Abstract
Butyrate induces cytodifferentiation in many tumor cells of different origin, suggesting that an as yet unidentified common mechanism inherent to malignant cells is the target of butyrate action. This study determined the role of different mitogen-activated protein (MAP) kinase signal transduction pathways in butyrate-induced erythroid differentiation of K562 human leukemia cells. Using a panel of anti-ERK, JNK, and p38 phosphospecific antibodies, the study showed that phosphorylation of ERK and JNK is decreased following treatment of cells with butyrate, whereas phosphorylation of p38 is increased. In contrast, a K562 subline defective in butyrate-mediated induction of erythroid differentiation did not reveal these changes in phosphorylation patterns. Inhibition of ERK activity by UO126 induces erythroid differentiation and acts synergistically with butyrate on hemoglobin synthesis and inhibition of cell proliferation, whereas inhibition of p38 activity by SB203580 completely abolished induction of hemoglobin expression by butyrate. Taken together, our data suggest a model in which butyrate induces erythroid differentiation of K562 cells by inhibition of ERK and activation of p38 signal transduction pathways.
Butyrate and its derivatives induce cytodifferentiation in a variety of tumor cells in vitro.1Subsequent reports of anecdotal clinical applications and phase I pharmacokinetic studies have been published following the idea of differentiation therapy of malignant disease.2-6 However, the cellular mechanism by which butyrate exerts its effects on tumor cells leading to inhibition of cell growth, induction of differentiation markers, and morphologic changes into a more benign phenotype are largely unknown. The mitogen-activated protein (MAP) kinase signaling cascade comprising the extracellular signal-regulated kinases (ERK), c-Jun N-terminal kinases (JNK), and p38 MAP kinase (p38) has been shown to regulate a wide variety of cellular events such as cell proliferation, differentiation, and development7-9 and may therefore be a potential target of butyrate action. This study investigated the role of these MAP kinase pathways in butyrate-induced erythroid differentiation of the human erythroleukemia cell line K562. Treatment of K562 cells with butyrate leads to erythroid differentiation as evidenced by inhibition of cell proliferation and induction of hemoglobin synthesis.10 Here we show that butyrate modulates parallel signal processing in K562 cells leading to inhibition of cell proliferation and induction of hemoglobin synthesis by down-regulation of ERK and activation of p38 signal transduction pathways.
Material and methods
Cell culture
The human leukemia cell line K562, referred to as K562s (sensitive to butyrate) in this paper, was obtained from ATCC, Philadelphia, PA (CCL-243). The K562 subline K562r (resistant to butyrate) was purchased from DSM, Braunschweig, Germany (DSM ACC 10). Cells were cultured in RPMI containing 10% fetal calf serum with addition of penicillin/streptomycin. For experiments, cells were seeded at a density of 4 × 105 cells/4 mL in 35-mm dishes and cultured for 4 days in the presence or absence of the inducing/inhibiting agents as indicated. The following concentrations were used: 0.6 mM sodium butyrate, 0.05 mM hemin, 100 μM hydroxyurea, 2 μM 5-azacytidine, and 0.6 mM sodium phenylacetate. All substances were purchased from Sigma (St. Louis, MO). Cell counts were determined using the trypan-blue dye exclusion test. For measurement of hemoglobin synthesis, cells were centrifuged and washed with phosphate-buffered saline (PBS); the cell pellet was resuspended in lysis buffer (100 mM potassium phosphate pH 7.8, 0.2% Triton X-100) and incubated 10 minutes at room temperature. After pelleting cellular debris, the supernatant was collected and hemoglobin concentration was determined using the plasma hemoglobin kit from Sigma, according to the manufacturer's instructions. After measurement of protein concentration of the lysate by the Coomassie method, nanograms of hemoglobin per micrograms of total cellular protein was calculated. For immunoblot analysis, cell pellets were directly resuspended in sodium dodecyl sulfate (SDS) sample buffer (62.5 mM Tris-HCl pH 6.8, 2% SDS, 10% glycerol, 50 mM dithiothreitol DTT, 0.1% bromphenol blue), incubated for 5 minutes at 95°C, cooled on ice for 5 minutes and stored at −20°C until further use.
Materials
The following antibodies were purchased from Calbiochem, San Diego, CA: anti-ERK1/2 phosphorylated, anti-JNK1/2 phosphorylated, and anti-p38 total. Antibodies obtained from Sigma were anti-p38 phosporylated, anti-ERK1/2 total, and JNK1/2 total. The p38-specific inhibitor SB203580 was from Calbiochem and the ERK1/2 specific inhibitors UO126/PD 98059 were from Promega, Madison, WI.
Immunoblot analysis
Cell lysates were subjected to SDS-polyacrylamide gel electrophoresis (PAGE) using 10% polyacrylamide gels and transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, Bedford, MA) using a semidry electroblot chamber. Transfer of proteins was assessed by ponceau-red staining. Membranes were blocked in Tris-buffered saline pH 7.4 containing 0.1% Tween-20 and 5% bovine serum albumin for 1 hour at room temperature. Incubations with primary antibodies were carried out at 4°C overnight using antibody dilutions as recommended by the manufacturer in Tris-buffered saline pH 7.4, 0.1% Tween-20. Following 1 hour of incubation with goat-antirabbit peroxidase-conjugated antibody (Promega) at room temperature, proteins were detected by the electrogenerated chemiluminescence method (Amersham-Pharmacia, Piscataway, NJ) according to the manufacturer's instructions. Blots were stripped at 50°C for 30 minutes in 100 mM 2-mercaptoethanol, 2% SDS, 62.5 mM Tris-HCl pH 6.7, and reprobed as indicated.
RNA preparation and Northern blot analysis
For isolation of total cellular RNA, 5 × 106cells, which had been cultured in the absence or presence of the inducing agents indicated, were harvested and RNA was prepared with the RNeasy-kit from Quiagen (Chatsworth, CA) according to the manufacturer's instructions. For detection of globin gene transcripts, a probe was generated from the 489 bp fragment of the γ-gene coding region. All probes were labeled with 32P-dCTP using the Rediprime kit (Amersham-Pharmacia). For Northern blot analysis, total RNA was transferred onto nylon membrane (Hybond-N, Amersham-Pharmacia). Hybridizations of blots were carried out in 50% deionized formamide, 5× SSPE (sodium chloride-sodium phosphate-ethylendiamine tetra acetic acid), 5× Denhardt's solution, and 0.1% SDS at 42°C for 16 hours. Transcript size calculations were based on electrophoretic mobility of ribosomal RNA bands.
Results
Figure 1 gives an overview of the 3 main MAP kinase pathways—ERK, JNK, and p38. Included are the different inhibitors and antibodies used in this work.
Schematic drawing of intracellular MAP kinase signaling pathways.
Indicated are different inhibitors and antibodies used in this study.
Schematic drawing of intracellular MAP kinase signaling pathways.
Indicated are different inhibitors and antibodies used in this study.
Butyrate modulates MAP kinase pathways in K562 cells
We first examined the influence of butyrate on ERK1/2, JNK1/2, and p38 phosphorylation. K562s cells were treated with butyrate for different times as indicated (Figure 2) and cellular extracts were subjected to immunoblot analysis using phosphospecific antibodies against the respective MAP kinases. These antibodies specifically recognize the activated, diphosphorylated form of ERK1/2,11 JNK1/2,12 and p38.13We observed a brief increase of ERK phosphorylation followed by a sustained dephosphorylation beginning 3 hours after butyrate treatment and lasting for the entire experimental period (Figure 2A). The predominating ERK isoform expressed in K562 cells was found to be the 42 kd protein corresponding to ERK2. In contrast to ERK signaling, p38 revealed a sustained phosphorylation starting 3 hours after butyrate addition, lasting for the entire observation period of 4 days (Figure 2A). JNK1/2 is down-regulated after 3 hours following butyrate addition (Figure 2A). All blots were stripped and reprobed with the respective antibodies recognizing ERK1/2, JNK1/2, and p38 proteins, demonstrating no changes in total MAP kinase expression. The same changes in phosphorylation patterns were observed in 4 experiments. Thus, butyrate down-regulates ERK and JNK pathways and at the same time activates p38 in K562 cells. This modulation occurs well before measurable induction of hemoglobin synthesis occurs (Figure 2A). To investigate the relationship of ERK and p38 pathways, K562s cells were treated with ERK inhibitor UO126 for 5 minutes up to 4 days and p38 and JNK phosphorylation was investigated by immunoblot analysis according to the experiments shown in Figure 2. These blots did not reveal changes of p38 or JNK phosphorylation (data not shown). Conversely, inhibition of p38 by SB203580 did not change ERK or JNK phosphorylation (data not shown). Thus, inhibition of ERK does not influence p38 signaling in K562 cells and vice versa.
Western blot analysis of the MAP kinase proteins ERK1/2, JNK1/2, and p38 in butyrate-treated K562 cells.
Cells were treated with butyrate for the various times indicated and harvested, and 20μg cell lysate was subjected to SDS-gel electrophoresis. After electroblotting, blots were incubated with specific antibodies against phosphorylated ERK1/2, JNK1/2, and p38, respectively, and detected by chemiluminescence. Blots were stripped and reprobed with antibodies against ERK1/2, JNK1/2, and p38, referred to as total in the figure. A shows blots of the butyrate-sensitive subline K562s. B shows blots of the butyrate-resistant subline K562r. Each experiment was repeated 4 times and similar results were obtained.
Western blot analysis of the MAP kinase proteins ERK1/2, JNK1/2, and p38 in butyrate-treated K562 cells.
Cells were treated with butyrate for the various times indicated and harvested, and 20μg cell lysate was subjected to SDS-gel electrophoresis. After electroblotting, blots were incubated with specific antibodies against phosphorylated ERK1/2, JNK1/2, and p38, respectively, and detected by chemiluminescence. Blots were stripped and reprobed with antibodies against ERK1/2, JNK1/2, and p38, referred to as total in the figure. A shows blots of the butyrate-sensitive subline K562s. B shows blots of the butyrate-resistant subline K562r. Each experiment was repeated 4 times and similar results were obtained.
Defective MAP kinase signaling in a K562r subline not responding to butyrate treatment
We have identified a K562 subline with a defect in butyrate-mediated erythroid differentiation. Globin gene expression in these cells is not induced on butyrate or phenylacetate treatment (Figure3B), whereas hemin, hydroxyurea, and 5-azacytidine are capable of inducing globin gene expression at the messenger RNA (mRNA) and protein level (Figure 3B). Because this subline is resistant to butyrate treatment, we referred to these cells as K562r. In contrast, the wild-type K562 cell line responds to all chemical inducers including butyrate (Figure 3A). We referred to this butyrate-sensitive line as K562s in this work. The nonresponsiveness of K562r cells to the action of butyrate and phenylacetate suggests that these cells are lacking factor(s) responsible for mediating erythroid differentiation by butyrate and other short-chain fatty acid derivatives. Because we observed a complex modulation of MAP kinase phosphorylation patterns after butyrate treatment of K562s cells, we asked whether K562r cells differ from K562s cells with respect to butyrate-associated changes in MAP kinase signaling. Interestingly, K562r cells revealed no changes in ERK phosphorylation following butyrate treatment of cells (Figure 2B). The overall amount of phosphorylated ERK was relatively low. However, total ERK expression in these cells is comparable to expression in K562s cells with ERK2 being the mainly expressed isoform. Thus, the butyrate-resistant subline lacks butyrate-associated changes of ERK phosphorylation patterns. Investigation of the JNK pathway revealed no detectable phosphorylation of JNK in K562r cells, no changes on butyrate treatment, but comparable expression of total JNK protein in K562r and K562s cells (Figure 2B). Examination of the p38 signal transduction showed that uninduced K562r cells contain phosphorylated p38 protein, but in contrast to K562s cells, we did not observe an increase in p38 phosphorylation following butyrate treatment of cells (Figure 2B). Taken together, these data demonstrate that a K562r subline with a specific defect in butyrate-mediated induction of hemoglobin expression lacks the modulation of MAP kinase signaling observed in K562s cells.
Induction of globin gene expression by different chemical agents in K562 cells.
Cells were treated with hemin, hydroxyurea, butyrate, phenylacetate, 5-azacytidine, or phenylacetate plus hydroxyurea for 4 days. For Northern blot analysis, total RNA was prepared, subjected to agarose gel electrophoresis and blotted on nylon membranes. Blots were hybridized with a 32P-labeled probe of the γ-globin coding region. After stripping, blots were rehybridized with a β-actin probe. Hemoglobin and protein concentrations of total cell extracts were determined as described. A shows results from butyrate-sensitive K562s cells and B results from butyrate-resistant K562r cells.
Induction of globin gene expression by different chemical agents in K562 cells.
Cells were treated with hemin, hydroxyurea, butyrate, phenylacetate, 5-azacytidine, or phenylacetate plus hydroxyurea for 4 days. For Northern blot analysis, total RNA was prepared, subjected to agarose gel electrophoresis and blotted on nylon membranes. Blots were hybridized with a 32P-labeled probe of the γ-globin coding region. After stripping, blots were rehybridized with a β-actin probe. Hemoglobin and protein concentrations of total cell extracts were determined as described. A shows results from butyrate-sensitive K562s cells and B results from butyrate-resistant K562r cells.
Influence of specific MAP kinase inhibitors on butyrate action
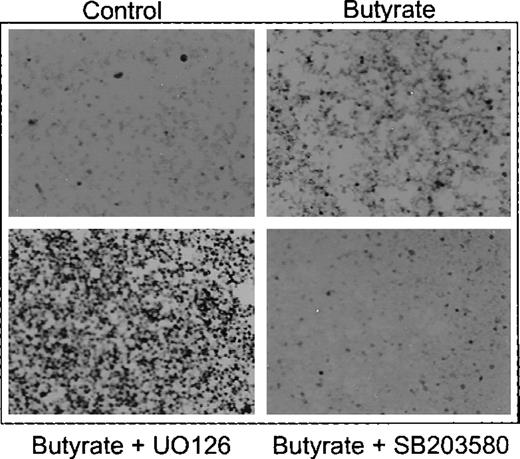
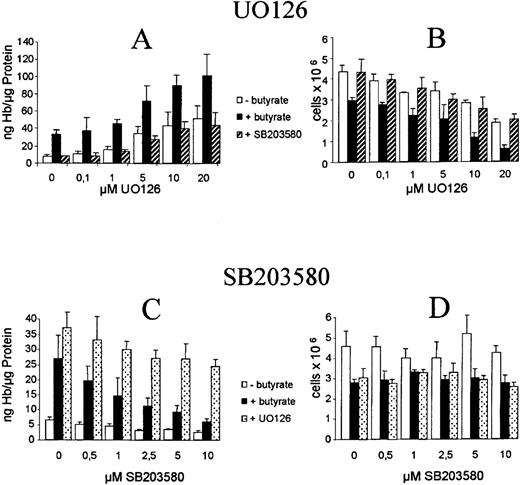
The Western blot experiments shown above suggested that inhibition of ERK/JNK and activation of p38 play a role in butyrate-mediated eythroid differentiation of K562 cells. To further prove this observation, we next examined the influence of the specific ERK inhibitors UO126/PD9805914-16 and p38 inhibitor SB20358017-19 on butyrate-induced erythroid differentiation (Figure 1). Figure 4 shows benzidine staining of butyrate-treated K562 cells that were cultured in the presence of UO126 and SB 203580, respectively. UO126 further increased benzidine positivity of butyrate-treated K562 cells, whereas SB203580 completely abolished induction of erythroid differentiation by butyrate (Figure 4). Quantification of these effects demonstrates a concentration-dependent synergistic effect of UO126 on butyrate induction of hemoglobin synthesis (Figure5A, black bars) and inhibition of cell growth (Figure 5B, black bars). UO126 alone mimics the effect of butyrate on K562 cells, that is, it induces hemoglobin synthesis (Figure 5A, white bars) and inhibits cell proliferation (Figure 5B, white bars) in a concentration-dependent manner. The same results were obtained with PD98059, another specific inhibitor of ERK signaling, and with genistin, a tyrosine kinase inhibitor (data not shown). In contrast, addition of SB203580, a specific inhibitor of p38, abrogated the hemoglobin-inducing effect of butyrate in a concentration-dependent manner (Figure 5C, black bars), whereas SB203580 alone slightly reduced basal hemoglobin synthesis (Figure 5C, white bars). Furthermore, SB203580 did not influence inhibition of cell proliferation by butyrate (Figure 5D). Thus, activation of p38 MAP kinase by butyrate induces hemoglobin expression in K562s cells, but has no influence on cell proliferation. To investigate the effect of simultaneous inhibition of ERK and p38 kinases, K562s cells were cultured in the presence of SB203580 plus increasing concentrations of UO126 (Figure 5A and B, dashed bars) and vice versa (Figure 5C and D, dotted bars). Inhibition of both kinases caused induction of hemoglobin synthesis and inhibition of cell proliferation, that is, simultaneous inhibition of ERK and p38 signaling promotes erythroid differentiation in K562 cells.
Benzidine staining of K562 cells treated with butyrate in the presence of ERK inhibitor UO126 and p38 inhibitor SB203580, respectively.
Cells were cultured in the absence (control) or presence of butyrate alone (butyrate) or with butyrate plus UO126 (butyrate + UO126) and SB203580 (butyrate + SB203580), respectively. After harvesting, intracellular hemoglobin was detected by benzidine-staining and cell smears were subjected to microscopy using 100× magnification.
Benzidine staining of K562 cells treated with butyrate in the presence of ERK inhibitor UO126 and p38 inhibitor SB203580, respectively.
Cells were cultured in the absence (control) or presence of butyrate alone (butyrate) or with butyrate plus UO126 (butyrate + UO126) and SB203580 (butyrate + SB203580), respectively. After harvesting, intracellular hemoglobin was detected by benzidine-staining and cell smears were subjected to microscopy using 100× magnification.
Effect of ERK inhibitor UO126 and p38 inhibitor SB203580 on butyrate-mediated erythroid differentiation of K562s cells.
Cells were cultured for 4 days in the absence (white bars) or presence (black bars) of butyrate. Simultaneous addition of both inhibitors are shown as dashed bars (A and B: 10 μM SB203580 plus increasing concentrations of UO126) or dotted bars (C and D: 10 μM UO126 plus increasing concentrations of SB203580). Inhibitors were added 1 hour prior to butyrate. Cell numbers were counted and cells were then harvested and lysed. Hemoglobin and protein concentrations were determined as described in “Materials and methods.” Each experiment was performed 4 times and standard errors were calculated as indicated.
Effect of ERK inhibitor UO126 and p38 inhibitor SB203580 on butyrate-mediated erythroid differentiation of K562s cells.
Cells were cultured for 4 days in the absence (white bars) or presence (black bars) of butyrate. Simultaneous addition of both inhibitors are shown as dashed bars (A and B: 10 μM SB203580 plus increasing concentrations of UO126) or dotted bars (C and D: 10 μM UO126 plus increasing concentrations of SB203580). Inhibitors were added 1 hour prior to butyrate. Cell numbers were counted and cells were then harvested and lysed. Hemoglobin and protein concentrations were determined as described in “Materials and methods.” Each experiment was performed 4 times and standard errors were calculated as indicated.
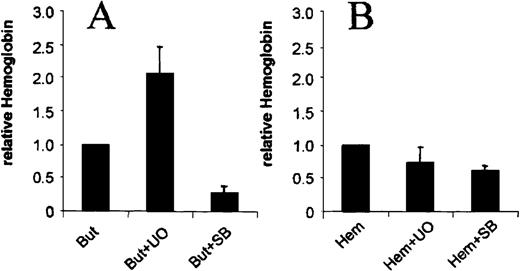
We next asked whether the observed involvement of ERK and p38 MAP kinase pathways is specific for butyrate-induced erythroid differentiation of K562 cells and investigated the influence of UO126 and SB203580 on hemin-induced erythroid differentiation. In contrast to butyrate-treated cells, addition of UO126 and SB203580 had no significant effect on hemin-induced hemoglobin synthesis (Figure6). These data demonstrate that the observed changes in MAP kinase signaling are indeed specific for the action of butyrate.
Effects of UO126 and SB203580 on hemin-induced erythroid differentiation of K562s cells.
Cells were induced with butyrate (A) or hemin (B) in the absence or presence of 10 μM UO126 (UO) and 10 μM SB203580 (SB), respectively. Shown are relative changes of hemoglobin expression compared to the inducing agent without inhibitor. Each experiment was performed 4 times and standard errors were calculated as indicated.
Effects of UO126 and SB203580 on hemin-induced erythroid differentiation of K562s cells.
Cells were induced with butyrate (A) or hemin (B) in the absence or presence of 10 μM UO126 (UO) and 10 μM SB203580 (SB), respectively. Shown are relative changes of hemoglobin expression compared to the inducing agent without inhibitor. Each experiment was performed 4 times and standard errors were calculated as indicated.
Discussion
Butyrate induces differentiation in a wide range of tumor cells in culture. In contrast to most other chemical inducers, the action of butyrate appears not to be limited to certain cell types and effective cytodifferentiation has been documented for malignant cells from different tumor types such as colon carcinoma,20,21neuroblastoma,22 hepatoma,23leukemias,24-27 prostatic carcinoma,28retinoblastoma,29 ovarian carcinoma,30 and breast cancer.31 This suggests that butyrate acts via a common mechanism inherent to many cancer cells. We investigated the role of components of the ubiquitous MAP kinase signal transduction system in butyrate-mediated induction of erythroid differentiation of K562 leukemia cells. Our data show that inhibition of ERK and activation of p38 signal transduction pathways play a critical role in butyrate-induced erythroid differentiation. This is based on the following observations:
1. Butyrate causes dephosphorylation of ERK, dephosphorylation of JNK, and phosphorylation of p38 MAP kinases.
2. A K562 subline with a defect in butyrate-mediated erythroid differentiation lacks these changes in phosphorylation patterns.
3. Inhibition of ERK signaling by specific inhibitors induces hemoglobin synthesis and inhibition of cell proliferation and acts synergistically with butyrate. Specific inhibition of p38 signaling causes down-regulation of basal hemoglobin expression and abrogates butyrate-mediated induction of hemoglobin synthesis but has no influence on cell proliferation.
4. ERK and p38 signaling are not involved in hemin-mediated induction of cytodifferentiation.
These data suggest a model of butyrate action in erythroid differentiation of K562 human leukemia cells as depicted in Figure7.
Hypothetical model of butyrate action in cytodifferentiation of K562 cells.
Preliminary data obtained in HL60 promyelocytic leukemia cells and HT29 colon carcinoma cells indicate that butyrate also inhibits ERK and JNK signaling, but influence on p38 signaling appears to be different (data not shown). This suggests that our model of butyrate action in K562 cells may partially hold true for other tumor cell lines.
Involvement of signal transduction pathways in mediating butyrate effects on cells has been described recently. In murine erythroleukemia cells, butyrate causes sustained activation of JAK/STAT signaling32 and inhibition of a serine-threonine-protein phosphatase is involved in butyrate-mediated differentiation of a hepatoma cell line.33 In K562 cells, activation of ERK signaling by phorbol-ester leads to megakaryocytic differentiation in K562 cells, whereas inhibition of the ERK pathway enhances the erythroid phenotype,34 35 consistent with our finding that ERK is down-regulated during butyrate-induced erythroid differentiation.
ERK is the archetypal pathway of the MAP kinase superfamily. It is activated in response to a wide variety of growth factors and mitogens and sustained activation eventually initiates cell proliferation and hallmarks of transformation.8,9,36,37 Conversely, inhibition of ERK has been shown to inhibit cell growth and growth factor–stimulated gene transcription.38,39 In PC12 rat pheochromocytoma cells, the duration of ERK activation appears to be critical for neuronal differentiation versus proliferation of cells.40,41 Among ERK substrates are cytoskeletal elements and their phosphorylation by ERK appears to regulate cytoskeletal rearrangements and cellular morphology.42,43 Furthermore, many of the cellular oncogenes involve mutations of receptor tyrosine kinases such as Src, Ras, Raf-1, and G proteins leading to constitutive activation of the ERK pathway.9 44 Thus, inhibition of ERK signaling by butyrate might be an attractive explanation for the well-documented biochemical and morphologic reverse of many cancer cells into a more benign phenotype following butyrate treatment.
JNK and p38 signaling are implicated in responses to cellular stress, inflammation, and apoptosis.8,9 Additionally, activation by several hematopoietic cytokines including granulocyte colony-stimulating factor, thrombopoietin, interleukin-3, and erythropoietin have also been reported.45-49 In a recent paper, activation of p38 and JNK MAP kinase pathways have been shown to be essential for erythropoietin-induced erythroid differentiation of mouse erythroleukemia cells.50 We also found that activation of p38 signaling is involved in induction of hemoglobin synthesis, but the role of the observed changes in JNK MAP kinase activity by butyrate remains to be clarified, because no specific inhibitor of this pathway is available.
To date there is only very limited experience in butyrate treatment of malignant disease2,3 mainly due to pharmacologic problems of this compound. Phase I clinical trials of butyrate derivatives with longer plasma half-lives have been published.4-6 A number of studies have demonstrated anticancer effects of butyrate derivatives in tumor models in rats and mice.51-55 Treatment of patients with β-thalassemia and sickle cell disease with arginine-butyrate has shown clinical benefit due to induction of fetal hemoglobin expression in these patients.56 57 Whether modulation of MAP kinase signal transduction also plays a role in butyrate-mediated induction of fetal hemoglobin synthesis during hematopoiesis remains to be shown.
Sponsored by grants from the Deutsche Forschungsgemeinschaft (Pe 374/2-3), the Dieter-Schlag-Stiftung, Hannover, and by the Elternhilfe für das krebskranke Kind, Göttingen, Germany.
Reprints:Olaf Witt, Children's Hospital, University of Göttingen, Robert- Koch-Str. 40, D-37075 Göttingen, Germany.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal