Abstract
Engagement of cell surface adhesion receptors with extracellular constituents and with cellular counter-receptors is crucial for the extravasation of blood-borne neoplastic cells and their seeding at distant sites; however, the early events of tumor dissemination—ie, the intravasation step(s)—have been largely neglected. A role for the 4β7 integrin was hypothesized to explain the high leukemogenicity exhibited by one (NQ22) among several T-cell lymphomas studied. To clarify the mechanisms of early aggressivity, the behavior of highly and poorly leukemogenic cell lines were compared in vitro. Cocultivation of physically separated leukemic cells with resting endothelial cells resulted in the up-regulation of VCAM-1 expression. NQ22 cells expressed mRNA of different cytokines that up-regulate VCAM-1 and at higher levels than cells of a nonaggressive lymphoma, and they migrated more efficiently through an activated endothelial cell layer. With the use of neutralizing antibodies against interferon-γ, granulocyte macrophage colony-stimulating factor, and tumor necrosis factor (TNF)-, it was determined that TNF- is one of the soluble factors released by NQ22 cells involved in the up-regulation of VCAM-1. The finding that vascular cells within the early local growth were strongly positive for VCAM-1 indicated that NQ22 cells could activate endothelial cells also in vivo. Finally, cocultivation of preleukemic 4−NQ22 cells with TNF--activated endothelial cells induced the expression of 4 integrins on the former cells. Reciprocal up-regulation and engagement of 4/VCAM-1 pairs determined the sequential transmigration and intravasation steps, and similar mechanisms might affect the aggressivity of human T lymphoblastic lymphomas.
Tumor progression is a complex biologic process for which cells must possess a series of traits that enable them to complete the multiple and sequential steps involved: detachment and emigration from the primary site, invasion of surrounding tissues, entrance into blood or lymphatic vessels (intravasation), escape from the microvasculature (extravasation), seeding, and metastatic growth at distant target sites.1,2 The interactions of migratory lymphoma cells with extracellular matrix (ECM) components, with stromal cell elements, and with the endothelial cell surfaces are mediated by the engagement of specific and multiple adhesion molecules expressed on the disseminating cells.3 In addition, soluble cell membrane- and ECM-bound chemotactic factors contribute to this complex phenomenon. Numerous studies in several model systems have been conducted in the attempt to clarify the mechanisms regulating the various steps in lymphoma dissemination,4 and these have revealed that transmigration, locomotion, and localization to target organs resemble, at least in part, the physiology of leukocyte adhesion and homing.5 6
Although much has been learned concerning the role of the endothelium to allow blood-borne neoplastic cells to seed at distant sites, the early events leading to intravasation of malignant lymphoma cells have only occasionally been addressed. Elucidation of this issue is, however, of relevance in light of the finding that intravasation and the subsequent leukemic evolution of malignant lymphocytes adversely affect the clinical outcome of patients with non-Hodgkin's lymphoma. In particular, the switch from a sessile to a migratory condition after intravasation is often detected at the onset of human T-lymphoblastic lymphoma (T-LBL). The dissemination process represents a good example of early intravasation and could be considered a model system for the more generalized metastatic process of malignant cells. We have previously developed and partially characterized an experimental model, suitable for unraveling of this phenomenon, which consists of a series of AKR T-lymphoma cell lines with different leukemic potential.7-9 After subcutaneous (SC) inoculation, one of these lines (NQ22) showed a unique leukemic phenotype with early peripheral blood invasion and widespread organ dissemination. A similar behavior was detected in a spontaneous T-lymphoma in an SJL mouse.9 In contrast to several of our other poorly leukemogenic and locally growing lymphomas, NQ22 cells were the only ones displaying de novo expression of the α4β7 integrin complexes on their surfaces concomitantly with in vivo spreading.8,9Furthermore, using a less leukemogenic NQ22 variant, a direct relationship was disclosed between the leukemic phenotype exhibited by the cells and the in vivo induction, among a number of other cell adhesion molecules investigated, such as Pgp-1/CD44, Mel 14, LFA-1, ICAM-1, and CD26,8 only of the α4β7 integrin complex.9 Interestingly, a similar correlation was found in a series of pediatric T-LBL in which the early bone marrow infiltration correlated with the expression of the α4β7 integrin on leukemic T blasts.9
The α4β1 and α4β7 integrins are expressed on most leukocytes and are well established to function as receptors for the vascular cell adhesion molecule 1 (VCAM-1), the mucosal addressin cell adhesion molecule 1 (MAdCAM-1), and fibronectin (FN).10-14 By virtue of their ability to interact with FN and with endothelial cells, α4β1 and α4β7 are believed to play an important role in leukocyte–endothelial interactions.15-18 VCAM-1 has a crucial role in supporting the capture and the immobilization of normal and neoplastic leukocytes in the bloodstream. Furthermore, the expression of VCAM-1 is induced by various stimuli including lipopolysaccharide and a number of cytokines: IL-1β, IL-4, IFN-γ, tumor necrosis factor (TNF)-α, granulocyte macrophage-colony stimulating factor (GM-CSF).19-25 In their extravascular condition, lymphoma cells interact with proteins of the ECM, with FN representing a prototype constituent of ECM, that could be considered inducers and regulators of the migration and transendothelial processes leading to massive dissemination to distant sites.
In the current study we hypothesized that the aggressive behavior of highly leukemogenic NQ22 cells, enabling their efficient and rapid migration from the inoculation site, could result from interactions with ECM and endothelial cells. NQ22 cells recognized and bound to VCAM-1 and up-regulated the expression of VCAM-1 on endothelial cell lines grown in vitro. This in vitro activity, at least partly dependent on TNF-α released from NQ22 cells, was reflected in the strong VCAM-1 positivity of endothelial cells in the SC inoculation site of mice injected with NQ22 cells but not with cells of other T-lymphoma cell lines. Thus, the engagement of α4/VCAM-1 pair(s) appear(s) to be involved in the controlled and sequential transmigration and intravasation of the highly malignant NQ22 lymphoma cells.
Materials and methods
Antibodies and proteins
The monoclonal antibodies (mAbs) used in this study were the following: L3T4 (CD4) and Lyt-2 (CD8) were purchased from Becton Dickinson (Milan, Italy); DATK-32 (α4β7)16 was obtained from Dr E. C. Butcher (Stanford University Medical Center, Stanford, CA); 2E7 (α4IEL),26 R1-2 (α4),13 NFR5 (5H10) (α5), and 429 (CD31/VCAM-1) were purchased from PharMingen (San Diego, CA); GoH3 (α6β1)27 was a gift of Dr A. Sonnenberg (The Netherlands Cancer Institute, Amsterdam, The Netherlands); M/K-2.7 (VCAM-1),15 FIB504 (β7),16 M298 (β7),17 M1/70 (αMβ2),28 and PS/2 (α4)15 were from ATCC (Rockville, MD); KM16 (β1)29 was from Dr P. Kinkade (Oklahoma Medical Research Foundation, Oklahoma City, OK); 9EG7 (β1)30 was from Dr D. Vestweber, Max Plank Institute for Immunobiology, Freiburg, Germany. A rabbit polyclonal antibody against the cytoplasmic portion of mouse β1 chain was provided by Dr G. Tarone (University of Turin, Turin, Italy). FN was purified from human plasma according to the procedures of Vuento and Vaheri.31
Cell lines and subcutaneous inoculations
The origin, establishment, growth behavior, and optimal culture conditions of MCF-247 virus-induced AKR T-cell lymphoma-derived cell lines NQ9, NQ22, NQ29, NQ35, and NQ36 have been described previously.7 8 The murine endothelioma cell lines H-end73 and H-end80, produced by immortalization of primary murine heart microvasculature endothelial cells with the polyoma virus, were a generous gift of Dr F. Bussolino (University of Turin, Turin, Italy) and were maintained in Dulbecco's minimum essential medium (DMEM) containing 10% fetal calf serum.
AKR/J mice of both sexes maintained in our laboratory were injected in the flank with 105 cells of each line suspended in 0.1 mL sterile phosphate-buffered saline. Injected animals were monitored daily, and tumors were allowed to grow until the death of the recipients.7 8 For the various types of experiments described, only neoplastic cell suspensions in which the DATK32-positive cells exceeded 90% were used.
Immunohistochemistry and flow cytometry
Fresh tissue samples from subcutaneous neoplastic masses were either embedded in OCT compound (Miles Laboratories, Naperville, IL), snap-frozen in liquid nitrogen-cooled isopentane, and stored at −70°C until the time of processing or directly cryosectioned. Sections 5-μm thick were stained with the antibodies indicated and then by anti-rat IgG and peroxidase ABC–Elite complex (Vector Laboratories, Burlingame, CA). For double-immunolabeling, the peroxidase ABC method developed in black with diaminobenzidine-nickel was used in the first reaction, followed by an alkaline phosphatase–biotin–streptavidin method developed in red with Vector-Red (Vector Laboratories). Other sections were incubated unfixed with the anti-VCAM-1 429 monoclonal antibody and examined under the confocal laser scanning microscope (Diaphot 200, Nikon; MRC-1024, Bio-Rad Laboratories, Hercules, CA) using the computerized serial optical sectioning function provided within the Lasersharp software (Bio-Rad).
Cell surface expression of putative adhesion receptors on cultured cells, on lymphoma cells grown subcutaneously, and on leukemic cells at dissemination sites was assessed by flow cytometry. Data were acquired using a FACScan flow cytometer and analyzed using PAINT-A-GATEPlus (Becton Dickinson) gated to exclude cell debris and nonviable elements.
Radiolabeling of cell surface proteins and immunoprecipitation
NQ22 cells from terminal stage leukemic mice were surface labeled with 125I as described.35 Different mAbs were added to cell lysates along with protein G-Sepharose, and the mixture was incubated overnight at 4°C under gentle shaking. For sequential immunoprecipitations, the cell lysates were incubated repeatedly with 1 antibody until no radioactivity was detected on the immunoprecipitates, and then the cell extract supernatants were sequentially incubated with the other antibodies. Samples were analyzed by SDS-PAGE on a 7% polyacrylamide gel, and dried gels were processed for autoradiography using MP films (Amersham, Buckinghamshire, UK).
RNA extraction and RT-PCR
Total cellular RNA was extracted from sorted cells by acid guanidinium thiocyanate-phenol-chloroform.32 cDNA was synthesized from 10 ng RNA using 20 U AMV reverse transcriptase (Promega, Madison, WI), 1 mmol/L dNTPs (each), 20 U RNAsin (Promega), and hexanucleotides (12.5 pmol). The following oligonucleotide primers were used: IL-1β (sense: 5′-GCAACTGTTCCTGAACTCA-3′; antisense: 5′-CTCGGAGCCTGTAGTGCAG-3′); IFN-γ (sense: 5′-AACGCTACACACTGCATCTTGG-3′; antisense: 5′-GACTTCAAAGAGTCTGAGG-3′); TNF-α (sense: 5′-CTCACACTCAGATCATCTTCTC-3′; antisense: 5′-GGCTACAGGCTTGTCACTCGA-3′); GM-CSF (sense: 5′-TTCCTGGGCATTGTGGTC-3′; antisense: 5′-TGGATTCAGAGCTGG CCTGG-33); HPRT (sense: 5′-GTTGGATACAGGCCAGACTTTGTTG-3′; antisense:5′-GATTCAACTTGCGCTCATCTTAGGC-3′) (40 cycles, annealing temperature 52°C). The amplified products were diluted 500-fold and then subjected to a nested polymerase chain reaction (PCR) using the following sense primers: IL-1β 5′-ATTGTGGCTGTGGAGAA-3′; IFN-γ 5′-GGAGGAACTGGCAAAAGGA-3′; TNF-α 5′-TCGAGTGACAAGCCTGTAGCC-3′; GM-CSF 5′-CACGTTGAATGAAGAGGT-3′ (40 cycles, annealing temperature 53°C). The amplification products of this second reaction were resolved by agarose gel and visualized by ethidium bromide and ultraviolet light.
Preparation of Fab fragments
The anti-α4 chain mAb PS/2 was purified from the 50% ammonium sulfate precipitate by loading this fraction on a protein G-Sepharose column (mAb Trap; Pharmacia, Uppsala, Sweden) and eluting the mAb with 50 mmol/L glycine-HCl, pH 2.7. The purified mAb was dialyzed for 16 hours at 4°C against phosphate-buffered saline. The Fab fragments were isolated by digestion of the purified antibody (1.5 mg/mL) with immobilized ficin (Immunopure preparation kit; Pierce Chemical, Rockford, IL) according to the manufacturer's instructions. Purification of the digested antibody was performed by loading the mixture on a protein G-Sepharose columns. The effluent contained only the Fab fragment as indicated by separation on a 10% polyacrylamide SDS gel under reducing conditions.
Cell adhesion assays
Cell adhesion to molecular substrates.
The cell adhesion assay was a centrifugation-based protocol originally introduced by Lotz et al33 and applied by us with the use of 35S-methionine-labeled34,35 and of calcein-labeled cells36 to measure cell adhesion to molecular and cellular substrates, respectively. For cell adhesion assays PVC 96-well plates (Microtiter flexible Falcon; Becton Dickinson) were coated for 16 hours at 4°C with 50 μL appropriate concentration of the substrate molecule in bicarbonate buffer, pH 9.6. The percentage of attached cells was calculated from those values. Assays were repeated at least 3 times, and average values that did not deviate more than 10% from the mean were plotted. In experiments aimed at examining the effects of competing antibodies, the various antibodies were added directly to the wells just before adding the cells under investigation.
Cell adhesion to cellular substrates.
H-end80 cells were brought to confluence into PVC 96-well plates (Microtiter flexible Falcon); cells were then stimulated for 16 hours with 10 ng/mL TNF-α, rinsed with DMEM containing 0.3% polyvinylpyrrolidone and incubated with calcein-labeled NQ cells. The plates were centrifuged for 5 minutes at 500 rpm and incubated at 37°C for 20 minutes. At the end of the incubation, nonadherent cells were removed by gently rinsing with DMEM containing 0.3% polyvinylpyrrolidone, and the extent of adherent cells was measured on a microplate fluorometer (SPECTRAFluor Plus; TECAN, Salzburg, Austria) at 485 nm excitation and 535 nm emission. The percentage of bound cells was determined by dividing the fluorescence of adherent cells by the fluorescence of the input cells.
Coculture of lymphoma and endothelial cells
H-end73 and H-end80 cells were grown in Transwell culture inserts (Costar, Cambridge, MA) measuring 2.4 cm in diameter bearing polycarbonate membranes with 3- to 5-μm diameter pores until they reached confluence. Lymphoma cells grown in vitro or cells isolated from in vivo growing tumors were plated directly onto the endothelial monolayers and cocultured for different time intervals. Alternatively, lymphoma cells were physically separated from the endothelial cell layers by a tissue culture insert bearing membranes with 0.02-μm diameter pores (Nunc, Roskilde, Denmark). At the end of the coculture period, lymphoma cells were removed by extensive washing, and the endothelial cells were recovered after trypsin-EDTA detachment. These were then processed for FACS analysis to determine the levels of VCAM-1 cell surface expression. Endothelial cells grown in the presence of 10 ng/mL TNF-α for 16 hours were adopted as a positive control for up-regulated VCAM-1 expression.
Neutralization of cytokine bioactivity
Cytokines released by NQ22 cells during the cocultivation assay with endothelial cells were neutralized by the addition, at the start of the assay, of saturating amounts of polyclonal antibodies against mouse IFN-γ, GM-CSF, and TNF-α (R&D Systems, Abingdon, UK). The final concentration of antibodies added was 10 μg/mL irrespective of whether a single antibody or a mixture of 3 different antibodies was used. Normal goat IgG was used as negative control. After 7 hours of coculture, H-end80 cells were processed for FACS analysis as above.
Cell migration
The ability of lymphoma cells to transmigrate through an endothelial cell layer was examined using Transwell (Costar) culture chambers bearing polycarbonate membranes with 3- to 5-μm diameter pores. At various time points, the number of cells migrated to the lower chamber was determined, and the mean ± SD was calculated in 9 microscope fields. In this series of experiments, not more than approximately 8% to 12% of the input cells were able to transmigrate within the timeframe allocated for the transmigration to occur.
Results
Integrin expression on NQ22 cells
In a previous immunophenotypic study, we had shown that the highly leukemogenic NQ22 cell line displayed a remarkable induction of the α4β7 integrin after in vivo inoculation.9 For the current study, a comparative analysis of the integrin expression of NQ22 and of poorly leukemogenic cells confirmed the in vivo up-regulation of α4, β7, and α4β7 of NQ22. In addition, the expression of the β1 integrin chain, Mac-1, and the α6 integrin chain were also induced in NQ22 cells; the α5 integrin chain was weakly expressed, whereas the αv and α4ΙEL chains were not detected at all. Instead, the poorly leukemogenic cells did not show the induction of the integrin chains α4, β1, β7, Mac-1, and α6 as detected in NQ22 cells (data not shown).
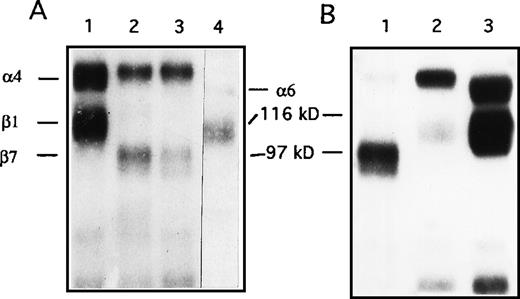
Integrin complexes identified by flow cytometry were further characterized immunochemically by precipitation of the iodinated complexes present on NQ22 cells from leukemic spleens (90% DATK-32 positive cells by FACS analysis) with several mAbs. As shown in Figure1A, SDS-PAGE under nonreducing conditions of such immunoprecipitates revealed 2 bands of approximately 150 and 110 kd (anti-α4 mAb) and of approximately 150 and 100 kd (anti-β7 and anti-α4β7), respectively (Figure 1), demonstrating that both α4β1 and α4β7 integrins were expressed on highly leukemogenic NQ22 cells. The direct physical association between the α4 and the β1 chains and the relative amounts of α4β7 and α4β1 was demonstrated by sequential immunoprecipitation: thus, NQ22 cells were first depleted of the α4β7 integrins (Figure 1B, lane 1); then the lysate supernatant was incubated with the anti-α4 mAb R1-2, and, in this case, a strong 150 kd polypeptide band associated with a 110 kd polypeptide band was immunoprecipitated (lane 2). Finally, the cell lysate supernatant was immunoprecipitated with a polyclonal anti-β1 antibody (lane 3) revealing the persistence of other β1 integrin complexes, likely α5β1 and α6β1.
Immunoprecipitation of integrins from NQ22 cells.
Approximately 2.5 × 107 cells were radiolabeled with 125I and extracted in lysis buffer. (A) Aliquots of the cell lysate were immunoprecipitated using the following antibodies: lane 1, anti-α4 (R1-2); lane 2, anti-β7 (M298); lane 3, anti-α4β7 (DATK-32); lane 4, anti-α6β1 (GoH3). (B) Sequential immunoprecipitations. NQ22 cell lysates were first depleted with 3 rounds of immunoprecipitation with anti-β7 (lane 1), followed by 3 rounds with anti-α4 (lane 2), and finally with the polyclonal anti-β1 antibody (lane 3). Samples were then analyzed by SDS-PAGE on a 7% gel under nonreducing conditions. The migration of standard molecular weight markers is shown in the center. The migration of integrin α and β chains is also indicated.
Immunoprecipitation of integrins from NQ22 cells.
Approximately 2.5 × 107 cells were radiolabeled with 125I and extracted in lysis buffer. (A) Aliquots of the cell lysate were immunoprecipitated using the following antibodies: lane 1, anti-α4 (R1-2); lane 2, anti-β7 (M298); lane 3, anti-α4β7 (DATK-32); lane 4, anti-α6β1 (GoH3). (B) Sequential immunoprecipitations. NQ22 cell lysates were first depleted with 3 rounds of immunoprecipitation with anti-β7 (lane 1), followed by 3 rounds with anti-α4 (lane 2), and finally with the polyclonal anti-β1 antibody (lane 3). Samples were then analyzed by SDS-PAGE on a 7% gel under nonreducing conditions. The migration of standard molecular weight markers is shown in the center. The migration of integrin α and β chains is also indicated.
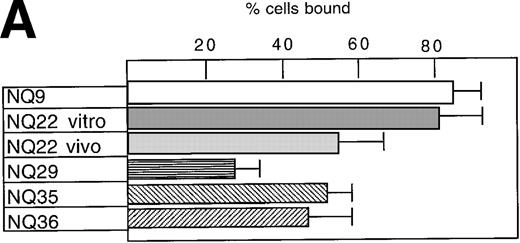
Adhesion of highly and poorly malignant cells to FN and to endothelial cells
Both pre-leukemic (ie, in vitro grown) and highly leukemogenic NQ22 cells that had infiltrated the spleen and lymph nodes bound strongly to FN, the latter with a slightly lower efficiency compared to the cells maintained in culture (Figure 2A). Cell adhesion to FN of several other in vitro grown cell lines, which are characterized by a prevalent local SC growth when transplanted in vivo, was variable: NQ9 cells displayed high binding to FN, whereas NQ29, NQ35, and NQ36 displayed intermediate or low levels of cell adhesion. Cell attachment of NQ22 cells to FN was mediated by its cell-binding domain comprising the RGD and the primary synergistic site within the 3Fn-9 repeat. In fact, the inhibition of cell adhesion by 80% and 40%, respectively, was attained with the addition of antibody 333, which binds near the RGD site,37 and antibody HFN-7 against the synergistic site (data not shown). Furthermore, cell adhesion to FN was not inhibited by anti-α4 (PS/2) or anti-α4β7 (DATK-32) mAbs (data not shown), suggesting that the α4β1 and α4β7 integrins contributed poorly to the overall interaction with FN and that binding was primarily mediated by other integrins (ie, α5β1).
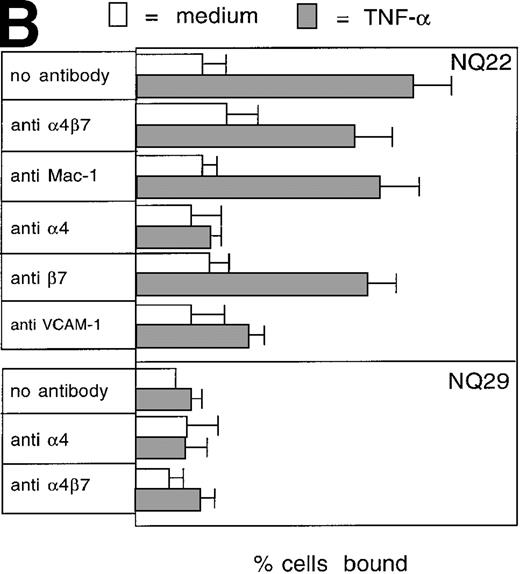
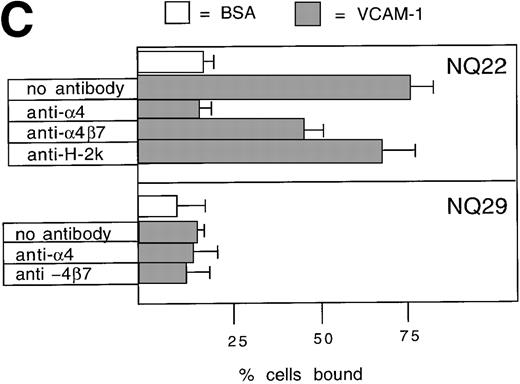
Cell adhesion.
(A) Cell adhesion of lymphoma cell lines to bovine serum albumin or FN coated at 10 μg/mL was carried out in the presence of 0.3 mmol/L Mg2+ or 20 μmol/L Mn2+. The values reported represent the average of 3 experiments. (B) Cell adhesion to endothelial cells. Confluent H-end80 monolayers were incubated with media alone or with media containing TNF-α for 16 hours and rinsed with DMEM. NQ22 and NQ29 cells (2 × 105) in DMEM were added for 20 minutes at 37°C under static conditions. At the end of the incubation period, the nonadherent cells were removed by gentle rinsing with DMEM and the percentage of bound cells was determined as detailed in “Materials and methods.” (C) NQ22 and NQ29 cell adhesion to purified VCAM-1 or bovine serum albumin coated at 10 μg/mL. For the inhibition of cell attachment to H-end80 cells and to purified VCAM-1, the different antibodies were added at 5 μg/mL just before the lymphoma cells were plated. The antibodies used were the following: DATK-32 (α4β7); M1/70 (Mac-1/αMβ2); PS/2 (α4); M298 (β7); and 429 (VCAM-1).
Cell adhesion.
(A) Cell adhesion of lymphoma cell lines to bovine serum albumin or FN coated at 10 μg/mL was carried out in the presence of 0.3 mmol/L Mg2+ or 20 μmol/L Mn2+. The values reported represent the average of 3 experiments. (B) Cell adhesion to endothelial cells. Confluent H-end80 monolayers were incubated with media alone or with media containing TNF-α for 16 hours and rinsed with DMEM. NQ22 and NQ29 cells (2 × 105) in DMEM were added for 20 minutes at 37°C under static conditions. At the end of the incubation period, the nonadherent cells were removed by gentle rinsing with DMEM and the percentage of bound cells was determined as detailed in “Materials and methods.” (C) NQ22 and NQ29 cell adhesion to purified VCAM-1 or bovine serum albumin coated at 10 μg/mL. For the inhibition of cell attachment to H-end80 cells and to purified VCAM-1, the different antibodies were added at 5 μg/mL just before the lymphoma cells were plated. The antibodies used were the following: DATK-32 (α4β7); M1/70 (Mac-1/αMβ2); PS/2 (α4); M298 (β7); and 429 (VCAM-1).
To assess whether adhesion to endothelial cells might represent a peculiar property of NQ22 cells in comparison to other lymphomas, we used endothelial cell lines. Although these cells are unique and likely lack a normal counterpart in vivo, they expressed several endothelial cell markers (data not shown) and proved suitable and highly reproducible. Very low binding was detected to nonactivated H-end80 cells (Figure 2B). Instead, NQ22 cells isolated from infiltrated spleen bound to TNF-α-activated H-end80 cells. Blocking studies with anti-α4, -β7, -α4β7, and -Mac-1 mAbs showed that only the PS/2 anti-α4 antibody abrogated cell adhesion (Figure 2B). In contrast, the overall effect of anti-β7, -α4β7, and -Mac-1 antibodies was negligible (Figure 2B). These results indicated a strong predominance of the α4β1 integrin in determining the adhesion of NQ22 cells to the activated endothelial cells. To demonstrate that the α4-integrin cognate ligand on TNF-α-activated endothelial cells was VCAM-1, endothelial cells were preincubated with a blocking anti-VCAM-1 mAb, and, in this case, NQ22 cell adhesion was to a great extent abrogated. Furthermore, we investigated the ability of NQ22 cells to adhere to surfaces coated with recombinant VCAM-1. NQ22 cells bound pronouncedly to VCAM-1 (75%), and this binding was completely inhibited by anti-α4 mAb (Figure 2C); the contribution of the α4β7 integrin to cell adhesion appeared to be much lower (approximately 30%). Therefore, as reported in other experimental systems involving lymphocytes expressing both α4β1 and α4β7,11α4β1-dependent binding to VCAM-1 was predominant also for NQ22 cells. NQ29 cells showed no binding to VCAM-1 (Figure 2B) even if plated at higher concentrations of the ligand (data not shown).
Induction of VCAM-1 on endothelial cells
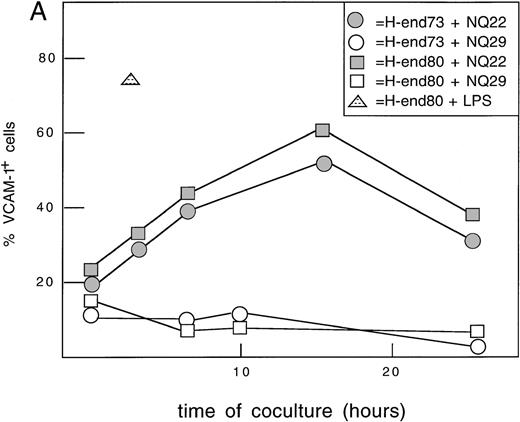
The in situ expression of VCAM-1 at local sites of the initial neoplastic growth of NQ22 and NQ29 cells was examined, and it was found that cells intensely positive for VCAM-1 were present as fully formed vessels and as single cells likely of endothelial or stromal origin if the infiltrating tumor cells were NQ22 (Figures3A, 3C); instead, weak positivity or a nonactivated pattern of staining for VCAM-1 in small locally growing NQ29 tumors was detected (Figure 3D). As reported previously for large tumor masses,9 few vascular spaces were engulfed by NQ22 cells highlighted by their strong positivity for the α4β7 marker; neoplastic cells could be seen protruding into the lumen and crossing the endothelial layer (Figures 3B, 3C). These morphologic and immunochemical findings suggested that the interactions between NQ22 leukemic cells and endothelial cells might indeed play an important and necessary role in the early leukemic evolution of this lymphoma. To try to recapitulate this phenomenon in vitro, leukemic cells were cultured for different lengths of time over a monolayer of endothelial cell lines (H-end73 and H-end80). These cells were rapidly induced to express high levels of VCAM-1 by LPS (Figure4A). The same result was obtained if either H-end73 or H-end80 cells were cocultured with NQ22 cells; in this case VCAM-1 expression peaked at approximately16 hours and decreased to near basal levels at 24 hours. On the contrary, NQ29 cells were not able significantly to up-regulate VCAM-1 expression on endothelial cells. The continuous presence of NQ22 cells was not necessary for the induction of VCAM-1 on H-end80 cells, as shown by the fact that if NQ22 cells were removed after 1 to 3 hours of coculture, H-end80 cells still showed a significantly increased VCAM-1 expression at 7 hours.
Immunohistochemical staining of SC tumor masses.
Immunolabeling of cryostat sections of masses at their initial growth was performed with anti-α4β7 and anti-VCAM-1 (A, C) or with anti-α4β7 and anti-CD31 (B) mAbs on NQ22 cells or with anti-VCAM-1 alone on NQ29 cells (D). Brown, α4β7; red, CD31 and VCAM-1. Magnification, × 200 (A, B) and × 400 (C, D).
Immunohistochemical staining of SC tumor masses.
Immunolabeling of cryostat sections of masses at their initial growth was performed with anti-α4β7 and anti-VCAM-1 (A, C) or with anti-α4β7 and anti-CD31 (B) mAbs on NQ22 cells or with anti-VCAM-1 alone on NQ29 cells (D). Brown, α4β7; red, CD31 and VCAM-1. Magnification, × 200 (A, B) and × 400 (C, D).
Induction of VCAM-1 on murine endothelial cells.
(A) Time-course. H-end73 (◍, ○) and H-end80 (□) cells were cocultured for different lengths of time with NQ22 (◍, ░) or NQ29 (○, □) cell suspensions obtained from frankly leukemic spleen (NQ22) or from a large SC tumor mass (NQ29), and the percentage of VCAM-1 positive cells was assessed by flow cytometry analysis using anti-VCAM-1 mAb 429. H-end80 stimulated with 100 ng/mL lipopolysaccharide (△) for 3 hours was used as positive control and similarly processed for flow cytometry analysis. (B) Induction of VCAM-1 by different cell types. H-end80 cells were cocultured for 7 hours with cell suspensions obtained from in vitro-grown NQ22 cells (NQ22 vitro), NQ22 cells isolated from an infiltrated spleen (NQ22 spleen), or a subcutaneous tumor mass (NQ22 SC mass) and from a subcutaneous mass of NQ29 cells (NQ29 SC mass). The expression of VCAM-1 was assessed with mAb 429. Control, isotype-matched unrelated primary antibody was added; basal, basal expression of VCAM-1 in H-end80 cells.
Induction of VCAM-1 on murine endothelial cells.
(A) Time-course. H-end73 (◍, ○) and H-end80 (□) cells were cocultured for different lengths of time with NQ22 (◍, ░) or NQ29 (○, □) cell suspensions obtained from frankly leukemic spleen (NQ22) or from a large SC tumor mass (NQ29), and the percentage of VCAM-1 positive cells was assessed by flow cytometry analysis using anti-VCAM-1 mAb 429. H-end80 stimulated with 100 ng/mL lipopolysaccharide (△) for 3 hours was used as positive control and similarly processed for flow cytometry analysis. (B) Induction of VCAM-1 by different cell types. H-end80 cells were cocultured for 7 hours with cell suspensions obtained from in vitro-grown NQ22 cells (NQ22 vitro), NQ22 cells isolated from an infiltrated spleen (NQ22 spleen), or a subcutaneous tumor mass (NQ22 SC mass) and from a subcutaneous mass of NQ29 cells (NQ29 SC mass). The expression of VCAM-1 was assessed with mAb 429. Control, isotype-matched unrelated primary antibody was added; basal, basal expression of VCAM-1 in H-end80 cells.
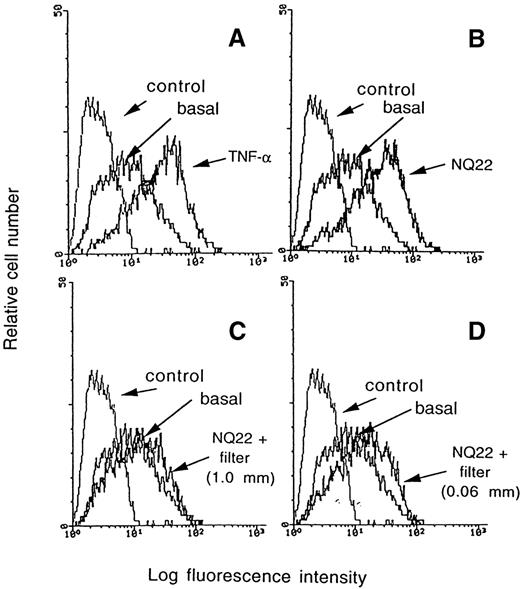
To assess more accurately whether the ability to up-regulate VCAM-1 in endothelial cells was an intrinsic property of NQ22 cells, coculture experiments were carried out between H-end80 and NQ22 leukemic cells from different sources. As shown in Figure 4B, though NQ29 cells from an SC mass did not up-regulate VCAM-1 within the timeframe of this experiment, in vitro cultured NQ22 cells weakly induced its expression, cells from a locally growing SC NQ22 mass had an intermediate capacity of induction, and NQ22 cells obtained from dissemination sites up-regulated VCAM-1 expression to levels comparable to those attained with TNF-α. Separation of the 2 cell populations with a membrane (0.02-μm pores) resulted in a much lower induction of VCAM-1 (Figure5C) than TNF-α-treated cells (Figure 5A). These types of filters allow the diffusion of soluble mediators but not cell–cell contacts and cell transmigration. However, the distance between the 2 cell types in the above experiment was such (approximately 1 mm) that the inductive cues eventually exerted by diffusible factors might not reach the target endothelial cells at functionally effective levels. Thus, a different experimental setup was developed to maintain the cocultured cells still physically separated, but by a much shorter distance (0.06 mm). This was achieved by first growing the H-end80 cells on the lower side of an inverted Transwell (Costar) and then positioning it in the culture wells in the correct upright position with the endothelial cells facing downward. In this case the up-regulation of VCAM-1, achieved after the coculture of NQ22 cells added in the upper chamber, reached intermediate levels (Figure5D) and was nearly comparable to the up-regulation attained by cocultivating the 2 cell types (Figure 5B), suggesting that soluble factors actively released by NQ22 cells likely contribute to the induction of VCAM-1 in endothelial cells.
Induction of VCAM-1 can be inhibited by a nitrocellulose membrane.
VCAM-1 expression was evaluated on H-end80 cells cocultured for 7 hours with NQ22 cells (B); with NQ22 cells separated by a tissue culture insert with an anopore membrane bearing 0.02-μm diameter pores in which the 2 cell types were separated by a distance of approximately1 mm (C); with NQ22 cells separated by a tissue culture insert with an anopore membrane bearing 0.02-μm diameter pores in which the 2 cell types were separated by a distance of approximately 0.06 mm (D). Other endothelial cells were grown for 16 hours in the presence of 10 ng/mL TNF-α (A). NQ22 cells were isolated from an infiltrated spleen; basal, basal expression of VCAM-1 in H-end80 cells.
Induction of VCAM-1 can be inhibited by a nitrocellulose membrane.
VCAM-1 expression was evaluated on H-end80 cells cocultured for 7 hours with NQ22 cells (B); with NQ22 cells separated by a tissue culture insert with an anopore membrane bearing 0.02-μm diameter pores in which the 2 cell types were separated by a distance of approximately1 mm (C); with NQ22 cells separated by a tissue culture insert with an anopore membrane bearing 0.02-μm diameter pores in which the 2 cell types were separated by a distance of approximately 0.06 mm (D). Other endothelial cells were grown for 16 hours in the presence of 10 ng/mL TNF-α (A). NQ22 cells were isolated from an infiltrated spleen; basal, basal expression of VCAM-1 in H-end80 cells.
That physical cell–cell interaction between endothelial and NQ22 cells was not a prerequisite for VCAM-1 up-regulation was further investigated: monovalent Fab fragments of the blocking PS/2 mAb (Figure6A, inset), prepared to avoid integrin cross-linking and further activation of NQ22 cells, were used to block NQ22 cell adhesion to TNF-α-stimulated H-end80 cells; Fab was almost as efficient as bivalent IgG (Figure 6A). In a parallel experiment nonactivated H-end80 cells were cocultured for 7 hours with NQ22 cells in the presence of saturating amounts of Fab fragments of PS/2 antibody. In this case, though effective in blocking the cell–cell interaction, Fab fragments did not prevent the up-regulation of VCAM-1 induced by the coculture (Figure 6B).
Inhibition of cell adhesion by anti-4 Fab fragments does not inhibit VCAM-1 induction on endothelial cells.
(A) Confluent H-end80 monolayers were incubated with media alone or with media containing 10 ng/mL TNF-α for 16 hours and rinsed with DMEM. NQ22 cells (2 × 105) in DMEM were added for 20 minutes at 37°C under static conditions either in the absence or in the presence of PS/2 anti-integrin α4 subunit IgG (10 μg/mL) or Fab fragment (5 or 15 μg/mL). At the end of the incubation period, the nonadherent cells were removed as detailed in “Materials and methods.” The purity of the IgG and of the Fab fragment, analyzed by SDS-PAGE on a 10% gel under reducing conditions, is shown in the inset, lane 1 and lane 2, respectively. (B) Expression of VCAM-1 on H-end80 cells was evaluated after 7 hours of cocultivation with NQ22 cells obtained from infiltrated spleen either in the presence or in the absence of 50 μg/mL Fab fragment.
Inhibition of cell adhesion by anti-4 Fab fragments does not inhibit VCAM-1 induction on endothelial cells.
(A) Confluent H-end80 monolayers were incubated with media alone or with media containing 10 ng/mL TNF-α for 16 hours and rinsed with DMEM. NQ22 cells (2 × 105) in DMEM were added for 20 minutes at 37°C under static conditions either in the absence or in the presence of PS/2 anti-integrin α4 subunit IgG (10 μg/mL) or Fab fragment (5 or 15 μg/mL). At the end of the incubation period, the nonadherent cells were removed as detailed in “Materials and methods.” The purity of the IgG and of the Fab fragment, analyzed by SDS-PAGE on a 10% gel under reducing conditions, is shown in the inset, lane 1 and lane 2, respectively. (B) Expression of VCAM-1 on H-end80 cells was evaluated after 7 hours of cocultivation with NQ22 cells obtained from infiltrated spleen either in the presence or in the absence of 50 μg/mL Fab fragment.
Expression of cytokines by NQ22 and NQ29 cells
Because it has been reported that a number of cytokines, including IL-1β, IFN-γ, TNF-α, IL-4, and GM-CSF, elicit a profound and relatively rapid up-regulation of VCAM-1 on several cell types,19-25 we investigated by RT-PCR whether the differential expression of mRNA of several cytokines might be a mechanism by which NQ22 cells up-regulate VCAM-1 on endothelial cells. Although TNF-α appeared to be expressed in all cell types analyzed (in NQ22 cells, it was already detectable after the first amplification step; data not shown), the expression of IFN-γ was detected only in NQ22 cells grown in vitro and in highly leukemogenic NQ22 cells isolated from infiltrated spleen; furthermore, IL-1β and GM-CSF were detected only in cells transplanted in vivo (Figure7). These data indicated that a set of cytokines (TNF-α, IFN-γ, IL-1β, and GM-CSF), expressed exclusively or predominantly in NQ22 cells, contributed to the up-regulation of VCAM-1 on local vessels on SC inoculation. To investigate this possibility further, a cocultivation between H-end80 and NQ22 cells was set up in the presence of saturating amounts of neutralizing antibodies against different mouse cytokines. Although the addition of normal goat IgG or of anti IFN-γ or of anti GM-CSF had no effect (76% VCAM-1 positive cells versus 71% when NQ22 cells alone were cocultured), the presence of anti TNF-α antibodies during the coculture prevented to a large extent (40% positive cells) the up-regulation of VCAM-1 on H-end80 cells (Figure8A). If the 3 antibodies were added together, keeping the total amount of immune IgG added still at 10 μg/mL, a slightly reduced inhibition was noticed (47% positive cells) (Figure 8B), very likely because of the lower amount of anti TNF-α in the coculture system.
Differential expression of cytokines in NQ22 and NQ29 lymphoma cells.
Total RNA was extracted from 107 NQ22 and NQ29 cells grown in vitro, from a spleen heavily infiltrated with NQ22 cells, from NQ22 cells cocultured with H-end80 cells, and from NQ29 cells from a subcutaneous tumor mass. RNA was reverse transcribed and amplified with primers specific for IL-1β, IFN-γ, TNF-α, GM-CSF, and HPRT as detailed in “Materials and methods.” Aliquots of the nested PCRs were separated by 2% agarose gel and visualized by ethidium bromide and ultraviolet light. Only the relevant part of the gel is shown.
Differential expression of cytokines in NQ22 and NQ29 lymphoma cells.
Total RNA was extracted from 107 NQ22 and NQ29 cells grown in vitro, from a spleen heavily infiltrated with NQ22 cells, from NQ22 cells cocultured with H-end80 cells, and from NQ29 cells from a subcutaneous tumor mass. RNA was reverse transcribed and amplified with primers specific for IL-1β, IFN-γ, TNF-α, GM-CSF, and HPRT as detailed in “Materials and methods.” Aliquots of the nested PCRs were separated by 2% agarose gel and visualized by ethidium bromide and ultraviolet light. Only the relevant part of the gel is shown.
Inhibition of VCAM-1 induction by TNF--neutralizing antibodies.
A polyclonal antibody (10 μg/mL) against TNF-α (A) or a mixture of polyclonal antibodies (3.3 μg/mL each antibody) against IFN-γ, GM-CSF, and TNF-α (B) were added to H-end80 cells during the coculture with NQ22 cells. The expression of VCAM-1 was assessed with mAb 429; basal, basal expression of VCAM-1 in H-end80 cells.
Inhibition of VCAM-1 induction by TNF--neutralizing antibodies.
A polyclonal antibody (10 μg/mL) against TNF-α (A) or a mixture of polyclonal antibodies (3.3 μg/mL each antibody) against IFN-γ, GM-CSF, and TNF-α (B) were added to H-end80 cells during the coculture with NQ22 cells. The expression of VCAM-1 was assessed with mAb 429; basal, basal expression of VCAM-1 in H-end80 cells.
Migration of NQ22 cells in vitro
The transendothelial migratory capability of NQ22 cells was compared to that of NQ29 cells. For this assay the Transwell (Costar) system was used with H-end80 cells grown to confluence in the upper side of the filter in either the presence or the absence of TNF-α. An increasing number of NQ22 cells was detected with time, migrating across the activated endothelial cell layer to reach the lower chamber (Figure9A). Significantly fewer NQ22 cells were detected in the lower chamber of each Transwell (Costar) in which nonactivated H-end80 cells were seeded, though a moderate increase with time was evident (compare the values at 3, 6, and 12 hours in Figure 9A). In contrast, few NQ29 cells transmigrated irrespective of whether the endothelial cell layer had been activated with TNF-α, and only at 24 hours (data not shown) a certain degree of equivalent migration was detected through both activated and nonactivated H-end80 cells.
NQ22 cells migrate more efficiently than NQ29 cells.
Transendothelial migration. H-end80 cells were grown to confluence on the upper side of uncoated wells of Transwell culture chambers and either were left untreated or were treated for 16 hours with 10 ng/mL TNF-α . NQ22 cells (•, ○) obtained from heavily infiltrated spleen and NQ29 cells from a subcutaneous mass (▪, □) (2 × 105) were seeded in the upper chamber of the Transwell, and cells that migrated through untreated (○, □) or through TNF-α-treated (•, ▪) H-end80 cells to the lower chamber were harvested and counted at the indicated time-points. The values shown represent the mean of 3 optical fields.
NQ22 cells migrate more efficiently than NQ29 cells.
Transendothelial migration. H-end80 cells were grown to confluence on the upper side of uncoated wells of Transwell culture chambers and either were left untreated or were treated for 16 hours with 10 ng/mL TNF-α . NQ22 cells (•, ○) obtained from heavily infiltrated spleen and NQ29 cells from a subcutaneous mass (▪, □) (2 × 105) were seeded in the upper chamber of the Transwell, and cells that migrated through untreated (○, □) or through TNF-α-treated (•, ▪) H-end80 cells to the lower chamber were harvested and counted at the indicated time-points. The values shown represent the mean of 3 optical fields.
VCAM-1 is induced on the apical and basolateral sides of endothelial cells
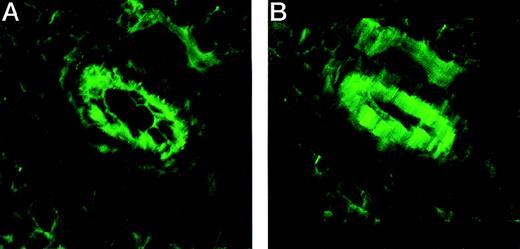
The adhesion to and the transmigration through an endothelial cell layer displayed by NQ22 cells in vitro provided some clues about how these cells could intravasate in vivo and then invade the bloodstream. To determine whether VCAM-1 was expressed also on the basolateral side of endothelial cells, nonactivated (which display a constitutive weak expression for VCAM-1) and activated H-end80 cells were grown to confluence and were stained with anti VCAM-1 antibodies. Permeabilization with saponin allowed the measurement of an increase in the fluorescence intensity compared to nonpermeabilized cells (data not shown). A confocal analysis of sections through a local site of neoplastic growth of NQ22 cells stained with VCAM-1 antibodies indeed showed that this ligand was expressed on the apical and the basolateral sides in vivo (Figure 10).
VCAM-1 is expressed on both the apical and the basolateral surfaces of endothelial cells in vivo.
Cryostat sections of NQ22 masses at their initial SC growth were fixed in acetone–methanol and stained with anti VCAM-1 mAb M/K-2 and visualized with fluorescein-conjugated goat antimouse IgG. (A) Detail of the 2-dimensional reconstruction. (B) Detail of the tridimensional reconstruction of a series of sections taken in 0.13-μm steps through a tissue section in which 2 blood vessels are clearly evident.
VCAM-1 is expressed on both the apical and the basolateral surfaces of endothelial cells in vivo.
Cryostat sections of NQ22 masses at their initial SC growth were fixed in acetone–methanol and stained with anti VCAM-1 mAb M/K-2 and visualized with fluorescein-conjugated goat antimouse IgG. (A) Detail of the 2-dimensional reconstruction. (B) Detail of the tridimensional reconstruction of a series of sections taken in 0.13-μm steps through a tissue section in which 2 blood vessels are clearly evident.
De novo expression of 4 integrins on pre-leukemic NQ22 cells
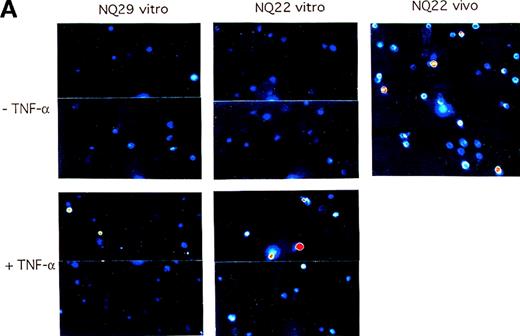
The data generated in the previous set of analyses outline a possible sequence of events that might explain the aggressive behavior of NQ22 cells: these cells spontaneously release, or are induced to release, TNF-α and perhaps a number of other cytokines that up-regulate the expression of VCAM-1 on endothelial cells of nearby small vessels and then reach the basolateral surface of a by-now activated endothelial cell layer, bind to these cells by an α4/VCAM-1-dependent mechanism, and finally transmigrate through the endothelium into the bloodstream. However, in vitro grown NQ22 cells do not express α4 integrins; thus, 1 fundamental element of this multistep process is still missing. To investigate whether activated endothelial cells might induce the expression of α4 integrins in NQ22 cells, TNF-α stimulated and nonstimulated H-end80 cells were cocultured with in vitro grown NQ22 or NQ29 cells for 24 hours, and the expression of the α4 subunit was measured with the PS/2 antibody. Nonactivated H-end80 cells were ineffective with both NQ22 and NQ29 cells; on the contrary, pretreatment with TNF-α resulted in de novo expression of α4 integrins in a low percentage (2% to 3%) of NQ22 cells. Fewer NQ29 cells were induced than NQ22 cells (Figure11).
Activated endothelium can up-regulate the expression of 4 integrins on NQ22 cells.
H-end80 cells grown to confluence were left untreated (upper panels) or were treated for 16 hours with 10 ng/mL TNF-α (lower panels) and then were cocultured for 24 hours with NQ22 or NQ29 cells grown in vitro. At the end of the incubation period, NQ cells were collected, the expression of α4 integrin subunit was determined with the use of PS/2 mAb, and the reactivity was examined by confocal fluorescence microscopy with pseudocolor enhancement. NQ22 cells from heavily infiltrated spleen (NQ22 vivo) were used as positive control. Selected fields with a relatively higher percentage of positive cells were chosen for display for NQ22 and NQ29 cells (A), whereas the percentage of positive cells is graphically depicted (B).
Activated endothelium can up-regulate the expression of 4 integrins on NQ22 cells.
H-end80 cells grown to confluence were left untreated (upper panels) or were treated for 16 hours with 10 ng/mL TNF-α (lower panels) and then were cocultured for 24 hours with NQ22 or NQ29 cells grown in vitro. At the end of the incubation period, NQ cells were collected, the expression of α4 integrin subunit was determined with the use of PS/2 mAb, and the reactivity was examined by confocal fluorescence microscopy with pseudocolor enhancement. NQ22 cells from heavily infiltrated spleen (NQ22 vivo) were used as positive control. Selected fields with a relatively higher percentage of positive cells were chosen for display for NQ22 and NQ29 cells (A), whereas the percentage of positive cells is graphically depicted (B).
Discussion
Malignant lymphomas may undergo transition from a nodal to an intravascular state, leading to the extravasation, dissemination, and accumulation of the malignant cells in other nodal and extranodal tissues. Several studies have analyzed the extravasation phenomena of these cells, and, in some cases, functional similarities with the process of leukocyte extravasation have been identified. However, little attention had been paid to the regulation of the early steps of this “metastatic” behavior apart from a recent study on carcinoma cells.38 To contribute to the understanding of the principles governing intravasation, we have used a murine model system based on the lymphoma cell line NQ22 that disseminates after a subcutaneous injection.7-9 The current study indicates that α4 integrins (α4β1 to a greater extent than α4β7) contribute mechanistically and in a substantial way to the process of leukemic dissemination of the NQ22 lymphoma cells. First, coculture of activated endothelium with the in vitro grown pre-leukemic α4−NQ22 cells induced α4 integrin expression on these cells, allowing an α4/VCAM-1-dependent cell–cell interaction. Second, the mRNA of cytokines known to up-regulate the expression of VCAM-1, such as TNF-α, IFN-γ, IL-1β, and GM-CSF, were differentially expressed in the aggressive NQ22 cells compared to the locally growing NQ29 cells. Consistent with this finding, only cocultivation of the highly leukemogenic NQ22 cells with nonactivated endothelial cells was followed by the up-regulation of VCAM-1 on these latter cells. Third, the NQ22-dependent up-regulation of VCAM-1 was prevented to a large extent by TNF-α-neutralizing antibodies. Fourth, leukemogenic NQ22 cells migrated in vitro more efficiently through an activated endothelial cell layer than nonleukemogenic NQ29 cells. Fifth, the endothelial cells of vascular spaces at SC sites of the initial neoplastic growth of NQ22 cells expressed high levels of VCAM-1. Finally, the demonstration that activated endothelial cells grown in vitro, as well as at local sites of NQ22 initial growth, also expressed VCAM-1 on the basolateral surface indicated a mechanism by which the α4/VCAM-1-dependent interaction between incoming NQ22 cells and the basolateral surface of vascular endothelial cells could be a prelude to the transendothelial migration occurring during the intravasation step. The possibility that similar mechanisms may also be used by intravasating aggressive experimental T lymphomas, such as those of the SJL mouse strain and by pediatric T-LBL9 and even by neoplastic cells of other cell lineages, represents an attractive hypothesis that deserves further investigation.
It has been reported that the use of the α5β1 integrin in the recognition of the FN ligand predominates over the use of other integrins when more FN-binding integrins are expressed on lymphoid cells.39 Accordingly, we could not affect binding to FN by the simple neutralization with anti-α4 and anti-α4β7 integrin antibodies. In contrast, the binding of NQ22 cells to purified recombinant VCAM-1 was fully inhibited by an anti-α4 integrin chain mAb and partly inhibited by an anti-α4β7 integrin mAb. The up-regulation of VCAM-1 observed after coculture of endothelial and NQ22 cells resulted in the acquisition of a pro-adhesive state by endothelial cells. This activity depended on the induction of VCAM-1 expression because the cell–cell adhesion of NQ22 to endothelial cells was fully inhibited with the use of functional anti-α4 and anti-VCAM-1 mAbs. Although the use of mAbs against the β7 subunit and the α4β7 complex suggested that α4β7 played only a minor role, it is plausible that both α4 integrins are instrumental in this cell adhesion step.
The finding that endothelial cells cocultured with NQ22 cells up-regulated VCAM-1 expression in vitro is consistent with the results of the immunohistochemical analysis showing that VCAM-1 was induced on the vessel wall at sites of early NQ22 local growth. On the contrary, the inability of NQ29 cells to up-regulate VCAM-1 expression on endothelial cells in vitro as well as in vivo was consistent with their prevalent local growth. This difference disappeared if NQ22 and NQ29 cells were injected intravenously; in those instances, mice injected with either type of lymphoma cells had equivalent life expectancies (Dolcetti et al, unpublished data). The inherent ability of NQ22 cells to up-regulate VCAM-1 expression was confirmed by the strong expression of VCAM-1 on endothelial cells observed at distant sites of dissemination whenever leukemic cells displayed perivascular growth.9
Although the main focus of recent studies has been the extravasation steps,4,40-46 other reports have suggested that the ability of transformed cells to metastasize at distant organ sites can be independent of the extravasation steps47 or can manifest itself at a stage subsequent to cell dissemination.18,48 In the current study, the pro-invasive role of α4 integrins appears of primary importance in the early steps. In a different tumor cell model, a high expression of transfected α4β1 integrin has instead resulted in reduced invasion resulting from the homotypic intercellular adhesion of α4+ cells in the early invasive stage of the metastatic cascade before intravasation.49 Thus, the contrasting involvement of α4 integrins observed in the various experimental systems studied points to the importance of the cellular context in determining the functional role of integrins. NQ22 cells did not need any further activation to adhere to FN or to adhere to and transmigrate across endothelial cells. In the context of NQ22 cells, α4 integrins, with the α4β1 playing a more fundamental role than the α4β7 integrin, can be used for efficient dissemination.
Using the H-end73 and the H-end80 in vitro endothelial cell model system explored herein, the need for a direct cell–cell contact did not seem to be a prerequisite for the activation of endothelial cells and the consequent up-regulation of VCAM-1 by NQ22 cells. Provided that the 2 cell types were not too far apart, consistent up-regulation of VCAM-1 was detected even when the cells were physically separated by a tissue-culture insert. Instead, in other experimental systems, the cell–cell contact seemed to be a prerequisite for cell surface molecule up-regulation or for cytokine release.50,51Because α4 integrins are not expressed on in vitro grown pre-leukemic NQ22 and NQ29 cells, the de novo expression of α4 integrins induced after the cocultivation with activated endothelial H-end80 cells was more pronounced in NQ22 than in NQ29 cells. Their differential expression of cytokines and the NQ22-dependent up-regulation of VCAM-1 on endothelial cells that is largely prevented by TNF-α neutralizing antibodies likely represent key elements in determining the aggressive behavior of NQ22 cells. In addition, it was ascertained that NQ22 cells or their α4 integrins are particularly sensitive to activation by still unknown signals generated by TNF-α-activated endothelial cells. This latter might represent another important influence that impinges on the intrinsic propensity of NQ22 cells to disseminate through the tissues. Although there is not yet direct evidence, it is likely that the action of soluble factors precedes the cell–cell interaction when NQ22 cells establish close contact with endothelial cells. This was suggested by the observation that in vitro grown NQ22 cells constitutively express the mRNA for IFN-γ, GM-CSF, and TNF-α. It could then be hypothesized that after the induction of cell adhesion molecules on NQ22 cells by the intimate interaction with endothelial cells, NQ22 cells may adhere to activated, VCAM-1 positive endothelial cells and produce increased amounts of cytokines. These would in turn further activate the microvasculature in a continuous loop. While we have demonstrated that VCAM-1 supports NQ22 cell adhesion to endothelial cells, it is likely that additional or synergistic mechanisms regulate the movement of migratory NQ22 cells toward the vasculature. For instance, CD40L,52 the TNF receptor,53 and Fas54 have been found to represent determining factors in the up-regulation of endothelial cell adhesion molecules. Similarly, the disruption of the vascular–endothelial cadherin complex, triggered by an efficient adhesion of the leukocytes to the activated endothelium, is thought to constitute a fundamental step in leukocyte transendothelial migration.55 In this context, NQ22 cells that adhere efficiently to H-end cells and transmigrate efficiently across cytokine-activated endothelial cells in vitro are found in vascular spaces at the site of inoculation shortly after injection.
In light of the possible application of our findings in a clinical setting, it is worth considering that in addition to the important role played by TNF-α, the cytokines involved in the intravasation of NQ22 cells might be even more than the ones we have studied, thus making particularly difficult any attempt to block VCAM-1 up-regulation therapeutically. Nevertheless, α4 integrins and VCAM-1 may still be considered appropriate molecular targets for the design of new therapeutic strategies aimed at counteracting the leukemic evolution of T-LBLs.
Acknowledgments
We thank Drs F. Bussolino, E. C. Butcher, P. J. Kilshaw, P. Kinkade, A. Sonnenberg, G. Tarone, and D. Vestweber for providing antibodies.
Supported by the Associazione Italiana Ricerca sul Cancro (A.C., R.D.) and by the FSN 1994-1995 grant (A.C.) of the Ministero della Sanità.
L.B. and E.G. contributed equally to this work.
Reprints:Alfonso Colombatti, Divisione di Oncologia Sperimentale 2, CRO, 33081 Aviano, Italy; e-mail: acolombatti@ets.it.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.









This feature is available to Subscribers Only
Sign In or Create an Account Close Modal