Abstract
Incidences of and risk factors for Streptococcus pneumoniaesepsis (SPS) after hematopoietic stem cell transplantation were analyzed in 1329 patients treated at a single center between 1973 and 1997. SPS developed in 31 patients a median of 10 months after transplantation (range, 3 to 187 months). The infection was fatal in 7 patients. The probability of SPS developing at 5 and 10 years was 4% and 6%, respectively. Age, sex, diagnosis, and graft versus host disease (GVHD) prophylaxis did not influence the development of SPS. Allogeneic transplantation (10-year probability, 7% vs 3% for nonallogeneic transplants; P = .03) and chronic GVHD (10-year probability, 14% vs 4%; P = .002) were associated with significantly higher risk for SPS. All the episodes of SPS were seen in patients who had undergone allograft or total body irradiation (TBI) (31 of 1202 vs 0 of 127;P = .07). Eight patients were taking regular penicillin prophylaxis at the time of SPS, whereas 23 were not taking any prophylaxis. None of the 7 patients with fatal infections was taking prophylaxis for Pneumococcus. Pneumococcal bacteremia was associated with higher incidences of mortality (6 of 15 vs 1 of 16;P = .04). We conclude that there is a significant long-term risk for pneumococcal infection in patients who have undergone allograft transplantation, especially those with chronic GVHD. Patients who have undergone autograft transplantation after TBI-containing regimens also appear to be at increased risk. These patients should receive lifelong pneumococcus prophylaxis. Consistent with increasing resistance to penicillin, penicillin prophylaxis does not universally prevent SPS, though it may protect against fatal infections. Further studies are required to determine the optimum prophylactic strategy in patients at risk.
Bone marrow transplantation for leukemia results in long-term survival in approximately 50% of patients who undergo it.1-4 These long-term survivors, otherwise cured of their disease, are at risk for complications that include infections.3-6 Immunodeficiency caused by chronic graft versus host disease (GVHD),7 functional hyposplenism induced by the conditioning regimen,8 and gradual loss of acquired immunity in the absence of reimmunization9 increase a patient's susceptibility to infection. This is particularly true of infections with encapsulated organisms because of the patient's impaired reactivity to polysaccharide antigens and IgG2 deficiency.9-12
Streptococcus pneumoniae is a common cause of community-acquired pneumonia; it accounts for up to 30% of all such infections.13 The numerous reports of pneumococcal infections in recipients of marrow3-6,14-22 and solid organ23,24 transplants raise the important issue of long-term prophylactic measures either with antimicrobial therapy or vaccination. Although the desirability of using prophylactic measures in asplenic patients has been emphasized,25 the duration of risk for S pneumoniae sepsis (SPS) and the need for pneumococcal prophylaxis have not been determined in recipients of blood and marrow transplants. This retrospective analysis was undertaken to determine the long-term risk for SPS in those who undergo bone marrow transplantation (BMT).
Patients and methods
The database26,27 of the Leukaemia Unit of the Royal Marsden Hospital was searched to identify 1329 consecutive patients who underwent autologous, syngeneic, or allogeneic BMT for leukemia or allogeneic BMT for any malignant disease between 1973 and 1997. Patients who underwent autograft transplantation for multiple myeloma, lymphoma, or solid tumors were not included. Patient characteristics are shown in Table 1. Details of conditioning regimens and GVHD prophylaxis have been published earlier.28-31
Patient characteristics
| . | All patients (n = 1329) . | Patients with S pneumoniae sepsis (n = 31) . |
|---|---|---|
| Median age (y) at BMT (range, y) | 25 (1-61) | 22 (2-49) |
| Sex (M/F) | 800/529 | 19/12 |
| Diagnosis | ||
| Acute myeloid leukemia | 615 | 18 |
| Acute lymphoblastic leukemia | 339 | 7 |
| Chronic myeloid leukemia | 143 | 2 |
| Other | 232 | 3 |
| Median time (mo) from BMT (range, mo) | 10 (3-187) | |
| Type of BMT | ||
| Allograft | 918 | 26 |
| Donor | ||
| Matched sibling | 754 | 24 |
| Mismatched family | 91 | 0 |
| Matched unrelated | 73 | 2 |
| Total-body irradiation | ||
| Yes | 755 | 22 |
| No | 163 | 4 |
| GVHD prophylaxis | ||
| Cyclosporine alone | 525 | 21 |
| Methotrexate ± cyclosporine | 393 | 5 |
| Autograft | 411 | 5 |
| Total-body irradiation | ||
| Yes | 284 | 5 |
| No | 127 | 0 |
| Conditioning regimen | ||
| Total-body irradiation | 1039 | 27 |
| Chemotherapy only | 290 | 4 |
| . | All patients (n = 1329) . | Patients with S pneumoniae sepsis (n = 31) . |
|---|---|---|
| Median age (y) at BMT (range, y) | 25 (1-61) | 22 (2-49) |
| Sex (M/F) | 800/529 | 19/12 |
| Diagnosis | ||
| Acute myeloid leukemia | 615 | 18 |
| Acute lymphoblastic leukemia | 339 | 7 |
| Chronic myeloid leukemia | 143 | 2 |
| Other | 232 | 3 |
| Median time (mo) from BMT (range, mo) | 10 (3-187) | |
| Type of BMT | ||
| Allograft | 918 | 26 |
| Donor | ||
| Matched sibling | 754 | 24 |
| Mismatched family | 91 | 0 |
| Matched unrelated | 73 | 2 |
| Total-body irradiation | ||
| Yes | 755 | 22 |
| No | 163 | 4 |
| GVHD prophylaxis | ||
| Cyclosporine alone | 525 | 21 |
| Methotrexate ± cyclosporine | 393 | 5 |
| Autograft | 411 | 5 |
| Total-body irradiation | ||
| Yes | 284 | 5 |
| No | 127 | 0 |
| Conditioning regimen | ||
| Total-body irradiation | 1039 | 27 |
| Chemotherapy only | 290 | 4 |
The autograft group included 19 recipients of syngeneic transplants, 1 of whom had pneumococcal sepsis.
Streptococcus pneumoniae prophylaxis
Patients were not routinely administered pneumococcal vaccine.9 Recipients of allogeneic transplants were recommended indefinite pneumococcal pharmaco-prophylaxis with penicillin or erythromycin from the late 1980s. After our initial analysis of SPS in transplant recipients in 1993,19 it was thought this should be extended to all patients who undergo transplantation.32 However, all patients were not receiving prophylaxis regularly, primarily because of compliance issues.21 Although details of drug prophylaxis were available for patients in whom SPS developed, these were not available in most patients in whom SPS did not develop.
Streptococcus pneumoniae sepsis
From the main group of 1329 patients, those who had a growth ofS pneumoniae in blood, sputum, upper respiratory tract, or normally sterile body fluids and who had symptoms of infection beyond 3 months after transplantation were identified. Serum immunoglobulin levels were unavailable after transplantation in most of the patients, including those with SPS. Patients in whom SPS developed were not always treated at our center, and they received the best available standard of care in terms of antimicrobial therapy and supportive therapy.
Statistical methods
The data were analyzed in September 1998. The probability for pneumococcal infection was estimated using the method of Kaplan and Meier.32 The following factors were analyzed in univariate and multivariate fashion to examine their influence on the probability for SPS: age at transplantation, sex, diagnosis, type of transplant (allograft vs autograft; syngeneic transplants included in the autograft group), conditioning regimen (TBI or no TBI), type of GVHD prophylaxis, acute GVHD, and chronic GVHD.
Results
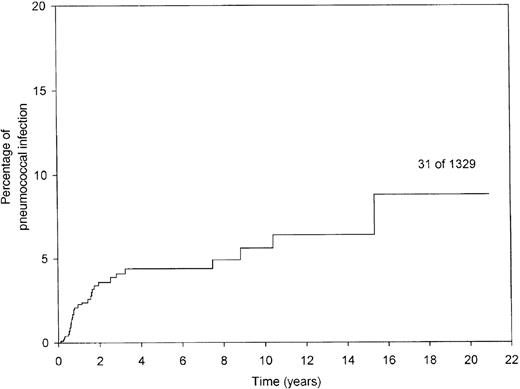
Thirty patients had 31 episodes of clinically and microbiologically documented SPS for a median of 10 months (range, 3 to 187 months) after transplantation. One patient had 2 episodes of SPS in 4 years (pneumonia followed by meningitis). Table2 shows clinical presentations and outcomes; Figure 1 shows the probability of developing SPS.
Clinical presentation and outcome (31 episodes in 30 patients)
| Clinical picture . | n . | Death (%) . |
|---|---|---|
| Pneumonia | 16 | 1 (6)* |
| Bacteremia | 15 | 6 (40)* |
| Bacteremia alone | 9 | 6 (67) |
| With meningitis | 3 | 0 (0) |
| With pneumonia | 3 | 0 (0) |
| Clinical picture . | n . | Death (%) . |
|---|---|---|
| Pneumonia | 16 | 1 (6)* |
| Bacteremia | 15 | 6 (40)* |
| Bacteremia alone | 9 | 6 (67) |
| With meningitis | 3 | 0 (0) |
| With pneumonia | 3 | 0 (0) |
P = .04 (Fisher exact test).
Occurrence of pneumococcal infection after blood or marrow stem cell transplantation.
Occurrence of pneumococcal infection after blood or marrow stem cell transplantation.
Eight episodes of SPS occurred while the patients were regularly taking penicillin, and 23 occurred when patients were not receiving any prophylaxis. All the patients were admitted to the hospital and received parenteral broad-spectrum antibiotics, including penicillin (n = 29), cephalosporin (n = 1), or clarithromycin (n = 1).
Seven (23%) patients died as a direct consequence of SPS; 6 received allografts and 1 received an autograft. Six of 15 (40%) patients with bacteremia died compared with 1 of 16 (6.3%) without bacteremia (P = .04; Fisher exact test). All the deaths occurred in patients not receiving pneumococcal prophylaxis (7 of 23 vs 0 of 8 on prophylaxis; P = .09; Fisher exact test). Age, sex, diagnosis, type of transplant, conditioning regimen, GVHD prophylaxis, prior acute GVHD, or chronic GVHD did not have any impact on mortality. Of the 3 patients with meningitis, 2 have neurologic sequelae—seizures requiring anticonvulsants (n = 1) and partial deafness (n = 2).
Factors influencing the risk for pneumococcal sepsis
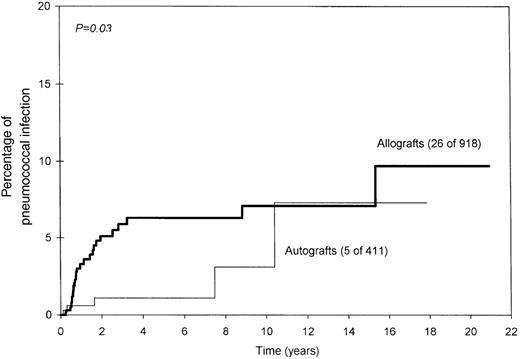
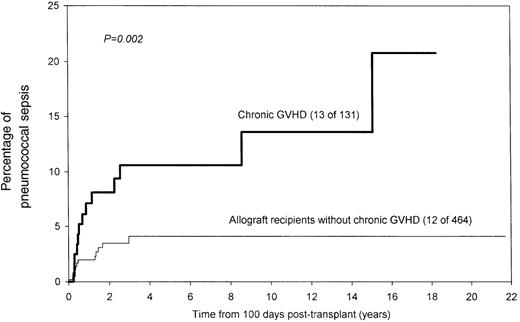
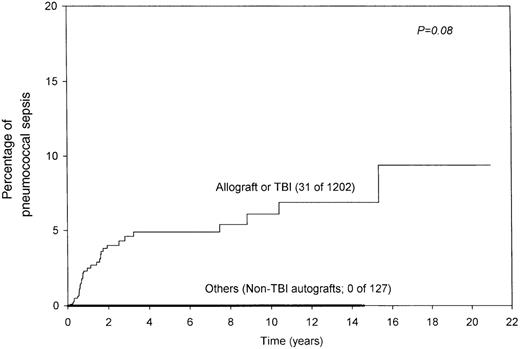
Age, sex, diagnosis, conditioning regimen, GVHD prophylaxis, or prior acute GVHD did not influence the risk for SPS. The risk was significantly higher in allograft recipients (7% vs 3% at 10 years;P = .03; Figure 2). Similarly, allograft recipients who had chronic GVHD were at higher risk (14% vs 4% for other allograft recipients at 10 years; P = .002; Figure 3). It was interesting that SPS was seen only in patients who had undergone allograft transplantation or received TBI (6% vs 0% at 10 years; P = .07; Figure4), even though this difference was not statistically significant. As stated earlier, it was difficult to ascertain prophylaxis compliance among patients who did not have SPS; therefore, the influence of prophylaxis on the incidence of SPS could not be assessed.
Significantly higher probability of pneumococcal infection among allograft recipients.
Significantly higher probability of pneumococcal infection among allograft recipients.
Significantly higher risk for pneumococcal sepsis among allograft recipients with chronic graft versus host disease.
Significantly higher risk for pneumococcal sepsis among allograft recipients with chronic graft versus host disease.
Combined effect of the type of transplant and TBI.
All instances of pneumococcal sepsis were seen in patients who had undergone allograft transplantation or TBI.
Combined effect of the type of transplant and TBI.
All instances of pneumococcal sepsis were seen in patients who had undergone allograft transplantation or TBI.
In multivariate analysis, the only factor independently associated with an increased risk for SPS was chronic GVHD (RR, 3.7;P = .001). The adverse influence of chronic GVHD persisted whether the whole group (all patients alive at day 100) or the allograft subpopulation (allograft recipients alive at day 100) were considered.
Discussion
The reported crude annual incidence of SPS in the general population varies between 1 and 100 per 100 000.33 In our series of BMT recipients, we identified 31 episodes in 1329 patients. Although a direct comparison with the general population is difficult, SPS seems to be a more noticeable problem in BMT recipients. Penicillin prophylaxis does not seem to be universally effective, but it may attenuate the severity of the infection and help prevent a fatal outcome.
The portal of entry for pneumococci is the respiratory tract, where the mucociliary lining facilitates their removal. Leukocyte margination and phagocytosis is important in containing the infection when the organism reaches the alveoli. Local appearance of antibody to capsular polysaccharide enhances phagocytosis and precedes the appearance of the antibody in the circulation. Failure of the local defense mechanisms allows spread of the organism to the hilar nodes, through the thoracic duct, and into the circulation, resulting in bacteremia. One or more of these mechanisms are defective in hematopoietic stem cell transplant recipients at some stage after transplantation, predisposing them to SPS.7-12 34
Hypogammaglobulinemia and the inability to react to polysaccharide antigens are common in allograft recipients, especially in those with chronic GVHD.7 These defects are less pronounced in autograft recipients. Almost all patients who have autografts retain protective levels of antipneumococcal antibodies after transplantation, whereas only 25% of those who have allografts do.11 Why then are autograft recipients also at risk for SPS? TBI-induced functional hyposplenism can contribute to defective opsonization and leukocyte margination. Consistent with this explantation, SPS was seen only in autograft recipients who had undergone TBI.
It is noteworthy that SPS developed in 8 patients while they were on regular penicillin prophylaxis. This raises the possibility of emerging penicillin resistance.33 Despite resistance, it is possible that penicillin may protect against fatal SPS, and it is still probably the agent of choice for prophylaxis. Patients in whom SPS develops despite penicillin prophylaxis ought to be switched to an alternative agent, such as a macrolide or a quinolone.
The mortality rate of community-acquired SPS in older patients is in the range of 20%. The 23% mortality rate seen by us is consistent and suggests that transplantation increases susceptibility without altering mortality. Mortality rates were significantly higher in patients with bacteremia than in those with other sites of infection. This is not surprising because hematogenous dissemination occurs when the infection is advanced and the defense mechanisms of the body have failed to confine the infection.
Pneumococcal vaccination in transplant recipients has been shown to be relatively ineffective early after transplantation and in the presence of chronic GVHD.9,35 Even in patients who do respond to the 23-valent vaccine, the IgG2 response is often poor.11,36The 23-valent polysaccharide vaccine, apart from being poorly immunogenic, also does not cover 20% of the commonly encountered pathogenic pneumococcal strains, and immunized persons remain susceptible to them.9 37
In conclusion, recipients of hematopoietic stem cell allografts, especially those with chronic GVHD, are at significant long-term risk for SPS and should receive lifelong penicillin prophylaxis. Autograft recipients who have undergone TBI also appear to be at risk and should receive lifelong prophylaxis. Prospective studies would help define the optimum duration of prophylaxis and would evaluate drugs other than penicillin, but they may have to be on a scale that is logistically difficult to manage. Autograft recipients (excluding patients with myeloma) who have not undergone TBI do not require lifelong prophylaxis.
Reprints:Ray Powles, Leukaemia and Myeloma Units, Royal Marsden Hospital, Downs Rd, Sutton, Surrey SM2 5PT, UK; e-mail:leukemia@clara.net.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal