Abstract
Multidrug resistance (MDR) is often characterized by the expression of P-glycoprotein (P-gp), a 170-kd ATP-dependent drug efflux protein. As well as effluxing xenotoxins, functional P-gp can confer resistance to caspase-dependent apoptosis induced by a range of different stimuli, including Fas ligand, tumor necrosis factor, UV irradiation, and serum starvation. However, P-gp-positive cells remain sensitive to caspase-independent death induced by cytotoxic T-cell granule proteins, perforin, and granzyme B. It is, therefore, possible that agents that induce cell death in a caspase-independent manner might circumvent P-gp-mediated MDR. We demonstrated here that hexamethylene bisacetamide (HMBA) induced equivalent caspase-independent cell death in both P-gp-positive and -negative cell lines at concentrations of 10 mmol/L and above. The HMBA-induced death pathway was marked by release of cytochrome c from the mitochondria and reduction of Bcl-2 protein levels. In addition, we show that functional P-gp specifically inhibits the activation of particular caspases, such as caspases-8 and -3, whereas others, such as caspase-9, remain unaffected. These studies greatly enhance our understanding of the molecular cell death events that can be regulated by functional P-gp and highlight the potential clinical use of drugs that function via a caspase-independent pathway for the treatment of MDR tumors.
Multidrug resistance (MDR) is a major clinical problem in treating human cancer and is often characterized by the expression of the MDR1 gene product, P-glycoprotein (P-gp).1 A member of the adenosine triphosphate-binding cassette transporter family, P-gp has traditionally been thought to confer resistance to chemotoxins by actively effluxing them from the cell.2,3However, recent reports by our group and others4-6 have indicated that, in addition to its role as an efflux pump, P-gp regulates programmed cell death mediated by antineoplastic agents, serum starvation, UV irradiation, and ligation of the cell surface death receptors Fas and tumor necrosis factor (TNF) receptor. Common to these diverse apoptotic stimuli is their dependence on the activation of caspases to initiate cell death. We, therefore, hypothesized that the successful treatment of P-gp+ MDR tumors might be enhanced using chemotherapeutic agents that can function in absence of caspase activation.
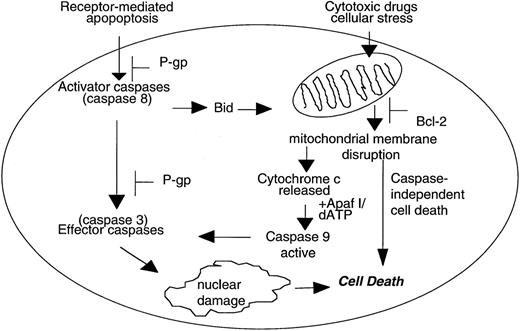
There are now at least two molecular pathways leading to caspase-dependent apoptosis (Figure 1). The best-defined pathway involves ligation of death receptors, typically members of the TNF superfamily such as Fas and TNF receptor, at the cell surface resulting in sequential activation of proximal caspases such as caspase-8 and downstream effector caspases such as caspase-3.7-9
Caspase-dependent and caspase-independent mechanisms of cell death.
Caspase-dependent apoptosis induced by death-receptor ligation can act independently of the mitochondria and is inhibited by P-glycoprotein (P-gp) at the level of caspase-8 and caspase-3 activation. In contrast, some cytotoxic drugs induce the disruption of mitochondrial membrane potential (ΔΨm), release of cytochrome c, and subsequent activation of caspase-9. In addition, disruption of ΔΨm can also lead to caspase-independent cell death that is not inhibited by P-gp.
Caspase-dependent and caspase-independent mechanisms of cell death.
Caspase-dependent apoptosis induced by death-receptor ligation can act independently of the mitochondria and is inhibited by P-glycoprotein (P-gp) at the level of caspase-8 and caspase-3 activation. In contrast, some cytotoxic drugs induce the disruption of mitochondrial membrane potential (ΔΨm), release of cytochrome c, and subsequent activation of caspase-9. In addition, disruption of ΔΨm can also lead to caspase-independent cell death that is not inhibited by P-gp.
The second, less well-defined caspase-dependent pathway involves the disruption of the mitochondrial transmembrane potential (ΔΨm) and the release of mitochondrial proteins such as cytochrome c10-12 and apoptosis-inducing factor.13 Cytochrome c cooperates with dATP and Apaf-1 to induce the activation of caspase-9 that can cleave and activate caspase-3.14,15 Importantly, release of mitochondrial cytochrome c is inhibited by anti-apoptotic members of the Bcl-2 family, such as Bcl-2 and Bcl-XL, and promoted by pro-apoptotic members such as Bid, Bax, and Bak.11 16-18
In addition to caspase-dependent apoptosis, there is now sufficient evidence to suggest that apoptosis can occur in the absence of active caspases. Caspase-independent cell death has been shown to be induced by perforin and granzyme B,19 anti-CD2,20 and the protein kinase C inhibitor staurosporine.20,21 In the absence of caspase activation, cytoplasmic events related to apoptosis, such as cell shrinkage, cytoplasmic condensation, and loss of mitochondrial membrane integrity and exposure of phosphatidylserine, are still induced by these stimuli.19-21Caspase-independent cell death has been shown to be inhibited by Bcl-2,22 23 suggesting that a loss of mitochondrial function is central to this death process.
Work by our group and others4-6 has demonstrated that functional P-gp can confer resistance to a wide range of caspase-dependent apoptotic stimuli, such as ligation of cell-surface death receptors, serum starvation, and UV irradiation. We demonstrated that functional P-gp-inhibited activation of caspase-3 following Fas ligation and that this inhibitory effect could be reversed using P-gp antagonists, such as specific anti-P-gp monoclonal antibodies (mAbs) or the pharmacological inhibitor verapamil.4 Many chemotherapeutic drugs, such as doxorubicin and vincristine, function in a caspase-dependent manner;4,5 therefore, P-gp may play a dual role in regulating cell death induced by these stimuli (i) by removing the toxins from the cell and (ii) by inhibiting the activation of caspases. Importantly, P-gp does not offer cells protection from death induced by lytic concentrations of the pore-forming protein perforin5 or by a combination of granzyme B and sublytic concentrations of perforin,4,5which together can function in a caspase-independent manner.4,5 19 We, therefore, reasoned that drugs that were not P-gp substrates and could mediate caspase-independent cell death might be clinically useful for the treatment of MDR. Given that caspase-independent cell death is comparatively poorly understood, and few cell-death stimuli have been shown to function in a caspase-independent manner, we undertook a search for a chemotherapeutic agent that could kill P-gp-expressing cells by this mechanism.
Hexamethylene bisacetamide (HMBA) is a hybrid polar compound that can induce terminal differentiation of transformed cells24,25 and has been used clinically to treat patients with acute myeloid leukemia and myelodysplastic syndrome.26Terminal differentiation induced by HMBA correlates with a dramatic decrease in cdk4-dependent kinase activity and hypophosphorylation of the retinoblastoma tumor suppressor protein.27Interestingly, HMBA also induced activation of the JAK/STAT signal transduction pathway via JAK2 and STAT5 but suppressed activation via SHC and GRB2.28 Although hypophosphorylation of retinoblastoma tumor suppressor protein and the related family member p107 is clearly involved in causing cells to arrest in the G1 phase of the cell cycle, it is unclear what role HMBA-mediated modulation of signaling pathways triggered by stimulation of cytokine and/or growth factor receptors plays in inducing cellular differentiation. A previous report29 indicated that HMBA could induce cell death in P-gp-expressing cells; however, no data were shown and the mechanism of cell death in P-gp-expressing cells was not explored. We report here that HMBA can induce cell death in P-gp-expressing human tumor cells, and, in agreement with our previous reports, HMBA induced activation of caspase-3 in P-gp- but not P-gp+ cells. However, in the absence of caspase activation, HMBA could still induce cell death that was marked by the caspase-independent release of cytochrome c from the mitochondria to the cytosol and a reduction in Bcl-2 levels. Importantly, overexpression of Bcl-2 inhibited HMBA-mediated cell death. Furthermore, we illustrate that P-gp-expressing cells have a reduced capacity to activate caspases involved in death receptor-mediated apoptosis, such as caspases-8 and-3, but not caspase-9, which is activated following release of cytochrome c from the mitochondria.
Materials and methods
Cell culture
The acute T-cell leukemia cell line, CEM-CCRF, and its doxorubicin-selected and -resistant P-gp+-derived line CEM-A7+ have been previously described.30 Colon carcinoma LoVo cell line and LoVo resistant to Adriamycin (LoVo-adr) have been previously described.31 K562 and vincristine selected P-gp+ KVIN2000 cells were kindly provided by Greg Woods, University of Tasmania, Hobart, Australia. CEM and FDC-P1 cells overexpressing human Bcl-2, Bid, and Bid-Bcl-2 were a gift from David Huang (Walter and Eliza Hall Institute, Melbourne, Victoria). All cells were grown in RPMI medium 1640 supplemented with 10% (vol/vol) fetal calf serum, 2 mmol/L glutamine, 100 units/mL of penicillin, and 100 μg/mL of streptomycin (GIBCO, Grand Island, NY), with the exception of FDC-P1 cell lines that were grown in DME with the additives listed above and interleukin-3 (IL-3). Cells were cultured for 4-72 hours with 0-30 mmol/L HMBA (Sigma, St. Louis, MS), 10-100 ng/mL doxorubicin, 5-100 ng/mL vincristine, or 100-500 ng/mL anti-Fas antibody clone CH-11 (Upstate Biotechnology, Lake Placid, NY). Doxorubicin and vincristine were obtained from Phillip Kantharidis, Peter MacCallum Cancer Institute, East Melbourne, Australia. To inhibit the activation of caspases, cells were pretreated for 30 minutes with peptidyl fluoromethylketones (ZFA-fmk, ZVAD-fmk, or Z-YVAD-fmk) (Enzyme System Products, Dublin, CA) (final 0-40 μmol/L).
Viability assays
Cells were cultured at 2 × 105 cells/mL in the presence or absence of cell death stimuli for varying times. Trypan blue exclusion assays were performed as previously described.5 In all assays, 150-300 cells were counted for each data point, and data were calculated as the mean ± SE of triplicate samples and are representative of at least three separate assays. The number of apoptotic or dead cells (blue cells) was expressed as a percentage of the total cell number.
Clonogenic assays
Colony assays were performed on cells treated for 24-72 hours with various apoptotic stimuli as previously described.5
DNA fragmentation
Cells (1 × 106) were washed with phosphate-buffered saline (PBS) and pelleted by centrifugation. Genomic DNA was prepared as previously described.32 DNA (10-15 μg) was run on a 2% agarose gel for 2 hours. The gel was stained in ethidium bromide (1 μg/mL) for 15 minutes and washed for 1 hour in water.
TUNEL assay
Cells (2 × 105) treated with various apoptotic stimuli were assayed by TUNEL (Boehringer Mannheim) as previously described.33
Propidium iodide (PI) staining
Cells (2 × 105) treated with various apoptotic stimuli were washed with PBS and fixed in 50% ice-cold EtOH/PBS for 20 minutes on ice. Cells were washed with PBS and incubated in PI solution (69 mmol/L PI/388 mmol/L Na Citrate/100 μg/mL Rnase A) for 15 minutes at 37°C. Cells were immediately analyzed by FACscan flow cytometer (Becton Dickinson).
Western blot analysis
Cells (2 × 105) were lysed in 50 mL of ice-cold Nonidet P-40 lysis buffer as previously described.4 Protein determinations were performed using the Bradford reaction.34 Proteins (10-20 μg) were separated on SDS-12% or SDS-15% polyacrylamide gels and electroblotted onto nylon membranes. Blots were probed with anti-human caspase-3 mAb (Transduction Laboratories, Lexington, KY), anti-human FLICE mAb (gift from Marcus Peter, University of Heidelberg, Germany), anti-human caspase-9 (gift from Xiaodong Wang, Howard Hughes Medical Institute and Department of Biochemistry, University of Texas Southwestern Medical Center, Dallas, TX; and Douglas Green, La Jolla Institute for Allergy and Immunology, San Diego, CA), anti-human Bcl-2 mAb (David Huang, WEHI, Melbourne Australia), anti-human PARP (Boehringer Mannheim), or anti-human a-tubulin mAb (Sigma, St. Louis, MO) and visualized by enhanced chemiluminescence (Amersham, UK).
Cytochrome c release
Cells (2 × 107) treated for 3 days with 10 or 15 mmol/L HMBA were washed twice in ice-cold PBS. Cytosolic lysates were prepared as previously described.21 Cytosolic proteins were separated on a SDS-15% polyacrylamide gel and transferred onto nylon membranes. Blots were probed with anti-human cytochrome c mAb (PharMingen, San Diego, CA; clone 7H8.2C12) and visualized as above. The same blots were reprobed with anti-α-tubulin to determine that equal amounts of protein were present in each well. Cytospin slides of CEM cells treated for 24-72 hours with 10 and 15 mmol/L HMBA in the presence or absence of 40 μmol/L ZVAD-fmk or ZFA-fmk were stained for cytochrome c as previously described35 and visualized by confocal microscopy.
Immunofluorescence and analysis of apoptotic nuclei
Cytospin slides of 1 × 105 cells treated for varying times with 0-20 mmol/L HMBA were fixed in EtOH/acetic acid (6/1) (vol/vol) for 5 minutes. Slides were stained with 10 ng/mL Hoechst 33 258 for 5 minutes and washed twice in citrate buffer pH 5.5. Cells were visualized by confocal and fluorescence microscopy, and 450-500 cells per sample were counted and apoptotic nuclei scored.
Results
P-gp does not protect tumor cells from HMBA-mediated death
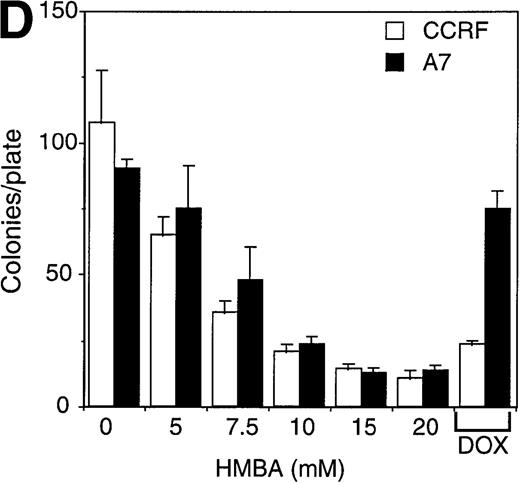
P-gp+ and P-gp- CEM, LoVo, and K562 cell lines were used to examine the effects of HMBA on cell viability. CEM cell lines were cultured in the presence of 0-20 mmol/L HMBA for 24-72 hours, and cell death was initially determined by trypan blue exclusion assays. HMBA-induced equivalent cell death in a dose- and time-dependent manner in both P-gp+ and P-gp-CEM (Figure 2A) and LoVo (Figure 2B) cell lines. However, the effects of HMBA on cell viability were cell specific. P-gp+ and P-gp- K562 cells treated with HMBA did not die (Figure 2C), but rather underwent G1 cell cycle arrest as measured by PI staining for DNA content (data not shown), consistent with previous reports24 that HMBA induces growth arrest in this erythroid cell line. As controls for drug sensitivity, P-gp- CEM, LoVo, and K562 cells were effectively killed by the chemotherapeutic agent, doxorubicin (100-200 ng/mL), whereas P-gp+ cells were resistant to doxorubicin-induced death (Figure 2A-C). To evaluate viability by a more sensitive method, and to correlate trypan blue staining with cell death, clonogenic assays were employed. HMBA treatment of P-gp+ or P-gp- CEM cells resulted in a dose-dependent decrease in colony formation (Figure2D). P-gp+ CEM cells survived doxorubicin-mediated apoptosis, whereas P-gp- CEM cells were sensitive to doxorubicin-induced cell death.
Hexamethylene bisacetamide (HMBA)-induced cell death in P-glycoprotein (P-gp) cell lines.
P-gp+ (A7) and P-gp- (CCRF) CEM cells (A), P-gp+ (LoVo-adr) and P-gp- (LoVo) LoVo colon carcinoma cells (B), and P-gp+ (KVIN) and P-gp-(K562) K562 myeloid cells (C) were cultured for 72 hours with 0-20 mmol/L HMBA or 100-200 ng/mL doxorubicin. Cells were counted at 72 hours by trypan blue exclusion as described in “Materials and methods.” (D) After 3 days in culture with 0-20 mmol/L HMBA or 100 ng/mL doxorubicin, A7 and CCRF cells were plated out in soft agar. Colonies were scored after 2 weeks in culture as described in Materials and methods section.
Hexamethylene bisacetamide (HMBA)-induced cell death in P-glycoprotein (P-gp) cell lines.
P-gp+ (A7) and P-gp- (CCRF) CEM cells (A), P-gp+ (LoVo-adr) and P-gp- (LoVo) LoVo colon carcinoma cells (B), and P-gp+ (KVIN) and P-gp-(K562) K562 myeloid cells (C) were cultured for 72 hours with 0-20 mmol/L HMBA or 100-200 ng/mL doxorubicin. Cells were counted at 72 hours by trypan blue exclusion as described in “Materials and methods.” (D) After 3 days in culture with 0-20 mmol/L HMBA or 100 ng/mL doxorubicin, A7 and CCRF cells were plated out in soft agar. Colonies were scored after 2 weeks in culture as described in Materials and methods section.
HMBA induces nuclear morphological changes consistent with apoptosis
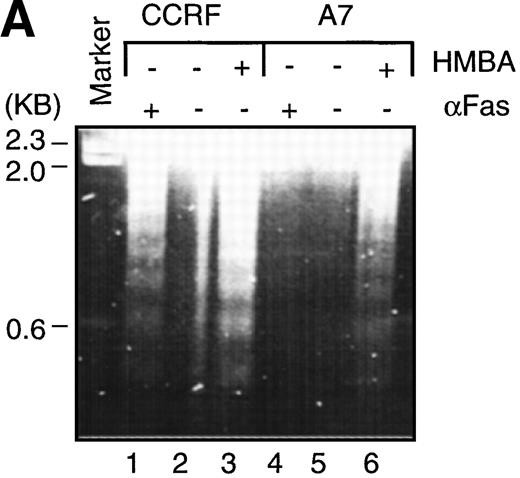
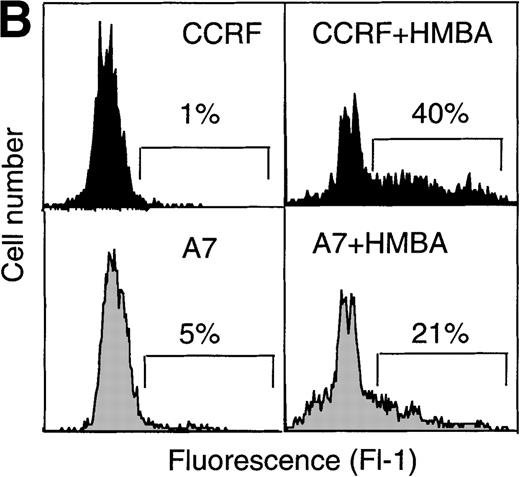
To characterize the biochemical and morphological changes in cells undergoing HMBA-induced cell death, CEM cells were cultured in the presence or absence of 10 mmol/L HMBA for 24-48 hours and subsequently assayed for classical hallmarks of apoptosis, such as chromatin condensation, DNA laddering, and cleavage of the DNA repair enzyme PARP.36-38 Treatment of P-gp+ and P-gp- CEM cells with HMBA resulted in DNA fragmentation as evidenced by the formation of DNA ladders (Figure3A) and by TUNEL staining (Figure 3B). In addition, CEM cells treated with HMBA and subsequently stained with Hoechst 33 258 displayed chromatin condensation indicative of apoptotic nuclei (Figure 3C). Apoptotic nuclei appeared within 6 hours after treatment with 10 mmol/L HMBA, and the number of apoptotic cells increased with time (>15% at 24 hours, >25% at 48 hours). In addition, PARP was cleaved in a dose-dependent manner in both P-gp+ and P-gp- CEM cells (Figure 3D).
Hexamethylene bisacetamide (HMBA)-induced nuclear damage indicative of apoptosis.
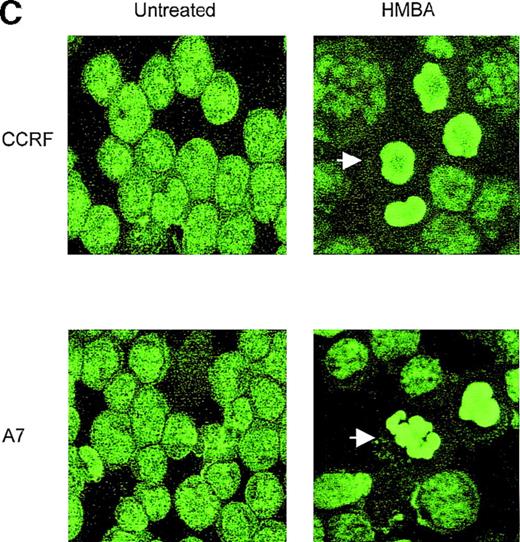
(A) CCRF and A7 CEM cells were untreated or cultured for 48 hours with 10 mmol/L HMBA or for 4 hours with 100 ng/mL anti-Fas antibody (CH-11). Genomic DNA was extracted and run on a 2% agarose gel. CCRF and A7 CEM cells were treated for 48 hours with 10 mmol/L HMBA and assayed by TUNEL for DNA fragmentation as denoted by an increase in mean channel fluorescence (B) and Hoechst 33 258 staining (C) for chromatin condensation. The percentage of TUNEL-positive cells are shown above each histogram, and cells displaying chromatin condensation are indicated by an arrow. Lysates from CCRF and A7 cells treated for 72 hours with 0-20 mmol/L HMBA were separated by SDS-PAGE, and Western blots were performed using polyclonal anti-PARP antibody (D). The active form of PARP migrates as a 118-kD protein and cleaved to an inactive form of approximately 89 kd.
Hexamethylene bisacetamide (HMBA)-induced nuclear damage indicative of apoptosis.
(A) CCRF and A7 CEM cells were untreated or cultured for 48 hours with 10 mmol/L HMBA or for 4 hours with 100 ng/mL anti-Fas antibody (CH-11). Genomic DNA was extracted and run on a 2% agarose gel. CCRF and A7 CEM cells were treated for 48 hours with 10 mmol/L HMBA and assayed by TUNEL for DNA fragmentation as denoted by an increase in mean channel fluorescence (B) and Hoechst 33 258 staining (C) for chromatin condensation. The percentage of TUNEL-positive cells are shown above each histogram, and cells displaying chromatin condensation are indicated by an arrow. Lysates from CCRF and A7 cells treated for 72 hours with 0-20 mmol/L HMBA were separated by SDS-PAGE, and Western blots were performed using polyclonal anti-PARP antibody (D). The active form of PARP migrates as a 118-kD protein and cleaved to an inactive form of approximately 89 kd.
Interestingly, P-gp+ cells typically displayed significantly less chromatin condensation and DNA fragmentation than P-gp- cells following HMBA treatment (Figure 3A-C). This finding is consistent with our previous studies showing that P-gp+ cells were less sensitive to those nuclear apoptotic events that occur following caspase activation.4 5
HMBA can induce caspase-independent cell death
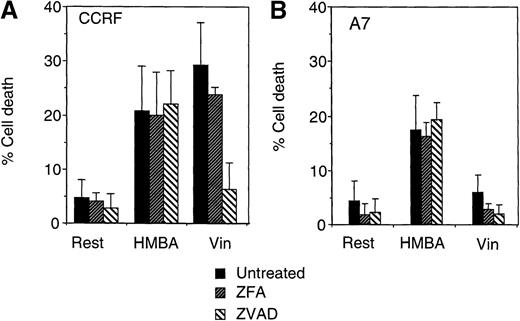
As HMBA induced many of the cell death events indicative of caspase-dependent apoptosis, we sought to determine whether this chemotherapeutic agent could still kill CEM cells in the absence of caspase activation. To examine if HMBA-induced death was caspase-independent, CEM cells were pretreated with the universal caspase inhibitor ZVAD-fmk or the control inhibitor ZFA-fmk and then cultured for 48 hours with 10 mmol/L HMBA. P-gp+ and P-gp- CEM cells treated with HMBA in the presence of ZVAD-fmk displayed equivalent cell death when compared with controls treated with HMBA alone, indicating that HMBA-induced cell death was caspase-independent (Figure 4A). By comparison, P-gp- CEM cells treated for 48 hours with 5 ng/mL vincristine in the presence of ZVAD-fmk were protected from cell death, indicating that, as previously demonstrated,5vincristine-induced cell death was dependent on active caspases (Figure4A). As expected, P-gp+ CEM cells were resistant to caspase-dependent cell death induced by vincristine (Figure 4A). These control experiments with vincristine as the apoptotic mediator also serve to confirm the effectiveness of the caspase inhibitory ZVAD-fmk peptide over the 48-hour time course of the experiment. These data were confirmed by clonogenic assays with equivalent HMBA-induced cell death in the presence of ZVAD-fmk and ZFA-fmk (data not shown).
Hexamethylene bisacetamide (HMBA)-induced caspase-independent cell death.
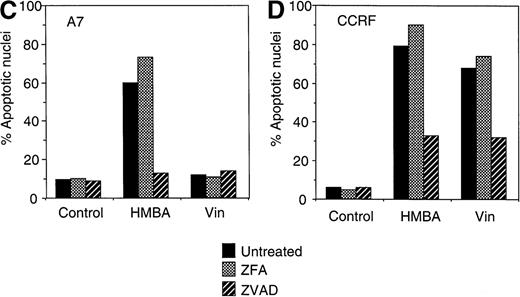
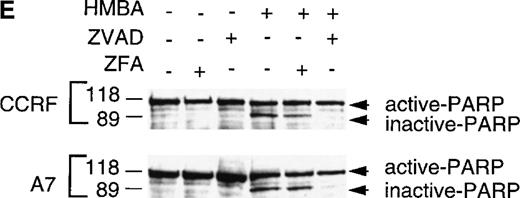
CCRF (A, C) and A7 (B, D) CEM cells were treated with 10 mmol/L HMBA or 5 ng/mL vincristine (Vin) for 48 hours. In some wells, cells were pre-incubated for 4 hours with 40 μmol/L ZFA-fmk or ZVAD-fmk. Cells were assayed by trypan blue exclusion as described in “Materials and methods” (A, B) or were stained with Hoechst 33 258 and visualized by confocal microscopy and scored for apoptotic nuclei as described in “Materials and methods” (C, D). Protein lystates were prepared from cells treated as above and separated by SDS-PAGE. Western blots were performed using polyclonal anti-PARP antibody (e). The active 118-kD and inactive 89-kD forms of PARP are indicated.
Hexamethylene bisacetamide (HMBA)-induced caspase-independent cell death.
CCRF (A, C) and A7 (B, D) CEM cells were treated with 10 mmol/L HMBA or 5 ng/mL vincristine (Vin) for 48 hours. In some wells, cells were pre-incubated for 4 hours with 40 μmol/L ZFA-fmk or ZVAD-fmk. Cells were assayed by trypan blue exclusion as described in “Materials and methods” (A, B) or were stained with Hoechst 33 258 and visualized by confocal microscopy and scored for apoptotic nuclei as described in “Materials and methods” (C, D). Protein lystates were prepared from cells treated as above and separated by SDS-PAGE. Western blots were performed using polyclonal anti-PARP antibody (e). The active 118-kD and inactive 89-kD forms of PARP are indicated.
Although HMBA-induced death was found to be caspase-independent, ZVAD-fmk inhibited chromatin condensation (Figure 4B), DNA fragmentation (data not shown), and cleavage of the caspase substrate PARP (Figure 4E) in HMBA-treated cells. These data indicate that, although HMBA induced equivalent caspase-independent cell death in P-gp+ and P-gp- CEM cells (Figures 2 and 3), most nuclear events associated with this form of apoptosis remained caspase-dependent (Figure 4B). Furthermore, the fact that ZVAD-fmk completely abrogated PARP cleavage indicates that caspases were not active under these conditions. Thus, although HMBA is capable of inducing cell death in the absence of caspase activity, HMBA does instigate activation of some caspases independent of P-gp expression.
HMBA induces activation of caspase-9
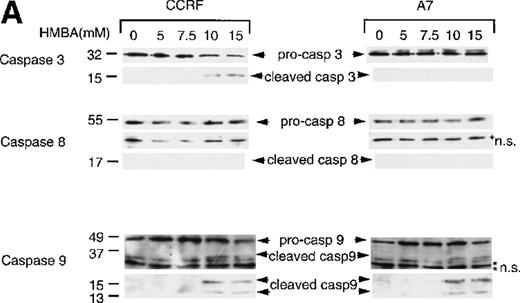
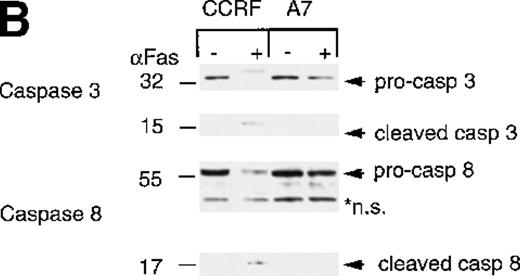
As we had previously determined that caspase-3 activation was inhibited in P-gp+ cells, we were surprised to find that the caspase substrate PARP was equivalently cleaved in both P-gp+ and P-gp- cells. Therefore, we determined which caspases were activated following HMBA treatment and potentially responsible for the observed PARP cleavage and apoptotic nuclear events. Caspase-3 was activated by HMBA in P-gp- cells (Figure 5A, top left panel) but not in P-gp+ cells (Figure 5A, top right panel). Similarly, and consistent with our earlier report,4 caspase-3 was activated following Fas ligation only in P-gp- but not P-gp+ CEM cells (Figure 5B, top panel). Inhibition of caspase-3 activation in P-gp-expressing CEM cells can be reversed by the P-gp inhibitor verapamil and mAbs to P-gp4, indicating that these cells do not express a mutated form of caspase-3 but rather that expression of functional P-gp inhibits caspase-3 activation. We then investigated whether key “activator” caspases upstream of caspase-3, such as caspase-8, typically involved in receptor-mediated cell death,7-9 or caspase-9, central to the mitochondrial death pathway,14,15 were activated during HMBA-induced death. Western analyses for caspases-8 and -9 were performed on lysates from cells treated with HMBA (Figure 5A). Even after 72 hours, no evidence of caspase-8 activation was observed in either P-gp+ or P-gp- cells treated with as much as 15 mmol/L HMBA (Figure 5A, middle panel). However, treatment with anti-Fas mAb resulted in a reduction in pro-caspase-8 and the appearance of active caspase-8 in P-gp- but not P-gp+ CEM cells (Figure 5B, middle panel), demonstrating for the first time that P-gp can inhibit caspase-8 activation. As with caspase-3, caspase-8 could be activated in Fas-ligated P-gp+ CEM cells following treatment with either specific antagonistic anti-P-gp mAbs or with verapamil (data not shown). In contrast, HMBA induced the typical cleavage and auto-activation39 of caspase-9 to p37, p15, and p13 subunits in a dose-dependent manner in both P-gp+and P-gp- CEM cells (Figure 5A, bottom panel), indicating that the cell death pathway that involves disruption of the mitochondrial membrane might be functioning in HMBA-mediated cell death and that the activation of this caspase is not affected by P-gp expression.
Hexamethylene bisacetamide (HMBA)-stimulated activation of caspase 9.
CCRF and A7 CEM cells were treated with (A) 0-15 mmol/L HMBA for 72 hours or (B) 100 ng/mL anti-Fas antibody (CH-11) for 12 hours. Protein lystates were separated by SDS/PAGE, and Western blots were performed using anti-caspase-3 (Caspase 3), anti-caspase-8 monoclonal antibody (Caspase 8), and anti-caspase-9 polyclonal antibody (Caspase 9). The inactive (pro) and active (cleaved) forms of caspases-3, -8, and -9 are indicated. Nonspecific protein bands are denoted by an asterisk.
Hexamethylene bisacetamide (HMBA)-stimulated activation of caspase 9.
CCRF and A7 CEM cells were treated with (A) 0-15 mmol/L HMBA for 72 hours or (B) 100 ng/mL anti-Fas antibody (CH-11) for 12 hours. Protein lystates were separated by SDS/PAGE, and Western blots were performed using anti-caspase-3 (Caspase 3), anti-caspase-8 monoclonal antibody (Caspase 8), and anti-caspase-9 polyclonal antibody (Caspase 9). The inactive (pro) and active (cleaved) forms of caspases-3, -8, and -9 are indicated. Nonspecific protein bands are denoted by an asterisk.
HMBA stimulates the loss of Bcl-2 and release of mitochondrial cytochrome c
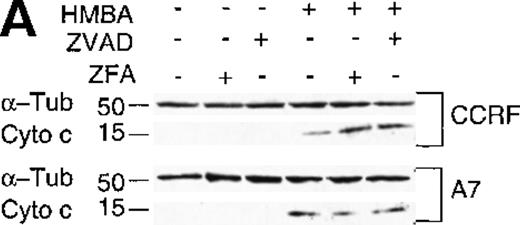
Activation of caspase-9 can be mediated via the interaction of mitochondrial cytochrome c with Apaf 1 in the presence of dATP/ATP in the cytosol.14 15 We, therefore, examined whether cytochrome c was released from the mitochondria during HMBA-mediated death. Cytochrome c was present in all cytosolic extracts of P-gp+ and P-gp- CEM cells treated with HMBA (Figure 6A) but was not detected in untreated control cells. In addition, HMBA-induced cytochrome c release was caspase-independent as it was not inhibited by addition of ZVAD-fmk (Figure 6A). These results were confirmed by confocal microscopy on cells treated as above and stained by immunofluorescence for mitochondrial and cytosolic cytochrome c (data not shown).
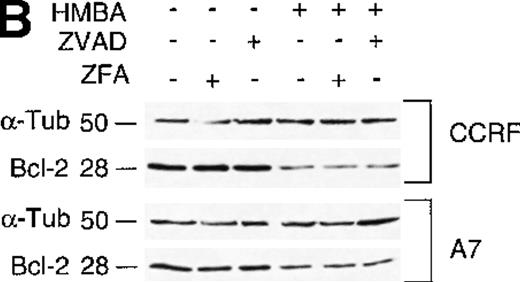
Hexamethylene bisacetamide (HMBA) stimulates the release of mitochondrial cytochrome c and loss of Bcl-2.
(A) Cytosolic extracts from CCRF and A7 CEM cells either untreated or treated with 10 mmol/L HMBA for 72 hours were separated by SDS/PAGE, and Western blots were performed using anti-cytochrome c monoclonal antibody (mAb) that migrates at approximately 15 kd. The same blots were reprobed with anti-α-Tubulin mAb denoted by a band of approximately 50 kd. In some wells, cells were pre-incubated for 4 hours with 40 μmol/L ZFA-fmk or ZVAD-fmk. (B) CCRF and A7 CEM cells were either untreated or treated with 10 mmol/L HMBA for 72 hours. In some wells, cells were pre-incubated for 4 hours with 40 mmol/L ZFA-fmk or ZVAD-fmk. Protein lystates were separated by SDS/PAGE, and Western blots were performed using anti-Bcl-2 mAb that detects a specific band of approximately 28 kd. The same blots were reprobed with anti-α-Tubulin mAb seen migrating as a 50-kD protein.
Hexamethylene bisacetamide (HMBA) stimulates the release of mitochondrial cytochrome c and loss of Bcl-2.
(A) Cytosolic extracts from CCRF and A7 CEM cells either untreated or treated with 10 mmol/L HMBA for 72 hours were separated by SDS/PAGE, and Western blots were performed using anti-cytochrome c monoclonal antibody (mAb) that migrates at approximately 15 kd. The same blots were reprobed with anti-α-Tubulin mAb denoted by a band of approximately 50 kd. In some wells, cells were pre-incubated for 4 hours with 40 μmol/L ZFA-fmk or ZVAD-fmk. (B) CCRF and A7 CEM cells were either untreated or treated with 10 mmol/L HMBA for 72 hours. In some wells, cells were pre-incubated for 4 hours with 40 mmol/L ZFA-fmk or ZVAD-fmk. Protein lystates were separated by SDS/PAGE, and Western blots were performed using anti-Bcl-2 mAb that detects a specific band of approximately 28 kd. The same blots were reprobed with anti-α-Tubulin mAb seen migrating as a 50-kD protein.
It has been reported that HMBA induces a reduction in the levels of the anti-apoptotic protein Bcl-2; however, the mechanism of Bcl-2 down-regulation was not investigated.29 To examine the affects of HMBA on Bcl-2 levels in P-gp+ and P-gp- cells, lysates from CEM cells treated with ZVAD-fmk or control inhibitor ZFA-fmk and HMBA were analyzed for Bcl-2 by Western analysis. As shown in Figure 6B, treatment with HMBA resulted in a caspase-independent reduction of Bcl-2. The decrease in Bcl-2 levels was specific as the levels of other proteins such as α-tubulin (Figure 6B) and caspase-8 (Figure 5A) were unchanged. These data indicate that HMBA induces a caspase-independent decrease in Bcl-2 protein levels in P-gp+ and P-gp- cells with concomitant release of cytochrome c from the mitochondria to the cytosol.
Overexpression of Bcl-2 inhibits HMBA-induced release of cytochrome c and apoptosis
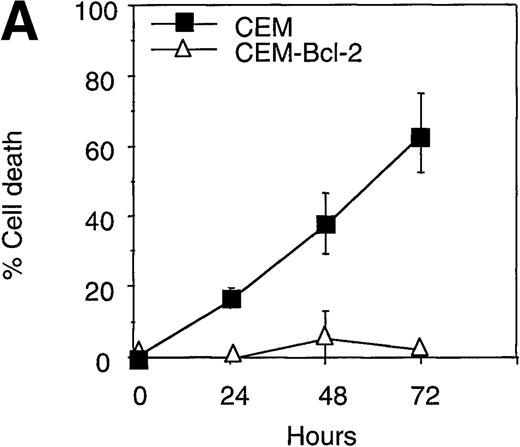
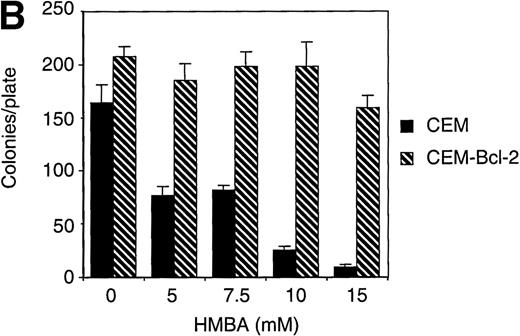
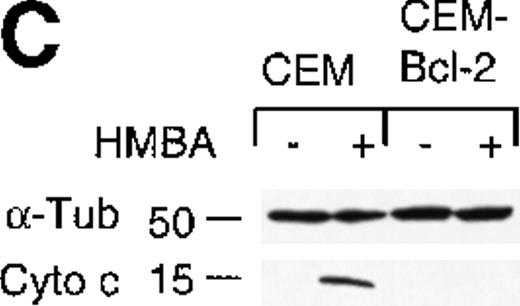
To further investigate the role of Bcl-2 in HMBA-induced cell death, we examined the effects of overexpression of Bcl-2 in CEM cells treated with HMBA. CEM cells overexpressing human Bcl-2 and parental CEM cells were treated with 10 mmol/L HMBA for 24-72 hours, and cell death was determined by trypan blue exclusion (Figure7A). After just 24 hours of treatment with 10 mmol/L HMBA, parental CEM cells underwent apoptosis (Figure 7A); however, Bcl-2 overexpressing cells were protected against HMBA-induced apoptosis at concentrations from 10 mmol/L (Figure 7A) to 30 mmol/L for as long as 72 hours (data not shown). Clonogenic assays were performed on cells treated for 72 hours with 0-15 mmol/L HMBA. As illustrated in Figure 7B, CEM-Bcl-2 cells formed significant numbers of colonies at HMBA concentrations as high as 15 mmol/L. These findings are consistent with an earlier report that overexpression of Bcl-2 inhibits HMBA-mediated cell death.29 In addition, cytochrome c was detected in cytosolic lysates from CEM cells treated for 72 hours with 10 mmol/L HMBA but was not present in lysates from CEM-Bcl-2 cells treated in the same manner (Figure 7C), illustrating that the overexpression of Bcl-2 can completely block HMBA-mediated cytochrome c release.
Overexpression of Bcl-2 inhibits hexamethylene bisacetamide (HMBA)-mediated cell death.
(A) CEM and CEM-Bcl-2 cells were cultured with 10 mmol/L HMBA for 72 hours, and cell death was assayed by trypan blue exclusion. These data were calculated as the mean± SE of duplicate samples and are representative of at least three separate experiments. (B) After incubation with 0, 1, 5, 7.5, 10, and 15 mmol/L HMBA for 72 hours, cells were plated out in soft agar. Colonies were scored after 7 days in culture as described in “Materials and methods.” (C) Cytosolic lysates from CEM (lanes 1-2) or CEM-Bcl-2 (lanes 3-4) cells either untreated (lanes 1 and 3) or treated for 72 hours with 10 mmol/L HMBA (lanes 2 and 4) were separated by SDS/PAGE and Western blotted for cytochrome c (15-kD protein) and then α-Tubulin (50-kD protein).
Overexpression of Bcl-2 inhibits hexamethylene bisacetamide (HMBA)-mediated cell death.
(A) CEM and CEM-Bcl-2 cells were cultured with 10 mmol/L HMBA for 72 hours, and cell death was assayed by trypan blue exclusion. These data were calculated as the mean± SE of duplicate samples and are representative of at least three separate experiments. (B) After incubation with 0, 1, 5, 7.5, 10, and 15 mmol/L HMBA for 72 hours, cells were plated out in soft agar. Colonies were scored after 7 days in culture as described in “Materials and methods.” (C) Cytosolic lysates from CEM (lanes 1-2) or CEM-Bcl-2 (lanes 3-4) cells either untreated (lanes 1 and 3) or treated for 72 hours with 10 mmol/L HMBA (lanes 2 and 4) were separated by SDS/PAGE and Western blotted for cytochrome c (15-kD protein) and then α-Tubulin (50-kD protein).
Discussion
P-gp-expressing, MDR T-cell leukemia, and colon carcinoma cell lines were shown to undergo cell death induced by HMBA, a potent differentiation agent of some hemopoietic cell lineages. P-gp+ and P-gp- cells treated with HMBA displayed classical morphological changes consistent with apoptosis, including chromatin condensation, DNA fragmentation, and membrane damage. Nuclear events, such as DNA fragmentation and chromatin condensation induced by HMBA treatment, were dependent on activation of caspases. However, in the absence of caspase activation, HMBA still mediated a decrease in Bcl-2 protein levels, cytochrome c release, cell membrane damage, and subsequent cell death. Although HMBA could activate caspases such as caspase-9 in both P-gp+ and P-gp- cells, consistent with our previous findings, activation of caspase-3 was inhibited in P-gp+ cells. In addition, we have now established that P-gp can affect activation of caspase-8 following ligation of cell surface Fas. We have previously shown that addition of P-gp-inhibitory antibodies MRK-16 or UIC2, or pharmacological inhibitor verapamil, can allow the Fas-mediated activation of caspase-3 in the same P-gp-positive cells used in this study,4,5 demonstrating that functional P-gp is affecting caspase-3 activation and there are not endogenous inhibitors of caspases in P-gp-expressing cells. We have demonstrated that HMBA activates two death-inducing pathways (Figure 1): (i) a caspase-dependent pathway that mediates nuclear apoptotic events and (ii) a caspase-independent cell death pathway involving a decrease in Bcl-2 protein levels, mitochondrial damage, and cytochrome c release that could be inhibited by the overexpression of Bcl-2. To date, few examples of caspase-independent death stimuli have been described, and this mechanism of cell death remains poorly understood. Those stimuli which have been described to induce caspase-independent cell death include CTL granule proteins,19 viral proteins,40anti-CD2,20 gangliosides,41staurosporine,20,21 and the Bcl-2 family member Bax.42 HMBA appears to be the first chemotherapeutic drug shown to induce this novel form of cell death.
We have previously reported that caspase-3 activation induced by Fas ligation was inhibited in P-gp-expressing cells. We demonstrate here that the cleavage of the upstream activator caspase-8 is also inhibited in P-gp-expressing cells. Surprisingly, caspase-9 was activated in both P-gp+ and P-gp- cells treated with HMBA, indicating that P-gp may not act as a universal caspase inhibitor. The fact that PARP was cleaved in both P-gp+ and P-gp- CEM cells treated with HMBA further suggests that “effector” caspases other than caspase-3 may be active in P-gp+ cells, such as caspase-7, or that active caspase-9 might directly cleave PARP. Indeed, it has been demonstrated that MCF-7 cells that lack functional caspase-3 still cleave PARP on addition of an apoptotic stimulus such as TNF.43 Taken together, these data strongly suggest that P-gp may only offer protection against cell death stimuli that rely on activation of caspase-8, such as receptor-mediated cell death and certain chemotherapeutic drugs that require activation of caspases-8 and -3 without ligation of cell surface Fas.44 Therefore, cell death stimuli that utilize the mitochondria-mediated death pathway, resulting in cytochrome c release and activation of caspase-9, may be more effective in the treatment of MDR tumors. It is interesting to note that P-gp+ cells treated with HMBA displayed a reduction in DNA fragmentation compared with parental P-gp- cell lines and that caspase inhibitors were more effective in blocking DNA fragmentation in P-gp+ cells. It is tempting to speculate that this phenomenon is due to the observed effect of P-gp on caspase-8 and -3 activation (4 5 and this study), therefore resulting in an overall decrease in activation of a number of different caspases. The molecular mechanisms underpinning P-gp-mediated inhibition of caspase-8 and -3 activation are not known and are currently under investigation in our laboratory.
These data further confirm our recent studies4,5demonstrating that P-gp cannot inhibit caspase-independent cell death. As with the CTL granule proteins perforin/granzyme B,19HMBA-induced cell death could occur in the absence of caspase activation and was inhibited by overexpression of the anti-apoptotic protein Bcl-2. Moreover, HMBA-induced cytochrome c release was independent of active caspases. Together these data support the concept that the disruption of the mitochondria membrane and subsequent release of mitochondrial proteins may be necessary to induce caspase-independent cell death.19,21,44 It is not known how HMBA mediates the release of cytochrome c from the mitochondria. Whether HMBA directly acts on the mitochondrial membrane and suppresses the membrane potential or indirectly enables cytochrome c release by reducing Bcl-2 levels remains to be elucidated. Because Bcl-2 had such an overwhelming inhibitory affect on HMBA-mediated cell death, it is likely that other pro-apoptotic Bcl-2 family members are central to this pathway. However, clearly not all chemotherapeutic agents function via this pathway. For example, Flavopiridol, which is a candidate drug for the treatment of chronic lymphocytic leukemia, induces cell death that correlates with cleavage and activation of caspase-3, but no changes in the levels of Bcl-2 were detected.45 Thus, the molecular events that lie between HMBA treatment and cytochrome c release have yet to be elucidated. Other pro-apoptotic Bcl-2 family members, such as Bax or Bad, or the recently identified apoptosis-inducing factor,13 which has been shown to induce apoptosis in a caspase-independent manner, may be more directly involved. We believe the pro-apoptotic Bcl-2 family member Bid is not likely to be involved in this mechanism of cell death since HMBA failed to cleave Bid to its p15 active subunit, whereas Fas ligation in CEM cells resulted in Bid cleavage (data not shown). As Bid is known to be cleaved to its active subunits by active caspase-8,16 17the lack of Bid cleavage is consistent with the fact that caspase-8 was not activated during HMBA-mediated cell death.
We demonstrated that HMBA can induce apoptosis or cell cycle arrest in a cell-type specific manner using the chemotherapeutic drug doses used in this study. CEM/A7 T-cell lines and LoVo/LoVo-adr colon cell lines were sensitive to HMBA-induced cell death, whereas the K562/KVIN erythroleukemia cells were arrested in the G1 phase of the cell cycle with no apparent induction of apoptosis. It is interesting to note that the CEM-Bcl-2 cells that were resistant to HMBA-induced cell death underwent a G1 arrest when incubated in 20-30 mmol/L HMBA. These results indicate that the relative levels of the anti-apoptotic Bcl-2 family of proteins may dictate whether a cell undergoes growth arrest or apoptosis following exposure to HMBA, independent of P-gp expression. A link between expression of Bcl-2 proteins and cell cycle regulation has been previously hypothesized based on the close correlation between cell cycle arrest in Go/G1 and upregulated expression of the anti-apoptotic protein Bcl-XL in murine F-MEL erythroleukemia cells induced to undergo terminal differentiation with DMSO.46
Our results suggest that a more effective way to treat P-gp+ MDR tumors may hinge on the selection of drugs that are capable of inducing caspase-independent apoptosis via mitochondrial membrane disruption and do not require activation of caspases central to the receptor-mediated cell death pathway. Although HMBA has limited value in the clinic because of the high dosage required (millimolar blood levels) and toxic side effects (thrombocytopenia),26we are currently testing more potent second-generation hybrid polar compounds47 for their effectiveness in inducing apoptosis in P-gp-expressing MDR tumor cells. Future drug design for the most effective treatment of P-gp+ MDR tumors could, therefore, focus on agents that meet two criteria: (i) They cannot be effluxed by P-gp, and (ii) they do not rely on the activation of caspases to induce cell death. Thus, the development of chemotherapeutic reagents that function in a similar manner to HMBA to perturb the mitochondrial membrane, mediate the release of cytochrome c, and induce caspase-independent cell death may lead to improved outcomes in the treatment for MDR tumors.
Acknowledgments
We thank Joseph Trapani, Vivien Sutton, and Sarah Russell for their critical review of this manuscript, and David Huang, Jerry Adams, Beni Wolf, Douglas Green, Xiaodong Wang, Carsten Scaffidi, Peter Krammer, and Marcus Peter for reagents.
Supported by project grants from the NH&MRC and the Anti-Cancer Council, Victoria.
R.W.J. is an R.D. Wright Fellow of the National Health and Medical Research Council of Australia (NH&MRC); M.J.S. is a Principal Research Fellow, NH&MRC; and A.A.R. is currently supported by the Dr Laurence LeWinn Post-graduate Research Scholarship.
Reprints:Ricky W. Johnstone, The Austin Research Institute, Austin Hospital, Studley Rd, Heidelberg 3084, Victoria, Australia; e-mail: r.johnstone@ari.unimelb.edu.au.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal