding of fragment D1 to immobilized Des-AA fm.
Des-AA fm (or fibrinogen) solution at 5 μg/mL in urea 4 mol/L was immobilized on glutaraldehyde-treated 96-well microplates, as described by Stanilawski et al.29 To the coated wells, 100 μL of a solution of fragment D1 at concentrations ranging from 1.5 to 25 μg/mL were added. After an incubation period of 2 hours at 37°C, the plates were washed 4 times. Then, in each well, 100 μL of a monoclonal antibody that specifically reacts with an antigenic neo-epitope on fragment D1 (anti-D neo-F2C5), previously described by Mirshahi et al,30 was added. After a 2-hour incubation period, the plates were washed, and bound anti-D neo-F2C5 was detected by adding 100 μL of 0.1% diluted peroxidase-labeled rabbit immunoglobulin anti-mouse to each well (Amersham France SA, Les Ulis, France). All the dilutions were performed in phosphate-buffered saline containing 0.05% Tween 20 and 0.1% serum bovine albumin. After an incubation period of 1 hour at 37°C, the plates were washed, and the amount of bound peroxidase was quantified by adding orthophenylene diamine and perhydrol (Sigma Chemical, St Quentin Fallavier, France) as described by Wolters et al.31
Binding of fibrinogen to immobilized Des-AA fm.
Microplates were coated with 100 μL per well of normal Des-AA fm solution at 5 μg/mL in 25 mmol/L acetic acid for 18 hours at 4°C. The plates were washed. Then 100 μL normal fibrinogen or fibrinogen Alès, at concentrations ranging from 0.1 to 10 μg/mL in Tris-buffer saline-Tween (TBST) containing 1 mmol/L CaCl2, were introduced into each well and incubated for 2 hours at room temperature. Identical incubations were performed using 10 μg/mL fibrinogen solution containing Gly-Pro-Arg-Pro peptide at concentrations ranging from 25 nmol/L to 1500 nmol/L. The amount of fibrinogen bound to Des-AA fm was measured using a peroxidase-conjugate monoclonal antibody Y18-peroxidase (Gaubius Laboratory, Leiden, The Netherlands) that reacts with FpA. Immunoconjugate bound to the plates was determined with tetra methyl benzidine and perhydrol as the chromogen (Organon Teknika, Boxtel, The Netherlands).
Isolation of genomic DNA, amplification, cloning, and sequencing
Genomic DNA was isolated from blood cells according to the manufacturer's instructions using genomic DNA isolation kit (Gentra System, Minneapolis, MN). A 385-bp fragment encoding exon VIII (amino acids 259 to 350) and an 823-bp fragment encoding the exon IX (amino acids 351 to 427) of the γ-chain were amplified by polymerase chain reaction (PCR) with the following primer pairs: VIIIγR 5′-AACTTGGAATCTAAGAAAGGAAAACTACC-3′, VIIIγF 5′-GAAGCATCCTACGAAAGAGGG-3′ and IXγR 5′-AAAAAAGGAATTCTCTTTTGAAACGGTC-3′, IXγF 5′-GAAGCATCCTACGAAAGAGGG-3′.
Amplification was performed in a 50-μL reaction volume containing 0.3 μg genomic DNA, 5 U Taq polymerase (Gibco BRL), 0.2 mmol/L each of dATP, dCTP, dGTP, and dTTP (Pharmacia, Orsay, France), and 0.2 mmol/L each of primer VIIIγR and VIIIγF or IXγR and IXγF in reaction buffer (20 mmol/L Tris-HCl, pH 8.4, at 25°C, 50 mmol/L KCl, and 2.5 mmol/L MgCl2). Samples were heated at 94°C for 4 minutes and were incubated for 37 cycles of 1 minute at 95°C, 1 minute at 57°C, and 2 minutes at 72°C. Twenty microliters amplified DNA mixture was run on a 1.5% (wt/vol) agarose gel (Boehringer Mannheim, Mannheim, Germany). Bands of the appropriate size, as predicted from the genomic DNA of the γ-chain sequence,32 were cut out and isolated by Jetsorb kit (Genomed, Uithoorn, The Netherlands). The purified double-stranded DNA fragment was then cloned into EcoRV-digested pMOSBlue T-vector (Amersham, Orsay, France), and individual clones were sequenced using a T7 DNA sequencing kit (Pharmacia).
Determination of endogenous thrombin potential
Endogenous thrombin potential determination was performed according to Hemker et al33 on the basis of the time course of amidolytic activity using chromothrombin (Stago, Asnières, France) after the triggering of thrombin generation by calcium. Experiments were performed using both native citrated plasma and normal plasma that was depleted in its fibrinogen by removing the clot formed by the addition of reptilase.
Results
Clinical and laboratory data
Fibrinogen Alès was discovered in a 25-year-old man admitted to the hospital for multiple traumas. During this period there were no signs of any unusual bleeding or thrombotic tendency. However, at the age of 30, the propositus suffered 2 episodes of thrombotic stroke, as demonstrated by angiography. No history of hemorrhage or thrombotic tendency was observed in the propositus' parents or in his sister (35 years old). The known risk factors for thrombosis, suggested by ISTH,16 were absent in the propositus and his sister. Levels of protein C, protein S, and antithrombin III were within the normal range; levels of triglycerides, total cholesterol, and LDL/ HDL cholesterol were normal; lupus anticoagulant and anticardiolipid findings were negative. The level of homocysteine was also found to be within the normal range. Neither the factor V Leiden nor prothrombin gene 20210 G→A polymorphisms were present in the propositus or his sister. Apo E genotype was found to be epsilon 3/epsilon 4 for the propositus and his sister.
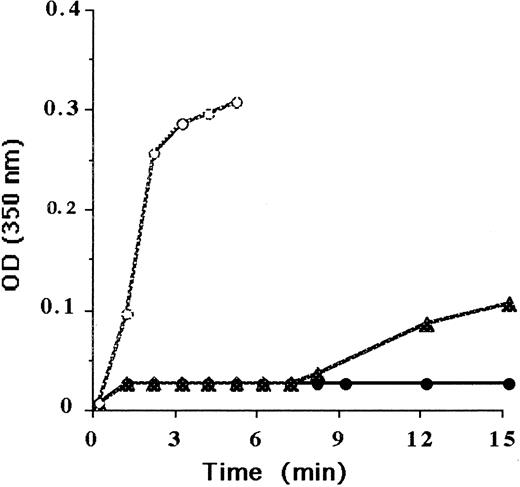
Results of the coagulation screening tests of the propositus and his family members are summarized in Table 1. He and his sister had the same profile. Prothrombin time was markedly prolonged, whereas the blood factors II, V, and VII + X were within normal range. Thrombin time was markedly prolonged, resulting in a small and weak clot. Reptilase time was infinite; no clot was observed with this enzyme. The presence of calcium chloride (from 25 to 100 mmol/L) did not induce any correction of the thrombin time. Polymerization curve, performed with the plasma of the propositus, did not show any increase of turbidity when thrombin was added to plasma diluted 1:10. However, using a lower dilution of the plasma (1:3), a prolonged lag time and a low polymerization rate were demonstrated in comparison with the polymerization curve of the normal plasma diluted to 1:10 (Figure 1).
Routine coagulation studies from the propositus and the members of his family
| . | Propositus . | Sister . | Father . | Mother . | Control . |
|---|---|---|---|---|---|
| Prothrombin time (sec)* | >180 | >180 | 16.5 | 15.5 | 13 |
| Thrombin time (sec) | |||||
| Without CaCl2 | 300 | 380 | 30 | 30 | 20 |
| With CaCl2 | 300 | 380 | ND | ND | 17 |
| Reptilase time (sec) | Unclottable | Unclottable | ND | ND | 20 |
| Reptilase (×10) | Unclottable | Unclottable | ND | ND | 10 |
| Fibrinogen (g/L) | |||||
| Functional assay | 0.20 | 0.20 | 0.80 | 0.80 | 2 ñ 4 |
| Heat precipitation | 3.15 | 3.30 | 3.70 | 4.00 | 2 ñ4 |
| Immunologic assay | 3.05 | 3.02 | 4.00 | 4.40 | 2 ñ4 |
| Fibrinogen-related antigen in serum (μg/mL) | 20 | 20 | ND | ND | 20 |
| . | Propositus . | Sister . | Father . | Mother . | Control . |
|---|---|---|---|---|---|
| Prothrombin time (sec)* | >180 | >180 | 16.5 | 15.5 | 13 |
| Thrombin time (sec) | |||||
| Without CaCl2 | 300 | 380 | 30 | 30 | 20 |
| With CaCl2 | 300 | 380 | ND | ND | 17 |
| Reptilase time (sec) | Unclottable | Unclottable | ND | ND | 20 |
| Reptilase (×10) | Unclottable | Unclottable | ND | ND | 10 |
| Fibrinogen (g/L) | |||||
| Functional assay | 0.20 | 0.20 | 0.80 | 0.80 | 2 ñ 4 |
| Heat precipitation | 3.15 | 3.30 | 3.70 | 4.00 | 2 ñ4 |
| Immunologic assay | 3.05 | 3.02 | 4.00 | 4.40 | 2 ñ4 |
| Fibrinogen-related antigen in serum (μg/mL) | 20 | 20 | ND | ND | 20 |
For each member of the family, factors V, VII+X, and II were in the normal range. ND, not determined.
Coagulation profiles of fibrinogen.
Normal plasma and plasma from the propositus, used at the same immunologic concentration of fibrinogen (4 mg/mL), were diluted in 150 mmol/L NaCl. After thrombin addition, the optical density (OD) was recorded at 350 nm. Normal plasma diluted to 1:10 (○), plasma from the propositus diluted to 1:10 (●), and plasma from the propositus diluted to 1:3 (▴).
Coagulation profiles of fibrinogen.
Normal plasma and plasma from the propositus, used at the same immunologic concentration of fibrinogen (4 mg/mL), were diluted in 150 mmol/L NaCl. After thrombin addition, the optical density (OD) was recorded at 350 nm. Normal plasma diluted to 1:10 (○), plasma from the propositus diluted to 1:10 (●), and plasma from the propositus diluted to 1:3 (▴).
Plasma fibrinogen level determined by the functional assay of Clauss12 was markedly decreased, but when it was determined by heat precipitation and the immunological method, the results were normal. Platelet count and platelet aggregation levels were both normal. The fibrinogen-related antigen, which remained in serum obtained after 18-hour clotting of the plasma with thrombin, was normal.
The father and the mother of the propositus, who are cousins, also had abnormal fibrin formation that was less severe than that observed in their children. These data indicate that the patient and his sister are homozygous and that his dysfibrinogenemia is congenital.
Coagulation of purified fibrinogen
The thrombin clotting time of purified fibrinogen Alès was markedly prolonged compared to normal fibrinogen (165 seconds; control, 15 seconds), and the reptilase time was infinite because fibrinogen Alès remained unclottable even when a high concentration of reptilase was used.
The HPLC profiles of FpA and FpB, released by thrombin from fibrinogen Alès, were normal with respect to both the initial rate and the total amount released. Furthermore, the rate of FpA released after the addition of reptilase to fibrinogen Alès was normal (data not shown).
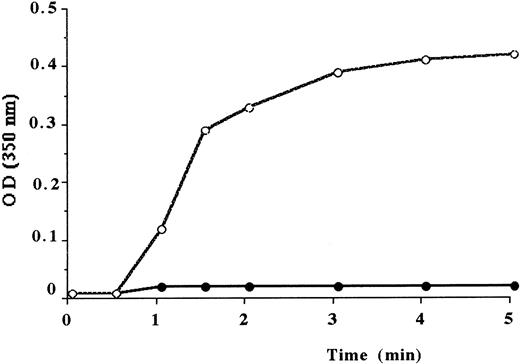
Fibrin monomer aggregation of Des-AABB-fm (fibrin monomers depleted in fibrinopeptides A and B), derived from the abnormal fibrinogen, did not show any increase of the turbidity (Figure2). The analysis of the cross-linking profiles of fibrin mediated by factor XIIIa showed that γ-chains from fibrinogen Alès were converted to γ-dimers and that all the α-chains were converted to α-polymers (data not shown).
Polymerization of isolated fibrin monomers.
To 2.8 mL phosphate buffer at pH 7.4 was added 0.2 mL fibrin monomers at 6 mg/mL. OD was recorded at 350 nm. Normal fibrin monomers (○), fibrin monomers from the propositus (●).
Polymerization of isolated fibrin monomers.
To 2.8 mL phosphate buffer at pH 7.4 was added 0.2 mL fibrin monomers at 6 mg/mL. OD was recorded at 350 nm. Normal fibrin monomers (○), fibrin monomers from the propositus (●).
Analysis of fibrinogen molecule
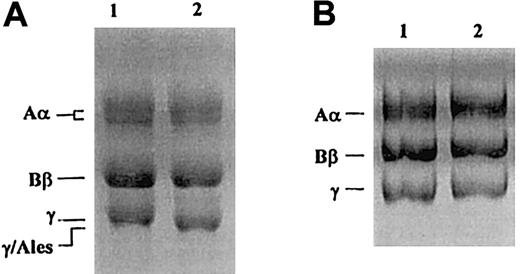
SDS-PAGE of purified fibrinogen Alès was carried out under reducing conditions according to the method of Laemmli.27A γ-chain with higher mobility than that of the normal γ-chain was observed (Figure 3A), and there was no γ-chain with a normal mobility. In contrast, SDS-PAGE, performed according to the method of Weber and Osborn,21showed no difference in electrophoretic migration between γ-chains of normal fibrinogen and those of fibrinogen Alès (Figure3B).
SDS-PAGE of purified fibrinogen after reduction with 2-mercaptoethanol.
(A) 10% SDS-PAGE according to Laemmli.27 (B) 10% SDS-PAGE according to Weber and Osborn.21 Lane 1, normal fibrinogen; lane 2, fibrinogen Alès.
Plasmin digestion of purified fibrinogen
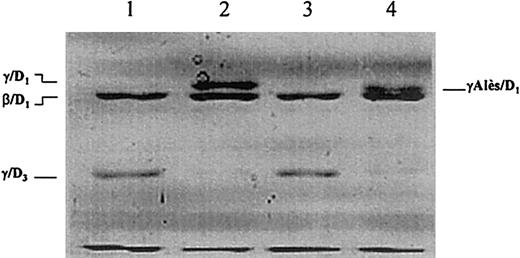
When plasmin digestion was performed in the presence of calcium and the profile of the plasmin digest of fibrinogen Alès was analyzed by SDS-PAGE under reducing conditions, only the γ-chain and the β-chain remnants of fragment D1 remained, and the Alès γ-chain remnant of fragment D1(γAlès/D1) had a higher mobility than that of normal fragment D1 (γ/D1) (Figure4, lanes 2, 4).
Plasmin degradation of fibrinogen analyzed by SDS-PAGE according to Laemmli after reduction with 2-mercaptoethanol.
Lanes 1 and 2, plasmin digest of normal fibrinogen in the presence of 10 mmol/L EDTA and 5 mmol/L CaCl2, respectively. Lanes 3 and 4, plasmin digest of fibrinogen Alès in the presence of 10 mmol/L EDTA and 5 mmol/L CaCl2, respectively.
Plasmin degradation of fibrinogen analyzed by SDS-PAGE according to Laemmli after reduction with 2-mercaptoethanol.
Lanes 1 and 2, plasmin digest of normal fibrinogen in the presence of 10 mmol/L EDTA and 5 mmol/L CaCl2, respectively. Lanes 3 and 4, plasmin digest of fibrinogen Alès in the presence of 10 mmol/L EDTA and 5 mmol/L CaCl2, respectively.
When plasmin digestion was performed in the presence of EDTA, the profile of fibrinogen digest from Alès revealed γ- and β-chain remnants of fragment D3. Under this condition, the Alès γ-chain remnant of fragment D3 did not differ from the normal one (Figure 4, lanes 1, 3).
Binding of fibrinogen and its derived fragment D1 to Des-AA fm
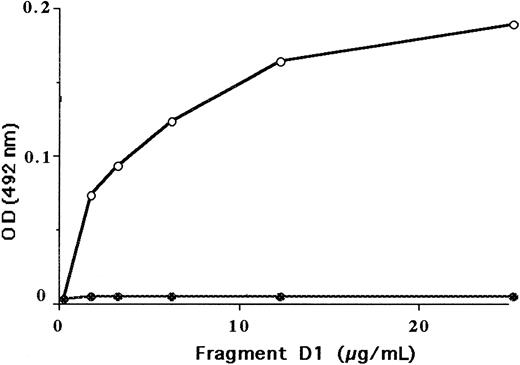
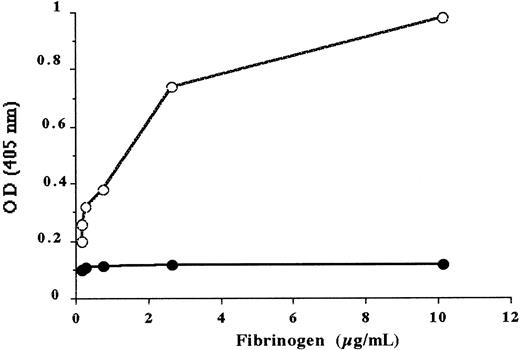
In contrast to normal fragment D1, the fragment D1 isolated from fibrinogen Alès did not bind to immobilized normal Des-AA fm (Figure 5). In the same way, fibrinogen Alès did not bind to Des-AA fm (Figure 6). As a control, it was shown that 750 nmol/L of the peptide Gly-Pro-Arg-Pro, (corresponding to the site A), inhibited the binding of normal fibrinogen to Des-AA fm by more than 80%.
Binding of purified normal fragment D1 and of purified fragment D1 from fibrinogen Alès to insolubilized normal Des-AA fm.
Fragment D bound to immobilized Des-AA fm was evaluated using an anti-D neo-monoclonal antibody. Normal fragment D1 (○), fragment D1 from fibrinogen Alès (●).
Binding of purified normal fragment D1 and of purified fragment D1 from fibrinogen Alès to insolubilized normal Des-AA fm.
Fragment D bound to immobilized Des-AA fm was evaluated using an anti-D neo-monoclonal antibody. Normal fragment D1 (○), fragment D1 from fibrinogen Alès (●).
Binding of normal fibrinogen and fibrinogen Alès to insolubilized normal Des-AA fm in the presence of 1 mmol/L CaCl2.
Fibrinogen bound to immobilized Des-AA fm was evaluated using an antibody against fibrinopeptide A. Normal fibrinogen (open circles), fibrinogen Alès (filled circles).
Binding of normal fibrinogen and fibrinogen Alès to insolubilized normal Des-AA fm in the presence of 1 mmol/L CaCl2.
Fibrinogen bound to immobilized Des-AA fm was evaluated using an antibody against fibrinopeptide A. Normal fibrinogen (open circles), fibrinogen Alès (filled circles).
Determination of the γ-chain defect in fibrinogen Alès
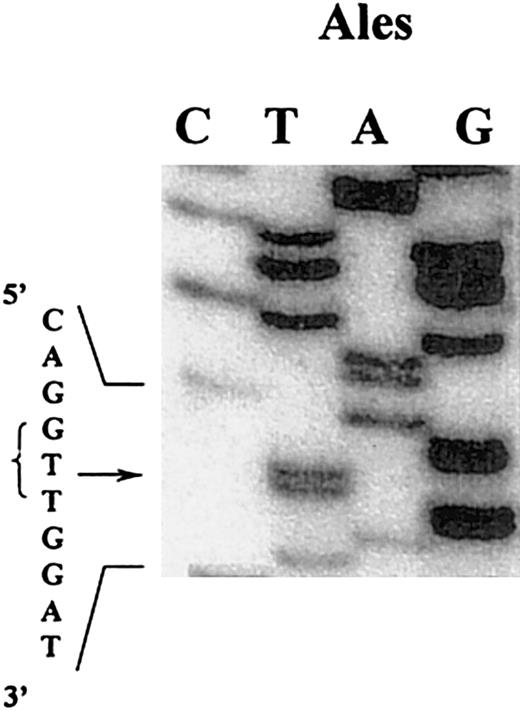
Considering the abnormal migration of the γ-chain as shown by SDS-PAGE and the localization of the abnormality to the carboxyl terminus part of the γ-chain by plasmic digest, 2 DNA fragments comprising exons VIII and IX encoding for amino acids 259 through 350 and 351 through 427 of the γ-chain were amplified by PCR. The DNA sequence of the amplified exon IX from our patient was indistinguishable from the DNA normal sequence. DNA sequence analysis of exon VIII from Alès demonstrated a single base substitution in the codon for Asp330 (GAT), changing this for the codon for Val (GTT) (Figure 7). Sequence analysis of 10 clones derived from DNA of the patient showed only the abnormal sequence, indicating that this patient is homozygous.
DNA sequence analysis of a mixture of clones containing the amplified exon VIII of the γ-chain gene coding for fibrinogen Alès.
An adenine was substituted by a thymine (arrow indicates the mutation), changing the triplet GAT (Asp) to GTT (Val).
DNA sequence analysis of a mixture of clones containing the amplified exon VIII of the γ-chain gene coding for fibrinogen Alès.
An adenine was substituted by a thymine (arrow indicates the mutation), changing the triplet GAT (Asp) to GTT (Val).
Analysis of endogenous thrombin potential
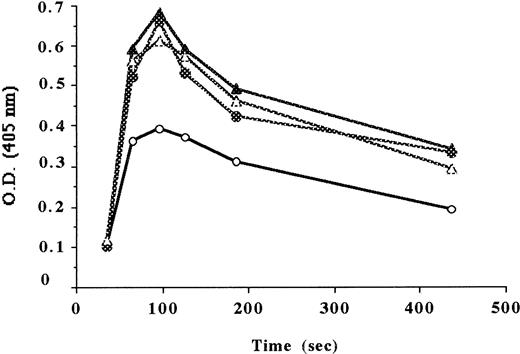
Endogenous thrombin potential (ETP), the area under the thrombin generation curve, was higher in normal fibrinogen-depleted plasma than in the corresponding untreated plasma. Thrombin measured at the peak from 5 normal healthy volunteers was 50% to 63% of that measured using the corresponding reptilase-treated plasmas, indicating that 50% to 37% of thrombin was bound to the clot.
In contrast, ETP in Alès plasma (experiment performed in triplicate) was found to be much higher than that observed using the pool of 5 normal untreated plasmas. Both plasmas, however, have the same immunologic level of fibrinogen. Furthermore, ETP was found to be similar in Alès samples, regardless of whether they were treated by reptilase (Figure 8). The coefficient of variation in thrombin evaluation measured at the peak was 8% in Alès plasma, 13% to 15% in untreated normal plasma, and 10% in reptilase-treated plasma (due to experimental error in sampling time).
Endogenous thrombin potential.
Time-course of amidolytic activity using chromothrombin (Stago, Asnières, France) after adding calcium to the plasma to trigger thrombin generation. Normal plasma (○), reptilase-treated normal plasma (▵), Alès plasma (●), reptilase-treated Alès plasma (▴).
Endogenous thrombin potential.
Time-course of amidolytic activity using chromothrombin (Stago, Asnières, France) after adding calcium to the plasma to trigger thrombin generation. Normal plasma (○), reptilase-treated normal plasma (▵), Alès plasma (●), reptilase-treated Alès plasma (▴).
Discussion
Homozygous dysfibrinogenemia was described in 2 members of the same family (the propositus and his sister), denoted as fibrinogen Alès. Each of the parents, who are cousins, had a heterozygous form of the anomaly. The abnormal fibrinogen of the propositus was unclottable by reptilase, and the thrombin-clotting time was dramatically delayed. Fibrinogen concentration was decreased when measured by functional assay, but it was normal when determined by heat precipitation or immunologic assay. Prolongation of the thrombin clotting time was not related to the defective release of FpA or FpB. In the same way, the absence of clotting by reptilase did not result from an abnormal release of FpA. Fibrin polymerization performed by adding thrombin to plasma was markedly impaired, and the aggregation of fm derived from thrombin-treated fibrinogen Alès did not show any increase in turbidity. In addition, with plasma diluted 1:10, there was no polymerization.
SDS-PAGE of purified fibrinogen Alès indicated that the structural defect was located in the γ-chain. Furthermore, because the anomaly was also detected in the γ-chain remnant of fragment D1 but not in the γ-chain remnant of fragment D3, it was evident that the anomaly was located in the peptide removed during the conversion of fragment D1 to fragment D3.34 Based on these results, genomic DNA of the propositus encoding exons VIII and IX of the γ-chain were amplified by PCR. Sequence analysis of the amplified fragments demonstrated that the propositus was homozygous for a single base substitution (GAT→GTT) in the codon γ-Asp330, converting this residue to a Val. The absence of normal γ-chain in fibrinogen Alès is consistent with the genetic analysis, which demonstrated that the patient was homozygous for the mutation. The difference in apparent molecular weight observed by SDS-PAGE27 between the γ-chain from Alès and that of the normal γ-chain was unexpected, but this has been reported35 previously for other fibrinogens with a single amino acid substitution. This change has been ascribed to an alteration in hydrophobicity or a local conformation that then affects the mobility of the protein-SDS complex. No difference was observed in SDS-PAGE as done by Weber and Osborn,21 which is more reliable for molecular weight, between the γ-chain from Alès and that of a normal γ-chain.
The A-a interaction occurring between the reptilase-activated E domain of one molecule of fibrin monomer and the D domain of another plays an essential role in fibrin formation because the cleavage of FpA alone is sufficient to induce the fibrin assembly process.36 In fibrinogen Alès, this type of interaction does not occur because fragment D1 from Alès, which contains the site a, does not bind to immobilized normal Des-AA fm, which has the site A available. Under the same conditions, normal fragment D1binds to Des-AA fm. Similarly, intact fibrinogen Alès does not bind to normal Des-AA fm, whereas normal fibrinogen does. As a control, it was shown that this binding process was inhibited by the addition of GPRP peptide (corresponding to the site A), which is known to compete for binding of normal fibrinogen or normal fragment D1 to Des-AA fm.
Therefore, these results demonstrate that the mutation of the residue γ-Asp330 to Val perturbs the conformation of the fibrin polymerization site a required for fibrin polymerization and leads to a nonfunctional polymerization site a in fibrinogen Alès. This same mutation was already described by Reber et al37 in fibrinogen Milano I, but in this family, the affected patients were heterozygous.
Another mutation at the same position, γ-chain 330, was reported in a different patient with heterozygous congenital dysfibrinogenemia, fibrinogen Kyoto III (Asp→Tyr),38 and was also characterized by impaired fibrin polymerization. The comparison of the sequence of the γ-chains of human,32 rat,39and lamprey40 fibrinogens showed that γ-Asp330 is totally conserved, suggesting that this residue is essential for the function of fibrinogen.
Furthermore, in determining by crystallography the structure of the complex GPRP-fragment D41 or that of the complex GPRP- rFbgC30 (recombinant 30-kd protein, corresponding to the carboxyl terminus region of the γ-chain), it has been shown that γ Asp330 forms a salt bridge with Arg of GPRP, providing an interaction essential for D:E binding. It has also been shown that the carboxyl group of Asp330 is hydrogen-bonded to the side chain of His340, amino acid, which also forms an additional hydrogen bond with the peptide amino terminus.42Therefore, it was hypothesized that the replacement of Asp330 by an uncharged amino acid residue such as Val would alter the electrostatic environment of the polymerization pocket and also the extensive and precise network of hydrogen bonds within this pocket.
The absence of interaction between the polymerization sites A and a explains the unclottability of fibrinogen Alès by reptilase. More interesting, the observation that thrombin induces clotting of fibrinogen Alès suggests that polymerization can occur only through B-b interaction because the polymerization site B is unmasked in Des-AABB fm and masked in Des-AA fm. This should be compared to the Metz fibrinogen data, reported by Soria et al,43 a homozygous abnormal fibrinogen characterized by a whole blood clot formation occurring only through B-b interaction, because the addition of thrombin to fibrinogen Metz induces only the release of FpB. Therefore, because FpA is not released, this leads to an unavailability of site A. This situation represents “the other side of the coin” to fibrinogen Alès. Given that no hemorrhagic disease was observed in the propositus or in his sister, it is assumed that the clot resulting from the interaction B-b is sufficient to stop bleeding.
With respect to the 2 episodes of arterial thrombosis it seems likely that, because clotting by thrombin is dramatically delayed in the patient, thrombin generated in the Alès sample is not trapped in fibrin, allowing platelet aggregation to occur. This hypothesis is consistent with endogenous thrombin potential, which has been proposed as a parameter for plasma-based hypercoagulability, being much higher in Alès plasma than in control. This difference was attributed to the fact that 37% to 50% of thrombin formed after calcium addition was trapped into the normal forming clot. The percentage of thrombin bound to the clot was deduced from the difference between thrombin activity in recalcified defibrinated plasma and that in recalcified plasma. Because clotting was dramatically delayed in Alès plasma, the thrombin generated remained in solution and its activity was similar to that found with fibrinogen-depleted normal plasma. Because reptilase added to plasma from Alès did not induce any clotting, it was shown, as a control, that endogenous thrombin potential was identical in Alès native and reptilase-treated plasmas. In all samples, a decay of thrombin activity that resulted from the neutralization of thrombin by antithrombin III was observed after 120 seconds, as already reported.44
However, it is important to know whether thrombin bound to Alès fibrin monomers is still accessible for platelet aggregation. In fact, it is well established that fibrin contains independent sites having affinity for thrombin. One of these sites, located in the E domain of the fibrin monomer, interacts with the anion binding site of thrombin. This binding exosite of thrombin is implicated in both fibrin and platelet binding. It has been shown that an equilibrium exists between thrombin bound to fibrin monomers and thrombin bound to platelets.45,46 Therefore thrombin bound to fibrin monomers is still active for platelet aggregation, but its activity is reduced in comparison to free thrombin. Thus, thrombin bound to fibrin monomers remains partly available for platelet aggregation. Furthermore, it has been reported that clot-associated thrombin induces platelet aggregation.47 48
In 2 other patients with congenital dysfibrinogenemia, an arterial thrombotic risk is reported that can be related to a defective thrombin binding to the clot, responsible for platelet aggregation.49,50 However, the sister of the propositus, though also homozygous for dysfibrinogenemia, is asymptomatic. We tried to determine whether the thrombotic risk could be linked to other risk factors associated with dysfibrinogenemia. The known risk factors for thrombosis, recommended by ISTH, were absent in the propositus and in his sister. The propositus is a smoker, but his sister is not. Both the patient and his sister also have apoE ε 3/ε 4 genotype, more frequent in stroke patients (21.1%) than in stroke-free controls (8.9%; P < .05), indicating that apoE ε 4 is a prominent lipid predictor for ischemic stroke among other risk factors.51 It is therefore suggested that the onset of thrombotic disease in this patient was multifactorial and was linked to a synergistic effect of dysfibrinogenemia, apoE ε 3/ε 4 genotype, and smoking.
The parents of this propositus and the affected members of the family Milano I are asymptomatic, but they are heterozygous for the mutation with a circulating mixture of normal and abnormal fibrinogens. Therefore, as already reported for other dysfibrinogenemias, the potential risk factor would explain the variability observed in the clinical symptoms associated with this homozygous dysfibrinogenemia because the mutation is associated with severe thrombophilia in the patient and appears to be silent in his sister.
In summary, a structural defect in a variant of fibrinogen, called fibrinogen Alès, was identified. Our results demonstrate that the substitution of the aspartic acid in position γ-330 by valine leads to a nonfunctioning polymerization site a, which is necessary for the normal polymerization of fibrinogen.
Acknowledgments
The authors thank Dr J. C. Gris, Centre Hospitalier Universitaire de Nimes, for performing the risk factors recommended by ISTH, and Mr Richard Medeiras for his advice in editing the manuscript.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Jeannette Soria, Laboratoire de Biochimie A, Hotel-Dieu de Paris, 1 Place du Parvis de Notre Dame, 75181 Paris cedex 04, France.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal