Abstract
Polymorphonuclear leukocyte (PMNL) phagocytosis mediated by FcγRII proceeds in concert with activation of the mitogen-activated protein (MAP) kinase, extracellular signal-regulated kinase ERK2. We hypothesized that myosin light chain kinase (MLCK) could be phosphorylated and activated by ERK, thereby linking the MAP kinase pathway to the activation of cytoskeletal components required for pseudopod formation. To explore this potential linkage, PMNLs were challenged with antibody-coated erythrocytes (EIgG). Peak MLCK activity, 3-fold increased over controls, occurred at 4 to 6 minutes, corresponding with the peak rate of target ingestion and ERK2 activity. The MLCK inhibitor ML-7 (10 μmol/L) inhibited both phagocytosis and MLCK activity to basal values, thereby providing further support for the linkage between the functional response and the requirement for MLCK activation. The MAPK kinase (MEK) inhibitor PD098059 inhibited phagocytosis, MLCK activity, and ERK2 activity by 80% to 90%. To directly link ERK activation to MLCK activation, ERK2 was immunoprecipitated from PMNLs after EIgG ingestion. The isolated ERK2 was incubated with PMNL cytosol as a source of unactivated MLCK and with MLCK substrate; under these conditions ERK2 activated MLCK, resulting in phosphorylation of the MLCK substrate or of the myosin light chain itself. Because MLCK activates myosin, we evaluated the effect of directly inhibiting myosin adenosine triphosphatase using 2,3-butanedione monoxime (BDM) and found that phagocytosis was inhibited by more than 90% but MLCK activity remained unaffected. These results are consistent with the interpretation that MEK activates ERK, ERK2 then activates MLCK, and MLCK activates myosin. MLCK activation is a critical step in the cytoskeletal changes resulting in pseudopod formation.
Polymorphonuclear leukocytes (PMNLs) are recruited by chemoattractants to sites of inflammation where they ingest, or phagocytose, opsonized particles. Two classes of PMNL receptors are involved in phagocytosis: the Fc receptor and the complement receptor.1 The sequence of signal transduction events from FcγRIIa receptor ligation leading to the ingestion of IgG-opsonized particles is partially known. One of the first steps after ligand binding to the Fc receptor is activation of several nonreceptor protein tyrosine kinases, such as Syk, p55fgr, and p59hck.2-6 Subsequently, phospholipase D is activated during PMNL phagocytosis7,8 and generates phosphatidic acid.9 Phosphatidate phosphohydrolase generates diglyceride from phosphatidic acid,10 thereby activating protein kinase C (PKC).8 The Ca++-independent PKCδ appears to be important to PMNL phagocytosis. Our group has recently shown that PKCδ and Raf-1 translocate to the plasma membrane during the first few minutes of PMNL phagocytosis.11 In most systems, Raf-1 then initiates the mitogen-activated protein (MAP) kinase cascade.12 Raf-1 activates MEK, which then phosphorylates ERK.13,14Previously we showed that activation of the MAP kinases ERK2 and ERK1, as well as phospholipase D, are required for the ingestion of IgG-opsonized particles.7,15 Others also have found ERK activity to increase during both FcR- and CR3-mediated phagocytosis16 and the inhibition of the ERK pathway to inhibit phagocytosis and the oxidative burst.17 The in vivo substrate for ERK2 during PMNL phagocytosis has not been determined.
The later part of the phagocytic signaling pathway involves activation of the cytoskeletal components actin and myosin. Actin polymerization is required for pseudopod extension and particle engulfment, whereas myosin furnishes propulsive force by its interaction with actin. Actin polymerization is initiated by many stimuli, including cross-linking of FcγRIIa.18 Myosin concentrates in the anterior pseudopod of phagocytosing PMNLs,19 where it binds F-actin and hydrolyzes adenosine triphosphate (ATP).20 Myosin converts ATP hydrolysis energy to movement through conformational change of the myosin molecule while it is bound to actin.21,22 Nonmuscle myosin II ATPase activity is up-regulated by light chain phosphorylation, relieving the inhibitory effect of the light chains,22 and this phosphorylation is accomplished by myosin light chain kinase (MLCK).23 24
PMNL chemotaxis and random migration involve MLCK activation and myosin phosphorylation.25,26 Klemke et al27 used COS-7 cells transfected with constitutively active MEK to show that MLCK is phosphorylated and activated by the MAP kinase ERK2, and these signals are required for chemotaxis. Because the MAP kinase cascade and cytoskeletal interactions are known to be critical to PMNL phagocytosis, we conjectured that these were similarly linked in phagocytic signaling. In this study, we demonstrate that ERK2, activated by MEK, activates MLCK during PMNL phagocytosis and that this is followed by the activation of myosin ATPase.
Materials and methods
Reagents
Peptide substrate specific for myosin light chain kinase, corresponding to residues 11-23 of smooth muscle myosin light chain, was obtained from Biomol (Plymouth Meeting, PA). The myosin light chain kinase inhibitor ML-7, (5-iodonaphthalene-1-sulfonyl) homopiperazine, was purchased from Calbiochem (San Diego, CA). 2,3-Butanedione 2-monoxime (BDM) was obtained from Sigma (St. Louis, MO). The MEK inhibitor PD098059 (2′-amino-3′-methoxyflavone) was a gift from Alan R. Saltiel, Parke-Davis (Ann Arbor, MI). Polyclonal antibody against ERK2 (p42; C-14) and secondary antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Polyclonal anti-ERK recognizing both p42 and p44 was a gift from Hua Yu, University of Southern Florida (Tampa, FL). [γ-32P]ATP was purchased from ICN Pharmaceuticals (Irvine, CA). Polyclonal antiplatelet myosin was a kind gift from Dr Robert Adelstein's laboratory (National Institutes of Health, Bethesda, MD).
Cells
PMNLs were isolated from peripheral venous blood from healthy volunteers as previously described.28 PMNLs were pretreated with 5 mmol/L diisopropyl fluorophosphate on ice for 5 minutes, then washed 3 times with phosphate-buffered saline (PBS). Incubation with inhibitors was performed before phagocytosis as follows: PMNLs were incubated with the indicated concentrations of ML-7 or BDM for 10 minutes at 37°C or with 50 μmol/L PD098059 for 30 minutes at 22°C.
Phagocytosis and preparation of PMNL lysates
Phagocytosis was conducted as previously described.7,29Briefly, sheep erythrocytes (E) were opsonized with anti-sheep E antibody (EIgG) (Cappel Organon Teknika, Durham, NC). PMNLs (2 × 106/mL) were warmed at 37°C for 3 minutes, and EIgG was added to PMNLs at a ratio of 50:1. No priming agent was used. Samples were removed at the indicated time points and microfuged for 7 seconds, then noningested EIgG were lysed with double-distilled water containing 1 mmol/L Na3VO4 and 50 mmol/L NaF. PMNLs were returned to isotonicity by adding KCl, microfuged, and suspended in MLCK kinase buffer (40 mmol/L HEPES pH 7.0, 5 mmol/L Mg acetate, 0.55 mmol/L CaCl2, and 0.1% Tween-80) containing freshly added 1 mmol/L Na3VO4, 50 mmol/L NaF, 1 mmol/L phenylmethylsulfonyl fluoride (PMSF), 10 μg/mL pepstatin, 10 μg/mL leupeptin, 10 μg/mL aprotinin, and 100 μg/mL soybean trypsin inhibitor. Samples were probe sonicated on ice for 12 seconds 2 times and microfuged for 5 minutes. Supernatants were used for the MLCK assay. Parallel samples were taken in each experiment to evaluate phagocytosis, as described previously.15 Significant differences in phagocytosis were assessed using 1-sample, 2-tailed Student t tests.
The binding of EIgG to PMNL was tested for possible effects of ML-7 and BDM by preincubating with inhibitors as described above, then placing the samples on ice. EIgG was added, and PMNLs were incubated for 30 minutes. No lysis step was performed; samples were fixed and evaluated by counting the number of PMNLs with and without bound EIgG.
Kinase assay
Each PMNL lysate sample was divided into 2 equal aliquots, 1 to receive substrate and 1 no substrate. A saturating concentration of substrate, 300 μmol/L, was chosen for this assay. A reaction mixture containing substrate peptide or buffer, 5 μCi [γ-32P] ATP, and 0.5 mmol/L unlabeled ATP was added to each tube, and samples were incubated for 10 minutes at 30°C. The reaction was terminated by filtering through Whatman P81 paper (Clifton, NJ). Filters were added to scintillation fluid and placed in a scintillation counter (Wallac, Gaithersburg, MD). Blanks were assay samples run without substrate. One-sample, 2-tailed Student t tests were used to assess statistical significance of increases in kinase activity.
Orthophosphate labeling and immunoprecipitation of myosin
PMNLs were suspended at 108/mL in 30 mmol/L HEPES pH 7.4, 140 mmol/L NaCl, 5 mmol/L KCl, 1 mmol/L MgCl2, 10 mmol/L glucose, 2 mg/mL bovine serum albumin, and 1 mCi/mL H3[32P]O4. They were incubated at 37°C for 30 minutes, then washed with PBS and suspended in PBS with 1 mmol/L Ca++ and 1 mmol/L Mg++. PMNLs were allowed to phagocytose EIgG for 4 minutes, then samples were lysed with buffer containing 1% NP-40, 250 mmol/L NaCl, 5 mmol/L EGTA, 20 mmol/L Tris pH 8, 40 mmol/L 4-nitrophenyl phosphate, and phosphatase inhibitors and protease inhibitors as described above. Lysates were incubated overnight with antiplatelet myosin, then for 2 hours with Protein A-Sepharose. Samples were subjected to 12% SDS-PAGE, and the gels were dried and exposed to X-ray film.
Preparation of cytosol
PMNLs were isolated and DFP-treated as described above. They were suspended at 108/mL in extraction buffer (50 mmol/L Tris pH 7.5, 2 mmol/L EGTA, 1 mmol/L PMSF, 1 μg/mL leupeptin, 10 μmol/L benzamidine, 10 μmol/L pepstatin, and 0.2 μg/mL aprotinin) and were probe sonicated for 12 seconds 2 times. Extracts were centrifuged for 10 minutes at 800g, and the supernatants were layered on 15% sucrose in 10 mmol/L HEPES, pH 7.5. The samples were centrifuged in a swinging bucket rotor at 150 000 × g for 30 minutes. Cytosol was removed from the top half of the upper layer and used within 2 hours.
ERK2 immunoprecipitation and coupled kinase assay
PMNLs were allowed to phagocytose for 5 minutes as described above; controls were incubated at 37°C for the same interval. PMNLs (1 × 107) were lysed in 800 μL buffer containing 50 mmol/L HEPES (pH 7.5), 100 mmol/L NaCl, 2 mmol/L EDTA, 1% Nonidet P-40, 1 μmol/L pepstatin, 1 μg/mL leupeptin, 0.2 mmol/L PMSF, 0.5 mmol/L sodium orthovanadate, 50 mmol/L NaF, 2 μg/mL aprotinin, and 40 mmol/L 4-nitrophenyl phosphate. Precleared lysates were incubated with 1 μg anti-ERK2 overnight at 4° C while rotating. Protein A-Sepharose was added to each sample and incubated for 1 hour at 4°C while rotating. Beads were washed twice with cold lysis buffer, then twice with cold MLCK kinase buffer. Cytosol from resting PMNLs, or buffer, was added to samples as indicated (106 cell equivalents/sample). Beads were then resuspended in MLCK kinase buffer containing 500 μmol/L ATP, 5 μCi/sample [γ-32P]ATP, and 300 μmol/L MLCK peptide substrate or buffer for blanks. Samples were incubated for 10 minutes at 30°C and microfuged for 30 seconds. The reaction was terminated by filtering supernatants through Whatman P81 paper. Filters were added to scintillation fluid and counted in a scintillation counter. Counts were expressed as percentage of controls from 22°C PMNLs. Paired, 2-tailed Student ttests were used to assess differences between treatments.
These experiments were also conducted using a crude PMNL myosin, prepared according to Boxer and Stossel,30 as substrate. After treatment with ERK2, the myosin was immunoprecipitated as described in “Orthophosphate Labeling and Immunoprecipitation of Myosin.” Incorporation of 32P was measured by scintillation counting of the immunoprecipitate.
Immunoblotting
To ensure equal ERK2 was immunoprecipitated from each sample, immunoblotting was performed on samples. Beads were combined with sample buffer,31 boiled 5 minutes, and run on 10% SDS-PAGE mini-gels. Proteins were transferred to polyvinylidene fluoride (PVDF) membrane (Schleicher and Schuell, Keene, NH) and then blocked with 5% nonfat dry milk in Tris-buffered saline containing 0.2% Tween-20. The membrane was probed with anti-ERK, which recognizes 42- and 44-kd MAP kinases diluted 1:1000 in blocking buffer, and washed 3 times with 0.2% Tween-20 in TBS. The membrane was then incubated with horseradish peroxide-conjugated sheep antirabbit antibody (Santa Cruz), diluted 1:10 000 in blocking buffer, and again washed 3 times. Phosphorylated bands were visualized using enhanced chemiluminescence (ECL) detection reagents (Amersham, Arlington Heights, IL) and exposing the membrane to Hyperfilm ECL (Amersham). Immunoblotting for tyrosine-phosphorylated Syk was performed as described previously.32
Intracellular calcium
PMNLs were loaded with the fluorescent calcium indicator fluo-3 as described previously.33 PMNLs were incubated with 10 μmol/L ML-7, 10 mmol/L BDM, or buffer for 10 minutes at 37°C, then stimulated with 100 nMN-formyl-methionyl-leucyl-phenylalanine (fMLP) and fluorescence monitored continuously.
Results
MLCK activity was measured using lysates from phagocytosing PMNL and peptide substrate specific for MLCK to determine the kinetics of MLCK activation. The peptide substrate was previously characterized to have an apparent Km of 7.5 μmol/L,34 comparable to the Km of 8.6 μmol/L for myosin light chain.35 MLCK activity increased more than 3-fold during phagocytosis, in contrast to control PMNLs (maintained for up to 10 minutes without phagocytic targets) which showed no increase in activity (Figure 1A). Unopsonized erythrocytes were not ingested by PMNL and did not stimulate MLCK activity (data not shown). The MLCK activity peaked at 4 to 6 minutes, corresponding to EIgG ingestion, which also reaches a maximum rate within 5 minutes of initiating phagocytosis.7 Paralleling the phagocytic rate, MLCK activity decreased to baseline by 10 minutes. To demonstrate phosphorylation of myosin light chain, we labeled PMNLs with 32P and immunoprecipitated myosin from detergent lysates using antiplatelet myosin. We observed a single band at approximately 20 kd whose intensity was increased in samples from phagocytosing PMNLs compared with controls (Figure 1B). This band was most likely myosin light chain based on its relative mobility and its recognition by antiplatelet myosin. In prior studies we observed ERK2 activity to peak by 3 minutes,15 consistent with the hypothesis that ERK2 resides upstream of MLCK in the phagocytic signaling pathway.
MLCK activation during PMNL phagocytosis.
(A) Kinase activity. PMNLs (2 × 106/mL) were challenged with EIgG (1 × 108/mL) (circles) or incubated with no EIgG (triangles) at 37°C for the indicated times. PMNL lysates were prepared by probe sonication as described in “Materials and methods.” MLCK was assayed in the lysates by phosphorylation of substrate peptide (myosin light chain, amino acids 11-23) in the presence of [γ-32P]ATP for 10 minutes at 30°C. Data represent the mean ± SEM of 4 experiments. Significantly different from time 0 sample; P < .05. (B) Phosphorylation of endogenous myosin light chain. PMNLs were labeled with 32P, challenged with EIgG (P) or not stimulated (U) for 4 minutes, and lysed. Samples were immunoprecipitated with antiplatelet myosin and subjected to 12% SDS-PAGE followed by autoradiography.
MLCK activation during PMNL phagocytosis.
(A) Kinase activity. PMNLs (2 × 106/mL) were challenged with EIgG (1 × 108/mL) (circles) or incubated with no EIgG (triangles) at 37°C for the indicated times. PMNL lysates were prepared by probe sonication as described in “Materials and methods.” MLCK was assayed in the lysates by phosphorylation of substrate peptide (myosin light chain, amino acids 11-23) in the presence of [γ-32P]ATP for 10 minutes at 30°C. Data represent the mean ± SEM of 4 experiments. Significantly different from time 0 sample; P < .05. (B) Phosphorylation of endogenous myosin light chain. PMNLs were labeled with 32P, challenged with EIgG (P) or not stimulated (U) for 4 minutes, and lysed. Samples were immunoprecipitated with antiplatelet myosin and subjected to 12% SDS-PAGE followed by autoradiography.
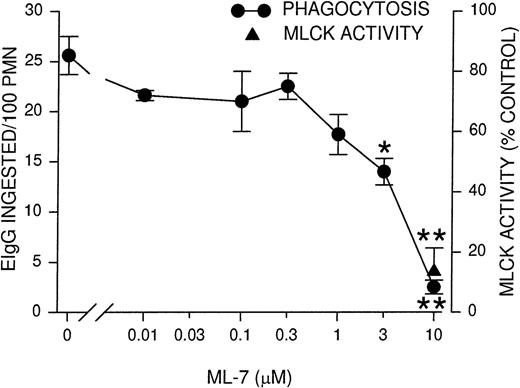
Because the time course of MLCK activity corresponded to the rate of phagocytosis, we next determined whether the inhibition of MLCK would block phagocytosis. PMNLs were incubated for 10 minutes with ML-7, a MLCK inhibitor,36 before assaying for phagocytosis and MLCK activity. Phagocytosis was inhibited partially (50%) using 3 μmol/L ML-7 and almost completely at 10 μmol/L ML-7 (Figure2). Similarly, MLCK activity was also inhibited to 13% of control rates in the presence of 10 μmol/L ML-7 (Figure 2). The observed inhibition of phagocytosis supports the notion that MLCK activation is linked to the functional response of particle ingestion. To test the specificity of ML-7 for phagocytosis and its link to MLCK, we conducted assays of fMLP-stimulated (100 nmol/L) intracellular Ca++ transients and phagocytosis-stimulated tyrosine phosphorylation of Syk. Neither response was significantly blocked by ML-7 (Table 1). In addition, the binding of EIgG to PMNLs, a preliminary step for phagocytosis, was tested at 4°C (to preclude ingestion). ML-7 had no effect on EIgG binding to PMNLs (Table 1). These data are consistent with the selective inhibition of MLCK activity by ML-7.
Phagocytosis and MLCK activity inhibition by MLCK inhibitor ML-7.
PMNLs were incubated for 10 minutes at 37°C with the indicated concentrations of ML-7, then challenged with EIgG for 30 minutes (phagocytosis) or 5 minutes (MLCK activity). EIgG that were not ingested were hypotonically lysed, and phagocytosis samples were fixed with glutaraldehyde for microscopic quantitation. MLCK activity was measured as described for Figure 1. Data points represent the mean ± SEM of 4 experiments. *Significantly different from control (no inhibitor); P < .05. **Significantly different from control; P < .005.
Phagocytosis and MLCK activity inhibition by MLCK inhibitor ML-7.
PMNLs were incubated for 10 minutes at 37°C with the indicated concentrations of ML-7, then challenged with EIgG for 30 minutes (phagocytosis) or 5 minutes (MLCK activity). EIgG that were not ingested were hypotonically lysed, and phagocytosis samples were fixed with glutaraldehyde for microscopic quantitation. MLCK activity was measured as described for Figure 1. Data points represent the mean ± SEM of 4 experiments. *Significantly different from control (no inhibitor); P < .05. **Significantly different from control; P < .005.
Effect of inhibitors on selected PMNL functions other than phagocytosis
| . | Syk phosphorylation . | Intracellular Ca++transient . | Binding of EIgG to PMNLs . |
|---|---|---|---|
| Control | 100% | 100% | 100% |
| ML-7 | 90.5 ± 30.5 | 87.0 ± 13.0 | 101.8 ± 3.0 |
| BDM | 86.0 ± 14.0 | 88.9 ± 6.4 | 104.6 ± 2.7 |
| . | Syk phosphorylation . | Intracellular Ca++transient . | Binding of EIgG to PMNLs . |
|---|---|---|---|
| Control | 100% | 100% | 100% |
| ML-7 | 90.5 ± 30.5 | 87.0 ± 13.0 | 101.8 ± 3.0 |
| BDM | 86.0 ± 14.0 | 88.9 ± 6.4 | 104.6 ± 2.7 |
PMNLs were incubated with 10 μmol/L ML-7 or 10 mmol/L BDM for 10 minutes at 37°C before stimulation. For Syk phosphorylation, PMNLs were challenged with EIgG for 3 minutes, lysed, and immunoblotted with anti-phosphotyrosine; blots were scanned with a densitometer. For measurement of Ca++ transients, PMNLs were loaded with fluo-3 and stimulated with 100 nmol/L fMLP, and fluorescence was monitored continuously; peak and duration of fluorescence were evaluated. Binding of EIgG was evaluated after 30 minutes at 4°C by counting the number of PMNLs with bound erythrocytes; each PMNL bound many erythrocytes. Data are expressed as mean ± SEM.
Previously we and others have found that ERK2 and ERK1 are activated by MEK during PMNL phagocytosis and in other cell systems.15-17,37 Activation of ERK2 is 20-fold higher than ERK1 during PMNL phagocytosis,15 leading us to believe that ERK2 is more relevant to phagocytosis. We also reported that the MEK inhibitor PD09805938 inhibits phagocytosis by 50% and the ERK2 activation required for phagocytosis by 90% in fMLP-primed PMNLs.15 Using nonprimed PMNLs, pretreatment with the MEK inhibitor PD098059 decreased EIgG phagocytosis and ERK2 activation by 80.1% ± 3.0% and 91.9% ± 2.7%, respectively (mean ± SEM of 3 experiments). MLCK activity was also inhibited by 82.1% ± 7.8% when PMNLs were pretreated with the MEK inhibitor before incubation with phagocytic targets. These results are consistent with the hypothesis that MLCK acts downstream of MEK and ERK2 activation.
To further assess the linkage between ERK2 and MLCK, a coupled reaction was performed to determine whether activated ERK2 could activate MLCK, thereby leading to phosphorylation of the peptide substrate. Using ERK2-specific antibody, ERK2 was immunoprecipitated from control and phagocytosing PMNLs to obtain inactive and active ERK2, respectively. An immunoblot with an ERK antibody that recognizes both ERK1 and ERK2 demonstrated that ERK2 was present in approximately equal amounts in our samples from control and phagocytosing PMNLs, while no ERK1 was observed (data not shown; consistent with our previous reports).15 The cytosol of resting PMNL was used as a source of inactive MLCK; though MLCK is found in association with the cytoskeleton,39 it is also distributed in cytosol, especially in resting cells.40 41 We combined the ERK2, PMNL cytosol, ATP, and peptide substrate to assay for MLCK activity. Four times as much activity was seen using active ERK2, immunoprecipitated from phagocytosing PMNLs, as using ERK2 derived from resting cells (Figure3). This result demonstrated that activation of ERK2 leads to activation of MLCK. To verify that the observed kinase activity was cytosolic, active ERK2 from phagocytosing PMNLs was incubated with peptide substrate in the absence of cytosol. MLCK activity under these conditions (Figure 3, fourth bar) was not significantly different from controls that contained cytosol and ERK2 from resting PMNLs (Figure 3, first bar). This observation confirms the specificity of the substrate for MLCK because active ERK2 was unable to directly phosphorylate the peptide substrate. Preincubating the cytosol with 10 μmol/L of the MLCK inhibitor ML-7 prevented MLCK from activation by active ERK2 (Figure 3, fifth bar). Similar results were obtained using exogenously added PMNL myosin instead of substrate peptide. In these experiments, activated ERK2 led to the phosphorylation of immunoprecipitated myosin that was 610% of unactivated ERK2 (control) (Figure 3, third bar); ML-7 inhibited phosphorylation to 133% of control (Figure 3, sixth bar). These data demonstrated that ERK2 activated during PMNL phagocytosis leads to activation of MLCK.
Activation of MLCK by ERK2.
PMNLs were allowed to undergo phagocytosis of EIgG for 5 minutes or incubated without EIgG, then lysed with NP-40. ERK2 was immunoprecipitated from lysates to obtain activated and unactivated ERK2, respectively. Cytosol isolated from resting PMNLs or buffer was added to samples along with [γ-32P]ATP and peptide substrate (plain bars), and kinase activity was quantitated as described for Figure 1, or PMNL myosin replaced peptide substrate (cross-hatched bars) and was immunoprecipitated. R, ERK2 immunoprecipitated from resting PMNLs incubated at 37°C; P, ERK2 immunoprecipitated from phagocytosing PMNLs. +, cytosol present; −, no cytosol; +ML-7, cytosol incubated before kinase assay with 10 μmol/L ML-7. Data represent the mean ± SEM of 3 experiments. MLCK activity in PMNLs at 22°C = 100%. *Significantly different from control (left bar); P < .005. Other data are not significantly different from each other.
Activation of MLCK by ERK2.
PMNLs were allowed to undergo phagocytosis of EIgG for 5 minutes or incubated without EIgG, then lysed with NP-40. ERK2 was immunoprecipitated from lysates to obtain activated and unactivated ERK2, respectively. Cytosol isolated from resting PMNLs or buffer was added to samples along with [γ-32P]ATP and peptide substrate (plain bars), and kinase activity was quantitated as described for Figure 1, or PMNL myosin replaced peptide substrate (cross-hatched bars) and was immunoprecipitated. R, ERK2 immunoprecipitated from resting PMNLs incubated at 37°C; P, ERK2 immunoprecipitated from phagocytosing PMNLs. +, cytosol present; −, no cytosol; +ML-7, cytosol incubated before kinase assay with 10 μmol/L ML-7. Data represent the mean ± SEM of 3 experiments. MLCK activity in PMNLs at 22°C = 100%. *Significantly different from control (left bar); P < .005. Other data are not significantly different from each other.
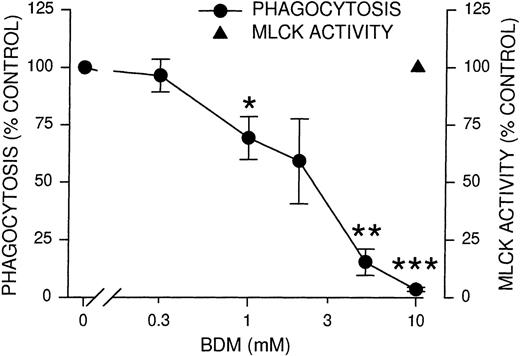
After phosphorylation of myosin light chains by MLCK, both the association of myosin with actin and the actin-stimulated adenosine triphosphatase activity of myosin increase.23,24 To determine whether myosin activation is required for phagocytosis, we used BDM, a known inhibitor of nonmuscle myosin II and myosin V adenosine triphosphatases including those of platelets42and macrophages.43 BDM has no effect on actin filaments in PtK2 cells42 or on the formation of actin-rich phagocytic cups in macrophages,43 implying it would not affect PMNL actin. PMNL phagocytosis was completely inhibited by 10 mmol/L BDM, whereas MLCK activity remained unaffected (Figure 4). This result indicated that the myosin ATPase activity lies downstream of MLCK and precluded nonspecific effects of BDM upstream of MLCK. To further evaluate the effects of BDM, we tested Ca++, the phosphorylation of Syk, and the binding of EIgG to PMNL in the presence of BDM. None of these processes were significantly inhibited (Table 1), demonstrating the relative specificity of BDM. The inhibition of phagocytosis by BDM was consistent with the interpretation that myosin ATPase activity is required for phagocytosis, supplying the energy to extend the pseudopod and to permit ingestion of the particles.
Inhibition of phagocytosis and MLCK activity by myosin adenosine triphosphatase inhibitor BDM.
PMNLs were incubated for 10 minutes at 37°C with the indicated concentrations of BDM, then challenged with EIgG for 30 minutes (phagocytosis) or 5 minutes (MLCK activity). EIgG that were not ingested were hypotonically lysed, and phagocytosis samples were fixed with glutaraldehyde for microscopic quantitation. MLCK activity was measured as described for Figure 1. Data points represent the mean ± SEM of 4 experiments. *Significantly different from control (100%); P < .05. **Significantly different from control (100%); P < .005. ***Significantly different from control (100%); P < .001.
Inhibition of phagocytosis and MLCK activity by myosin adenosine triphosphatase inhibitor BDM.
PMNLs were incubated for 10 minutes at 37°C with the indicated concentrations of BDM, then challenged with EIgG for 30 minutes (phagocytosis) or 5 minutes (MLCK activity). EIgG that were not ingested were hypotonically lysed, and phagocytosis samples were fixed with glutaraldehyde for microscopic quantitation. MLCK activity was measured as described for Figure 1. Data points represent the mean ± SEM of 4 experiments. *Significantly different from control (100%); P < .05. **Significantly different from control (100%); P < .005. ***Significantly different from control (100%); P < .001.
Discussion
This study demonstrated that the activation of MLCK during PMNL phagocytosis is a result of ERK2 activation. Both the increase in phosphorylation of a specific MLCK substrate during phagocytosis and the concurrent inhibition of this phosphorylation and phagocytosis by an inhibitor of MLCK implicate MLCK activation as a requirement for the ingestion of IgG-opsonized particles. The ability of ERK2 immunoprecipitated from phagocytosing, but not resting, PMNLs to activate cytosolic MLCK demonstrated that ERK2 activation is necessary for MLCK activation. However, our data did not rule out the presence of a cytosolic intermediate between ERK2 and MLCK.
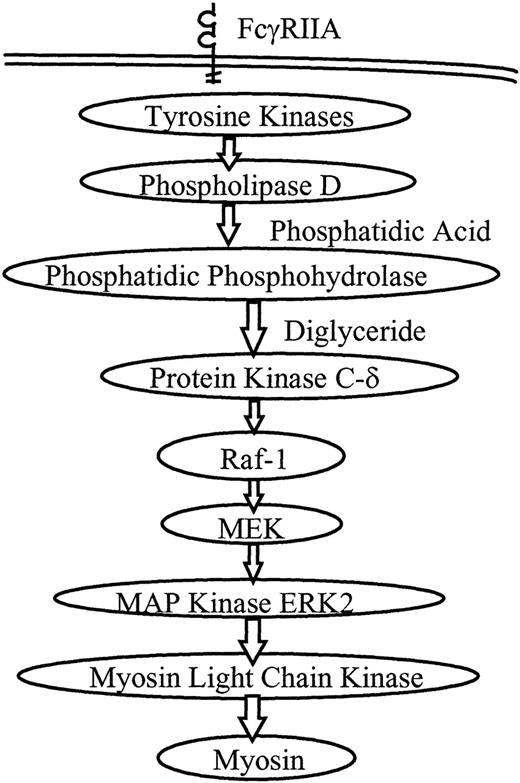
A proposed model of PMNL signaling during FcR-mediated phagocytosis is shown in Figure 5. We and others have investigated several aspects of this model. Our observations suggest that most of the signaling activity occurs in the first few minutes of phagocytosis, corresponding with the maximum rate of ingestion. Phospholipase D activity rises by 1 minute after initiation of ingestion and remains elevated for 30 minutes.7 Translocation of PKCδ and Raf-1 occurs at 3 minutes.11 ERK2 phosphorylation rises at 1 to 5 minutes and ERK2 activity peaks at 3 to 5 minutes.15 Syk phosphorylation similarly peaks at 3 to 5 minutes.32Translocation of PKCδ and Raf-1 and the phosphorylation of ERK2 and Syk all decrease by 10 minutes into phagocytosis, a time at which the ingestion rate has steeply declined. Consistent with these observations, in the current study MLCK activity peaked at 4 to 6 minutes and decreased to baseline by 10 minutes. The temporal proximity of activation of these kinases suggested that they were closely linked signaling steps in the phagocytic signaling cascade.
Proposed model for PMNL signaling through the MAP kinase pathway during FcR-mediated phagocytosis.
Receptor ligation directly or indirectly initiates tyrosine phosphorylation, followed by activation of phospholipase D. Phosphatidic phosphohydrolase generates diglyceride, a cofactor for PKC. PKCδ and Raf-1 are known to translocate to the plasma membrane during phagocytosis, and the MEK–MAP kinase pathway is activated. In this study we show that activation of ERK2 leads to activation of MLCK, which then phosphorylates myosin, and myosin ATPase activation is required for phagocytosis.
Proposed model for PMNL signaling through the MAP kinase pathway during FcR-mediated phagocytosis.
Receptor ligation directly or indirectly initiates tyrosine phosphorylation, followed by activation of phospholipase D. Phosphatidic phosphohydrolase generates diglyceride, a cofactor for PKC. PKCδ and Raf-1 are known to translocate to the plasma membrane during phagocytosis, and the MEK–MAP kinase pathway is activated. In this study we show that activation of ERK2 leads to activation of MLCK, which then phosphorylates myosin, and myosin ATPase activation is required for phagocytosis.
Inhibition of phagocytosis, ERK2 activation, and MLCK activity by a specific MEK inhibitor demonstrates that these phenomena are regulated by MEK. Immunoprecipitated ERK2, which was activated during PMNL phagocytosis, could activate cytosolic MLCK, and this activity was inhibitable by ML-7. Our results implicate a pathway similar to that described for COS-7 cells by Klemke et al,27 who showed that MEK-dependent ERK2 activation led to the activation of MLCK to phosphorylate myosin light chain. Furthermore, Klemke et al27 demonstrated direct phosphorylation of MLCK by ERK2.
Some studies have suggested possible mediators of MLCK activity or myosin light chain phosphorylation other than ERK2. A cyclic adenosine monophosphate (cAMP)-dependent protein kinase phosphorylates and inactivates MLCK in fibroblasts.39 However, results from our laboratory suggest that cAMP has no significant role in PMNL phagocytosis of EIgG (data not shown). PI 3-kinase is activated during phagocytosis.44,45 Recent work in our laboratory suggests that PI 3-kinase activation, though essential for PMNL phagocytosis, acts downstream of the MAP kinase cascade,32 making it a possible activator of MLCK. However, in macrophages PI 3-kinase is involved in the closure of phagosomes into intracellular vesicles and not in pseudopod extension,46 thus placing it downstream of MLCK as well. Protein kinase C may phosphorylate myosin light chain directly, but it does so on sites not included within the peptide we used as MLCK substrate.47 More recently, the small GTPase Rho has been shown to mediate myosin light chain phosphorylation.48 49 However, when PMNLs were treated withClostridium difficile toxin B to inhibit Rho, phosphorylation of myosin light chain still occurred during phagocytosis (data not shown).
Formation and movement of the pseudopod by actin–myosin interaction occur during various forms of cell movement, including phagocytosis and chemotaxis. MLCK is involved in granulocyte macrophage colony-stimulating factor-stimulated random migration and fMLP-stimulated chemotaxis of PMNLs.26 Activation of platelets by thrombin, leading to aggregation and granule release, results in diphosphorylation of myosin light chain by MLCK.50 Phosphorylation of myosin II light chain does not increase during phagocytosis of antibody-opsonized yeast particles by macrophage-like J774 cells51; however, HeLa cell phagocytosis appears to involve myosin II.52 In addition, myosin is phosphorylated during T-lymphocyte receptor capping,53 implicating MLCK. Thus MLCK activation may be dependent on cell type and function.
During PMNL phagocytosis, as part of the ERK2 pathway MLCK is activated to phosphorylate and activate myosin. Inhibition of phagocytosis by a myosin ATPase inhibitor revealed phagocytosis depends not only on MLCK but on the subsequent myosin–actin interaction arising from the stimulation of myosin ATPase activity. It is highly likely that the myosin ATPase activity translates into propulsive force to form the pseudopod, thereby permitting PMNLs to surround and ingest foreign particles.
Supported by National Institutes of Health grants AI20065 (L.A.B.) and DK41487 and DK39255 (J.A.S.).
Reprints:Laurence A. Boxer, Department of Pediatrics, Hematology/Oncology, University of Michigan, L2110 Women's Hospital, Box 0238, 1500 E. Medical Center Drive, Ann Arbor, MI 48109; e-mail: laboxer@umich.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Fig. 1. MLCK activation during PMNL phagocytosis. / (A) Kinase activity. PMNLs (2 × 106/mL) were challenged with EIgG (1 × 108/mL) (circles) or incubated with no EIgG (triangles) at 37°C for the indicated times. PMNL lysates were prepared by probe sonication as described in “Materials and methods.” MLCK was assayed in the lysates by phosphorylation of substrate peptide (myosin light chain, amino acids 11-23) in the presence of [γ-32P]ATP for 10 minutes at 30°C. Data represent the mean ± SEM of 4 experiments. Significantly different from time 0 sample; P < .05. (B) Phosphorylation of endogenous myosin light chain. PMNLs were labeled with 32P, challenged with EIgG (P) or not stimulated (U) for 4 minutes, and lysed. Samples were immunoprecipitated with antiplatelet myosin and subjected to 12% SDS-PAGE followed by autoradiography.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/7/10.1182_blood.v95.7.2407/5/m_bloo00702001ax.jpeg?Expires=1769254580&Signature=uRK2VfXO0VMJFGKQU3Ltt3KQoBnli6kP9UOYJWBHMJd1p3f8bi5ZRb5ekDwgj-YrYrLZ697oC4m21GMcWS~Vg820mR6uJoLJ3-O1rkcR21MvdJjXiqjYDrEVNECH6gZ-VJlUjRvezBpUgMdqPCtipc2uplU9JYHAb1keiTZjYTn7AxecJ~L7JD0VOXaFePg28-~51zLGvYTdJCRsiNIaYs5meRlsRoJrBBqxQBJO5gUqkbm65AF-XwaKO7rE1hYgm8eG2cgggCeSZO~6DNSr5eQ1-AeYtpz0jWpbJ9YlJiKwpGGgexLN1tg5B79qXJtgs8XTPrDmoobEvS645FEk~A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 1. MLCK activation during PMNL phagocytosis. / (A) Kinase activity. PMNLs (2 × 106/mL) were challenged with EIgG (1 × 108/mL) (circles) or incubated with no EIgG (triangles) at 37°C for the indicated times. PMNL lysates were prepared by probe sonication as described in “Materials and methods.” MLCK was assayed in the lysates by phosphorylation of substrate peptide (myosin light chain, amino acids 11-23) in the presence of [γ-32P]ATP for 10 minutes at 30°C. Data represent the mean ± SEM of 4 experiments. Significantly different from time 0 sample; P < .05. (B) Phosphorylation of endogenous myosin light chain. PMNLs were labeled with 32P, challenged with EIgG (P) or not stimulated (U) for 4 minutes, and lysed. Samples were immunoprecipitated with antiplatelet myosin and subjected to 12% SDS-PAGE followed by autoradiography.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/7/10.1182_blood.v95.7.2407/5/m_bloo00702001bw.jpeg?Expires=1769254580&Signature=tVWD99yPtU3Akjo5P0sR6j0HT15MhO7Qg9M4xo0jN-ln-xuH9tpUFfbpvkPL8q4PRmFIriUHpTkUlE7aiDBU2-iJ9dF71s6IbqL2ovVq2eWHJ5u0okE2AByVY2lBk90WBFPApZdS1RTGNBqA5pNXyRP6RmlGQJE2HV0y-ovYaf6WkF6CaDjYL8SrZmQ0nvfOO~bnwiEalzJFMtMfwC5224T0wgtnx5jIY527Z8g2rZ9HBKry8vjSlbku2auR91t1B5KpzrMunHu1B01~xGIloApw5N4UJ7myPGOnXmG5ZFNwDhHW-6bBQ97ImoiM6~R1XdiUVzizmSS2tCeQzf~obQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. Activation of MLCK by ERK2. / PMNLs were allowed to undergo phagocytosis of EIgG for 5 minutes or incubated without EIgG, then lysed with NP-40. ERK2 was immunoprecipitated from lysates to obtain activated and unactivated ERK2, respectively. Cytosol isolated from resting PMNLs or buffer was added to samples along with [γ-32P]ATP and peptide substrate (plain bars), and kinase activity was quantitated as described for Figure 1, or PMNL myosin replaced peptide substrate (cross-hatched bars) and was immunoprecipitated. R, ERK2 immunoprecipitated from resting PMNLs incubated at 37°C; P, ERK2 immunoprecipitated from phagocytosing PMNLs. +, cytosol present; −, no cytosol; +ML-7, cytosol incubated before kinase assay with 10 μmol/L ML-7. Data represent the mean ± SEM of 3 experiments. MLCK activity in PMNLs at 22°C = 100%. *Significantly different from control (left bar); P < .005. Other data are not significantly different from each other.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/7/10.1182_blood.v95.7.2407/5/m_bloo00702003x.jpeg?Expires=1769254580&Signature=m9qmCCGAc7Ypz6f6W~pXzF50aiMoLjkAWf0reBweFMugGZFL5QCpKN6SfaCuENi2FgG6SC-T1bhBj7WlR9XCfwRP1M0wuouvDLsFiFRW8sVR2PRSIH5ZCIiOTlneiVeI8ozJ~RD8~N1-c5Wa0F4mjRkPbe~gXJ0x04YJQ0bWKRfquSPXyrI9dWlcT0RnA0Y7uwt-GcBHMWzHOILdJqGvXvcIh8BhlJ4ozu0Gs03bE2vqL7zyvP4n8wSAuCGCWvZ8HwKi73KxkX5fJSg0vcVqT-3W3U1oWxJVU9o7faNXkFAyXOUPEZn7WApDOEjRu6GpEFzrNSvFZUcK4Pn7O3LL1A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal