Abstract
Shear-induced binding of von Willebrand factor (vWf) to the platelet glycoprotein (GP) Ib/V/IX complex plays a key role in initiating platelet adhesion and aggregation at sites of vascular injury. This study demonstrated that pretreating human platelets with inhibitors of actin polymerization, cytochalasin D or latrunculin B, dramatically enhances platelet aggregation induced by vWf. The effects of these inhibitors were specific to the vWf-GPIbα interaction because they enhanced vWf-induced aggregation of Glanzmann thrombasthenic platelets and Chinese hamster ovary (CHO) cells transfected with GPIb/V/IX. Moreover, cytochalasin D enhanced the extent of platelet aggregation induced by high shear stress (5000 s−1) and also lowered the shear threshold required to induce aggregation from 3000 s−1 to as low as 500 s−1. Studies of CHO cells expressing GPIbα cytoplasmic tail truncation mutants that failed to bind actin-binding protein-280 (deletion of residues 569-610 or 535-568) demonstrated that the linkage between GPIb and actin-binding protein-280 was not required for vWf-induced actin polymerization, but was critical for the enhancing effects of cytochalasin D on vWf-induced cell aggregation. Taken together, these studies suggest a fundamentally important role for the cytoskeleton in regulating the adhesive function of GPIb/V/IX.
Introduction
The ability of platelets to adhere to sites of blood vessel injury is essential for the arrest of bleeding and subsequent vascular repair. A key adhesive protein initiating platelet-vessel wall interactions is the large multimeric protein, von Willebrand factor (vWf). Once immobilized at the site of vessel wall injury, the A1 domain of vWf “captures” platelets from rapidly flowing blood by a specific interaction with the platelet glycoprotein (GP) Ib/V/IX complex. This tethering mechanism decelerates platelet velocity relative to free-flowing blood as a prerequisite step for integrin-mediated irreversible platelet adhesion.1-3
Growing evidence suggests that this multistep adhesion mechanism is not only important for platelet-vessel wall interactions but also for platelet aggregation, particularly at elevated shear rates. This was initially demonstrated from studies of platelet aggregation using a cone-plate viscometer. With this device, exposing platelets in suspension to pathologic levels of shear stress (≥ 3000 s−1) induces platelet aggregation independent of the addition of an exogenous stimulus.4-7 Shear-induced platelet aggregation is initiated by the binding of soluble vWf to GPIb/V/IX. This interaction not only tethers platelets to one another but also triggers platelet activation, converting the major platelet integrin αIIbβ3 from a low-affinity to a high-affinity receptor capable of binding fluid-phase adhesive proteins such as soluble vWf or fibrinogen.8-10 More recent studies using in vitro flow chambers, in which platelets in flowing blood adhere to the surface of platelets immobilized on a reactive surface, suggest that this dual-step aggregation mechanism also operates at physiologically relevant shear stresses.11 12
Despite the fundamental importance of the vWf-GPIb interaction in initiating both platelet adhesion and aggregation under flow, the physiologic mechanisms regulating this adhesive event remain poorly defined. In contrast to the integrins, which undergo affinity modulation as a result of conformational changes or receptor clustering in the plane of the plasma membrane,13-16 no current evidence suggests a similar mode of regulation for GPIb/V/IX. One potential mechanism regulating the ligand-binding function of GPIb is through its association with the membrane skeleton. In the resting platelet, the GPIb/V/IX complex is anchored to the membrane skeleton through a specific interaction between the cytoplasmic tail of GPIbα and actin-binding protein-280 (ABP-280).17-21 This interaction may limit lateral mobility of the receptor complex in the plane of the plasma membrane,22 thereby influencing the number of active bonds formed between GPIb and multimeric vWf. However, to date, there is limited evidence that the cytoplasmic tail of GPIbα or the cytoskeleton plays a major role in regulating the vWf-GPIb interaction particularly under physiologically relevant shear conditions.
In this study we have defined an important role for the cytoskeleton in regulating the vWf-GPIb interaction. We demonstrate that pretreating platelets with inhibitors of actin polymerization dramatically increased the rate and extent of platelet aggregation induced by vWf. Pretreating platelets with cytochalasin D (CD) also enhanced shear-induced platelet aggregation and dramatically reduced the shear threshold required to induce platelet aggregation from 3000 s−1 down to as low as 500 s−1. Studies of GPIbα cytoplasmic tail mutants expressed on the surface of Chinese hamster ovary (CHO) cells demonstrated that the linkage between GPIbα and the membrane skeleton is not required for GPIb/vWf-induced actin polymerization, but is essential for the enhancing effects of CD on GPIb/V/IX-mediated cell aggregation. These studies suggest that the link between GPIb and the membrane skeleton plays a key role in regulating the adhesive function of the GPIb/V/IX complex.
Materials and methods
Materials
Jasplakinolide and Alexa488-conjugated phalloidin were from Molecular Probes (Eugene, OR). Apyrase was purified from potatoes according to the method of Molnar and Lorand.23 Human vWf (HvWf), bovine vWf (BvWf), and fibrinogen were purified as previously described.24,25 Monoclonal antibody (mAb) ALMA12 against GPIbα was generated and characterized as previously reported.26 Full-length complementary DNAs (cDNAs) for GPIbα, Ibβ, and IX cloned into the pDX vector and CHO cells expressing GPIbβ and GPIX (CHOβIX) were generous gifts from Dr J. Lopez (Houston, TX). All other reagents and antibodies were from sources described previously.27-29
Platelet preparation and aggregation studies
Blood was collected from healthy donors or individuals with Glanzmann thrombasthenia (containing < 1% αIIbβ3) or type III von Willebrand disease (vWD) (< 1% plasma and platelet vWf), and the platelet-rich plasma (PRP) and washed platelets were prepared as described previously.27 Platelet aggregation studies using BvWf or HvWf and ristocetin were performed according to previously published methods.29 Thrombin-induced aggregations were performed in the presence of 1 mmol/L CaCl2; aggregation induced by collagen or adenosine diphosphate (ADP) was performed in the presence of 0.5 mg/mL fibrinogen and 1 mmol/L CaCl2. Shear-induced platelet aggregation (SIPA) was performed in a cone-and-plate viscometer (Carrimed rheometer, CSL100, Carri-Med, Dorking, United Kingdom). In these studies, PRP or washed platelets in the presence of 10 μg/mL HvWf were exposed to the indicated shear rate for 2 minutes at room temperature (22°C). In some experiments, platelets were pretreated for 10 minutes with the indicated concentrations of cytochalasin D (CD), latrunculin B (LB), jasplakinolide, apyrase, or prostaglandin E1(PGE1). In control experiments, 0.5 U/mL apyrase completely inhibited PRP or washed platelet aggregation induced by 10 μmol/L ADP and preincubation of platelets with 0.5μg/mL PGE1 totally blocked washed platelet aggregation induced by 1 U/mL thrombin. Pretreatment of platelets with 5 μmol/L jasplakinolide, a concentration that has been demonstrated to inhibit actin filament disassembly,30 completely inhibited platelet spreading on a vWf matrix. In other studies, washed platelets were preincubated for 10 minutes with either 2 mmol/L EDTA or the anti-αIIbβ3 c7E3 Fab (20 μg/mL) to block ligand binding to integrin αIIbβ3. Following aggregation, platelets were fixed for 60 minutes in 1% paraformaldehyde, stained overnight with 1 μmol/L DiOC6, and mounted in Permafluor. Platelet aggregates were imaged using confocal fluorescence microscopy (Leica TCS SP Confocal microscope, Leica Microsystems, Heidelberg, Germany), and volumetric analysis of 3-dimensional reconstructed platelet aggregates was performed using the Microcomputer Imaging Device (MCID, Imaging Research, St Catherine's, Ontario, Canada) or Voxblast (Vaytek, Fairfield, IA).
Generation of GPIbα mutants and expression of the GPIb/V/IX complex on the surface of Chinese hamster ovary cells
The GPIbαΔ535-568 deletion mutant was generated as described previously.31 GPIbαΔ569-610 was generated using a similar protocol, with the following primers flanking the designed deletion region: 5′ GAA GAG GCT GGA GCG GAA AGA 3′ (4718-4698) and 5′ TGA GGG TGG GAG GTT TGG GGA 3′ (4947-4967) (Genbank accession number M22403). Both GPIbαΔ535-568 and GPIbαΔ569-610 deletions were confirmed by DNA sequence analysis. Transfection of CHO cells with cDNAs for GPIbα (CHO-Ib/IX), GPIbαΔ569-610 (CHO-IbΔ569), or GPIbαΔ535-568 (CHO-IbΔ535), and their subsequent characterization by FACS analysis, were performed as described previously.31
Chinese hamster ovary cell aggregation studies
CHO-Ib/IX, CHO-IbΔ569, or CHO-IbΔ535 cells (1 × 106 cells/mL) were resuspended in Tyrode buffer containing 2 mmol/L EDTA. CHO cell aggregation was initiated using the indicated concentrations of HvWf and 1 mg/mL ristocetin, or BvWf, at 37°C with constant stirring. Aggregation was monitored using a Chronolog Dual Channel Aggrometer, Chromo-Log, Havertown, PA. In some studies, CHO cells were preincubated with 5 μmol/L CD for 10 minutes prior to initiation of aggregation. Cells were processed and subjected to confocal fluorescence microscopy and volumetric analysis, as described above for platelets.
Estimation of filamentous-actin content
Washed platelets (1 × 109/mL) were stimulated with either 10 μg/mL HvWf in the presence of 1 mg/mL ristocetin, or 1 U/mL thrombin for the indicated times, with stirring. Alternatively, GPIb/V/IX-transfected CHO cells (3 × 106/mL) were aggregated with 10 μg/mL BvWf for 20 minutes. Cells were lysed with an equal volume of 2 × Triton X-100 lysis buffer (40 mmol/L Tris-HCl, pH 7.4, 2% Triton X-100, 10 mmol/L EDTA, 2 mmol/L phenylmethylsulfonyl fluoride, 2 mmol/L Na3VO4, 4 mmol/L benzamidine, and 0.1 μmol/L phallacidin). Filamentous (F)-actin content was determined using the DNaseI inhibition assay32,33 or by the sedimentation method described by Fox.17
Chinese hamster ovary cell adhesion studies
Results
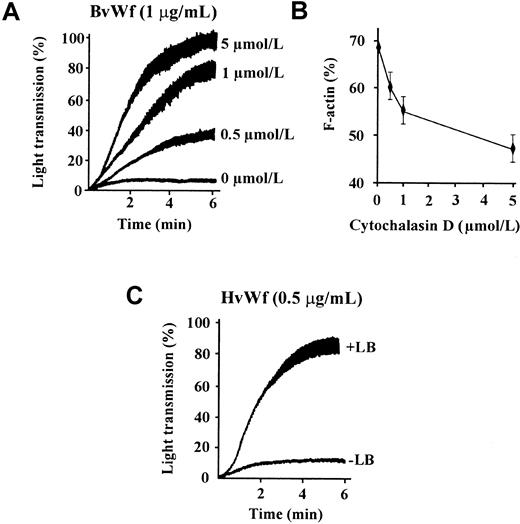
We have recently demonstrated that vWf binding to GPIb/V/IX is sufficient to induce actin polymerization and cytoskeletal reorganization in human platelets and GPIb/V/IX-transfected CHO cells.29 To investigate the possibility that these cytoskeletal changes may influence the adhesive function of GPIb/V/IX, we examined the effect of pretreating platelets with CD on vWf-induced platelet aggregation. As demonstrated in Figure1A, pretreating platelets with CD dramatically enhanced the rate and extent of platelet aggregation induced by HvWf in the presence of 1 mg/mL ristocetin. The extent of enhancement of platelet aggregation induced by CD was most evident when using low threshold concentrations of HvWf (0.25-1 μg/mL), although even at high concentrations of HvWf (5-10 μg/mL) an increase in aggregation was also observed. The enhancing effects of CD on ristocetin-induced platelet aggregation were observed using either washed platelets (Figure 1A) or PRP (Figure 1B). In control studies, preincubating platelets with a blocking antibody against GPIbα completely eliminated platelet aggregation induced by HvWf, in the presence or absence of CD (data not shown), demonstrating that aggregation was GPIb dependent. Moreover, pretreating platelets with CD alone or with CD in the presence of HvWf (without ristocetin) failed to induce platelet aggregation, demonstrating that CD was unlikely to be directly inducing vWf binding to GPIb (data not shown). Ristocetin dose-response studies were performed to investigate whether CD could enhance platelet aggregation at ristocetin concentrations less than 1 mg/mL, to produce a phenotype similar to that observed with platelet-type vWD. As demonstrated in Figure 1B, CD enhanced platelet aggregation when ristocetin was added to the reaction at 1 mg/mL, but it failed to induce aggregation at lower ristocetin concentrations (0.25-0.75 mg/mL). Studies using BvWf, which can bind GPIb and induce aggregation independent of artificial modulators, demonstrated CD enhancement of platelet aggregation induced by both low (1 μg/mL) and high concentrations (10 μg/mL) of BvWf (Figure 1A). These latter studies demonstrate that the effects of CD are not ristocetin dependent.
Cytochalasin D enhances vWf-induced platelet aggregation.
(A) Washed platelets (3 × 108/mL) were incubated with vehicle alone (−CD) or 5 μmol/L CD (+CD), then aggregated with the indicated concentrations of HvWf in the presence of 1 mg/mL ristocetin (i,ii) or BvWf alone (iii,iv). The aggregation tracings are from 1 experiment, representative of 10 independent experiments. (B) PRP was incubated with 5 μmol/L CD for 10 minutes followed by the addition of ristocetin at the indicated concentrations to initiate aggregation under stirred conditions.
Cytochalasin D enhances vWf-induced platelet aggregation.
(A) Washed platelets (3 × 108/mL) were incubated with vehicle alone (−CD) or 5 μmol/L CD (+CD), then aggregated with the indicated concentrations of HvWf in the presence of 1 mg/mL ristocetin (i,ii) or BvWf alone (iii,iv). The aggregation tracings are from 1 experiment, representative of 10 independent experiments. (B) PRP was incubated with 5 μmol/L CD for 10 minutes followed by the addition of ristocetin at the indicated concentrations to initiate aggregation under stirred conditions.
In further studies we confirmed that the enhancing effect of CD on vWf-induced aggregation was due to inhibition of actin polymerization rather than a direct effect on either vWf or GPIb/V/IX. As demonstrated in Figure 2A, concentrations of CD between 0.5 and 5 μmol/L enhanced platelet aggregation induced by BvWf in a dose-dependent manner that correlated closely with the inhibition of actin polymerization (Figure 2B). Moreover, treating platelets with a structurally unrelated inhibitor of actin polymerization, LB, also dramatically enhanced platelet aggregation induced by HvWf/ristocetin (Figure 2C) or BvWf (data not shown). LB is a potent marine toxin that sequesters actin monomers and prevents elongation of pre-existing actin filaments.34 As with CD, the effects of LB on vWf-induced platelet aggregation were dose-dependent and correlated closely with its ability to inhibit actin polymerization (data not shown). The importance of metabolically active platelets for the enhancing effects of CD or LB was demonstrated using formalin-fixed platelets. In these studies, neither CD or LB enhanced vWf-mediated agglutination of fixed platelets (data not shown).
Inhibition of actin polymerization enhances vWf-induced platelet aggregation.
(A) Washed platelets (3 × 108/mL) were treated with the indicated concentration of CD prior to the performance of aggregation studies using BvWf (1 μg/mL). The aggregation tracings are from 1 experiment, representative of 5. (B) Washed platelets were treated with the indicated concentrations of CD prior to platelet stimulation with thrombin (1 U/mL) for 10 minutes. Cells were then lysed and F-actin content in the whole-cell lysates determined by actin filament sedimentation assays as described under “Materials and methods.” Results are the mean ± SE from 4 independent experiments. (C) Washed platelets were incubated with either vehicle alone (−LB) or 200 ng/mL latrunculin B (+LB), prior to the initiation of platelet aggregation with HvWf (0.5 μg/mL) in the presence of ristocetin (1 mg/mL). The aggregation tracings are from 1 experiment, representative of 3 performed in duplicate.
Inhibition of actin polymerization enhances vWf-induced platelet aggregation.
(A) Washed platelets (3 × 108/mL) were treated with the indicated concentration of CD prior to the performance of aggregation studies using BvWf (1 μg/mL). The aggregation tracings are from 1 experiment, representative of 5. (B) Washed platelets were treated with the indicated concentrations of CD prior to platelet stimulation with thrombin (1 U/mL) for 10 minutes. Cells were then lysed and F-actin content in the whole-cell lysates determined by actin filament sedimentation assays as described under “Materials and methods.” Results are the mean ± SE from 4 independent experiments. (C) Washed platelets were incubated with either vehicle alone (−LB) or 200 ng/mL latrunculin B (+LB), prior to the initiation of platelet aggregation with HvWf (0.5 μg/mL) in the presence of ristocetin (1 mg/mL). The aggregation tracings are from 1 experiment, representative of 3 performed in duplicate.
A recent report has suggested that pretreating platelets with CD can induce fibrinogen binding to integrin αIIbβ3,35 raising the possibility that inhibiting actin polymerization may lead to a general enhancement in platelet aggregation induced by threshold concentrations of agonists. To investigate the specificity of the effects of CD on vWf-induced platelet aggregation, aggregation studies were performed with multiple physiologic agonists including thrombin, collagen, or ADP. As demonstrated in Figure 3, pretreating platelets with CD failed to enhance platelet aggregation induced by either low or high concentrations of the indicated agonist. In general, inhibiting actin polymerization resulted in a reduction in the rate and extent of platelet aggregation induced by each agonist. Similar observations were made with platelets pretreated with LB (data not shown), confirming that the enhancing effect of these inhibitors was specific to vWf-mediated platelet aggregation.
Cytochalasin D specifically enhances vWf-induced platelet aggregation.
Washed platelets (3 × 108/mL) were incubated with either vehicle alone (−) or 5 μmol/L CD (+) prior to the initiation of platelet aggregation with the indicated concentrations of thrombin (A), collagen (B), ADP (C), HvWf (0.5 μg/mL) and ristocetin (1 mg/mL), or BvWf (1 μg/mL). These aggregation tracings are from 1 experiment, representative of 5. The accompanying bar graph is a quantitative representation of the effects of CD on the rate of platelet aggregation induced by low concentrations of the indicated agonist. Results are presented as the fold increase in the initial rate of aggregation relative to Me2SO-treated platelets. Results are the mean ± SE from 5 experiments.
Cytochalasin D specifically enhances vWf-induced platelet aggregation.
Washed platelets (3 × 108/mL) were incubated with either vehicle alone (−) or 5 μmol/L CD (+) prior to the initiation of platelet aggregation with the indicated concentrations of thrombin (A), collagen (B), ADP (C), HvWf (0.5 μg/mL) and ristocetin (1 mg/mL), or BvWf (1 μg/mL). These aggregation tracings are from 1 experiment, representative of 5. The accompanying bar graph is a quantitative representation of the effects of CD on the rate of platelet aggregation induced by low concentrations of the indicated agonist. Results are presented as the fold increase in the initial rate of aggregation relative to Me2SO-treated platelets. Results are the mean ± SE from 5 experiments.
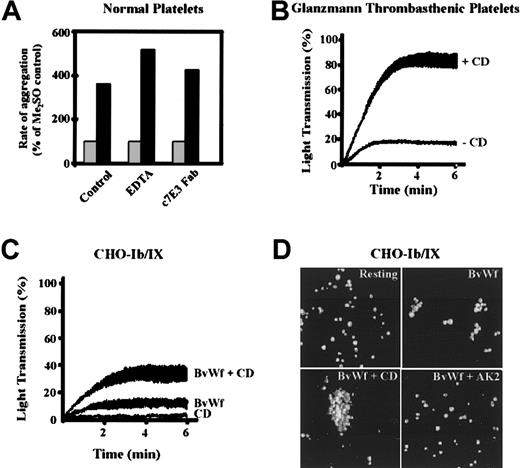
To determine whether the enhancing effect of CD on platelet aggregation was primarily due to effects on the vWf-GPIb interaction or involved integrin αIIbβ3, ligand binding to αIIbβ3 was prevented by preincubating washed platelets with either the anti-β3 integrin antibody c7E3 Fab or EDTA. As demonstrated in Figure4A, inhibiting ligand binding to integrin αIIbβ3 did not inhibit the ability of CD to enhance the rate and extent of platelet aggregation induced by HvWf/ristocetin or BvWf (data not shown). Similarly, studies using Glanzmann thrombasthenic platelets also demonstrated that the rate and extent of vWf-induced platelet aggregation was enhanced by CD (Figure4B), confirming that the effects of CD occurred independent of integrin αIIbβ3. To definitively establish that the enhancing effects of CD were specific to the vWf-GPIb interaction and did not involve other platelet adhesion receptors or endogenous platelet stimuli, studies were performed on CHO cells transfected with the GPIb/V/IX complex. Consistent with previous reports,36the addition of HvWf/ristocetin or BvWf to a stirred suspension of CHO-Ib/IX cells induced the formation of macroscopic CHO cell aggregates. The formation of these aggregates could be analyzed in real time on the basis of changes in light transmission using a platelet aggregometer. Similar to platelets, pretreating CHO-Ib/IX cells with CD enhanced both the rate and extent of CHO cell aggregation (Figure 4C). Overall the extent of CHO cell aggregation was less than that observed with platelets, presumably due to the lower GPIb/V/IX receptor density on CHO cells. As with platelets, the enhancing effects of CD were most obvious when using threshold concentrations of vWf to aggregate the cells. Confocal imaging of CHO cell aggregates demonstrated that BvWf alone induced small aggregates consisting of approximately 5 to 15 cells (Figure 4D), whereas the aggregates formed in the presence of CD consisted of more than 50 to 100 cells (Figure 4D). In control studies, CHO-Ib/IX cells preincubated with the anti-GPIb antibody, AK2, or CHO cells transfected with GP Ibβ/IX failed to aggregate in response to BvWf, in the presence or absence of CD (Figure 4D and data not shown). In all studies, CHO-Ib/IX cells were aggregated in the presence of EDTA to exclude a role for endogenous CHO cell integrins in the aggregation process. Taken together, these studies provide strong evidence that the enhancing effect of CD on vWf-induced aggregation is due to modulation of the vWf-GPIb/V/IX interaction.
Cytochalasin D enhances vWf-induced platelet and CHO-Ib/IX cell aggregation independent of integrin αIIbβ3.
(A) Washed platelets (3 × 108/mL) from healthy donors were incubated with vehicle alone (0.25% Me2SO, ░) or CD (5 μmol/L, ▪). Platelet aggregation was induced with HvWf (0.5 μg/mL) and ristocetin (1 mg/mL), in the presence of buffer alone (control), EDTA (2 mmol/L), or c7E3 Fab (20 μg/mL). Results are presented as percent change in aggregation rate (percent relative to Me2SO control, arbitrarily defined as 100%). (B) Glanzmann thrombasthenic platelets were aggregated with HvWf (0.5 μg/mL) and ristocetin, in the absence (−CD) or presence of 5 μmol/L CD (+CD). The aggregation tracings are from one experiment performed in triplicate. (C) CHO-Ib/IX cells (1 × 106/assay) were stirred for 6 minutes in the presence 5 μmol/L CD alone (CD), 10 μg/ml BvWf (BvWf), or BvWf and CD (BvWf + CD) in a platelet aggregometer. The aggregation tracings are from 1 experiment representative of 5. (D) CHO-Ib/IX cells were stirred in the presence of vehicle alone (resting), 10 μg/mL BvWf (BvWf), BvWf and 5 μmol/L CD (BvWf + CD), or BvWf in the presence of the anti-GPIb mAb, AK2 (BvWf + AK2). The cells were then fixed, stained with DiOC6 (1 μmol/L), mounted onto glass slides and subjected to confocal microscopy (10 × objective) as described under “Materials and methods.” The images of aggregated cells were reconstructed in 3-dimension using Voxblast (Vaytek Inc). Results presented are from 1 experiment, representative of 5.
Cytochalasin D enhances vWf-induced platelet and CHO-Ib/IX cell aggregation independent of integrin αIIbβ3.
(A) Washed platelets (3 × 108/mL) from healthy donors were incubated with vehicle alone (0.25% Me2SO, ░) or CD (5 μmol/L, ▪). Platelet aggregation was induced with HvWf (0.5 μg/mL) and ristocetin (1 mg/mL), in the presence of buffer alone (control), EDTA (2 mmol/L), or c7E3 Fab (20 μg/mL). Results are presented as percent change in aggregation rate (percent relative to Me2SO control, arbitrarily defined as 100%). (B) Glanzmann thrombasthenic platelets were aggregated with HvWf (0.5 μg/mL) and ristocetin, in the absence (−CD) or presence of 5 μmol/L CD (+CD). The aggregation tracings are from one experiment performed in triplicate. (C) CHO-Ib/IX cells (1 × 106/assay) were stirred for 6 minutes in the presence 5 μmol/L CD alone (CD), 10 μg/ml BvWf (BvWf), or BvWf and CD (BvWf + CD) in a platelet aggregometer. The aggregation tracings are from 1 experiment representative of 5. (D) CHO-Ib/IX cells were stirred in the presence of vehicle alone (resting), 10 μg/mL BvWf (BvWf), BvWf and 5 μmol/L CD (BvWf + CD), or BvWf in the presence of the anti-GPIb mAb, AK2 (BvWf + AK2). The cells were then fixed, stained with DiOC6 (1 μmol/L), mounted onto glass slides and subjected to confocal microscopy (10 × objective) as described under “Materials and methods.” The images of aggregated cells were reconstructed in 3-dimension using Voxblast (Vaytek Inc). Results presented are from 1 experiment, representative of 5.
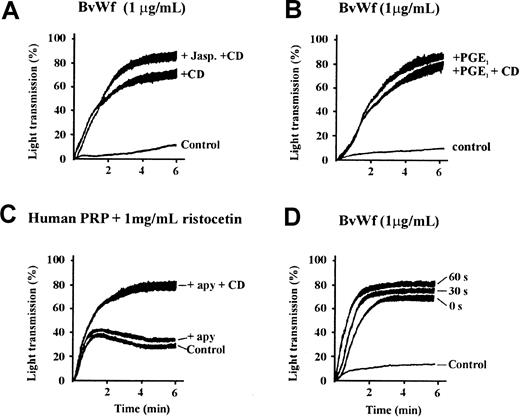
To investigate further the mechanism by which CD enhances vWf-induced platelet aggregation, in particular the role of actin filament depolymerization in this process, we examined the effect of the actin filament-stabilizing reagent, jasplakinolide. This reagent is a membrane-permeable cyclic peptide derived from the marine spongeJaspis johnstoni, which binds to and stabilizes pre-existing actin filaments, preventing filament depolymerization. Jasplakinolide has recently been demonstrated to effectively inhibit CD-induced integrin αIIbβ3 activation on the surface of human platelets.35 As demonstrated in Figure5A, pretreating washed platelets with jasplakinolide at concentrations that have previously been demonstrated to inhibit actin filament disassembly and completely inhibit platelet spreading (see “Materials and methods”) did not prevent the ability of CD to enhance vWf-induced platelet aggregation. Similar findings were apparent using LB (data not shown). These findings suggest that the primary mechanism by which CD and LB promote vWf-induced platelet aggregation is through inhibition of actin polymerization, rather than as a result of actin filament depolymerization. To investigate further the relationship between inhibition of actin polymerization and enhanced vWf-induced platelet aggregation, we examined the effect of the platelet activation inhibitor, PGE1. This reagent is a potent activator of the adenyl cyclase signaling pathway resulting in down-regulation of multiple platelet functional responses, including vWf-induced actin polymerization.29 As demonstrated in Figure 5B, pretreating platelets with PGE1 dramatically enhanced platelet aggregation induced by a threshold concentration of vWf, similar in magnitude to that observed with CD or LB. CD did not promote PGE1-enhanced aggregation further, indicating that the effects of these reagents were not additive. The ability of PGE1 to enhance vWf-induced platelet aggregation is consistent with its ability to inhibit actin polymerization, because a number of other signal transduction inhibitors, including inhibitors of tyrosine kinases (genistein, tyrphostin, or erbstatin), phosphoinositide (PI) 3-kinase (wortmannin or LY294002), protein kinase C (PKC) (calphostin C or bisindoylmaleimide), or prostaglandin metabolism (aspirin), that do not inhibit vWf-induced actin polymerization,29 failed to enhance the aggregation response (data not shown). These latter findings are consistent with the jasplakinolide data, suggesting enhanced platelet aggregation is due to inhibition of actin polymerization rather than disassembly of pre-existing actin filaments.
Cytochalasin D enhances vWf-induced platelet aggregation independent of actin filament severing or ADP.
(A) Washed platelets (3 × 108/mL) were preincubated with vehicle alone (control) or 5 μmol/L jasplakinolide (+ Jasp), and/or 5 μmol/L CD (+CD) for 10 minutes, prior to aggregation with 1 μg/mL BvWf. All aggregations were performed in the presence of 2 mmol/L EDTA. The aggregation tracings are from 1 experiment representative of 4 independent experiments performed in triplicate. (B) Washed platelets were preincubated with vehicle alone (control), 0.5 μg/mL PGE1 (+ PGE1) or PGE1 and 5 μmol/L CD (+ PGE1 + CD), then aggregated with BvWf (1 μg/mL). The tracings shown are from 1 experiment, representative of 5 independent experiments. (C) PRP was preincubated with either vehicle alone (control) or 5 μmol/L CD (+CD) for 10 minutes in the presence (+ apy) or absence of 0.5 U/mL apyrase. Aggregation of platelets was initiated with 1 mg/mL ristocetin. All aggregations were performed in the presence of anti-β3 antibody, c7E3 Fab (20 μg/mL), to prevent vWf binding to integrin αIIbβ3. The aggregation tracings are from 1 experiment representative of 4 individual experiments performed in duplicate. (D) Washed platelets were preincubated with either vehicle alone (control) or 5 μmol/L CD for 0, 30, or 60 seconds, then aggregated with 1 μg/mL BvWf. All aggregations were performed in the presence of 2 mmol/L EDTA to block ligand binding to integrin αIIbβ3. The aggregation tracings are from 1 experiment representative of 5 individual experiments performed in duplicate.
Cytochalasin D enhances vWf-induced platelet aggregation independent of actin filament severing or ADP.
(A) Washed platelets (3 × 108/mL) were preincubated with vehicle alone (control) or 5 μmol/L jasplakinolide (+ Jasp), and/or 5 μmol/L CD (+CD) for 10 minutes, prior to aggregation with 1 μg/mL BvWf. All aggregations were performed in the presence of 2 mmol/L EDTA. The aggregation tracings are from 1 experiment representative of 4 independent experiments performed in triplicate. (B) Washed platelets were preincubated with vehicle alone (control), 0.5 μg/mL PGE1 (+ PGE1) or PGE1 and 5 μmol/L CD (+ PGE1 + CD), then aggregated with BvWf (1 μg/mL). The tracings shown are from 1 experiment, representative of 5 independent experiments. (C) PRP was preincubated with either vehicle alone (control) or 5 μmol/L CD (+CD) for 10 minutes in the presence (+ apy) or absence of 0.5 U/mL apyrase. Aggregation of platelets was initiated with 1 mg/mL ristocetin. All aggregations were performed in the presence of anti-β3 antibody, c7E3 Fab (20 μg/mL), to prevent vWf binding to integrin αIIbβ3. The aggregation tracings are from 1 experiment representative of 4 individual experiments performed in duplicate. (D) Washed platelets were preincubated with either vehicle alone (control) or 5 μmol/L CD for 0, 30, or 60 seconds, then aggregated with 1 μg/mL BvWf. All aggregations were performed in the presence of 2 mmol/L EDTA to block ligand binding to integrin αIIbβ3. The aggregation tracings are from 1 experiment representative of 5 individual experiments performed in duplicate.
Recent studies by Bennett and colleagues35 have suggested that the cytoskeletal regulation of integrin αIIbβ3 requires a subthreshold concentration of ADP to induce slow actin filament turnover. We therefore examined the effects of the ADP scavenging enzyme, apyrase, on vWf-induced platelet aggregation. As demonstrated in Figure 5C, pretreating platelets with apyrase at concentrations that eliminate the stimulatory effects of ADP (see “Materials and methods”) did not significantly inhibit platelet aggregation induced by vWf. Moreover, apyrase did not affect the ability of CD to enhance vWf-induced platelet aggregation (Figure 5C). Further evidence suggesting that slow actin filament turnover was not responsible for the effects of the cytoskeleton on GPIb/V/IX was derived from CD time-course experiments. As demonstrated in Figure 5D, the enhancing effect of CD on platelet aggregation was extremely rapid, occurring within seconds of CD addition to the reaction mixture and reaching a maximum after 30 to 60 seconds of preincubation. Taken together, the results presented in Figure 5 suggest that the mechanism of cytoskeletal regulation of GPIb/V/IX is distinct from that reported for integrin αIIbβ3 (see “Discussion”).
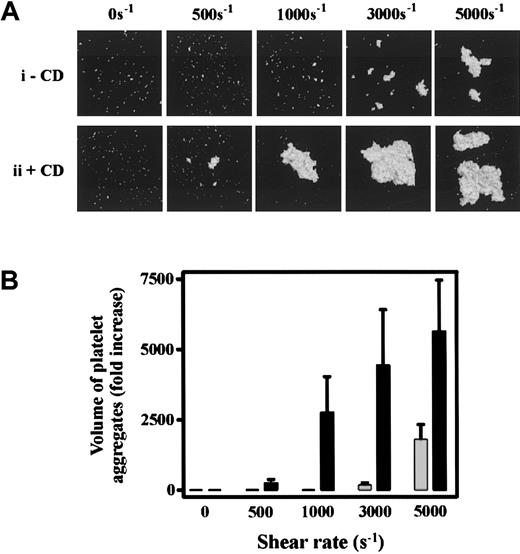
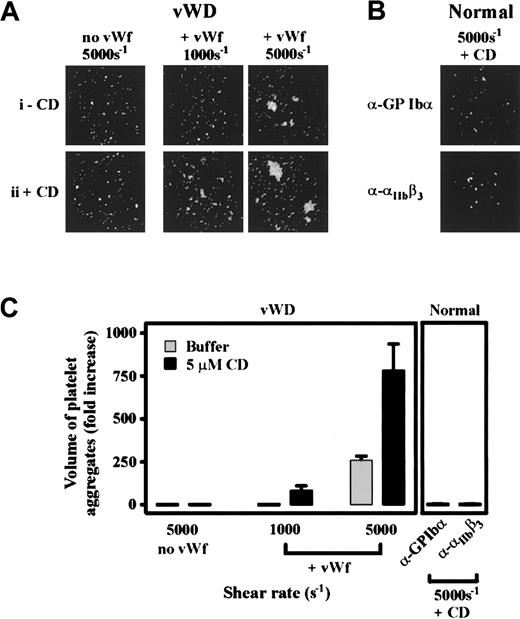
To investigate the significance of cytoskeletal regulation of GPIb/V/IX under pathophysiologically relevant shear conditions, we examined the effect of CD on SIPA. Consistent with previous reports,4-7exposure of PRP, or washed platelets in the presence of soluble vWf (10 μg/mL), to pathologic levels of shear (3000-5000 s−1) resulted in platelet aggregation. As demonstrated in Figure 6, the size of these aggregates increased as a function of shear (Figure 6Ai) and was dependent on ligand binding to both GPIb or integrin αIIbβ3, because pretreating platelets with blocking antibodies against either receptor inhibited SIPA (data not shown). Pretreating washed platelets or PRP with CD greatly enhanced the formation of platelet aggregates under high shear (Figure 6Aii,B) and also lowered the shear threshold required to induce platelet aggregation from pathologic (3000 s−1) to physiologic (500 s−1) levels of shear (Figure 6Aii). It is well established that at lower shear rates soluble agonists can induce platelet aggregation independent of vWf.11,37 To investigate the requirement for vWf in CD-enhanced platelet aggregation, studies were performed on platelets derived from an individual with type III vWD. Washed platelets or PRP failed to aggregate in response to intermediate (500-1000 s−1) or high shear (5000 s−1) in the presence or absence of CD (Figure 7A and data not shown). The addition of soluble vWf (20 μg/mL) to vWD- washed platelets or PRP restored SIPA, as well as their heightened responsiveness to shear in the presence of CD (Figure 7A). Interestingly, these aggregates were substantially smaller than aggregates from normal platelets, consistent with the potentially important role for platelet vWf in promoting SIPA.37 Moreover, under all shear conditions (500-5000 s−1) CD-enhanced aggregation of platelets was prevented by pretreating platelets with antibodies against GPIb or integrin αIIbβ3 (Figure 7B). Taken together, these findings suggest that inhibiting actin polymerization reduces the shear threshold by which the vWf-GPIb interaction induces platelet activation and integrin αIIbβ3-mediated irreversible aggregation.
Cytochalasin D enhances shear-induced platelet aggregation.
(A) Washed platelets (1.5 × 108/mL) were incubated with either vehicle alone (−CD, i) or 5 μmol/L CD (+CD, ii) in the presence of HvWf (10 μg/mL). Platelets were then exposed to the indicated shear rates in a cone-and-plate viscometer as described under “Materials and methods.” Platelets were then fixed, stained with 1 μmol/L DiOC6, mounted onto glass slides, and subjected to confocal microscopy (20 × objective). The 3-dimensional images of aggregated platelets were reconstructed using Voxblast (Vaytek Inc). Results presented are from 1 experiment, representative of 5 independent experiments. (B) Platelet aggregates from 8 random fields (10 × objective) were subjected to volumetric analysis, and the extent of aggregation was expressed as the fold increase in the volume of aggregates over that detected for Me2SO-treated control platelets. Results are the mean ± SE from 5 experiments. ░, buffer; ▪, 5 μmol/L CD.
Cytochalasin D enhances shear-induced platelet aggregation.
(A) Washed platelets (1.5 × 108/mL) were incubated with either vehicle alone (−CD, i) or 5 μmol/L CD (+CD, ii) in the presence of HvWf (10 μg/mL). Platelets were then exposed to the indicated shear rates in a cone-and-plate viscometer as described under “Materials and methods.” Platelets were then fixed, stained with 1 μmol/L DiOC6, mounted onto glass slides, and subjected to confocal microscopy (20 × objective). The 3-dimensional images of aggregated platelets were reconstructed using Voxblast (Vaytek Inc). Results presented are from 1 experiment, representative of 5 independent experiments. (B) Platelet aggregates from 8 random fields (10 × objective) were subjected to volumetric analysis, and the extent of aggregation was expressed as the fold increase in the volume of aggregates over that detected for Me2SO-treated control platelets. Results are the mean ± SE from 5 experiments. ░, buffer; ▪, 5 μmol/L CD.
An essential role for vWf, GPIb/V/IX, and integrin αIIbβ3 in mediating shear-induced platelet aggregation in the presence or absence of cytochalasin D.
(A) Platelet-rich plasma (PRP) from an individual with type III vWD was incubated with either vehicle alone (−CD, i) or 5 μmol/L CD (+CD, ii) prior to exposing platelets to a shear rate of 5000 s−1 (top panel). Alternatively, purified HvWf (20 μg/mL) was added to vWD PRP (+ vWf) prior to their exposure to the indicated shear rates. The size of platelet aggregates formed under the various experimental conditions was determined as described in Figure6. (B) Washed platelets from a normal donor were incubated with an anti-GPIb (ALMA12) or anti-β3 integrin mAb (c7E3 Fab) for 10 minutes in the presence of 5 μmol/L CD (+ CD) prior to the exposure of platelets to shear (5000 s−1). Platelets were then imaged as described in Figure 6. (C) Platelet aggregates from 8 random fields (10 × objective) were subjected to volumetric analysis, and the extent of aggregation was expressed as the fold increase in the volume of aggregates over that detected for Me2SO-treated control platelets. Results for vWD platelets are from a single vWD patient and results for normal platelets are the mean ± SE from 5 experiments. ░, buffer; ▪, 5 μmol/L.
An essential role for vWf, GPIb/V/IX, and integrin αIIbβ3 in mediating shear-induced platelet aggregation in the presence or absence of cytochalasin D.
(A) Platelet-rich plasma (PRP) from an individual with type III vWD was incubated with either vehicle alone (−CD, i) or 5 μmol/L CD (+CD, ii) prior to exposing platelets to a shear rate of 5000 s−1 (top panel). Alternatively, purified HvWf (20 μg/mL) was added to vWD PRP (+ vWf) prior to their exposure to the indicated shear rates. The size of platelet aggregates formed under the various experimental conditions was determined as described in Figure6. (B) Washed platelets from a normal donor were incubated with an anti-GPIb (ALMA12) or anti-β3 integrin mAb (c7E3 Fab) for 10 minutes in the presence of 5 μmol/L CD (+ CD) prior to the exposure of platelets to shear (5000 s−1). Platelets were then imaged as described in Figure 6. (C) Platelet aggregates from 8 random fields (10 × objective) were subjected to volumetric analysis, and the extent of aggregation was expressed as the fold increase in the volume of aggregates over that detected for Me2SO-treated control platelets. Results for vWD platelets are from a single vWD patient and results for normal platelets are the mean ± SE from 5 experiments. ░, buffer; ▪, 5 μmol/L.
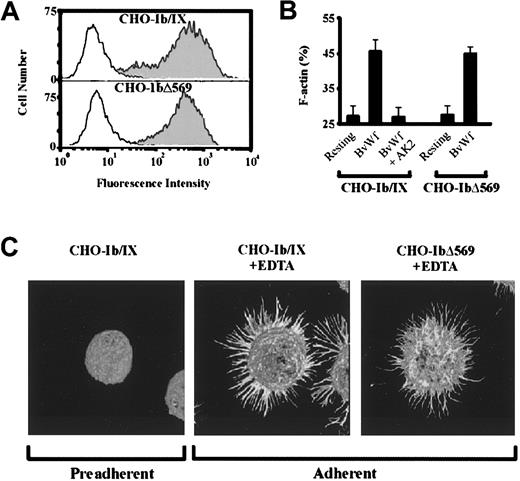
To investigate the relationship between the cytoskeletal linkage of GPIb/V/IX and the ability of CD to enhance cell aggregation, studies were performed on CHO cells expressing mutant forms of the GPIb/V/IX receptor complex. We initially generated a GPIbα cytoplasmic tail truncation mutant lacking the C-terminal 41 amino acids (CHO-IbΔ569), which has previously been demonstrated not to associate with the cytoskeleton.38 Our immunoprecipitation studies using biotin-labeled CHO cells have confirmed that this deletion is sufficient to disrupt the interaction between GPIbα and ABP (data not shown). Flow cytometric analysis of CHO cells expressing mutant GPIbΔ569 demonstrated efficient expression of the glycoprotein on the surface of CHOβIX cells (CHO-IbΔ569), similar to the level of wild-type GPIbα on CHO-Ib/IX (Figure8A). We also confirmed that GPIbΔ569 was able to bind vWf by the ability to tether and roll on a BvWf matrix under flow conditions in a similar manner to CHO-Ib/IX (data not shown). Previous studies have demonstrated that CHO cells expressing GPIbΔ569 extend filopodia and spread on a vWf matrix,38suggesting that the linkage between GPIb and ABP-280 is uncoupled from vWf-induced cytoskeletal reorganization. However, this conclusion is complicated by the observations that cytoskeletal reorganization in GPIb/V/IX-transfected CHO cells is not strictly dependent on vWf binding to GPIb/V/IX but also involves activation of endogenous CHO cell integrins.29 39 To investigate the ability of vWf to induce actin polymerization in CHO cells expressing a truncated form of GPIbα (CHO-Δ569), we performed DNaseI inhibition assays on whole-cell lysates prepared from nonaggregated or vWf-aggregated CHO-IbΔ569 cells (Figure 8B). As demonstrated, vWf-induced aggregation of CHO-IbΔ569 was associated with a 60% increase in the level of F-actin from a resting level of 27% up to a maximum of 45%. This increase was similar to that observed with CHO-Ib/IX and was abolished by preincubating these cells with the anti-GPIb mAb, AK2. A similar increase in F-actin was observed in vWf-aggregated CHO-Ib/IX and CHOΔ569 cells by monitoring changes in F-actin through FACS analysis of cells labeled with Alexa488-phalloidin (data not shown). Moreover, adhesion of CHO-IbΔ569 cells to immobilized HvWf in the presence of botrocetin was associated with cytoskeletal reorganization leading to filopodial extension, similar to that observed with CHO cells expressing the wild-type receptor (Figure 8C). It should be noted that all experiments were performed in the presence of EDTA, excluding a role for endogenous integrins in mediating these cytoskeletal changes. Similar cytoskeletal changes were obtained with an additional GPIbα cytoplasmic tail mutant that lacks the primary ABP-280 binding domain (deletion of residues 535-568, CHO-IbΔ535, data not shown), confirming that vWf-induced actin polymerization and cytoskeletal reorganization do not require physical linkage between GPIb and the cytoskeleton.
vWf induces actin polymerization in CHO-Ib/IX and CHO-IbΔ569 cells.
(A) Surface expression of GPIbα on CHO-Ib/IX and CHO-IbΔ569 cells was examined by FACS analysis using the anti-GPIb mAb, ALMA12 (filled histogram), as detailed under “Materials and methods.” (B) CHO-Ib/IX and CHO-IbΔ569 cells (3 × 106/mL) were stirred in the presence of buffer (resting) or BvWf (10 μg/mL) for 20 minutes. Where indicated, cells were also incubated with an anti-GPIb mAb, AK2 (5 μg/mL), prior to the initiation of aggregation. The cells were then lysed and F-actin contents in the whole lysates determined using the DNaseI inhibition assay. Results are the mean ± SE from 3 experiments, performed in duplicate. (C) CHO-Ib/IX cells (1 × 106/mL) were fixed in suspension (preadherent) with 3.7% formaldehyde for 10 minutes, prior to adhesion to poly-l-lysine (100 μg/mL)–coated coverslips. Alternatively, CHO-Ib/IX or CHO-IbΔ569 cells were adhered to a HvWf matrix (10 μg/mL) in the presence of 1 μg/mL botrocetin and 2 mmol/L EDTA for 60 minutes (adherent). Adherent cells were fixed, permeabilized, stained with fluorescein isothiocyanate-conjugated phalloidin and subjected to confocal fluorescence microscopy (100 × objective) as described under “Materials and methods.” The images presented were reconstructed using VoxBlast software and are representative of 5 independent experiments.
vWf induces actin polymerization in CHO-Ib/IX and CHO-IbΔ569 cells.
(A) Surface expression of GPIbα on CHO-Ib/IX and CHO-IbΔ569 cells was examined by FACS analysis using the anti-GPIb mAb, ALMA12 (filled histogram), as detailed under “Materials and methods.” (B) CHO-Ib/IX and CHO-IbΔ569 cells (3 × 106/mL) were stirred in the presence of buffer (resting) or BvWf (10 μg/mL) for 20 minutes. Where indicated, cells were also incubated with an anti-GPIb mAb, AK2 (5 μg/mL), prior to the initiation of aggregation. The cells were then lysed and F-actin contents in the whole lysates determined using the DNaseI inhibition assay. Results are the mean ± SE from 3 experiments, performed in duplicate. (C) CHO-Ib/IX cells (1 × 106/mL) were fixed in suspension (preadherent) with 3.7% formaldehyde for 10 minutes, prior to adhesion to poly-l-lysine (100 μg/mL)–coated coverslips. Alternatively, CHO-Ib/IX or CHO-IbΔ569 cells were adhered to a HvWf matrix (10 μg/mL) in the presence of 1 μg/mL botrocetin and 2 mmol/L EDTA for 60 minutes (adherent). Adherent cells were fixed, permeabilized, stained with fluorescein isothiocyanate-conjugated phalloidin and subjected to confocal fluorescence microscopy (100 × objective) as described under “Materials and methods.” The images presented were reconstructed using VoxBlast software and are representative of 5 independent experiments.
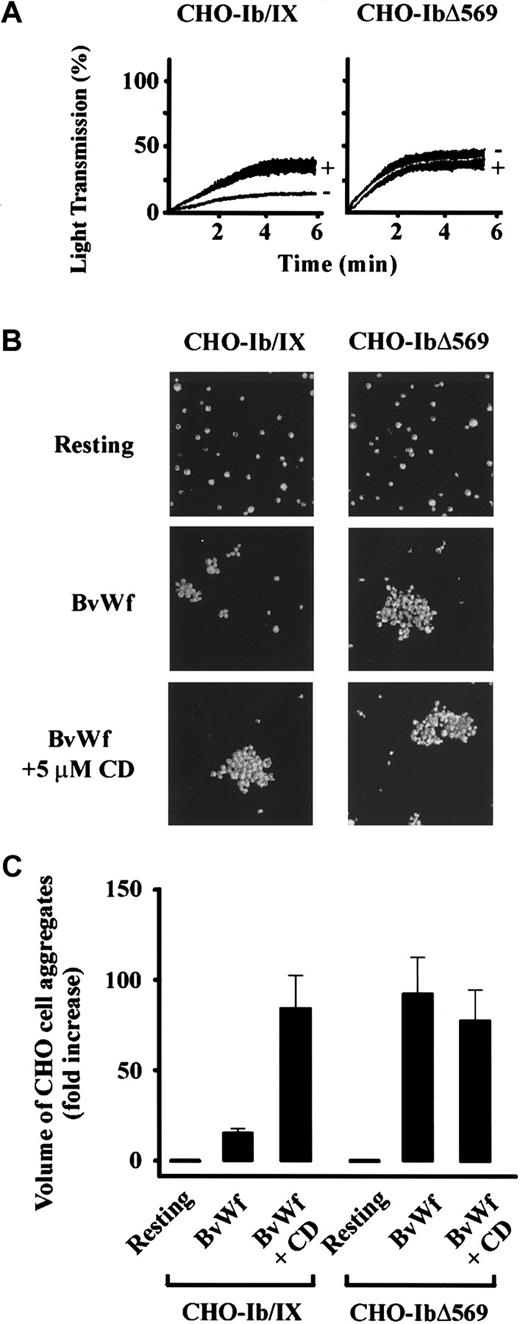
To determine the effects of disrupting the link between GPIb and the cytoskeleton on the ability of CD to enhance vWf-induced cell aggregation, comparative aggregation studies were performed on CHO-IbΔ569 and CHO-Ib/IX. As demonstrated in Figure9A, CHO-IbΔ569 stirred in the presence of BvWf aggregated more rapidly and to a much greater extent than CHO-Ib/IX. In contrast to CHO-Ib/IX, pretreating CHO-IbΔ569 with CD failed to enhance the aggregation process. It was unlikely that the inability of CD to enhance CHO-IbΔ569 cell aggregation was because these cells were maximally aggregated, because CD also failed to enhance aggregation of these cells when using threshold concentrations of BvWf (data not shown). Volumetric analysis of CHO-IbΔ569 cell aggregates following confocal imaging demonstrated that these aggregates were about 5-fold larger than CHO-Ib/IX in the absence of CD (Figure 9C), but were of similar size to CHO-Ib/IX cell aggregates formed in the presence of CD (Figure 9B,C). The increased aggregation response and insensitivity to CD was not unique to CHO-IbΔ569 because it was also observed with CHO-IbΔ535 (data not shown), confirming an important role for the GPIb–ABP-280 linkage in enabling cytoskeletal regulation of the vWf-GPIb interaction.
Disrupting the physical link between GPIbα and the cytoskeleton abolishes the ability of CD to enhance vWf-induced cell aggregation.
(A) CHO-Ib/IX and CHO-IbΔ569 cells (3 × 106/mL) were incubated with vehicle alone (−) or 5 μmol/L CD (+). The cells were then aggregated in a platelet aggregometer using BvWf (5 μg/mL). The aggregation traces are from 1 experiment, representative of 5 independent experiments. (B) CHO-Ib/IX and CHO-IbΔ569 cells (3 × 106/mL) were incubated with vehicle alone or 5 μmol/L CD, then stirred for 5 minutes in the presence of control buffer (resting) or BvWf (5 μg/mL). Cells were then fixed, stained, mounted onto glass slides, and subjected to confocal microscopy (10 × objective) as described for Figure 4D. Images were obtained from 1 experiment, representative of 5. (C) Cells from 8 random fields (10 × objective) were subjected to volumetric analysis, and the extent of cell aggregation was expressed as the fold increase in the volume of cell aggregates over that detected for Me2SO-treated control cells. Results are the mean ± SE from 5 experiments.
Disrupting the physical link between GPIbα and the cytoskeleton abolishes the ability of CD to enhance vWf-induced cell aggregation.
(A) CHO-Ib/IX and CHO-IbΔ569 cells (3 × 106/mL) were incubated with vehicle alone (−) or 5 μmol/L CD (+). The cells were then aggregated in a platelet aggregometer using BvWf (5 μg/mL). The aggregation traces are from 1 experiment, representative of 5 independent experiments. (B) CHO-Ib/IX and CHO-IbΔ569 cells (3 × 106/mL) were incubated with vehicle alone or 5 μmol/L CD, then stirred for 5 minutes in the presence of control buffer (resting) or BvWf (5 μg/mL). Cells were then fixed, stained, mounted onto glass slides, and subjected to confocal microscopy (10 × objective) as described for Figure 4D. Images were obtained from 1 experiment, representative of 5. (C) Cells from 8 random fields (10 × objective) were subjected to volumetric analysis, and the extent of cell aggregation was expressed as the fold increase in the volume of cell aggregates over that detected for Me2SO-treated control cells. Results are the mean ± SE from 5 experiments.
Discussion
A unique feature of the vWf-GPIb interaction is its positive regulation by high shear forces. Shear-induced binding of vWf to GPIb is a key step in the normal hemostatic process and may also promote pathologic thrombus formation in stenosed arteries and the microcirculation. Despite the fundamental importance of the vWf-GPIb interaction in hemostasis and thrombosis, the factors negatively regulating this adhesion event, particularly under elevated shear conditions, remain poorly defined. The studies presented here suggest for the first time a potentially important role for the cytoskeleton in regulating the adhesive function of GPIb/V/IX. Our studies have demonstrated that pretreating platelets or GPIb/V/IX-transfected CHO cells with inhibitors of actin polymerization selectively enhances cell aggregation induced by vWf. Moreover, our studies of CHO cells expressing mutant GPIb/V/IX complexes have demonstrated that the physical linkage between GPIb and the membrane skeleton is not required for vWf-induced actin polymerization but is essential for the enhancing effect of actin polymerization inhibitors on the aggregation process. Finally, studies of shear-induced platelet aggregation demonstrated that inhibiting actin polymerization not only enhanced the extent of platelet aggregation but also dramatically lowered the shear threshold required to induce the aggregation process. These latter findings raise the intriguing possibility that cytoskeletal regulation of the vWf-GPIb interaction plays a key role in preventing shear-induced platelet aggregation in the normal circulation.
Studies on Glanzmann thrombasthenic platelets and GPIb/V/IX-transfected CHO cells have demonstrated that CD enhances vWf-induced cell aggregation through regulation of GPIb/V/IX and does not require integrin αIIbβ3 or endogenous platelet stimuli. The recent studies of Bennett and coworkers35demonstrated that CD induces platelet integrin αIIbβ3 activation through a process requiring ADP-dependent actin filament turnover. These studies postulate that ADP-induced actin filament severing relieves cytoskeletal constraints on integrin αIIbβ3in resting platelets, leading to receptor activation. In contrast, our studies suggest that actin polymerization induced by vWf (as opposed to depolymerization of pre-existing filaments), is the primary mechanism by which the cytoskeleton regulates the adhesive function of GPIb/V/IX and is distinct from that observed with integrin αIIbβ3. For example, pretreating platelets with jasplakinolide to stabilize actin filaments does not prevent CD enhancement of vWf-induced aggregation. Moreover, the ability of PGE1 to enhance vWf-induced aggregation suggests that actin filament disassembly is not the primary mechanism by which CD and LB enhance the adhesive function of GPIb/V/IX. Two other lines of evidence indicate that slow actin filament turnover in resting platelets is not the principal mechanism of cytoskeletal regulation of GPIb/V/IX. First, removing the cellular effects of ADP did not inhibit CD enhancement of aggregation, and second, the effects of CD on aggregation were very rapid, occurring within seconds of its addition to the assay. Taken together, these studies suggest that the adhesive function of GPIb/V/IX is regulated by actin filament polymerization induced by vWf or other agonists, rather than depolymerization of pre-existing actin filaments.
Our studies of CHO-IbΔ569 cells demonstrate an important role for the cytoplasmic tail of GPIbα in regulating the adhesive function of the GPIb/V/IX receptor complex. We have demonstrated that CHO-IbΔ569 cells aggregate to a much greater extent in response to vWf than CHO cells expressing the wild-type receptor. Moreover, the aggregation response of CHO-IbΔ569 cells was not sensitive to the enhancing effects of CD. The simplest interpretation of these findings is that the mutant receptors are not subjected to the normal constraints imposed by the cytoskeleton, presumably as a consequence of the physical disruption of the link between GPIbα and the membrane skeleton. An alternative possibility is that mutating the GPIbα cytoplasmic tail alters the intrinsic ligand-binding properties of the receptor complex. We do not, however, favor this latter possibility for 3 reasons. First, we have not detected a major difference in the ability of CHO cells expressing GPIbΔ569, GPIbΔ535, or wild-type GPIb/V/IX to tether and roll on a vWf matrix under shear conditions (reference 31 and unpublished observations). Second, previous studies have demonstrated normal vWf binding to CHO cells expressing GPIbΔ569.38 Third, changes in the intrinsic binding characteristics of GPIb would not easily explain the inability of CD to enhance the aggregation process.
The ability of GPIbΔ569 (or GPIbΔ535) to support vWf-induced actin polymerization distinguishes GPIb/V/IX from other adhesion receptors, such as the integrins, which require physical linkage with the cytoskeleton to induce cytoskeletal remodelling.40 It remains to be established which structural domains of the GPIb/V/IX receptor complex are required for vWf-induced cytoskeletal reorganization and whether other surface receptors are involved in this process. For example, there is evidence for colocalization of GPIb/V/IX with FcγRIIA,41 and a number of recent reports suggest a contribution of this latter receptor to vWf-induced signaling.42-44 Our studies with CHO cells indicate that FcγRIIA is not essential for vWf-induced cytoskeletal changes because these cells totally lack Fc receptors.45 It should be noted that the GPIbΔ569-610 mutant lacks the 14-3-3ζ binding site,46 raising the interesting possibility that vWf-induced actin polymerization does not involve 14-3-3ζ. There is, however, evidence suggesting that 14-3-3ζ can associate with the tail of GPIbβ47,48 and that this binding is regulated by phosphorylation of serine-166 in the cytoplasmic tail of GPIbβ by protein kinase A (PKA).49 Studies are currently underway in our laboratory to examine the contribution of the cytoplasmic tail of GPIbβ to vWf-induced cytoskeletal reorganization, and in particular, the importance of phosphorylation of GPIbβ in this process.
A major finding from our studies is the ability of actin polymerization inhibitors to reduce the shear threshold required to induce platelet aggregation from about 3000 s−1 down to shear rates as low as 500 s−1. By performing studies on vWD platelets and using antibodies against GPIb and integrin αIIbβ3, we have established that CD-induced platelet aggregation at arterial shear rates (500-1000 s−1) is mediated by vWf engagement of both GPIb/V/IX and integrin αIIbβ3. The mechanism by which CD promotes vWf-induced platelet activation at physiologic shear rates is an important issue for future investigation. It is possible that pretreating platelets with CD alters the adhesive properties of GPIb/V/IX such that it has a higher “affinity” for soluble vWf at arterial shear rates. Alternatively, vWf binding to GPIb may be a normal ongoing process in the arterial circulation and inhibiting actin polymerization removes the inhibitory effects of the cytoskeleton on the vWf-GPIb interaction, thereby promoting platelet activation. Evidence supporting the hypothesis that vWf binding to GPIb is a normal ongoing process in the arterial circulation has been derived from studies of patients with elevated platelet counts.50 These individuals typically have reduced levels of high- molecular-weight vWf multimers in the circulation, presumably as a consequence of vWf binding to the expanded pool of GPIb. The ability of high-molecular-weight multimers to interact with GPIb under normal physiologic flow conditions is also supported by the observation that the plasma pool of vWf does not contain the very high-molecular-weight multimers present in platelet α-granules51 and in the Weibel-Palade bodies of endothelial cells.52 These multimers appear to be subjected to proteolytic regulation by a plasma metalloproteinase activity.53-56 Deficiency of this activity leads to vWf-induced platelet aggregation throughout the microcirculation, leading to organ failure and possibly death, highlighting the pathologic significance of unchecked shear-induced platelet aggregation in vivo.
A key issue for future investigation is to define the precise mechanism by which the cytoskeleton regulates the vWf-GPIb interaction. It is possible that actin polymerization induces a conformational change in the receptor that changes the kinetics of the vWf-GPIb bond. This may lead to a slower on-rate, rapid off-rate, or a combination of these events, leading to a reduced “affinity” of interaction. However, this seems unlikely for 2 reasons. First, pretreating platelets with CD did not induce platelet aggregation by vWf at low concentrations of ristocetin (< 1 mg/mL), as has been demonstrated for platelets expressing the mutant forms of GPIbα associated with platelet-type vWD57 that exhibit increased affinity for vWf. Second, previous vWf-binding studies on CHO cells expressing GPIbΔ569 did not reveal an increased affinity for vWf.38 An alternative possibility is that actin polymerization regulates GPIb receptor distribution on the cell surface, thereby influencing the number or “avidity” of the bonds formed between GPIb and the A1 domains of multimeric vWf. The anchorage of GPIb to the membrane skeleton is thought to play an important role in regulating the mobility of the receptor complex in the plane of the plasma membrane.22Thus, it is conceivable that vWf-induced cytoskeletal changes may alter receptor distribution on the cell surface such that a reduced number of GPIb molecules engage vWf. This may not only reduce the overall “avidity” of the vWf-GPIb adhesion interaction but also reduce the ability of these receptors to transduce signals necessary for integrin αIIbβ3 activation and irreversible platelet aggregation. A precedent for such cytoskeletal regulation of receptor distribution has been established from the studies of leukocyte integrin αLβ2 (LFA-1), in which inhibitors of actin polymerization induce receptor clustering leading to an increase in receptor avidity.58,59 A third possibility is that the cytoskeleton may mediate rapid GPIb/V/IX internalization following engagement of vWf. It is well established that platelet activation by physiologic agonists such as thrombin or ADP can induce internalization of GPIb/V/IX60,61 after 30 to 60 seconds of agonist stimulation.62 This internalization appears to require actin polymerization and cytoskeletal reorganization because it is completely prevented by pretreating platelets with CD.60 62 We have demonstrated that the effects of CD occur rapidly (within seconds of addition to platelets), suggesting that internalization of GPIb/V/IX is unlikely to be the primary mechanism regulating the vWf-GPIb/V/IX interaction. Studies are currently underway in our laboratory to address these various possibilities.
The ability of the cytoskeleton to regulate the vWf-GPIb interaction also has potentially important clinical considerations. A recent study37 has demonstrated that platelets from patients suffering an acute myocardial infarction have heightened reactivity to shear, leading to enhanced shear-induced platelet aggregation. This increased shear sensitivity appears to be due, at least in part, to an increase in the plasma level of vWf. Although purely speculative, our studies raise the interesting possibility that defects in the cytoskeletal regulation of GPIb/V/IX may represent a novel mechanism of promoting platelet reactivity in vivo, leading to excessive platelet adhesion and aggregation at sites of vascular injury. Unraveling the molecular mechanism by which vWf induces actin polymerization may not only improve our understanding of the mechanisms regulating platelet-platelet and platelet-vessel wall interactions, but may also have potentially important therapeutic implications, because pharmacologic modulation of this event may represent a novel approach to influence platelet reactivity in vivo.
Acknowledgments
We thank Prof Michael Berndt and Dr Robert Andrews for both their helpful discussions and for their generous donations of botrocetin and monoclonal antibodies. We would also like to thank Dr Jose Lopez for the GPIb/V/IX receptor constructs and CHOβ/IX cell line, Dr Corinne de la Salle for technical assistance, and Dr Simone Schoenwaelder for helpful advice.
Supported by a grant from the National Health and Medical Research Council of Australia. N.M. is a recipient of a Monash Graduate Scholarship.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Shaun P. Jackson, Australian Centre for Blood Diseases, Department of Medicine, Monash Medical School, Box Hill Hospital, Arnold St, Box Hill, Victoria 3128, Australia; e-mail:Shaun.Jackson@med.monash.edu.au.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal