Abstract
Evaluating high-dose therapy (HDT) with autologous stem cell transplantation (ASCT) in term of both duration and quality of life (QOL) presents major interests for patients with non-Hodgkin lymphoma. The quality-adjusted time without symptom and toxicity (Q-TWiST) methodology was applied to the LNH87-2 trial comparing HDT with ASCT versus sequential chemotherapy in 541 patients in first complete remission (CR). Overall survival (OS) and disease-free survival (DFS) curves were used to estimate duration of 4 health states: acute short-term toxicity (Tox1), secondary toxicity (Tox2), time without symptom and toxicity (TWiST), and relapse (Rel). Areas under survival curves (AUC) were retrospectively weighted according to QOL coefficients. HDT increased, but not significantly, TWiST (+2.4 months in AUC, P = .17) and decreased Rel (−3 months,P < .01). Survival estimates did not differ between the 2 treatments (AUC 47.7 months for OS, 39.7 months for DFS). High-risk patients treated by HDT versus chemotherapy had a significant benefit in DFS (AUC 28.8 versus 24.9 months, P < .01) but not in OS (AUC 37.3 versus 36 months, P = .27). Sensitivity analysis, performed by varying QOL coefficients, demonstrated significant quality-adjusted survival gain in high-risk patients treated by HDT. In low-risk patients, a diagram provided an aid to clinical decision-making. This analysis supports the use of HDT in these patients with adverse prognostic factors in the first CR, even after adjusting for QOL using the Q-TWiST method.
Aggressive non-Hodgkin lymphoma (NHL) is a chemosensitive malignancy that displays a steep dose-response curve. The disease is curable by first-line conventional chemotherapy in 50% to 60% of patients. The use of high-dose chemotherapy (HDT) and autologous stem cell transplantation (ASCT) for chemotherapy-sensitive relapsed aggressive NHL is now the gold standard.1,2 What is not clear is whether HDT should be withheld until the patient relapses or used up-front as part of first-line therapy, particularly for patients who have achieved remission. Three randomized studies have shown some benefit of HDT over sequential chemotherapy in patients in complete remission (CR).3-6
The importance of secondary end points, such as the impact of treatment on functional status and quality of life (QOL), has been recently recognized, particularly when alternative treatment options with similar potential for long-term survival become available. Autotransplantation treatment has an intense toxicity period but a short duration. On the other hand, chemotherapy is usually moderate, but the entire consolidative procedure generally takes longer. Therefore, QOL factors may contribute to the therapeutic decision between HDT or conventional chemotherapy. The methodologic challenges posed by the assessment of QOL are substantial. Although QOL research is progressing, most published studies of patients with ASCT are small, nonrandomized, or retrospective.7 In addition, comparisons among studies are complicated by differences in definitions and methods of QOL assessment. Ideally, detailed QOL assessment is made prospectively using standardized questionnaires, validated for transplantation trials. Given the relatively recent recognition of QOL as an important study end point, many mature clinical trials lack prospective information on QOL. Fortunately, techniques have been developed for retrospective analysis of the tradeoffs between quantity and quality of life in clinical trials. In particular, the Quality-adjusted Time Without Symptoms and Toxicity (Q-TWiST) method evaluates the risks and benefits of treatments by estimating the duration of health states that may affect QOL (eg, toxicity, disease relapse) and weighting these health state durations to arrive at a QOL-adjusted end point.
The Q-TWiST method was developed initially for comparing adjuvant chemotherapy regimens in solid tumors.8-11 It has also been useful in hematologic malignancies such as myeloma and NHL12,13 and was recently used in a transplantation trial in pediatric patients.14 To explore, in a large homogenous study population, the benefit of ASCT in high- and low- risk NHL patients, weighting the impact of treatment-related toxicity versus potentially improved survival, we performed a Q-TWiST analysis on the results of the Groupe d'Etude des Lymphomes de l'Adulte (GELA) LNH87-2 trial that compared ASCT with sequential chemotherapy for intermediate-grade and high-grade NHL in first CR.3 4
Patients and methods
Patients
LNH87-2 is a randomized, multicenter trial conducted in 35 European centers to compare ASCT versus chemotherapy for the treatment of aggressive NHL in adults. Details regarding study design and data management have been previously published.3 Brief descriptions of the patient population and treatment regimens are provided below.
Newly diagnosed patients, aged 16 to 55 years, with intermediate- or high-grade lymphoma, defined as group D to J in the Working Formulation, were eligible if they presented at least 1 of the following adverse factors: 2 or more extranodal sites, performance status of 2 to 4, maximal tumor burden of 10 cm or more, and bone marrow or central nervous system involvement. Patients with histologic progression of a previous low-grade lymphoma, those having positive serology for human immunodeficiency virus (HIV), or any contraindication for high-dose doxorubicin or cyclophosphamide were excluded. Initial staging procedures included bone marrow biopsy, cerebrospinal fluid examination, and computed tomography (CT) of the thorax and abdomen. Patients were staged according to the Ann Arbor classification. Performance status was assessed according to the Eastern Cooperative Oncology Group scale (ECOG). Serum lactate dehydrogenase (LDH) was expressed as a percentage of the maximal normal value. Patients were admitted to the trial between October 1, 1987, and February 28, 1993. Written informed consent was obtained from each patient before enrollment. A total of 916 patients were eligible, but the study population for this analysis consists of the 541 patients who achieved CR.
Treatment
The induction treatment consisted of 4 courses of chemotherapy with open randomization for the anthracycline: doxorubicin 75 mg/m2 (ACVBP arm) or mitoxantrone 12 mg/m2(NCVBP arm) day 1, cyclophosphamide 1200 mg/m2 day 1, vindesine 2 mg/m2 days 1 and 5, bleomycin 10 mg days 1 and 5, methylprednisolone 60 mg/m2 days 1 to 5, and IT methotrexate 15 mg. Courses were delivered every 2 weeks. Patients who achieved CR were subsequently randomized between HDT with CBV (cyclophosphamide 1500 mg/m2 day 7 to day 4, carmustine 300 mg/m2 day 4, and etoposide 250 mg/m2 day 7 to day 4) followed by ASCT (marrow infusion day 0) versus sequential consolidation regimen with 2 courses of high-dose methotrexate (3 g/m2) with leucovorin rescue, 2 courses of ifosfamide (1.5 g/m2 days 1 and 2) plus etoposide (300 mg/m2 days 1 and 2), and then asparaginase and cytarabine every 2 weeks for a total of 8 courses. After completion of therapy, patients had clinical examination and routine blood count every 3 months during the first 2 years, every 6 months during another 2 years, and annually thereafter. Thoracic and abdominal CT scans were performed at least annually during the first 4 years.
Q-TWiST method
The QOL-adjusted treatment comparison was performed using the Q-TWiST method, which compares treatments by defining relevant clinical health states and estimating their respective durations.15-18 The health state durations are then weighted according to patient utility, and the Q-TWiST end point is determined by the sum of the weighted health state durations. Four clinical health states were defined for this analysis:
• Tox1—the time period with acute, treatment-related symptomatic toxicities requiring hospitalization • Tox2—the time period with secondary toxicity requiring out patient treatment • TWiST—the time following toxicity but before relapse; and period representing the best possible QOL after the treatment of NHL, during which patients experience no toxicities of treatment or symptoms of disease • Rel—all time following disease relapse until death (regardless of whether the patient recovers from the relapse)
The TWiST health state is assumed to represent the best possible QOL for a patient with NHL because it is not associated with either treatment toxicity or disease relapse.
Detailed data regarding the duration of toxicity after transplantation was not available for individual patients. Therefore, following a methodology similar to that used by Parsons et al,14 we constructed a surrogate for the toxicity period as follows: the total duration of toxicity (Tox = Tox1 + Tox2) was assumed to be 100 days to reflect the usual period of peritransplant complications. The durations of Tox1 and Tox2 were assumed to be 30 days and 70 days, respectively. The 100-day period was also selected to incorporate the total consolidation chemotherapy duration (14 weeks).
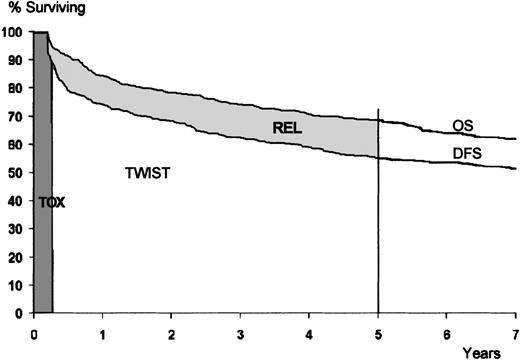
The mean health state durations were obtained by first plotting survival curves, up to the median follow-up duration, for disease-free survival (DFS) and overall survival (OS) on the same graph according to treatment group. The mean duration of TWiST for each treatment group was computed as the area under the DFS curve, less the assumed duration of toxicity of 100 days. The mean duration of Rel for each treatment group was computed as the area between the survival curves for DFS and OS (Figure 2).
The mean durations of the clinical health states were weighted using utility coefficients denoted by uTox1,uTox2 and uRel, to reflect the average value (relative to TWiST) of time in the health states Tox1, Tox2 and Rel, respectively. Each utility coefficient is a scale from 0 to 1, where 0 represents QOL as bad as death, 1 represents QOL as good as TWiST, and values between 0 and 1 represent degrees between these extremes.
The Q-TWiST end point was calculated as Q-TWiST = uTox1 × Tox1 +uTox2 × Tox2 + TWiST +uRel × Rel where Tox1, Tox2, TWiST, and Rel represent the respective mean clinical health state durations in months. Treatment comparisons were made by subtracting the mean Q-TWiST for the chemotherapy group from the mean Q-TWiST for the transplantation group, and these differences were compared using a Z test.
To investigate how prognostic factors, based on the International Prognostic Index (IPI),19 affect the treatment comparison, we used a parametric regression model for Q-TWiST.20 A brief technical description is given in the . Risk groups were defined according to the number of significant adverse factors. Using this model, we obtained predictions of the Q-TWiST treatment effect for various risk factor profiles.
Sensitivity analysis of utility
Because the LNH87-2 study did not collect QOL objective data, patient assessments of the utility coefficients are not available. In their absence, we performed a sensitivity analysis in which the Q-TWiST treatment effect was evaluated for all possible combinations of utility weight values. The results are presented graphically in a 2-dimensional plot that shows, as the utility coefficient values vary, the magnitude of the Q-TWiST treatment effect as well as whether the effect is statistically significant. This graph provides an aid to clinical decision-making by illustrating the QOL-adjusted treatment effect according to varying values of the utility coefficients. We also performed sensitivity analysis to assess the stability of the results when different Tox1 and Tox2 durations (ranging from 2-6 weeks and 2-4 months, respectively) were used for HDT.
All statistical analyses were computed with SAS 6.12 for PC (SAS Institute, Cary, NC).
Results
Univariate analysis
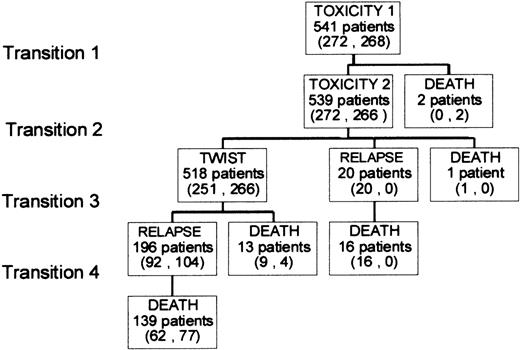
The study included 312 men and 229 women, with a median age of 40. Clinical characteristics are presented in Table1. Figure 1illustrates the possible health state transitions as well as the number of patients in each treatment group making each transition. Three toxic deaths were reported; 2 patients died of transplant-related complications and 1 of thromboembolic disease following asparaginase treatment. Among the 13 patients who died without relapsing, the cause of death was known in 9; 2 second cancers, 3 cardiac events, 2 infections, and 2 accidents. Figure 2 shows survival curves drawn out to the median follow-up of 5 years for the entire patient sample. The mean durations of Tox, TWiST and Rel were computed as areas under the curves (AUC). They were, respectively, 3.3 months, 36.6 months, and 7.8 months. ASCT increased, but not significantly, the mean time in TWiST (AUC 39.7 versus 37.3 months,P = .17) but decreased the time in Rel (6.4 versus 9.4 months, P < .01). The survival estimates did not differ significantly between the 2 consolidation treatments either in OS (47.7 versus 47.8 months, P = .8) or in DFS (41 versus 38.4 months,P = .17).
Clinical characteristics of the study population
| Characteristics . | Frequency n = 541 . | 5 y OS . | 5 y DFS . |
|---|---|---|---|
| LDH | |||
| ≤ Normal | 43% | 73 ± 6% | 61 ± 6% |
| > Normal | 57% | 64 ± 5%* | 56 ± 6%* |
| Stage | |||
| Localized (I-II) | 39% | 76 ± 6% | 66 ± 6% |
| Disseminated (III-IV) | 61% | 63 ± 5%* | 51 ± 6%* |
| Performance status | |||
| 0-1 | 80% | 70 ± 5% | 59 ± 6% |
| 2-4 | 20% | 62 ± 8%† | 53 ± 9% |
| IPI Score | |||
| 0 | 15% | 84 ± 8% | 76 ± 10% |
| 1 | 40% | 70 ± 7% | 58 ± 7% |
| 2 | 35% | 61 ± 8% | 55 ± 8% |
| 3 | 10% | 58 ± 14%* | 47 ± 14%* |
| Consolidative treatment | |||
| Sequential chemotherapy | 50.5% | 67 ± 6% | 54 ± 7% |
| HDT with ASCT | 49.5% | 69 ± 6% | 62 ± 6% |
| Characteristics . | Frequency n = 541 . | 5 y OS . | 5 y DFS . |
|---|---|---|---|
| LDH | |||
| ≤ Normal | 43% | 73 ± 6% | 61 ± 6% |
| > Normal | 57% | 64 ± 5%* | 56 ± 6%* |
| Stage | |||
| Localized (I-II) | 39% | 76 ± 6% | 66 ± 6% |
| Disseminated (III-IV) | 61% | 63 ± 5%* | 51 ± 6%* |
| Performance status | |||
| 0-1 | 80% | 70 ± 5% | 59 ± 6% |
| 2-4 | 20% | 62 ± 8%† | 53 ± 9% |
| IPI Score | |||
| 0 | 15% | 84 ± 8% | 76 ± 10% |
| 1 | 40% | 70 ± 7% | 58 ± 7% |
| 2 | 35% | 61 ± 8% | 55 ± 8% |
| 3 | 10% | 58 ± 14%* | 47 ± 14%* |
| Consolidative treatment | |||
| Sequential chemotherapy | 50.5% | 67 ± 6% | 54 ± 7% |
| HDT with ASCT | 49.5% | 69 ± 6% | 62 ± 6% |
Log-rank test:
P < .01;
P < .05.
ASCT indicates autologous stem cell transplantation; DFS, disease-free survival; HDT, high-dose therapy; IPI, International Prognostic Index; LDH, lactate dehydrogenase; OS, overall survival.
Transitions between health states.
The number of patients making each transition is given in parentheses, first for chemotherapy, second for ASCT.
Transitions between health states.
The number of patients making each transition is given in parentheses, first for chemotherapy, second for ASCT.
Survival curves for the study population.
Areas under the curves correspond to the mean duration of Tox (darkly shaded area), TWiST (white area), and Rel (lightly shaded area). Vertical line indicates the median follow-up.
Survival curves for the study population.
Areas under the curves correspond to the mean duration of Tox (darkly shaded area), TWiST (white area), and Rel (lightly shaded area). Vertical line indicates the median follow-up.
Multivariate analysis
To investigate how prognostic factors affect the treatment comparison, we performed a multivariate regression analysis for Q-TWiST. Its other interest was to examine separately prognostic factors for competing events such as relapse or death. The predictive value of clinical characteristics used by the age-adjusted IPI was investigated for each transition between health states. Table2 shows relative risk estimates. After the treatment period, performance status evaluated at diagnosis had no independent predictive value. By contrast, factors reflecting tumor invasive potential at diagnosis, such as disseminated stage or elevated LDH, remained predictive for relapse (relative risk respectively 2.44, IC 95% [1.25; 4.74] and 1.77, IC 95% [1.15; 2.72]). After relapse, first-line consolidative high-dose therapy was isolated as independent adverse prognostic factor (relative risk 1.40 IC 95% [1.08; 1.81]). This was partially due to an inability to tolerate intensive salvage regimens for patients receiving HDT as first-line treatment. Among the 112 patients who relapsed after consolidation chemotherapy, 48 (42%) were treated by HDT in second-line therapy, whereas only 24 (23%) of 104 who relapsed in the high-dose group could benefit from a second HDT. No statistically significant interaction was found between treatment effects and IPI factors.
Relative risk estimates for transition between health states
| . | Tox → Rel . | TWiST → Rel . | TWiST → Death . | Rel → Death . |
|---|---|---|---|---|
| ASCT vs Chemo | 0.65 | 0.94 | 0.8 | 1.37* |
| LDH > N vs ≤N | 1.85* | 1.77* | 0.75 | 1.40* |
| Stage III-IV vs I-II | 1.36 | 2.44* | 1.1 | 1.15 |
| PS 2-4 vs 0-1 | 0.7 | 1.2 | 0.82 | 1.17 |
| . | Tox → Rel . | TWiST → Rel . | TWiST → Death . | Rel → Death . |
|---|---|---|---|---|
| ASCT vs Chemo | 0.65 | 0.94 | 0.8 | 1.37* |
| LDH > N vs ≤N | 1.85* | 1.77* | 0.75 | 1.40* |
| Stage III-IV vs I-II | 1.36 | 2.44* | 1.1 | 1.15 |
| PS 2-4 vs 0-1 | 0.7 | 1.2 | 0.82 | 1.17 |
Likelihood ratio test, P < .05.
ASCT indicates autologous stem cell transplantation; LDH, lactate dehydrogenase; PS, performance status; Rel, relapse period; Tox, toxicity period; TWiST, time without symptom and toxicity (TWiST).
Because ECOG had no independent prognostic value in the study population, the low-risk group was defined by the conjunction of LDH less than N and localized stage. On the other hand, the high-risk group was defined by LDH more than N and disseminated stage. Intermediate-risk patients had only 1 adverse factor. Table3 shows the health state durations, within the median follow-up time, according to prognostic profile and treatment group. HDT systematically increased the time in TWiST but decreased the time in Rel. The OS did not differ significantly between the 2 consolidation treatments. However, in high-risk patients, HDT had a significant DFS gain (28.8 versus 24.9 months, P < .01).
Mean times spent in each health state according to treatment group
| . | ASCT . | Chemotherapy . | Difference . |
|---|---|---|---|
| Low-risk patients | |||
| TWiST | 54.5 (1.7) | 53.4 (1.7) | 1.1 (1.7) |
| Rel | 3.6 (0.3) | 5.2 (0.5) | −1.6 (0.4)3-150 |
| Overall Survival | 61.4 (1.7) | 62.2 (1.9) | −0.8 (1.9) |
| High-risk patients | |||
| TWiST | 25.5 (1.3) | 21.6 (1.5) | 3.9 (1.5)3-150 |
| Rel | 8.5 (0.8) | 11.1 (1) | −2.6 (0.9)3-150 |
| Overall survival | 37.3 (1.5) | 36 (1.4) | 1.3 (1.4) |
| . | ASCT . | Chemotherapy . | Difference . |
|---|---|---|---|
| Low-risk patients | |||
| TWiST | 54.5 (1.7) | 53.4 (1.7) | 1.1 (1.7) |
| Rel | 3.6 (0.3) | 5.2 (0.5) | −1.6 (0.4)3-150 |
| Overall Survival | 61.4 (1.7) | 62.2 (1.9) | −0.8 (1.9) |
| High-risk patients | |||
| TWiST | 25.5 (1.3) | 21.6 (1.5) | 3.9 (1.5)3-150 |
| Rel | 8.5 (0.8) | 11.1 (1) | −2.6 (0.9)3-150 |
| Overall survival | 37.3 (1.5) | 36 (1.4) | 1.3 (1.4) |
Z test P < .05.
LDH indicates lactate dehydrogenase; Rel, relapse period; TWiST, time without symptom and toxicity. Mean times are in months, with SE in parentheses.
Sensitivity analysis
To focus on tradeoff between quantity and quality of life, each health state duration was weighted by a coefficient measuring QOL and utility analysis was performed by varying QOL coefficient values.
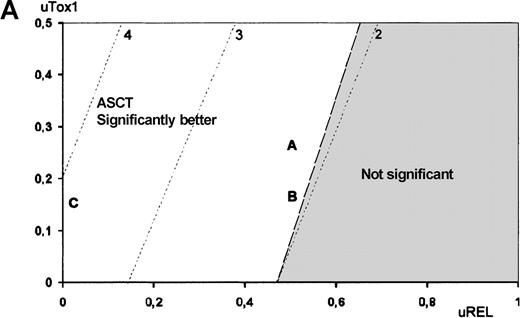
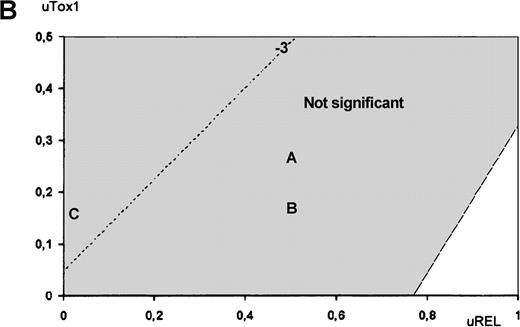
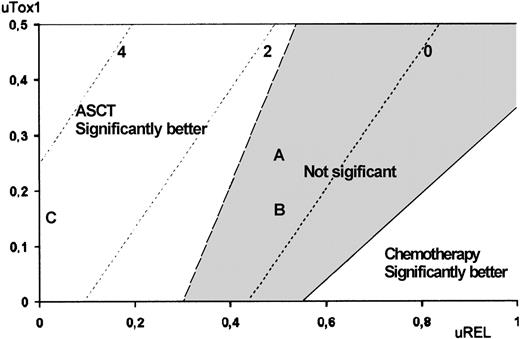
The utility analysis applied to high-risk patients revealed that ASCT was preferred over chemotherapy for all possible utility values, although this difference was not statistically significant for any of the utility value combinations. In contrast, treatment comparison among low-risk patients revealed that chemotherapy was preferred over ASCT. To illustrate the impact of QOL on treatment choice, Figures3 and 4 show Q-TWiST estimates according to various coefficients sets. In set A, ASCT QOL during Tox1 was assumed 2 times worse than chemotherapy. In set B, ASCT QOL during Tox1 was assumed 4 times worse than chemotherapy. In set C, lifetime after relapse was not taken into account (uRel = 0, eg DFS). For high- and low-risk patients (Figure 3A and 3B, respectively), the preferred therapy did not vary (ASCT and chemotherapy, respectively). But, in case of intermediate-risk patients, Figure 4 provides an aid to clinical decision-making. The physician may graphically evaluate tradeoff between QOL and survival. Then, optimal treatment may be proposed with respect to individual preferences regarding toxicity and disease relapse. When considering the coefficients sets A or B, ASCT showed no significant benefit. Conversely, if QOL during Rel was not taken into account (set C), then ASCT presented significant benefit.
Utility analysis.
Utility analysis is shown for high-risk (A) and low-risk (B) patients, assuming that QOL during Tox1 was better for chemotherapy than for ASCT (uTox1 for ASCT ranging from 0 to 0.5) and that QOL during Tox2 was better for ASCT than for chemotherapy (uTox2 respectively set to 0.75 and 0.5). Dashed lines indicate same amount of Q-TWiST (eg, line 2 indicates ASCT provides 2 more months than chemotherapy). Letters refer to examples given in text.
Utility analysis.
Utility analysis is shown for high-risk (A) and low-risk (B) patients, assuming that QOL during Tox1 was better for chemotherapy than for ASCT (uTox1 for ASCT ranging from 0 to 0.5) and that QOL during Tox2 was better for ASCT than for chemotherapy (uTox2 respectively set to 0.75 and 0.5). Dashed lines indicate same amount of Q-TWiST (eg, line 2 indicates ASCT provides 2 more months than chemotherapy). Letters refer to examples given in text.
Utility analysis.
Utility analysis is shown for intermediate-risk patients, assuming that QOL during Tox1 was better for chemotherapy than for ASCT (uTox1 for ASCT ranging from 0 to 0.5) and that QOL during Tox2 was better for ASCT than for chemotherapy (uTox2 respectively set to 0.75 and 0.5). Dashed lines indicate same amount of Q-TWiST (eg, line −3 indicates ASCT provides 3 fewer months than chemotherapy). Letters refer to examples given in text.
Utility analysis.
Utility analysis is shown for intermediate-risk patients, assuming that QOL during Tox1 was better for chemotherapy than for ASCT (uTox1 for ASCT ranging from 0 to 0.5) and that QOL during Tox2 was better for ASCT than for chemotherapy (uTox2 respectively set to 0.75 and 0.5). Dashed lines indicate same amount of Q-TWiST (eg, line −3 indicates ASCT provides 3 fewer months than chemotherapy). Letters refer to examples given in text.
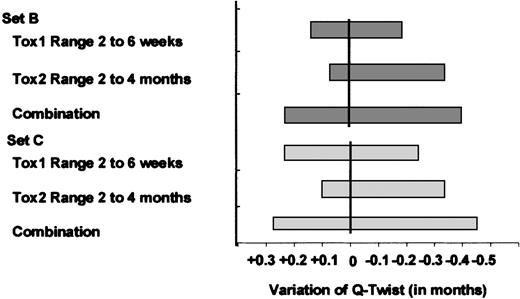
Lastly, because our surrogate for the toxicity period was set at 100 days, we had to assess the stability of our results when different Tox1 and Tox2 durations were used. We reiterated the analysis with Tox1 and Tox2 ranging, respectively, from 2 to 6 weeks and from 2 to 4 months for ASCT. Conclusions were insensitive to changes in toxicity duration. Figure 5 shows the effect of Tox1 and Tox2 variations in the case of intermediate-risk patients for coefficient sets B and C. The amount of variation of Q-TWiST ranged between −0.45 and +0.25 months but did not reach significance.
Effect of variation in toxicity duration for ASCT in intermediate-risk patients.
The vertical lines figure the initial Tox1 = 30 days and Tox2 = 70 days. The gray bars figure effects of Tox1 and Tox2 variation on amount of Q-TWiST (in months).
Effect of variation in toxicity duration for ASCT in intermediate-risk patients.
The vertical lines figure the initial Tox1 = 30 days and Tox2 = 70 days. The gray bars figure effects of Tox1 and Tox2 variation on amount of Q-TWiST (in months).
Discussion
In the absence of a large international randomized trial with uniform inclusion criteria, the benefit of ASCT in aggressive NHL has to be assessed on the basis of a few trials. It seems unlikely, at present, that ASCT will be appropriate for indiscriminate application to all patients in first CR. However, QOL studies might offer additional information. The difficulty is then to identify a group of patients with a poor prognosis who could benefit from high-dose consolidation regimens. Although it lacks biologic markers, the IPI permits a standardized approach. Previous univariate subset analyses of the LNH87-2 trial have demonstrated the benefit of ASCT over sequential chemotherapy in high and high-intermediate (IPI score 2-3) patients.4 The present multivariate analysis clarified these results investigating separately prognostic factors for competing events during the disease course. Among the IPI factors, only stage III to IV and elevated LDH were retained as independent prognostic factors for relapse. This agrees with the point that performance status at diagnosis is a marker of a patient's response to the tumor but not of the invasive potential of the tumor, and so, has no longer predictive value after the treatment period.3,21 More interesting was the negative impact of first-line ASCT treatment on survival after relapse. Compared with consolidation chemotherapy, ASCT diminished the mean time spent in relapse. It might be explained by the difficulties to tolerate intensive salvage therapy. Haioun et al4 have previously found a marginally significant quantitative interaction between treatment effect on OS and IPI score, suggesting that, for low-risk patients, the benefit of ASCT was smaller than for high-risk patients. In fact, autografting in first CR jeopardized their outcome after the relapse, if even it occurred.
The QOL-adjusted treatment comparison was based on generalization of the Q-TWiST method originally developed to evaluate adjuvant therapies for breast cancer and later extended to therapies for HIV and various solid tumors.8-11 The method is appropriate when the disease course has well-defined health states, indicative of QOL valuation. It provides logistical interests because computations are based on standard survival curves and do not require QOL questionnaires. However, it should be noted that Q-TWiST estimates are expressed on a QOL weighted time scale, with weighting coefficients less than 1, and so appear lower than on a real-time scale. There was only 1 previous Q-TWiST application to ASCT clinical issues in pediatric hematology by Parsons et al.14 But this study did not consider prognostic factors. Our fully parametric Q-TWiST approach overcame this limitation providing predictions of the Q-TWiST treatment effect for various risk factor profiles.
The more complex problem was to determine an appropriate quality function for transplantation issues. Following a methodology similar to that used by Parsons et al,14 we have to use a surrogate for individual Tox duration after completion of therapy. With the usual 100-day cutoff point, we focused on short-term toxicities because of their likely negative impact on QOL. But, in ASCT trials, the impact of long-term toxicity has to be discussed. Infertility, endocrinologic disturbances, cataracts, and second cancers are commonly associated with bone marrow transplantation.22-24 Psychosocial factors have also to be considered with special attention for those heavily treated patients.25,26 However, Chao et al27have shown that, globally, during the first year after autotransplantation, 90% of surviving patients reported an above average to excellent QOL. Moreover, in a recent study, Duell et al22 have reported that more than 90% of long-term survivors of allogeneic transplantation are in good health and had returned to full-time work. On the other hand, standard-dose chemotherapy has also shown late sequelae.28 In the work by Hjermstad et al,29 only cognitive function at 1 year was different between patients receiving ASCT and chemotherapy. We share the point that it may diminish QOL and that our approach was not sensitive enough to discriminate. But the aim of our study was to evaluate the tradeoff between quantity and quality of life in the LNH87-2 trial. For such analysis, the impact of long-term fatigue seems negligible when compared with relapse of the underlying malignancy or second tumor. In our study population, we only found 1 secondary leukemia but, among the other 12 deaths in patients in CR, cardiac events and infection cannot be definitively ruled out. So, in absence of prospective comparative data with long-term follow-up, our restriction of the toxicity period to 100 days seems to be a good clinical cutoff point. In addition, sensitivity analyses showed no change in conclusion when Tox1 and Tox2 duration for HDT varied.
In future studies, our approach will be easy to adapt. Tox1 should estimate acute grade III to IV toxicities, and Tox2 chronic low-grade toxicities. In addition, because most of the QOL questionnaire may be reduced to a single score, mean QOL patterns may further be combined with survival curves to improve Q-TWiST sensitivity.18
This study demonstrated that aggressive NHL patients in first remission might benefit from HDT with ASCT, even after adjusting for QOL. As in other Q-TWiST analyses, the gain seems small but mean Q-TWiST difference of 2 or 3 months on a QOL-weighted time scale, aimed at a large population, shows an important intervention. Between sequential chemotherapy and high-dose regimen, the best consolidation treatment was influenced by stage and LDH level as well as the relative valuation placed on treatment toxicity and relapse times. In the LNH87-2, our Q-TWiST analysis supports the use of ASCT for higher risk patients over a wide range of QOL coefficient values. Although we did not observe significant long-term toxicity of treatment in both arms, a longer follow-up of the study is necessary to rule out imbalances in late events. Future research may permit quality-adjusted survival comparisons in transplantation fields, especially by integrating direct assessment of long-term toxicity into traditional efficacy trials.
Parametric Q-TWiST method20
The first step was to fit a model of the transitions between health states, with each transition governed by competing risk and survival given by the sum of the time spent in each health state. The events studied were end of treatment toxicity, disease recurrence, and overall survival. All the patients were assumed to initially present toxic effects of treatment. As in other Q-TWiST analyses, the patients were assumed to progress forward through the health states but not backward. If a state was skipped, its duration was set at zero. If a transition time was censored, all the subsequent transition times were similarly censored. So, from each of the 541 patients, the amount of time spent in Tox, TWiST, and Rel was computed.
Then, the health state durations were analyzed using separate accelerated failure time models. Transition from TWiST to Rel had a hazard function that corresponded to a Weibull distribution. Transition from Tox to Rel and from Rel to death had an exponential distribution. To have a sufficient sample size, we assumed that the hazard for moving from Rel to death was the same for both the second and the third transition. For each state, the duration was modeled parametrically and the covariates were included in the model to adjust for the next state entry time. The transition from TWiST to Rel was modeled with a Weibull hazard function to account for the particular pattern of aggressive NHL where relapses happen early on and then level off, late death being attributed to a return to the underlying force of mortality of the population at large. Other transitions were modeled with an exponential hazard function. In addition to the factors used by the IPI,19 the treatment group was also included as covariable. The model parameters were estimated by maximum likelihood.
Lastly, model parameters were used in a simulation program to estimate the amount of time spent in each state for various covariate profiles. Independent random deviates were generated for each competing risk and gave the latent transition time for each possible health state. Minimum was taken and the process iterated until death was selected at one of the transitions. Mean times were computed on the simulated data (n = 500). Standard errors were obtained using 1000 bootstrap samples.30
Supported by grants from the Ligue Nationale contre le Cancer, the Fond d'Etudes et de Recherche du Corps Médical des Hôpitaux de Paris and the Direction de la Recherche Clinique de l'Assistance Publique, Hôpitaux de Paris (AP-HP), France.
Reprints:Eric Lepage, Département d'information hospitalier, CHU Henri Mondor 51, avenue du Maréchal De Lattre de Tassigny, 94010 Créteil, France; e-mail:eric.lepage@hmn.ap-hop-paris.fr.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal